Nylon Powder Composites with High Leveling Property and Toughness Prepared via Filler-Modified Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
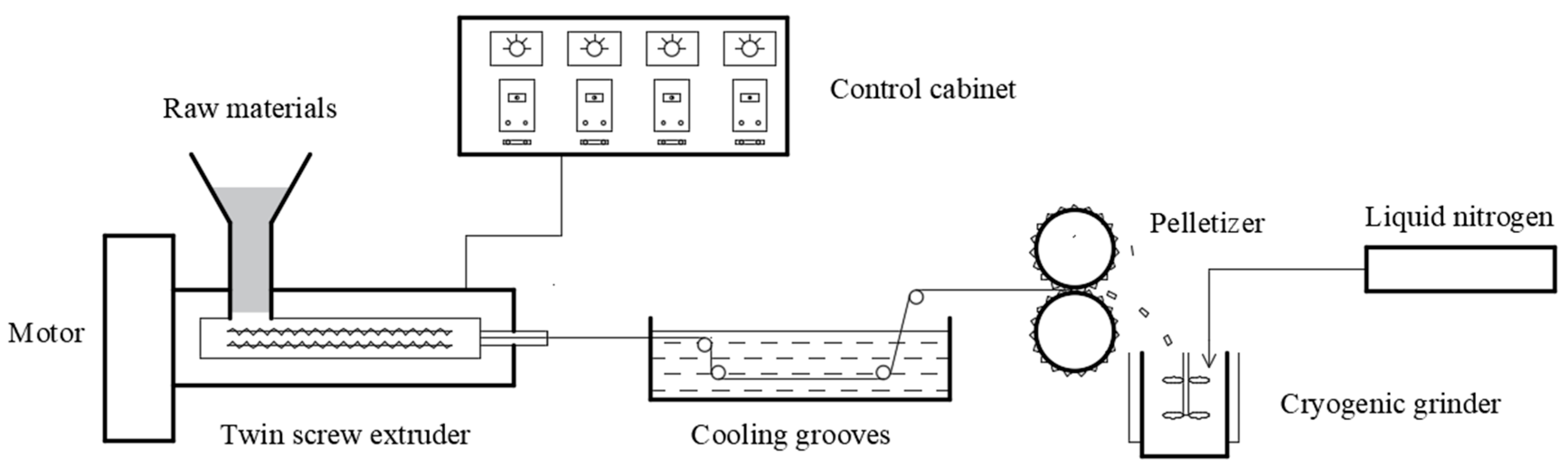
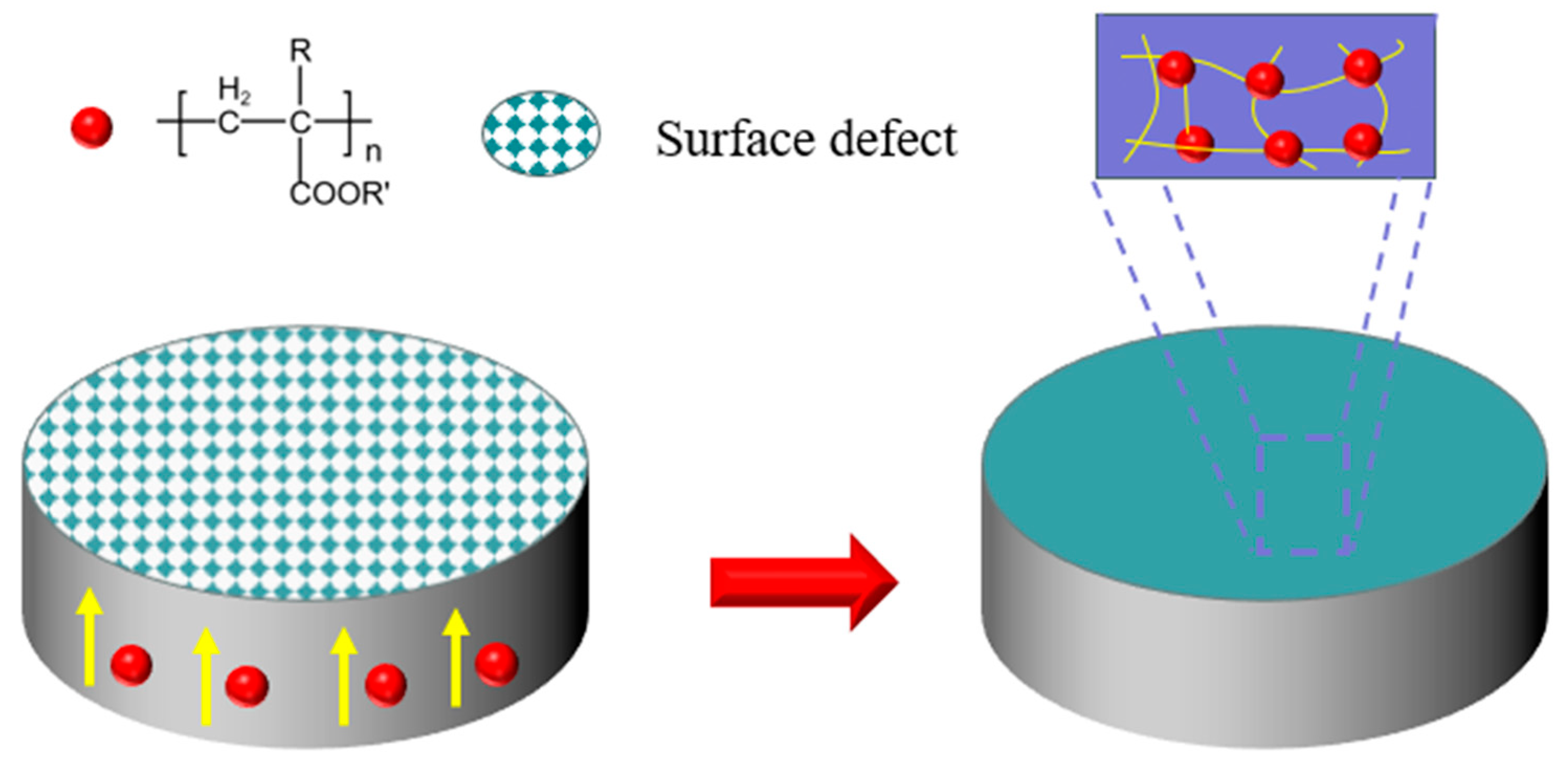
2.2. Synthesis of PA-12 Modified Particles
2.3. Preparation of PA-12 Powder
2.4. Characterization
2.5. Leveling Performance Test
3. Results and Discussion
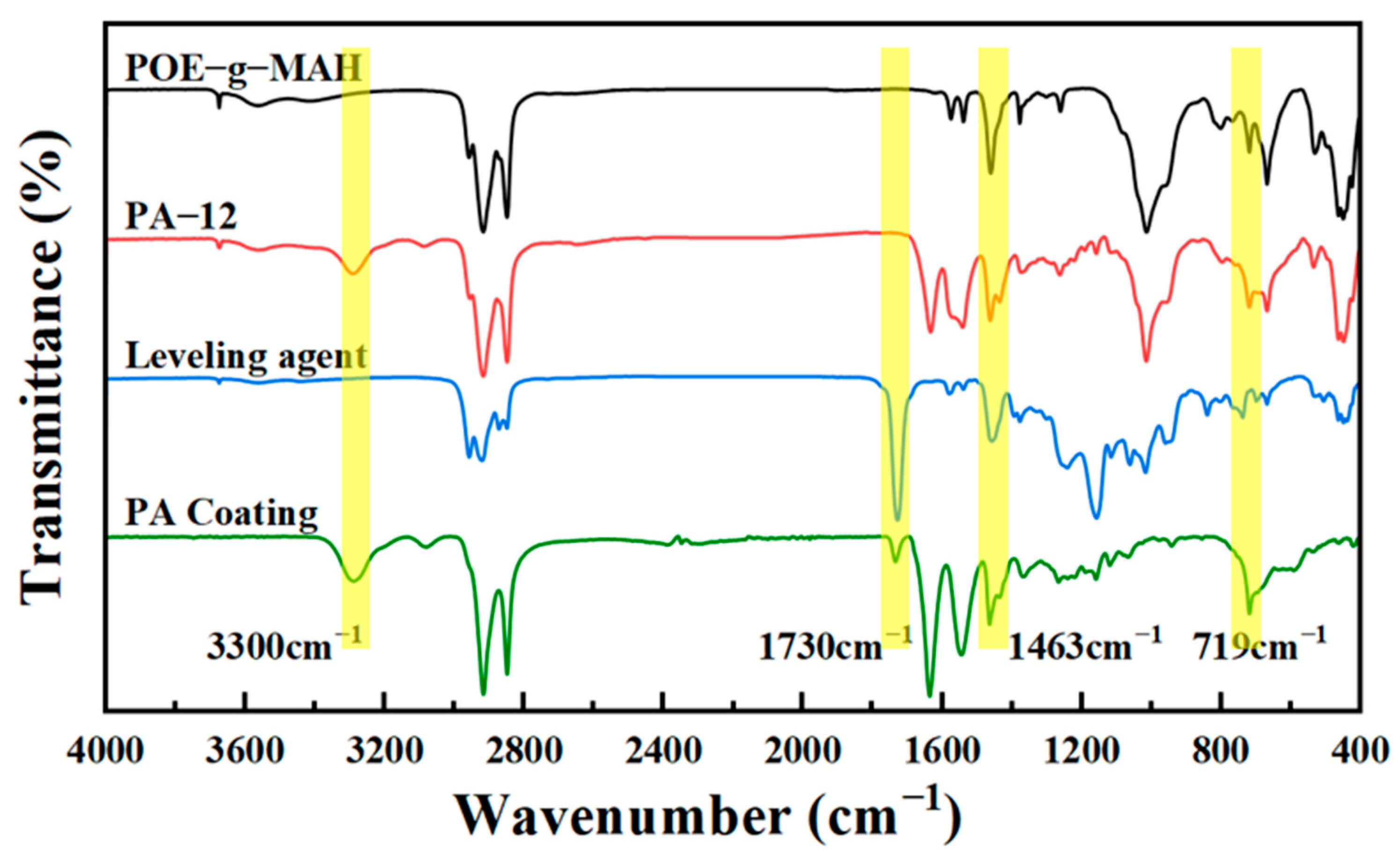
3.1. Chemical Structural Analysis
3.2. Thermal Analysis
3.3. Microscopic Morphology and Particle Size Analysis
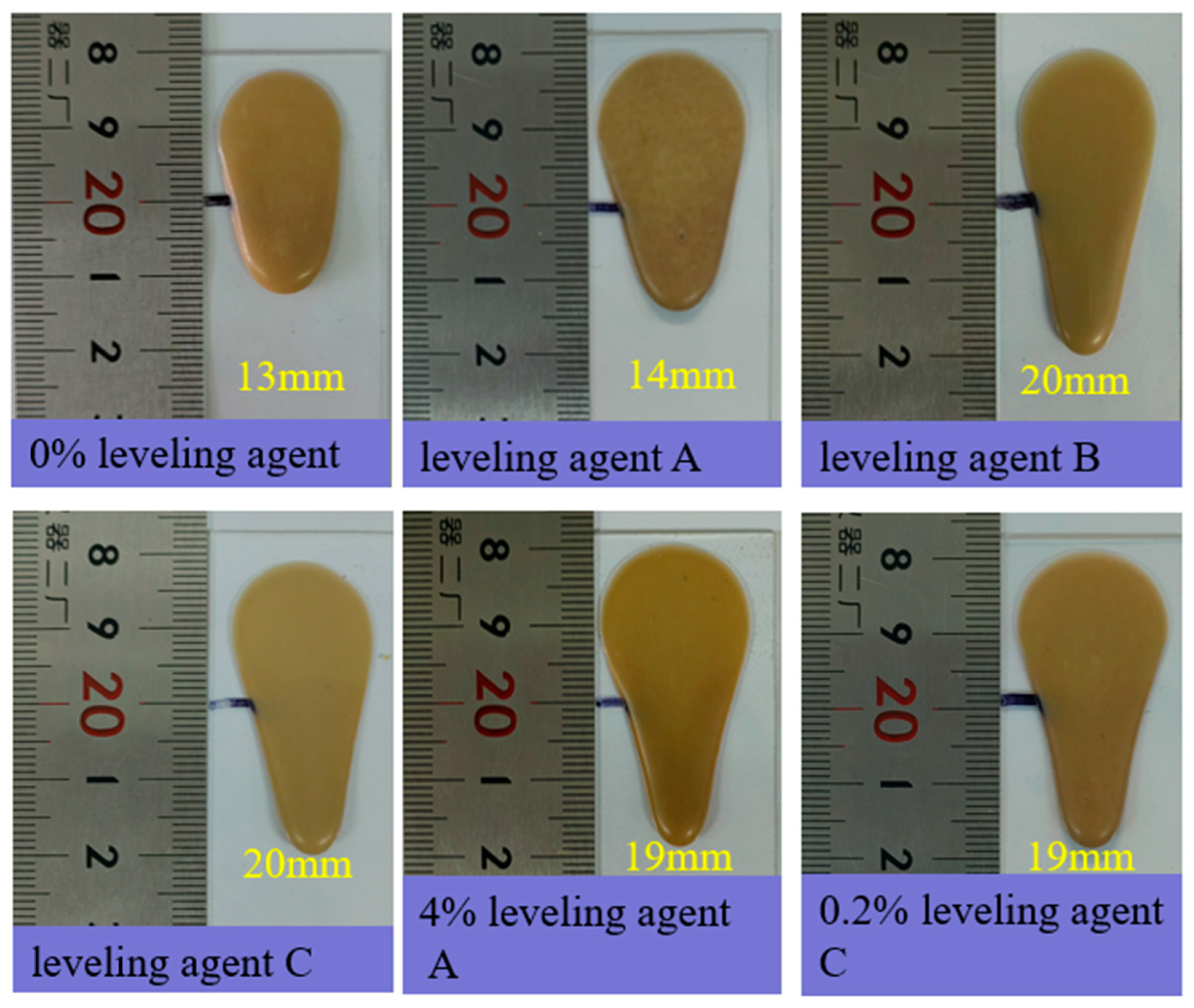
3.4. Leveling Performance Testing
3.5. Melt Flow Performance Analysis
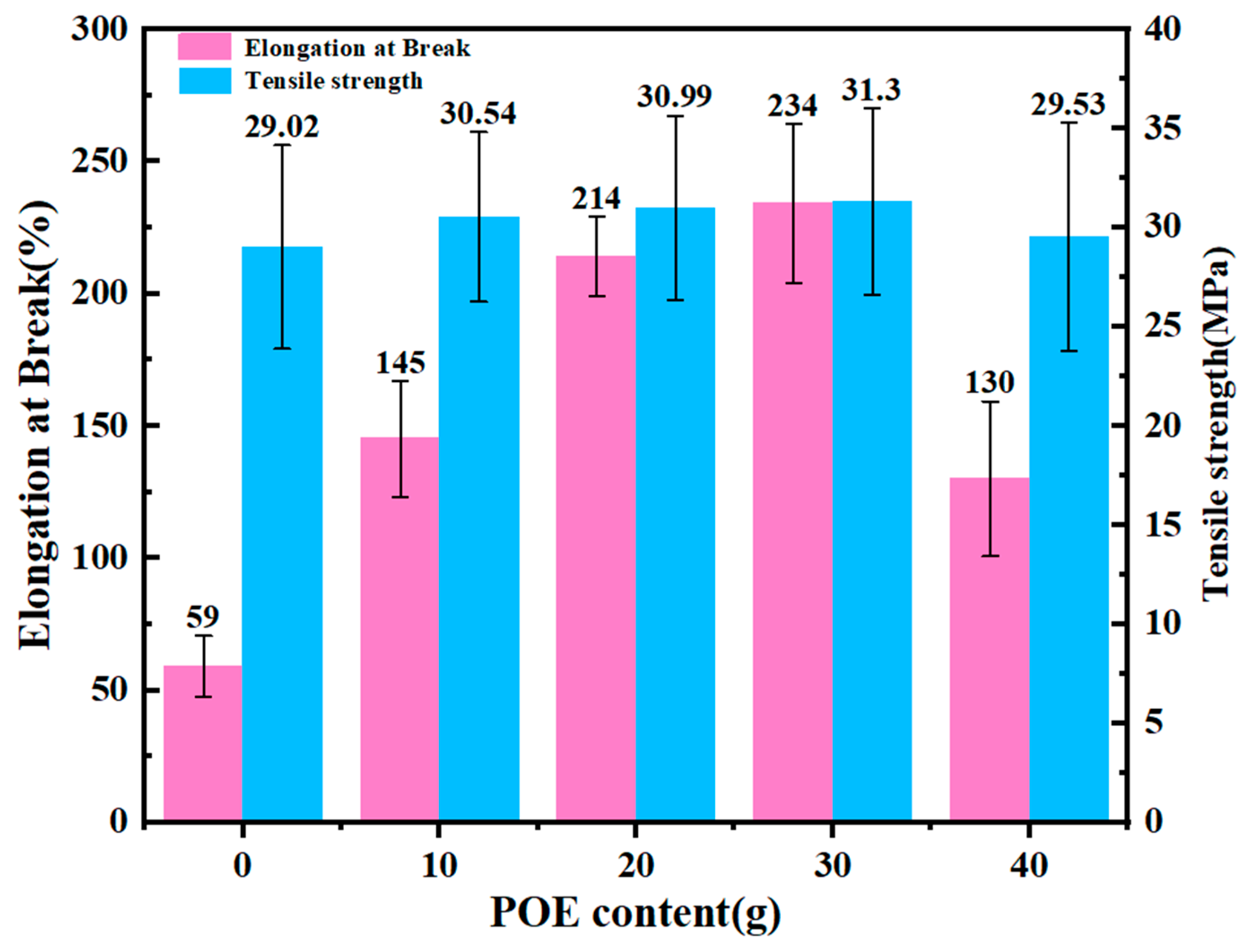
3.6. Mechanical Performance Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farshchi, N.; Gedan-Smolka, M.; Stommel, M. Preparation and Characterization of Self-Healing Polyurethane Powder Coating Using Diels–Alder Reaction. Polymers 2021, 13, 3803. [Google Scholar] [CrossRef]
- Lüddecke, A.; Hantke, N.; Zetzener, H.; Sehrt, J.T.; Kwade, A. Nanoparticle-coated X2CrNiMo17-12-2 Powder for Additive Manufacturing—Part I: Surface, Flowability, and Optical Properties of SiC, Si, and Si3 N4 Coated Metal Powders. Adv. Eng. Mater. 2025, 27, 2402297. [Google Scholar] [CrossRef]
- Farshchi, N.; Gedan-Smolka, M. Polyurethane Powder Coatings: A Review of Composition and Characterization. Ind. Eng. Chem. Res. 2020, 59, 15121–15132. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Biller, K.; Pilch-Pitera, B. Recent Development Advances in Bio-Based Powder Coatings: A Review. J. Coat. Technol. Res. 2024, 21, 435–444. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhang, J.; Guo, J.; Li, Y.; Xin, S.; Xu, Z.; Yuan, Y.; Zhang, D. Continuous Preparation of Carbon Nanotubes/Carbon Fiber Reinforcement Using Fe-Ni Bimetallic Catalyst. Surfaces 2025, 8, 60. [Google Scholar] [CrossRef]
- Ayrilmis, N. A Review on Electrostatic Powder Coatings for the Furniture Industry. Int. J. Adhes. Adhes. 2022, 113, 103062. [Google Scholar] [CrossRef]
- Liu, W.; Bhuiyan, M.T.I.; Zhang, H.; Zhu, J. Boosting Flowability and Curing Reaction of Ultra-Fine Powder Coatings Using Facile-Fabrication Al2O3/C4H6N2 Nanocomposite. Prog. Org. Coat. 2023, 185, 107836. [Google Scholar] [CrossRef]
- Andrei, D.C.; Hay, J.N.; Keddie, J.L.; Sear, R.P.; Yeates, S.G. Surface Levelling of Thermosetting Powder Coatings: Theory and Experiment. J. Phys. Appl. Phys. 2000, 33, 1975–1981. [Google Scholar] [CrossRef]
- Guan, M.; Li, J.; Fang, Y.; Dai, K.; Liu, X.; Tang, G. Fabrication and Performance Investigation of Flame Retardant Flexible Polyurethane Composites via Polyethyleneimine/Phytic Acid LBL Self-assembly Coatings. J. Appl. Polym. Sci. 2025, 142, 56535–56545. [Google Scholar] [CrossRef]
- He, X.; Cui, C.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D. MXene and Polymer Collision: Sparking the Future of High-performance Multifunctional Coatings. Adv. Funct. Mater. 2024, 34, 2409675. [Google Scholar] [CrossRef]
- Ahmad, S.; Habib, S.; Nawaz, M.; Shakoor, R.A.; Kahraman, R.; Mohammed, A.T.T. The Role of Polymeric Matrices on the Performance of Smart Self-Healing Coatings: A Review. J. Ind. Eng. Chem. 2023, 124, 40–67. [Google Scholar] [CrossRef]
- Franzen, V.; Trompeter, M.; Brosius, A.; Tekkaya, A.E. Finishing of Thermally Sprayed Tool Coatings for Sheet Metal Forming Operations by Roller Burnishing. Int. J. Mater. Form. 2010, 3, 147–150. [Google Scholar] [CrossRef]
- Dee, G.T.; Sauer, B.B. The Surface Tension of Polymer Liquids. Adv. Phys. 1998, 47, 161–205. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Zhang, H.; Zhang, H.; Zhu, J. Narrowing Particle Size Distributions to Enhance Powder Coating Performance by Improved Classifying. Powder Technol. 2024, 435, 119443. [Google Scholar] [CrossRef]
- Huang, J.; Yang, M.; Wan, L.; Tang, K.; Zhang, H.; Chen, J.; Noël, J.J.; Barker, I.; Zhang, H.; Zhu, J. Ultrafine Powder Coating: Smooth Surface, Dense Structure and Enhanced Corrosion Resistance. Chem. Eng. J. 2023, 455, 140815. [Google Scholar] [CrossRef]
- Wulf, M.; Uhlmann, P.; Michel, S.; Grundke, K. Surface Tension Studies of Levelling Additives in Powder Coatings. Prog. Org. Coat. 2000, 38, 59–66. [Google Scholar] [CrossRef]
- Pan, S.; Ouyang, Y.; Zhao, Y.; Wang, Q.; Qian, Y.; He, C. Optimization of a Low Surface Energy Coating for Enhanced Water Resistance and Condensation Suppression. Materials 2024, 17, 5238. [Google Scholar] [CrossRef]
- Bhavsar, R.; Shreepathi, S. Evolving Empirical Rheological Limits to Predict Flow-Levelling and Sag Resistance of Waterborne Architectural Paints. Prog. Org. Coat. 2016, 101, 15–23. [Google Scholar] [CrossRef]
- Grundke, K.; Michel, S.; Osterhold, M. Surface Tension Studies of Additives in Acrylic Resin-Based Powder Coatings Using the Wilhelmy Balance Technique. Prog. Org. Coat. 2000, 39, 101–106. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Zhu, J. Flow Properties of Fine Powders in Powder Coating. Particuology 2010, 8, 19–27. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Gu, Y.; Xie, J.; Zhang, J.; Qu, J. Improve Surface Levelling of Powder Coating with Semi-Crystalline Polyester Resin. Prog. Org. Coat. 2016, 99, 191–196. [Google Scholar] [CrossRef]
- Jain, P.K.; Pandey, P.M.; Rao, P.V.M. Selective Laser Sintering of Clay-Reinforced Polyamide. Polym. Compos. 2010, 31, 732–743. [Google Scholar] [CrossRef]
- Yi, J.; Li, S.; Xia, J.; Li, M.; Ding, H.; Xu, L.; Yang, X. Preparation and Properties of Polyether Aliphatic Polymerized Amide as a Vegetable Oil-Based Epoxy Curing Agent. ACS Omega 2019, 4, 6238–6244. [Google Scholar] [CrossRef]
- Chiririwa, H. Characterization of Polymer Powders and Effects of Powder Reuse in Selective Laser Sintering. Asian J. Chem. 2021, 33, 658–664. [Google Scholar] [CrossRef]
- Kaba, O.; Shah, A.T.; Dudaško, D.; Jain, N.; Bertz, T.; Görke, O.; Gurlo, A. Additive Manufacturing of a Polyamide 12 and Silica Nanocomposite: A Route for the Reusability of a Thermoplastic Selective Laser Sintering Powder. Polym. Compos. 2025, 46, 5964–5981. [Google Scholar] [CrossRef]
- An, R.; Hou, Y.; Tan, P.; Chen, M.; Zhao, L.; Zhou, K. Multi Jet Fusion of Surface-Modified ZnO Nanorod–Reinforced PA12 Nanocomposites. Virtual Phys. Prototyp. 2023, 18, e2273309. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Sun, C.; Wang, J.; Cao, Y.; Yang, Y.; Wang, W. Reactive Nano-Fe3O4 Compatibilized Magnetic Super-Tough PA1212/POE-g-MAH Composites with a Filler-Network Structure. Compos. Sci. Technol. 2021, 202, 108561. [Google Scholar] [CrossRef]
- Jaimes, K.Y.P.; Caldona, E.B.; Kim, E.M.; Lambert, J.; Winn, E.C.; Ribeiro, E.L.; Jiang, Y.; Ferdousi, S.; Hicks, B.; Wang, Y. Distinct Mechanism of Anti-Corrosion and Swelling-Adhesion Modeling of Low-Dimensional Nylon-Fluoropolymer Composite Coatings. ACS Appl. Polym. Mater. 2024, 6, 3049–3059. [Google Scholar] [CrossRef]
- Hong, P.H.; Moon, G.; Kim, J.; Choi, K.; Ko, M.J.; Yoon, H.G.; Hong, S.W. Highly Self-Healable Polymeric Coating Materials Based on Charge Transfer Complex Interactions with Outstanding Weatherability. Polymers 2023, 15, 4544. [Google Scholar] [CrossRef]
- Chong, Y.; Wang, L.; Xu, X.; Zhuang, X.; Zheng, R.; Cao, D.; Chen, Y. Preparation and Property Study of PA56/POE-g-MAH-GMA/PPO Ternary Alloy. Adv. Polym. Technol. 2023, 2023, 3114639. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Geng, J.; Xia, L. Structural Optimization of PA12/MVQ@POE-g-MAH Ternary Composite for Superior Toughness and Low-temperature Resistance. Macromol. Rapid Commun. 2024, 45, e2400228. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, L.; Gao, S.; Zhuge, X.; Hui, D.; Xu, F. The Toughening Mechanism of 3D Carbon Fiber Reinforced Polyetheretherketone Composites with Ultrahigh Interlaminar Fracture Toughness. Eng. Fract. Mech. 2025, 325, 111300. [Google Scholar] [CrossRef]



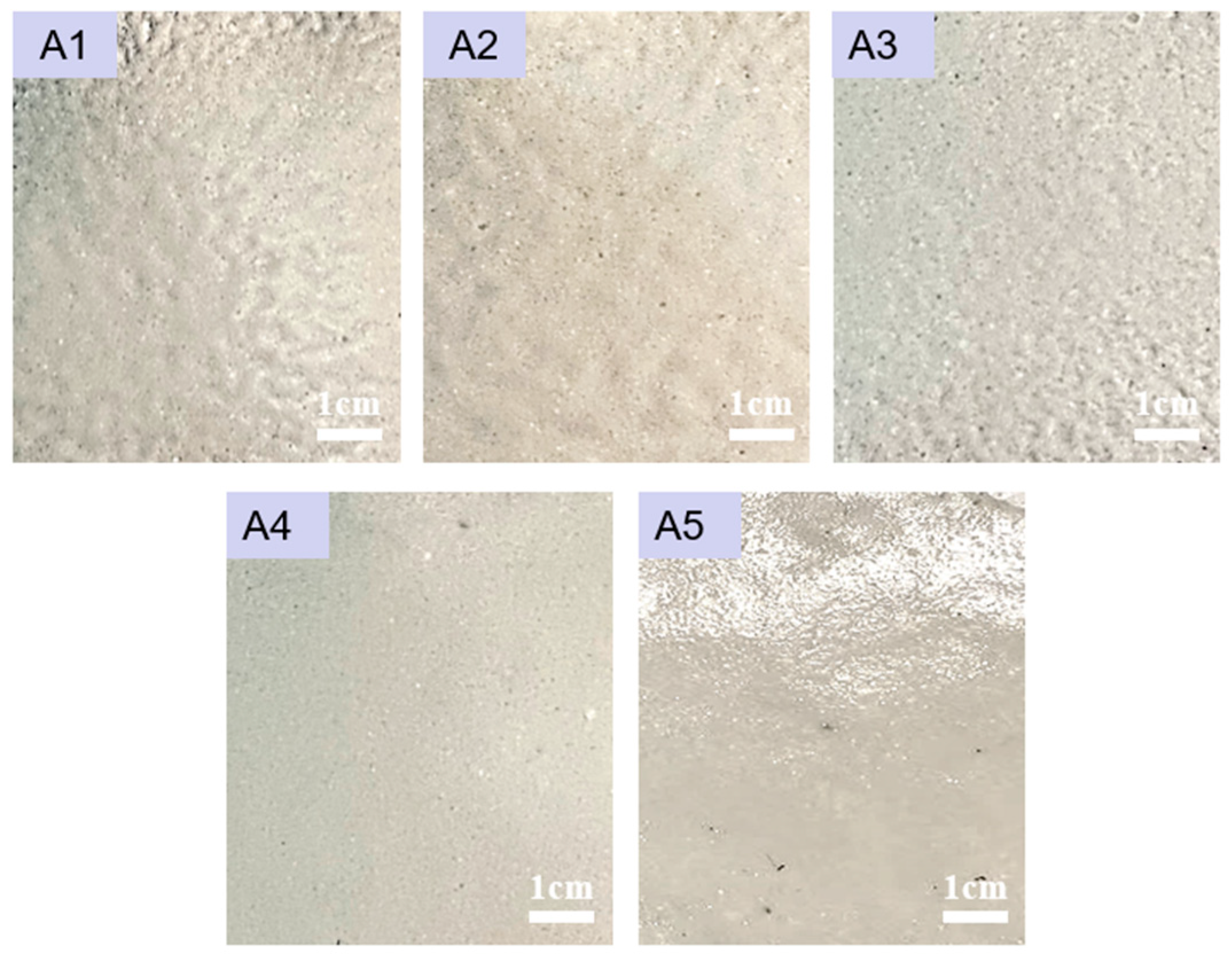

| Sample Designation | Leveling Agent Contents (%) | Toughener Contents (g) |
|---|---|---|
| A 1 | 0 | 0 |
| A 2 | 0.2 | 0 |
| A 3 | 0.4 | 0 |
| A 4 | 0.6 | 0 |
| A 5 | 0.8 | 0 |
| B 1 | 0.6 | 10 |
| B 2 | 0.6 | 20 |
| B 3 | 0.6 | 30 |
| B 4 | 0.6 | 40 |
| Sample Designation | Flow Length (mm) |
|---|---|
| 0% leveling agent | 13.0 ± 0.7 |
| Leveling agent A | 14.0 ± 1.6 |
| Leveling agent B | 20.0 ± 1.9 |
| Leveling agent C | 20.0 ± 1.2 |
| 4% leveling agent A | 19.0 ± 0.7 |
| Sample Designation | Average Quality (g) | MFR (g/10 min) |
|---|---|---|
| A 1 | 0.230 ± 0.006 | 27.60 ± 0.72 |
| A 2 | 0.250 ± 0.006 | 30.00 ± 0.72 |
| A 3 | 0.260 ± 0.014 | 31.20 ± 1.68 |
| A 4 | 0.260 ± 0.006 | 31.20 ± 0.72 |
| A 5 | 0.240 ± 0.012 | 28.80 ± 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, S.-A.; Wang, Y.; Xu, S.; Zhu, Y.; Xu, Z.; Yuan, Y.; Zhang, D.; Li, Y.; Hu, S. Nylon Powder Composites with High Leveling Property and Toughness Prepared via Filler-Modified Method. Surfaces 2025, 8, 80. https://doi.org/10.3390/surfaces8040080
Xin S-A, Wang Y, Xu S, Zhu Y, Xu Z, Yuan Y, Zhang D, Li Y, Hu S. Nylon Powder Composites with High Leveling Property and Toughness Prepared via Filler-Modified Method. Surfaces. 2025; 8(4):80. https://doi.org/10.3390/surfaces8040080
Chicago/Turabian StyleXin, Si-Ao, Yanxiang Wang, Shanshan Xu, Yanying Zhu, Ziyi Xu, Yanru Yuan, Dong Zhang, Yingfan Li, and Shaoao Hu. 2025. "Nylon Powder Composites with High Leveling Property and Toughness Prepared via Filler-Modified Method" Surfaces 8, no. 4: 80. https://doi.org/10.3390/surfaces8040080
APA StyleXin, S.-A., Wang, Y., Xu, S., Zhu, Y., Xu, Z., Yuan, Y., Zhang, D., Li, Y., & Hu, S. (2025). Nylon Powder Composites with High Leveling Property and Toughness Prepared via Filler-Modified Method. Surfaces, 8(4), 80. https://doi.org/10.3390/surfaces8040080





