Sleep Loss, Daytime Sleepiness, and Neurobehavioral Performance among Adolescents: A Field Study
Abstract
1. Introduction
2. Results
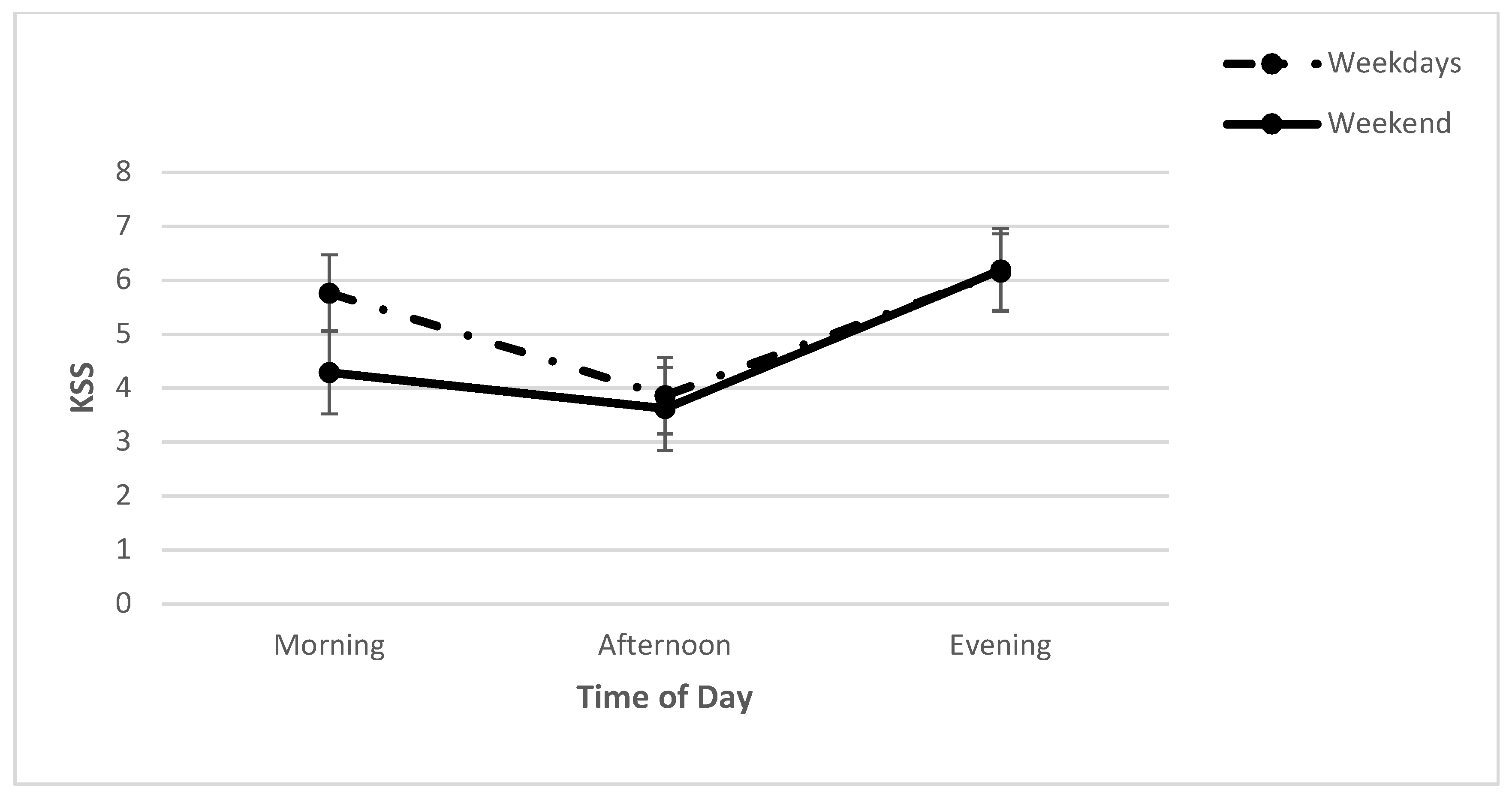
2.1. Subjective Sleepiness: KSS
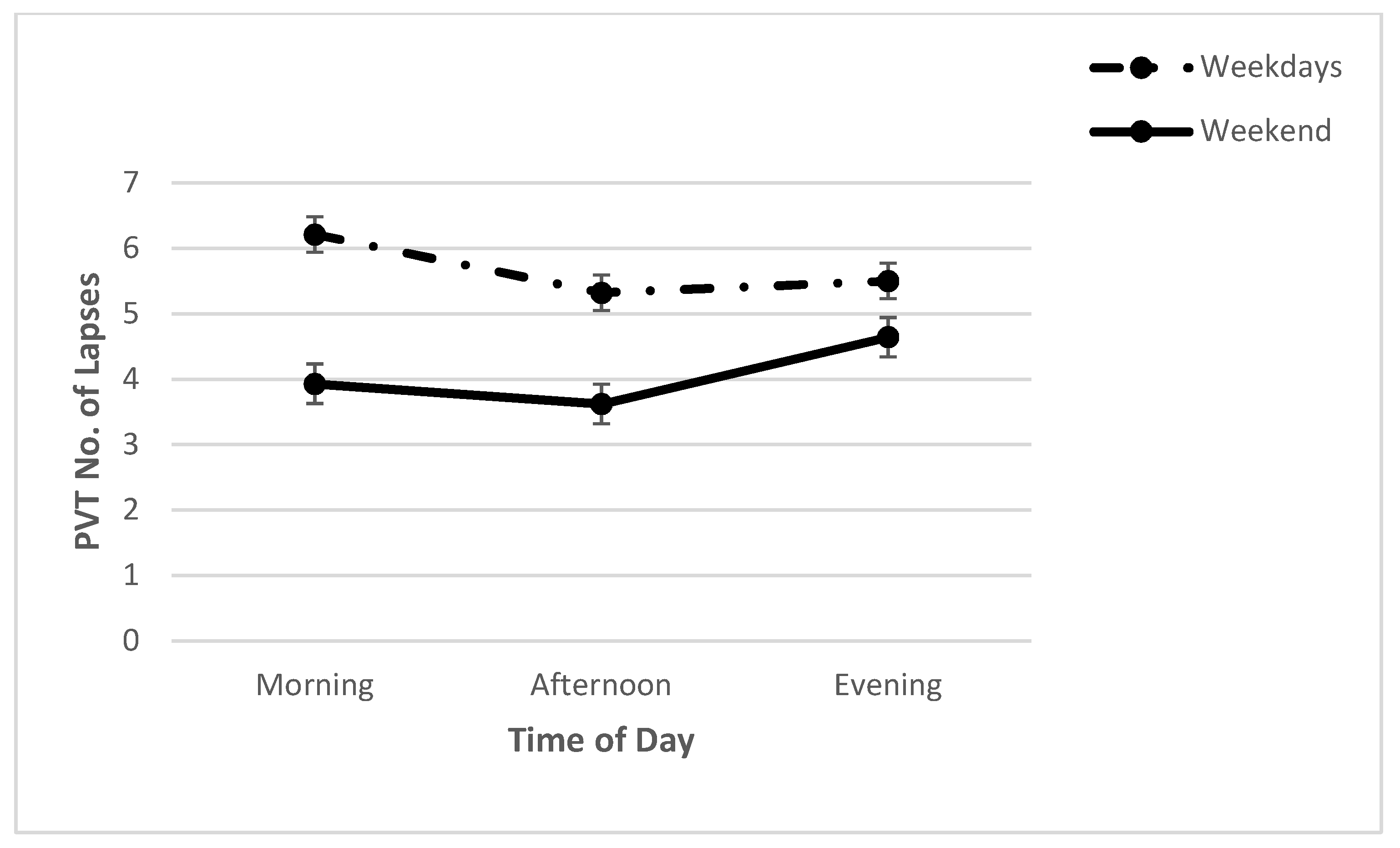
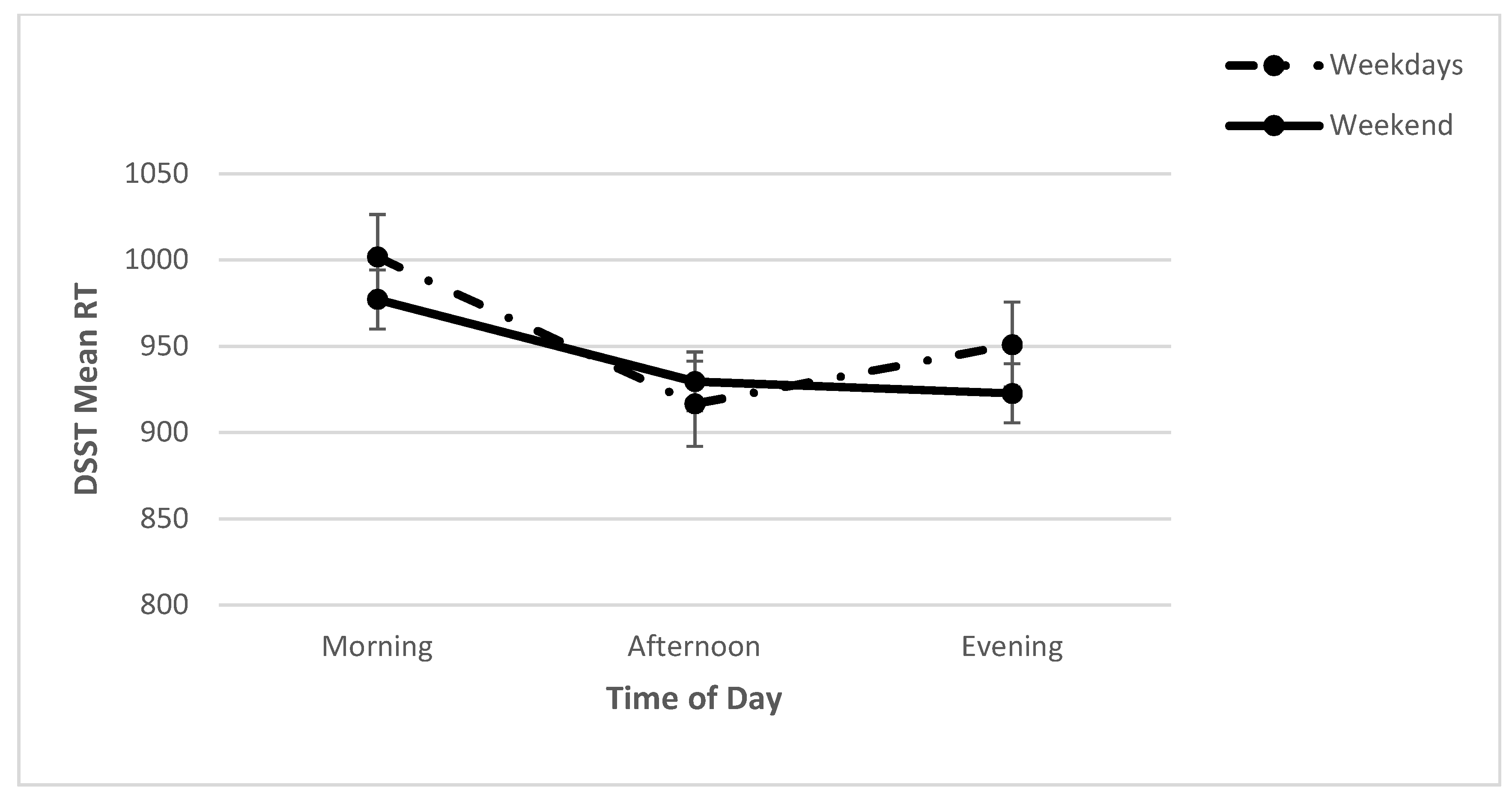
2.2. Cognitive Performance: PVT and DSST
3. Discussion
4. Material and Methods
4.1. Participants
4.2. Materials
4.2.1. Cognitive Performance
Psychomotor Vigilance Test (PVT)
Digit Symbol Substitution Test (DSST)
4.3. Sleep Measures
4.3.1. Sleep Patterns
4.3.2. The Modified School Sleep Habits Survey (SSHS)
4.3.3. Karolinska Sleepiness Scale (KSS)
4.4. Procedure
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, R.M. Adolescent maturational changes and psychosocial development: A dynamic interactional perspective. J. Youth Adolesc. 1985, 14, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Paus, T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005, 9, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, M.; D’Atri, A.; Scarpelli, S.; Reda, F.; De Gennaro, L. Sleep electroencephalography and brain maturation: Developmental trajectories and the relation with cognitive functioning. Sleep Med. 2020, 66, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.E.; Lewin, D.S. Pathways to adolescent health sleep regulation and behavior. J. Adolesc. Health 2002, 31, 175–184. [Google Scholar] [CrossRef]
- Gradisar, M.; Gardner, G.; Dohnt, H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Med. 2011, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Rao, H.; Durmer, J.; Dinges, D. Neurocognitive Consequences of Sleep Deprivation. Semin. Neurol. 2009, 29, 320–339. [Google Scholar] [CrossRef]
- Franzen, P.L.; Siegle, G.J.; Buysse, D.J. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J. Sleep Res. 2008, 17, 34–41. [Google Scholar] [CrossRef]
- Goldstein, A.N.; Walker, M.P. The Role of Sleep in Emotional Brain Function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef]
- Fredriksen, K.; Rhodes, J.; Reddy, R.; Way, N. Sleepless in Chicago: Tracking the effects of adolescent sleep loss during the middle school years. Child Dev. 2004, 75, 84–95. [Google Scholar] [CrossRef]
- Yeo, S.C.; Jos, A.M.; Erwin, C.; Lee, S.M.; Lee, X.K.; Lo, J.C.; Chee, M.; Gooley, J.J. Associations of sleep duration on school nights with self-rated health, overweight, and depression symptoms in adolescents: Problems and possible solutions. Sleep Med. 2019, 60, 96–108. [Google Scholar] [CrossRef]
- Baglioni, C.; Spiegelhalder, K.; Lombardo, C.; Riemann, D. Sleep and emotions: A focus on insomnia. Sleep Med. Rev. 2010, 14, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Shochat, T.; Cohen-Zion, M.; Tzischinsky, O. Functional consequences of inadequate sleep in adolescents: A systematic review. Sleep Med. Rev. 2014, 18, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Hussain, S.; Hubert Lam, K.B.; Lily Yao, G.; Neil Thomas, G.; Taheri, S. Exploring the complex pathways among specific types of technology, self-reported sleep duration and body mass index in UK adolescents. Int. J. Obes. 2013, 37, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Y.; Doi, S.; Mamun, A.A. Longitudinal impact of sleep on overweight and obesity in children and adolescents: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2015, 16, 137–149. [Google Scholar] [CrossRef]
- de Bruin, E.J.; van Run, C.; Staaks, J.; Meijer, A.M. Effects of sleep manipulation on cognitive functioning of adolescents: A systematic review. Sleep Med. Rev. 2017, 32, 45–57. [Google Scholar] [CrossRef]
- Beebe, D.W.; Fallone, G.; Godiwala, N.; Flanigan, M.; Martin, D.; Schaffner, L.; Amin, R. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J. Child Psychol. Psychiatry 2008, 49, 915–923. [Google Scholar] [CrossRef]
- Lin, L.; Somerville, G.; Boursier, J.; Santisteban, J.A.; Gruber, R. Sleep Duration Is Associated with Academic Achievement of Adolescent Girls in Mathematics. Nat. Sci. Sleep 2020, 12, 173–182. [Google Scholar] [CrossRef]
- Short, M.A.; Blunden, S.; Rigney, G.; Matricciani, L.; Coussens, S.; Reynolds, C.M.; Galland, B. Cognition and objectively measured sleep duration in children: A systematic review and meta-analysis. Sleep Health 2018, 4, 292–300. [Google Scholar] [CrossRef]
- Wolfson, A.R.; Carskadon, M.A. Understanding adolescent’s sleep patterns and school performance: A critical appraisal. Sleep Med. Rev. 2003, 7, 491–506. [Google Scholar] [CrossRef]
- Cohen-Zion, M.; Shabi, A.; Levy, S.; Glasner, L.; Wiener, A. Effects of Partial Sleep Deprivation on Information Processing Speed in Adolescence. J. Int. Neuropsychol. Soc. 2016, 22, 388–398. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Harvey, K.; Dement, W.C. Sleep Loss in Young Adolescents. Sleep 1981, 4, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Horne, J.A. Sleepiness Enhances Distraction During a Monotonous Task. Sleep 2006, 29, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Dinges, D.F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010, 136, 375–389. [Google Scholar] [CrossRef]
- Unsworth, N.; Robison, M.K. Working memory capacity and sustained attention: A cognitive-energetic perspective. J. Exp. Psychol. Learn. Mem. Cogn. 2020, 46, 77–103. [Google Scholar] [CrossRef]
- Dinges, D.F.; Mallis, M.M. Managing fatigue by drowsiness detection: Can technological promises be realized? In Managing Fatigue in Transportation; Hartley, L., Ed.; Pergamon: Oxford, UK, 1998; pp. 209–229. [Google Scholar]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The Cumulative Cost of Additional Wakefulness: Dose-Response Effects on Neurobehavioral Functions and Sleep Physiology from Chronic Sleep Restriction and Total Sleep Deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Doran, S.M.; Van Dongen, H.P.; Dinges, D.F. Sustained attention performance during sleep deprivation: Evidence of state instability. Arch. Ital. Biol. 2001, 139, 253–267. [Google Scholar]
- Tucker, A.M.; Whitney, P.; Belenky, G.; Hinson, J.M.; Van Dongen, H.P. Effects of Sleep Deprivation on Dissociated Components of Executive Functioning. Sleep 2010, 33, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Knowles, E.M.E.; Weiser, M.; David, A.; Glahn, D.C.; Davidson, M.; Reichenberg, A. The Puzzle of Processing Speed, Memory, and Executive Function Impairments in Schizophrenia: Fitting the Pieces Together. Biol. Psychiatry 2015, 78, 786–793. [Google Scholar] [CrossRef]
- Louca, M.; Short, M.A. The Effect of One Night’s Sleep Deprivation on Adolescent Neurobehavioral Performance. Sleep 2014, 37, 1799–1807. [Google Scholar] [CrossRef]
- Wu, L.J.; Acebo, C.; Seifer, R.; Carskadon, M.A. Sleepiness and Cognitive Performance among Younger and Older Adolescents across a 28-Hour Forced Desynchrony Protocol. Sleep 2015, 38, 1965–1972. [Google Scholar] [CrossRef][Green Version]
- Merdad, R.A.; Akil, H.; Wali, S.O. Sleepiness in Adolescents. Sleep Med. Clin. 2017, 12, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.S.; Powles, A.C.P.; Thabane, L.; O’Brien, S.; Molnar, D.S.; Trajanovic, N.; Ogilvie, R.; Shapiro, C.; Yan, M.; Chilcott-Tanser, L. “Sleepiness” is serious in adolescence: Two surveys of 3235 Canadian students. BMC Public Health 2006, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Boergers, J.; Gable, C.J.; Owens, J.A. Later School Start Time Is Associated with Improved Sleep and Daytime Functioning in Adolescents. J. Dev. Behav. Pediatr. 2014, 35, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Belon, K.; Moss, P. Impact of Delaying School Start Time on Adolescent Sleep, Mood, and Behavior. Arch. Pediatr. Adolesc. Med. 2010, 164, 608–614. [Google Scholar] [CrossRef]
- Lufi, D.; Tzischinsky, O.; Hadar, S. Delaying School Starting Time by One Hour: Some Effects on Attention Levels in Adolescents. J. Clin. Sleep Med. 2011, 7, 137–143. [Google Scholar] [CrossRef]
- Lowe, C.J.; Safati, A.; Hall, P. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef]
- Beebe, D.W.; Rose, D.; Amin, R. Adolescent health brief: Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J. Adolesc. Health 2010, 47, 523–525. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Jalilolghadr, S.; Hashemi, H.J.; Hashemi, F.; Nozari, H.; Yazdi, Z. Sleep duration and its relationship with school performance in Iranian adolescents. J. Prev. Med. Hyg. 2021, 62, E54–E59. [Google Scholar] [CrossRef]
- Lim, J.; Dinges, D.F. Sleep Deprivation and Vigilant Attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef]
- Lim, J.; Tan, J.C.; Parimal, S.; Dinges, D.F.; Chee, M.W.L. Sleep Deprivation Impairs Object-Selective Attention: A View from the Ventral Visual Cortex. PLoS ONE 2010, 5, e9087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Posner, M.I. Measuring Alertness. Ann. N. Y. Acad. Sci. 2008, 1129, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, L.T.; Kornguth, S.; Schnyer, D.M. An ERP Examination of the Different Effects of Sleep Deprivation on Exogenously Cued and Endogenously Cued Attention. Sleep 2009, 32, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, J. Digit Symbol Substitution Test. The case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- Thomas, M.L.; Sing, H.C.; Belenky, G.; Holcomb, H.H.; Mayberg, H.S.; Dannals, R.F.; Wagner, H.N., Jr.; Thorne, D.R.; Popp, K.A.; Rowland, L.M.; et al. Neural basis of alertness and cognitive performance impairments during sleepiness II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus Relat. Syst. 2003, 2, 199–229. [Google Scholar] [CrossRef]
- Galván, A. The Need for Sleep in the Adolescent Brain. Trends Cogn. Sci. 2020, 24, 79–89. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Gillberg, M. Subjective and Objective Sleepiness in the Active Individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- O’Malley, E.B.; O’Malley, M.B. School start time and its impact on learning and behavior. In Sleep and Psychiatric Disorders in Children and Adolescents; Ivenko, A., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 79–94. [Google Scholar]
- Minges, K.E.; Redeker, N. Delayed school start times and adolescent sleep: A systematic review of the experimental evidence. Sleep Med. Rev. 2016, 28, 86–95. [Google Scholar] [CrossRef]
- Banks, S.; Dinges, D.F. Behavioral and Physiological Consequences of Sleep Restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar] [CrossRef]
- Voyer, D.; Voyer, S.D.; Saint-Aubin, J. Sex differences in visual-spatial working memory: A meta-analysis. Psychon. Bull. Rev. 2017, 24, 307–334. [Google Scholar] [CrossRef]
- Palmer, M.A.; Brewer, N.; Horry, R. Understanding gender bias in face recognition: Effects of divided attention at encoding. Acta Psychol. 2013, 142, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; Hull, J.T.; Czeisler, C.A. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R1370–R1377. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Dinges, D.F. Maximizing Sensitivity of the Psychomotor Vigilance Test (PVT) to Sleep Loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Duffy, J.F.; Dijk, D.J.; Ronda, J.M.; Dyal, C.M.; Czeisler, C.A. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J. Sleep Res. 1992, 1, 24–29. [Google Scholar] [CrossRef]
- Stuss, D.T.; Murphy, K.J.; Binns, M.; Alexander, M.P. Staying on the job: The frontal lobes control individual performance variability. Brain 2003, 126, 2363–2380. [Google Scholar] [CrossRef]
- Shochat, T.; Flint-Bretler, O.; Tzischinsky, O. Sleep patterns, electronic media exposure and daytime sleep-related behaviours among Israeli adolescents. Acta Paediatr. 2010, 99, 1396–1400. [Google Scholar] [CrossRef]


| (a) | |||
|---|---|---|---|
| Weekdays | Weekend | t | |
| Bedtime (hh:mm) | 23:47 (1.05) | 01:07 (1.45) | 5.57 *** |
| Wake-up time (hh:mm) | 07:08 (0.49) | 09:13 (1.45) | 9.07 *** |
| Sleep duration (min) | 441.16 (55.89) | 486.46 (92.73) | 2.63 * |
| WASO (min) | 48.29 (21.58) | 46.33 (28.55) | 2.03 * |
| Sleep latency (min) | 19.78 (15.58) | 34.88 (39.79) | 2.87 ** |
| Sleep efficiency (min) | 84.42 (4.21) | 80.70 (13.81) | 2.05 * |
| (b) | |||
| Weekdays | Weekend | t | |
| Bedtime (hh:mm) | 22:48 (3:13) | 01:03 (1:23) | 5.54 *** |
| Wake-up time (hh:mm) | 6:55 (0:44) | 10:29 (1:29) | 18.12 *** |
| Sleep duration (min) | 430 (34.8) | 554 (79.8) | 9.9 *** |
| Latency (min) | 18.3(13.3) | 18.3 (21.6) | 0.07 |
| Weekdays | Weekend | |
|---|---|---|
| KSS | 5.30 (0.16) | 4.72 (0.17) |
| PVT | ||
| Mean RTlog10 | 2.61 (0.01) | 2.59 (0.01) |
| Lapseslog10 | 0.78 (0.06) | 0.60 (0.07) |
| False Startslog10 | 0.54 (0.04) | 0.56 (0.04) |
| DSST | ||
| Correct responses | 83.93 (1.49) | 86.14 (1.43) |
| Mean RT | 942.86 (18.38) | 908.82 (15.32) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orna, T.; Efrat, B. Sleep Loss, Daytime Sleepiness, and Neurobehavioral Performance among Adolescents: A Field Study. Clocks & Sleep 2022, 4, 160-171. https://doi.org/10.3390/clockssleep4010015
Orna T, Efrat B. Sleep Loss, Daytime Sleepiness, and Neurobehavioral Performance among Adolescents: A Field Study. Clocks & Sleep. 2022; 4(1):160-171. https://doi.org/10.3390/clockssleep4010015
Chicago/Turabian StyleOrna, Tzischinsky, and Barel Efrat. 2022. "Sleep Loss, Daytime Sleepiness, and Neurobehavioral Performance among Adolescents: A Field Study" Clocks & Sleep 4, no. 1: 160-171. https://doi.org/10.3390/clockssleep4010015
APA StyleOrna, T., & Efrat, B. (2022). Sleep Loss, Daytime Sleepiness, and Neurobehavioral Performance among Adolescents: A Field Study. Clocks & Sleep, 4(1), 160-171. https://doi.org/10.3390/clockssleep4010015






