The Effect of the Metal Impurities on the Stability, Chemical, and Sensing Properties of MoSe2 Surfaces
Abstract
1. Introduction
2. Computational Method
3. Results and Discussions
3.1. Lattice Stability and Electronic Structure
3.2. Sensing Properties
3.3. Catalytic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Wang, Y.H.; Li, P.L.; Xiong, Z.Z. Research status of MoSe2 and its composites: A review. Superlattices Microstruct. 2020, 139, 106388. [Google Scholar] [CrossRef]
- Kline, G.; Kam, K.; Canfield, D.; Parkinson, B.A. Efficient and stable hotoelectrochemical cells constructed with WSe2 and MoSe2 photoanodes. Sol. Energy Mater. 1981, 4, 301–308. [Google Scholar] [CrossRef]
- Bollero, A.; Kaupmees, L.; Raadik, T.; Grossberg, M.; Fernandez, S. Thermal stability of sputtered Mo/polyimide films and formation of MoSe2 and MoS2 layers for application in flexible Cu (In, Ga) (Se, S)2 based solar cells. Thin Solid Film. 2012, 520, 4163–4168. [Google Scholar] [CrossRef]
- D’Olimpio, G.; Genuzio, F.; Menteş, T.O.; Paolucci, V.; Kuo, C.-N.; Al Taleb, A.; Lue, C.S.; Torelli, P.; Farías, D.; Locatelli, A.; et al. Charge redistribution mechanisms in SnSe2 surfaces exposed to oxidative and humid environments and their related influence on chemical sensing. J. Chem. Phys. Lett. 2020, 11, 9003–9011. [Google Scholar] [CrossRef]
- D’Olimpio, G.; Farias, D.; Kuo, C.-N.; Ottaviano, L.; Lue, C.S.; Boukhvalov, D.W.; Politano, A. Tin diselenide (SnSe2) Van der Waals semiconductor: Surface chemical reactivity, ambient stability, cemical and optical Sensors. Materials 2022, 15, 1154. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.S.; Zhang, J. Mini-review: Recent progress in the development of MoSe2 based chemical sensors and biosensors. Microelectron. Eng. 2020, 225, 111279. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.C.; Sharma, S. Room temperature ammonia sensing using MoSe2 nanostructures. Mater. Today Proc. 2020, 28, 11–13. [Google Scholar] [CrossRef]
- Li, Q.; Meng, J.; Li, Z. Recent progress on Schottky sensors based on two-dimensional transition metal dichalcogenides. J. Mater. Chem. A 2022, 10, 8107–8128. [Google Scholar] [CrossRef]
- Thayil, R.; Parne, S.R. Recent advances and prospects on MoX2 (X=S, Se, Te) nanostructure-based sensors for room temperature gas detection: A review. Surf. Interfaces 2024, 52, 104966. [Google Scholar] [CrossRef]
- Zappa, D. Molybdenum Dichalcogenides for Environmental Chemical Sensing. Materials 2017, 10, 1418. [Google Scholar] [CrossRef]
- Guo, S.; Yang, D.; Zhang, S.; Dong, Q.; Li, B.; Tran, N.; Li, Z.; Xiong, Y.; Zaghloul, M.E. Development of a cloud-based epidermal MoSe2 device for hazardous gas sensing. Adv. Funct. Mater. 2019, 29, 1900138. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Han, Y.; Su, C.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z. Two-dimensional MoSe2 nanosheets via liquid-phase exfoliation for high-performance room temperature NO2 gas sensors. Nanotechnology 2019, 30, 445503. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liao, Y.; Liu, Y.; Zeng, W.; Zhou, Q. Room temperature detection of nitrogen dioxide gas sensor based on Pt-modified MoSe2 nanoflowers: Experimental and theoretical analysis. Appl. Surf. Sci. 2023, 610, 155527. [Google Scholar] [CrossRef]
- Kumar, S.; Mirzaei, A.; Kumar, A.; Lee, M.H.; Ghahremani, Z.; Kim, T.U.; Kim, J.Y.; Kwoka, M.; Kumar, M.; Kim, S.S.; et al. Nanoparticles anchored strategy to develop 2D MoS2 and MoSe2 based room temperature chemiresistive gas sensors. Coord. Chem. Rev. 2024, 503, 215657. [Google Scholar] [CrossRef]
- Panigrahi, P.; Hussain, T.; Karton, A.; Ahuja, R. Elemental substitution of two-dimensional transition metal dichalcogenides (MoSe2 and MoTe2): Implications for enhanced gas sensing. ACS Sens. 2019, 4, 2646–2653. [Google Scholar] [CrossRef]
- Mishra, N.; Pandey, B.P.; Kumar, S. Impact of N2O gas adsorption upon electronic properties of 2D MoSe2 monolayer: A DFT approach. IEEE Sens. J. 2021, 21, 9756–9762. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tian, F.H.; Sun, L.; Zhang, J. Oriented adsorption and efficient sensing of NO2 on MoSe2 monolayer: A comparative study with WSe2 monolayer. Mater. Today Chem. 2023, 28, 101354. [Google Scholar] [CrossRef]
- Shelke, N.T.; Late, D.J. Hydrothermal growth of MoSe2 nanoflowers for photo- and humidity sensor applications. Sens. Actuators A Phys. 2019, 295, 160–168. [Google Scholar] [CrossRef]
- Jha, R.K.; D’Costa, J.V.; Sakhuja, N.; Bhat, N. MoSe2 nanoflakes based chemiresistive sensors for ppb-level hydrogen sulfide gas detection. Sens. Actuators B Chem. 2019, 297, 126687. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. MoSe2 nanosheets for detection of methanol and ethanol vapors: A DFT study. J. Mol. Graph. Model. 2018, 81, 97–105. [Google Scholar] [CrossRef]
- Vinturaj, V.P.; Yadav, A.K.; Singh, R.; Garg, V.; Bhardwaj, R.; Ajith, K.M.; Pandey, S.K. DFT study of the adsorption behavior and sensing properties of CO gas on monolayer MoSe2 in CO2-rich environment. J. Mol. Model. 2024, 30, 250. [Google Scholar] [CrossRef]
- Ayesh, A.I. Investigation of NH3 adsorption on noble metal modified MoSe2. Phys. E Low-Dimens. Syst. Nanostructures 2022, 139, 115188. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Li, P.; Pang, M.; Luo, Y. Fabrication of Pd-decorated MoSe2 nanoflowers and density functional theory simulation toward ammonia sensing. IEEE Electron Device Lett. 2019, 40, 616–619. [Google Scholar] [CrossRef]
- Liu, T.; Cui, Z.; Li, X.; Cui, H.; Liu, Y. Al-doped MoSe2 monolayer as a promising biosensor for exhaled breath analysis: A DFT study. ACS Omega 2021, 6, 988–995. [Google Scholar] [CrossRef]
- Li, T.; Yu, S.; Li, Q.; Chi, M.; Li, P. Room temperature ethanol gas-sensing properties based on Ag-doped MoSe2 nanoflowers: Experimental and DFT investigation. New J. Chem. 2021, 45, 21423–21428. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hazra, A. Oxygen-functionalized MoSe2 nanoflowers for selective detection of xylene at room temperature. Surf. Interfaces 2023, 43, 103523. [Google Scholar] [CrossRef]
- Ayesh, A.I. H2S and SO2 adsorption on Cu doped MoSe2: DFT investigation. Phys. Lett. A 2022, 422, 127798. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Ayesh, A.I. DFT investigation of H2S and SO2 adsorption on Zn modified MoSe2. Superlattices Microstruct. 2022, 162, 107098. [Google Scholar] [CrossRef]
- Barzegar, M.; Berahman, M.; Asgari, R. First-principles study of molecule adsorption on Ni-decorated monolayer MoS2. J. Comput. Electron. 2019, 18, 826–835. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Zhang, X.; Xu, Z.; Liu, G.; Hussain, S.; Qiao, G. Tailoring the sensing capability of 2H-MoSe2 via 3d transition metal decoration. Appl. Surf. Sci. 2023, 610, 155399. [Google Scholar] [CrossRef]
- D’Olimpio, G.; Boukhvalov, D.W.; Galstyan, V.; Occhiuzzi, J.; Vorochta, M.; Amati, M.; Milosz, Z.; Gregoratti, L.; Istrate, M.C.; Kuo, C.-N.; et al. Unlocking superior NO2 sensitivity and selectivity: The role of sulfur abstraction in indium sulfide (InS) nanosheets-based sensor. J. Mater. Chem. A 2024, 12, 10329–10340. [Google Scholar] [CrossRef]
- Kuraganti, V.; Jain, A.; Bar-Ziv, R.; Ramasubramaniam, A.; Bar-Sadan, M. Manganese Doping of MoSe2 Promotes Active Defect Sites for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2019, 11, 25155–25162. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.S.; Kim, S.J.; Song, W.; Myung, S.; Lim, J.; Lee, S.S.; Jung, H.-K.; Hwang, J.; An, K.-S. One-Step Synthesis of Zn-Doped MoS2 Nanosheets with Tunable Doping Concentration Using Dopants-Loaded Seeding Promoters for Visible-Light Flexible Photodetectors. J. Alloys Compd. 2020, 835, 155383. [Google Scholar] [CrossRef]
- Eftekhari, A. Tungsten Dichalcogenides (WS2, WSe2, and WTe2): Materials Chemistry and Applications. J. Mater. Chem. A 2017, 5, 18299–18325. [Google Scholar] [CrossRef]
- Mohl, M.; Rautio, A.-R.; Asres, G.A.; Wasala, M.; Patil, P.D.; Talapatra, S.; Kordas, K. 2D Tungsten Chalcogenides: Synthesis, Properties and Applications. Adv. Mater. Interf. 2020, 7, 2000002. [Google Scholar] [CrossRef]
- Rahm, M.; Hoffmann, R.; Ashcroft, N.W. Corrigendum: Atomic and Ionic Radii of Elements 1–96. Chem.–Eur. J. 2017, 23, 4017. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Barone, V.; Casarin, M.; Forrer, D.; Pavone, M.; Sambi, M.; Vittadini, A. Role and effective treatment of dispersive forces in materials: Polyethylene and graphite crystals as test cases. J. Comput. Chem. 2009, 30, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalov, D.W.; Politano, A. Unveiling the origin of room-temperature ferromagnetism in monolayer VSe2: The role of extrinsic effects. Nanoscale 2020, 12, 20875–20882. [Google Scholar] [CrossRef]
- Ahn, E.C. Tailoring the sensing capability of 2D materials for spintronic devices. npj 2D Mater. Appl. 2020, 4, 17. [Google Scholar] [CrossRef]
- Edla, R.; Kuo, C.N.; Torelli, P.; Lue, C.S.; Boukhvalov, D.W.; Politano, A. Interaction of VSe2 with ambient gases: Stability and chemical reactivity. physica status solidi. (RRL)–Rapid Res. Lett. 2020, 14, 1900332. [Google Scholar] [CrossRef]
- Yu, W.; Li, J.; Herng, T.S.; Wang, Z.; Zhao, X.; Chi, X.; Fu, W.; Abdelwahab, I.; Zhou, J.; Dan, J.; et al. Chemically Exfoliated VSe2 Monolayers with Room-Temperature Ferromagnetism. Adv. Mater. 2019, 31, 1903779. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Zhou, X.; Wang, C.; Ji, W. In-Plane Epitaxy-Strain-Tuning Intralayer and Interlayer Magnetic Coupling in CrSe2 and CrTe2 Monolayers and Bilayers. Phys. Rev. B 2022, 106, L081401. [Google Scholar] [CrossRef]
- Li, B.; Wan, Z.; Wang, C.; Chen, P.; Huang, B.; Cheng, X.; Qian, Q.; Li, J.; Zhang, Z.; Sun, G.; et al. Van der Waals epitaxial growth of air-stable CrSe2 nanosheets with thickness-tunable magnetic order. Nat. Mater. 2021, 20, 818–825. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Duchamp, M.; Zeng, Q.; Wang, X.; Tsang, S.H.; Li, H.; Jing, L.; Yu, T.; Teo, E.H.T.; et al. Large-area atomic layers of the charge-density-wave conductor TiSe2. Adv. Mater. 2018, 30, 1704382. [Google Scholar] [CrossRef]
- Nappini, S.; Boukhvalov, D.W.; D’Olimpio, G.; Zhang, L.; Ghosh, B.; Kuo, C.N.; Zhu, H.; Cheng, J.; Nardone, M.; Ottaviano, L.; et al. Transition-metal dichalcogenide NiTe2: An ambient-stable material for catalysis and nanoelectronics. Adv. Funct. Mater. 2020, 30, 2000915. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Suna, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Shi, S.Q.; Zhang, H. High-Temperature Gas Sensors for Harsh Environment Applications: A Review. CLEAN—Soil Air Water 2019, 47, 1800491. [Google Scholar] [CrossRef]
- Janet, J.P.; Liu, F.; Nandy, A.; Duan, C.; Yang, T.; Lin, S.; Kulik, H.J. Designing in the Face of Uncertainty: Exploiting Electronic Structure and Machine Learning Models for Discovery in Inorganic Chemistry. Inorg. Chem. 2019, 58, 10592–10606. [Google Scholar] [CrossRef] [PubMed]
- Kulik, H.J.; Hammerschmidt, T.; Schmidt, J.; Botti, S.; Marques, M.A.L.; Boley, M.; Scheffler, M.; Todorović, M.; Rinke, P.; Oses, C. Roadmap on Machine Learning in Electronic Structure. Electron. Struct. 2022, 4, 023004. [Google Scholar] [CrossRef]
- Pushkarev, G.V.; Mazurenko, V.G.; Mazurenko, V.V.; Boukhvalov, D.W. Nature of interlayer bonds in two-dimensional materials. J. Phys. Chem. C 2023, 127, 8148–8158. [Google Scholar] [CrossRef]
- Ren, X.; Wei, Q.; Ren, P.; Wang, Y.; Chen, R. Synthesis of flower-like MoSe2@MoS2 nanocomposites as the high efficient water splitting electrocatalyst. Mater. Lett. 2018, 231, 213–216. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Sun, B.; Liu, J.; Xia, Y.; Li, W.; Jiang, C.; He, D.; Xiao, X. In-situ structural evolution of Bi2O3 nanoparticle catalysts for CO2 electroreduction. Int. J. Extrem. Manuf. 2022, 4, 035002. [Google Scholar] [CrossRef]
- Yi, J.-d.; Gao, X.; Zhou, H.; Chen, W.; Wu, Y. Design of Co-Cu Diatomic Site Catalysts for High-efficiency Synergistic CO2 Electroreduction at Industrial-level Current Density. Angew. Chem. Int. Ed. 2022, 61, e202212329. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Dreyer, D.R.; Bielawski, C.W.; Son, Y.-W. A Computational Investigation of the Catalytic Properties of Graphene Oxide: Exploring Mechanisms by using DFT Methods. ChemCatChem 2012, 4, 1844–1849. [Google Scholar] [CrossRef]
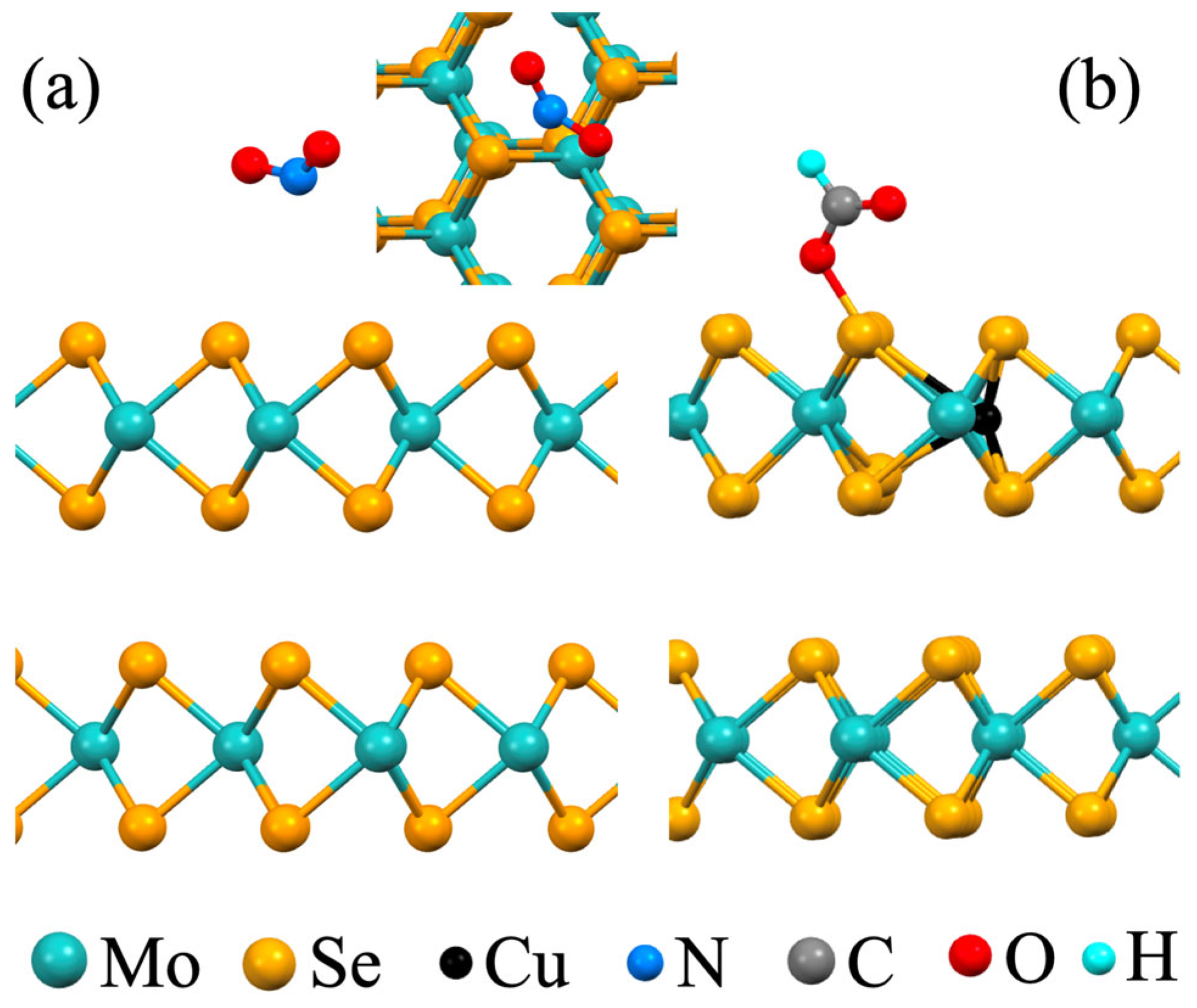
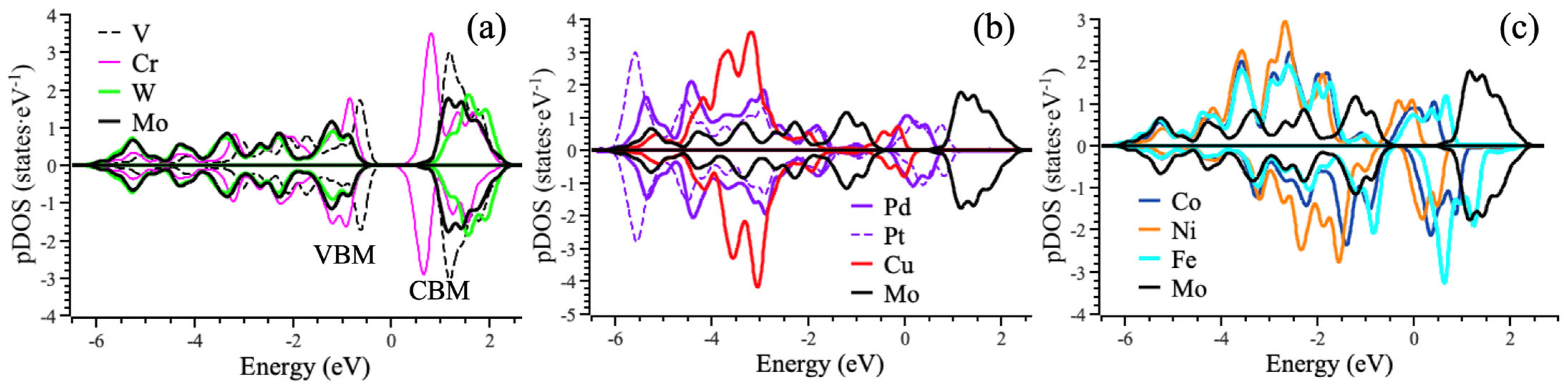
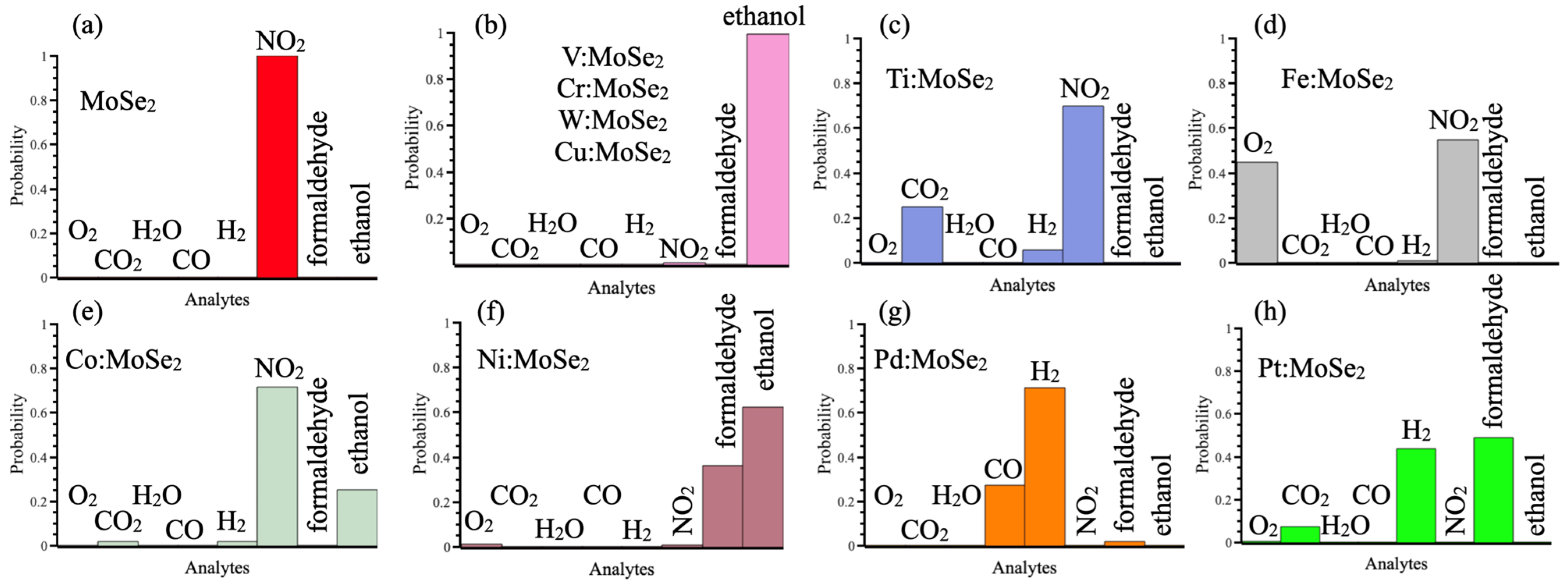
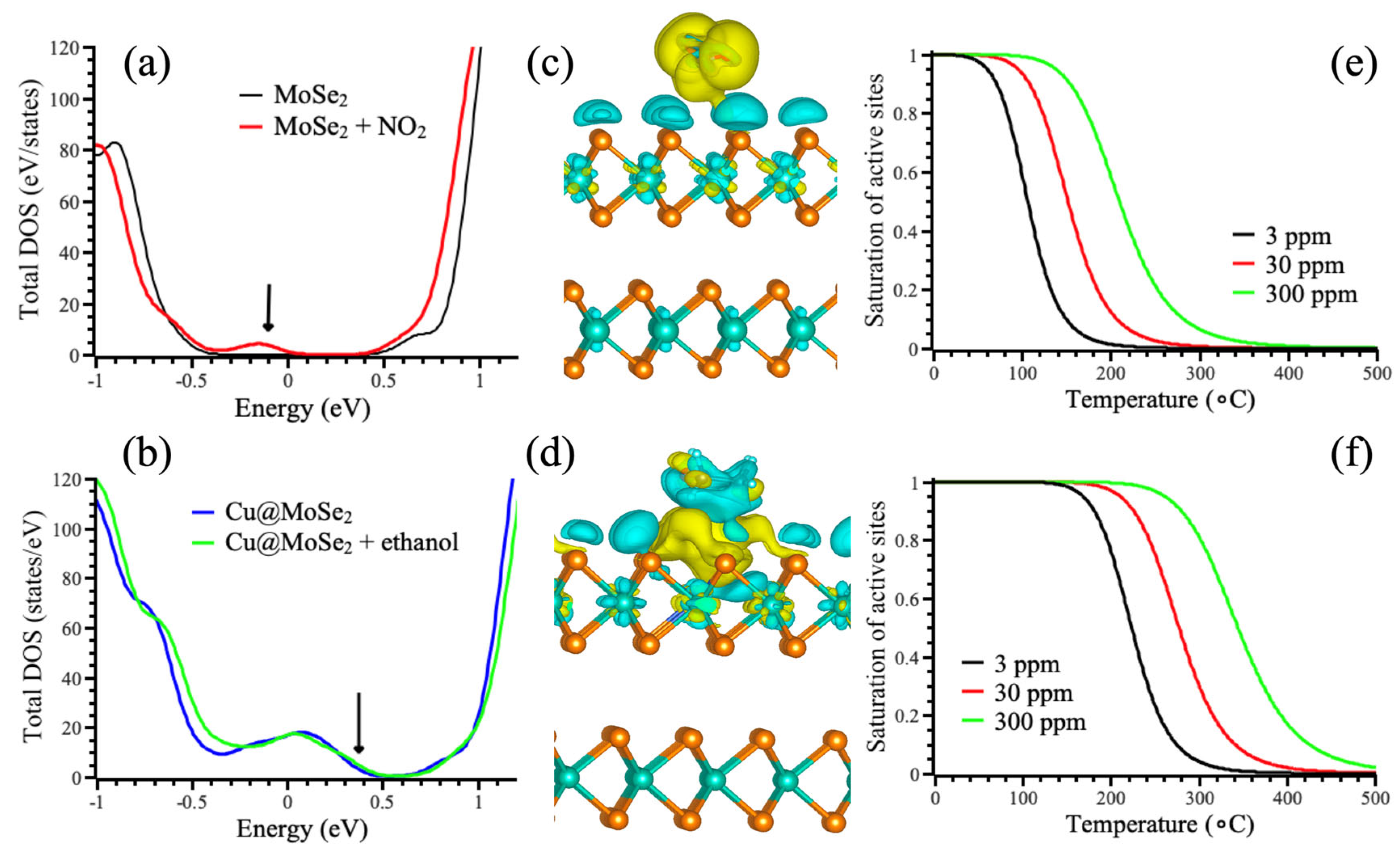

| Dopant | Efom(vSe) − E0form(vSe), kJ mol−1 | Analyte | ΔH, kJ mol−1 | ΔG, kJ mol−1 | |
|---|---|---|---|---|---|
| 400 °C | 800 °C | ||||
| Pure MoSe2 | 0.0 | O2 | −127.4 | −94.9 | −10.7 |
| CO2 | −157.1 | −139.8 | −93.4 | ||
| H2O | −35.1 | −2.5 | +114.6 | ||
| CO | −27.3 | −7.9 | +42.5 | ||
| H2 | −67.9 | −59.7 | −38.4 | ||
| NO2 | −195.2 | −162.1 | −76.0 | ||
| CH2O | −43.0 | −12.3 | +70.1 | ||
| C2H5OH | −150.1 | −117.3 | −32.0 | ||
| Ti | +46.5 | O2 | −132.8 | −100.4 | −16.1 |
| CO2 | −241.2 | −223.9 | −177.5 | ||
| H2O | −74.3 | −41.7 | +75.4 | ||
| CO | −22.2 | −2.8 | +47.6 | ||
| H2 | −194.8 | −186.6 | −165.3 | ||
| NO2 | −282.8 | −249.7 | −163.6 | ||
| CH2O | −55.1 | −24.4 | +58.0 | ||
| C2H5OH | −79.5 | −46.7 | +38.6 | ||
| V | +109.4 | O2 | −126.8 | −94.4 | −10.1 |
| CO2 | −48.0 | −30.7 | +15.7 | ||
| H2O | −43.1 | −10.5 | +106.6 | ||
| CO | −20.0 | −0.6 | +49.8 | ||
| H2 | −20.8 | −12.6 | +8.7 | ||
| NO2 | −74.7 | −41.6 | +44.5 | ||
| CH2O | −55.4 | −24.7 | +57.7 | ||
| C2H5OH | −155.9 | −123.1 | −37.8 | ||
| Cr | +149.1 | O2 | −117.0 | −84.6 | −0.3 |
| CO2 | −176.4 | −159.1 | −112.7 | ||
| H2O | −28.4 | +4.2 | +121.3 | ||
| CO | −25.9 | −6.5 | +43.9 | ||
| H2 | −21.5 | −13.3 | +8.0 | ||
| NO2 | −81.3 | −48.2 | +37.9 | ||
| CH2O | −46.7 | −16.0 | +66.4 | ||
| C2H5OH | −230.4 | −197.6 | −112.3 | ||
| Fe | +88.9 | O2 | −72.0 | −39.6 | +44.7 |
| CO2 | −39.9 | −22.6 | +23.8 | ||
| H2O | −33.0 | −0.4 | +116.7 | ||
| CO | −42.9 | −23.5 | +26.9 | ||
| H2 | −37.6 | −29.4 | −8.1 | ||
| NO2 | −73.2 | −40.1 | +46.0 | ||
| CH2O | −38.7 | −8.0 | +74.4 | ||
| C2H5OH | −43.0 | −10.2 | +75.1 | ||
| Co | +150.1 | O2 | −31.9 | +0.5 | +84.8 |
| CO2 | −38.6 | −21.3 | +25.1 | ||
| H2O | −23.0 | +9.6 | +126.7 | ||
| CO | −28.5 | −9.1 | +41.3 | ||
| H2 | −29.5 | −21.3 | 0.0 | ||
| NO2 | −63.6 | −30.5 | +55.6 | ||
| CH2O | −38.9 | −8.2 | +74.2 | ||
| C2H5OH | −60.7 | −27.9 | +57.4 | ||
| Ni | +220.3 | O2 | −166.2 | −133.8 | −49.5 |
| CO2 | −43.8 | −26.5 | +19.9 | ||
| H2O | −32.5 | +0.1 | +117.2 | ||
| CO | −56.1 | −36.7 | +13.7 | ||
| H2 | −61.5 | −53.3 | −32.0 | ||
| NO2 | −153.9 | −120.8 | −34.7 | ||
| CH2O | −255.5 | −224.8 | −142.4 | ||
| C2H5OH | −271.0 | −238.2 | −152.9 | ||
| Cu | +166.7 | O2 | −28.5 | +3.9 | +88.2 |
| CO2 | −89.3 | −72.0 | −25.6 | ||
| H2O | −67.9 | −35.3 | +81.8 | ||
| CO | −20.9 | −1.5 | +48.9 | ||
| H2 | −64.1 | −55.9 | −34.6 | ||
| NO2 | −113.8 | −80.7 | +5.4 | ||
| CH2O | −30.3 | +0.4 | +82.8 | ||
| C2H5OH | −125.7 | −92.9 | −7.6 | ||
| W | +20.1 | O2 | −119.3 | −86.9 | −2.6 |
| CO2 | −51.7 | −34.4 | +12.0 | ||
| H2O | −26.8 | +5.8 | +122.9 | ||
| CO | −23.8 | −4.4 | +46.0 | ||
| H2 | −38.2 | −30.0 | −8.7 | ||
| NO2 | −79.7 | −46.6 | +39.5 | ||
| CH2O | −46.9 | −16.2 | +66.2 | ||
| C2H5OH | −195.7 | −162.9 | −77.6 | ||
| Pd | +190.6 | O2 | −43.6 | −11.2 | +73.1 |
| CO2 | −95.4 | −78.1 | −31.7 | ||
| H2O | −64.7 | −32.1 | +85.0 | ||
| CO | −247.1 | −227.7 | −177.3 | ||
| H2 | −259.8 | −251.6 | −230.3 | ||
| NO2 | −113.1 | −80.0 | +6.1 | ||
| CH2O | −189.6 | −158.9 | −76.5 | ||
| C2H5OH | −110.7 | −77.9 | +7.4 | ||
| Pt | +206.8 | O2 | −147.1 | −114.7 | −30.4 |
| CO2 | −222.3 | −205.0 | −158.6 | ||
| H2O | −93.7 | −61.1 | +56.0 | ||
| CO | −50.8 | −31.4 | +19.0 | ||
| H2 | −258.1 | −249.9 | −228.6 | ||
| NO2 | −106.5 | −73.4 | +12.7 | ||
| CH2O | −283.5 | −252.8 | −170.4 | ||
| C2H5OH | −138.4 | −105.6 | −20.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhvalov, D.W.; Rakhimzhanov, M.K.; Shongalova, A.; Serikkanov, A.S.; Chuchvaga, N.A.; Osipov, V.Y. The Effect of the Metal Impurities on the Stability, Chemical, and Sensing Properties of MoSe2 Surfaces. Surfaces 2025, 8, 56. https://doi.org/10.3390/surfaces8030056
Boukhvalov DW, Rakhimzhanov MK, Shongalova A, Serikkanov AS, Chuchvaga NA, Osipov VY. The Effect of the Metal Impurities on the Stability, Chemical, and Sensing Properties of MoSe2 Surfaces. Surfaces. 2025; 8(3):56. https://doi.org/10.3390/surfaces8030056
Chicago/Turabian StyleBoukhvalov, Danil W., Murat K. Rakhimzhanov, Aigul Shongalova, Abay S. Serikkanov, Nikolay A. Chuchvaga, and Vladimir Yu. Osipov. 2025. "The Effect of the Metal Impurities on the Stability, Chemical, and Sensing Properties of MoSe2 Surfaces" Surfaces 8, no. 3: 56. https://doi.org/10.3390/surfaces8030056
APA StyleBoukhvalov, D. W., Rakhimzhanov, M. K., Shongalova, A., Serikkanov, A. S., Chuchvaga, N. A., & Osipov, V. Y. (2025). The Effect of the Metal Impurities on the Stability, Chemical, and Sensing Properties of MoSe2 Surfaces. Surfaces, 8(3), 56. https://doi.org/10.3390/surfaces8030056








