Hierarchical Manganese-Doped Nickel–Cobalt Oxide Electrodes with Graphene for Use as High-Energy-Density Supercapacitors
Abstract
1. Introduction
2. Experiment
2.1. Chemicals
2.2. Preparation of G@MNCO Electrodes
2.3. Characterization of G@MNCO Electrodes
2.4. Electrochemical Measurements
3. Results and Discussion
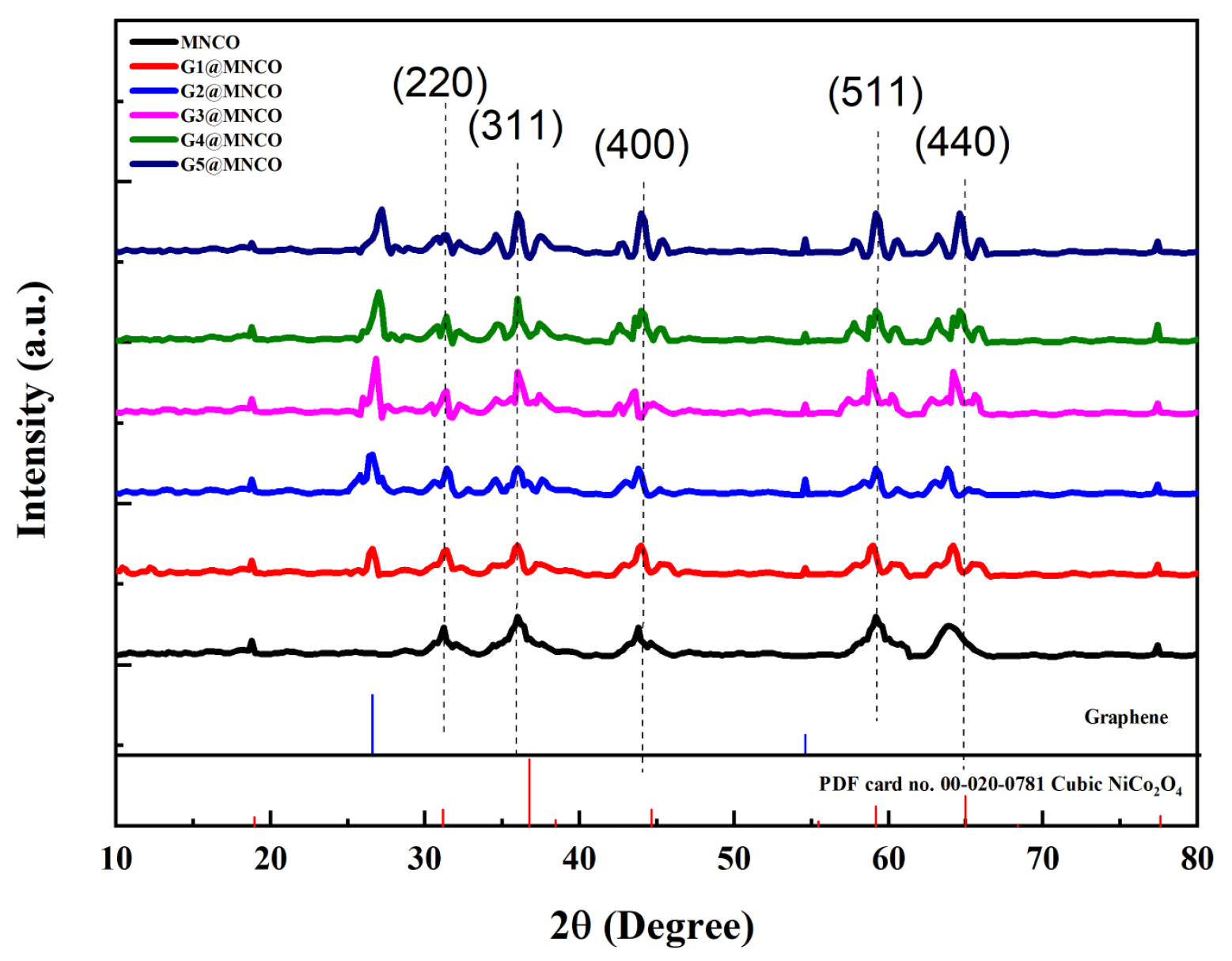
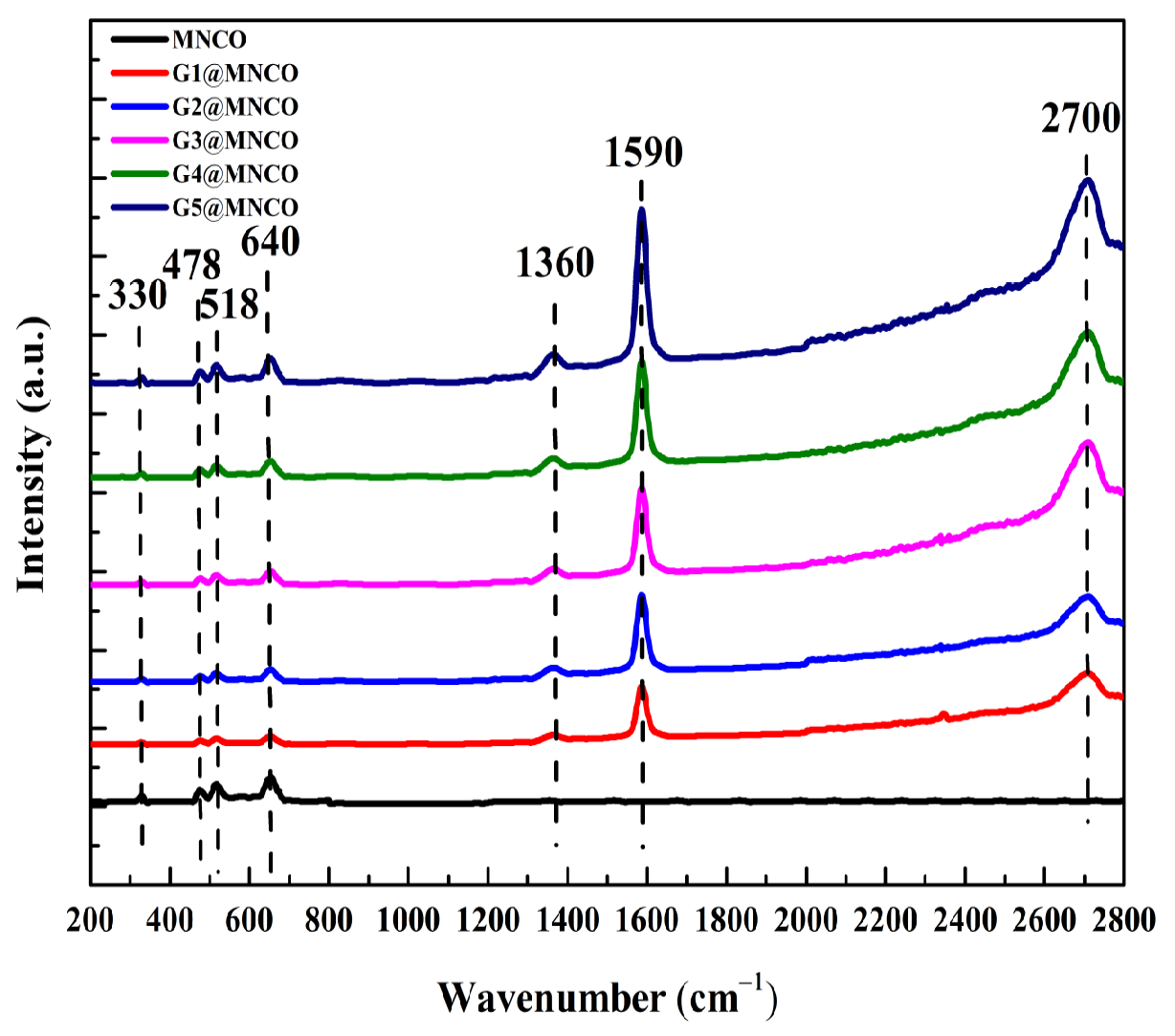
3.1. Crystalline-Phase and Raman Analyses
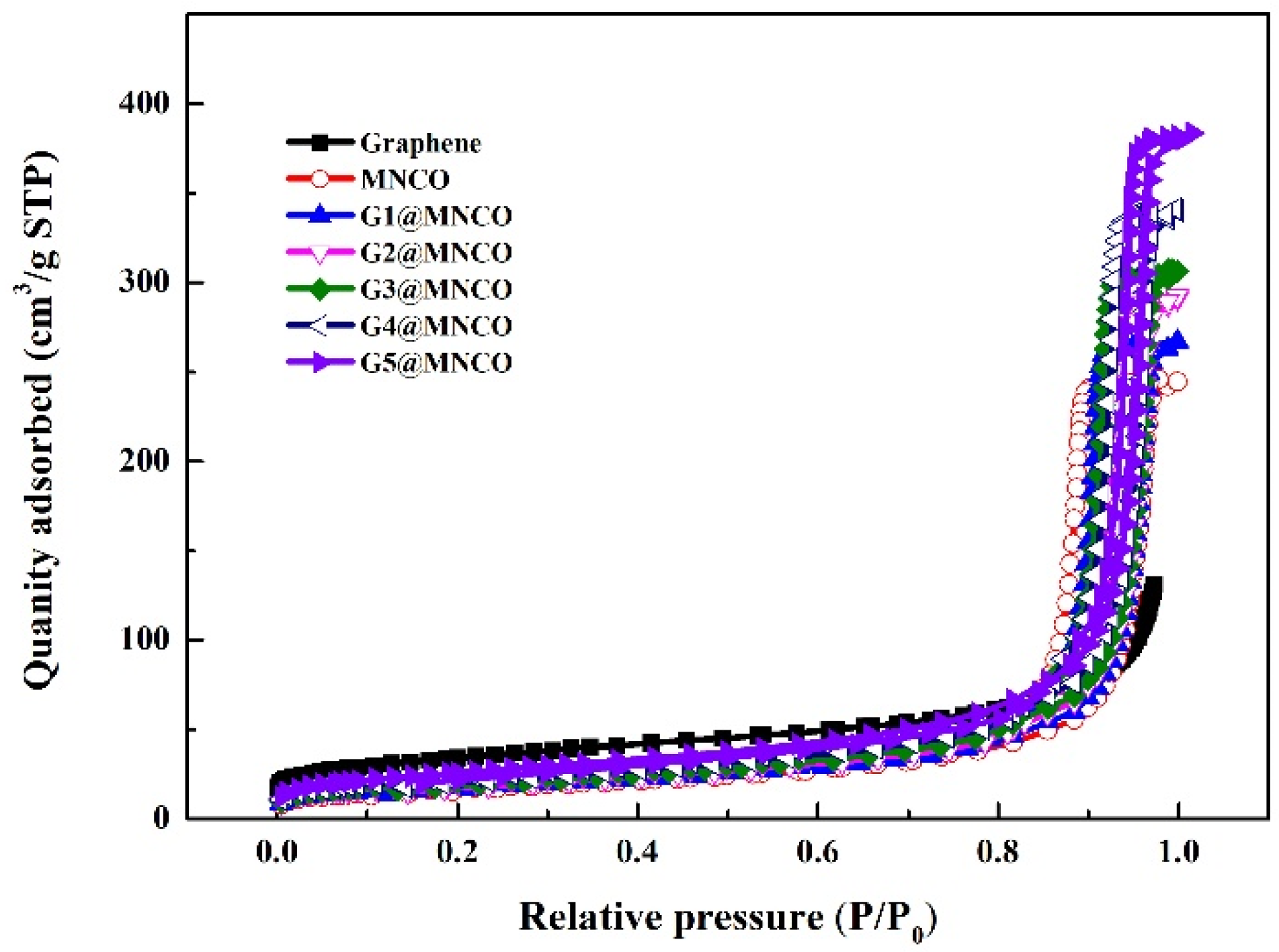
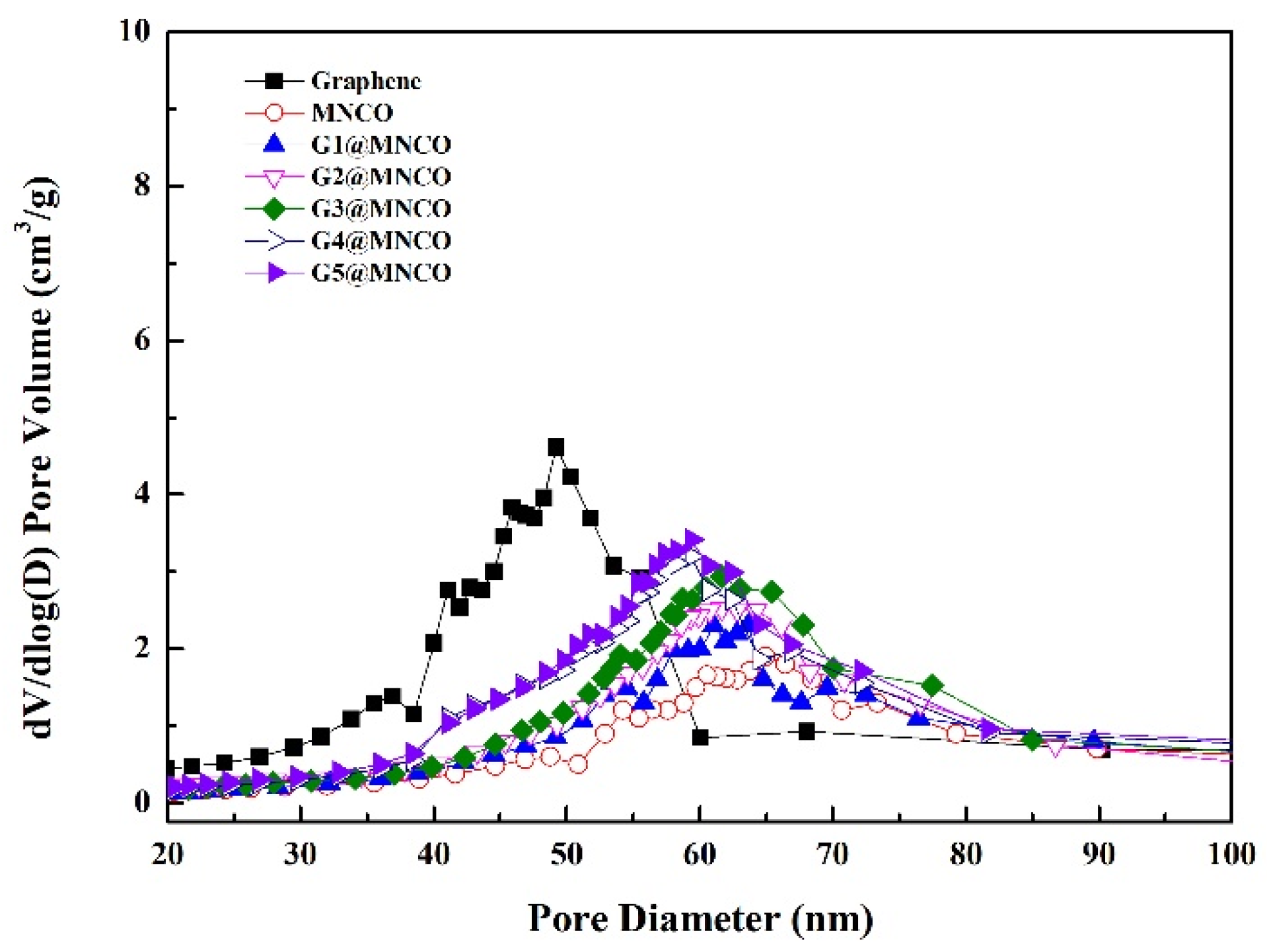
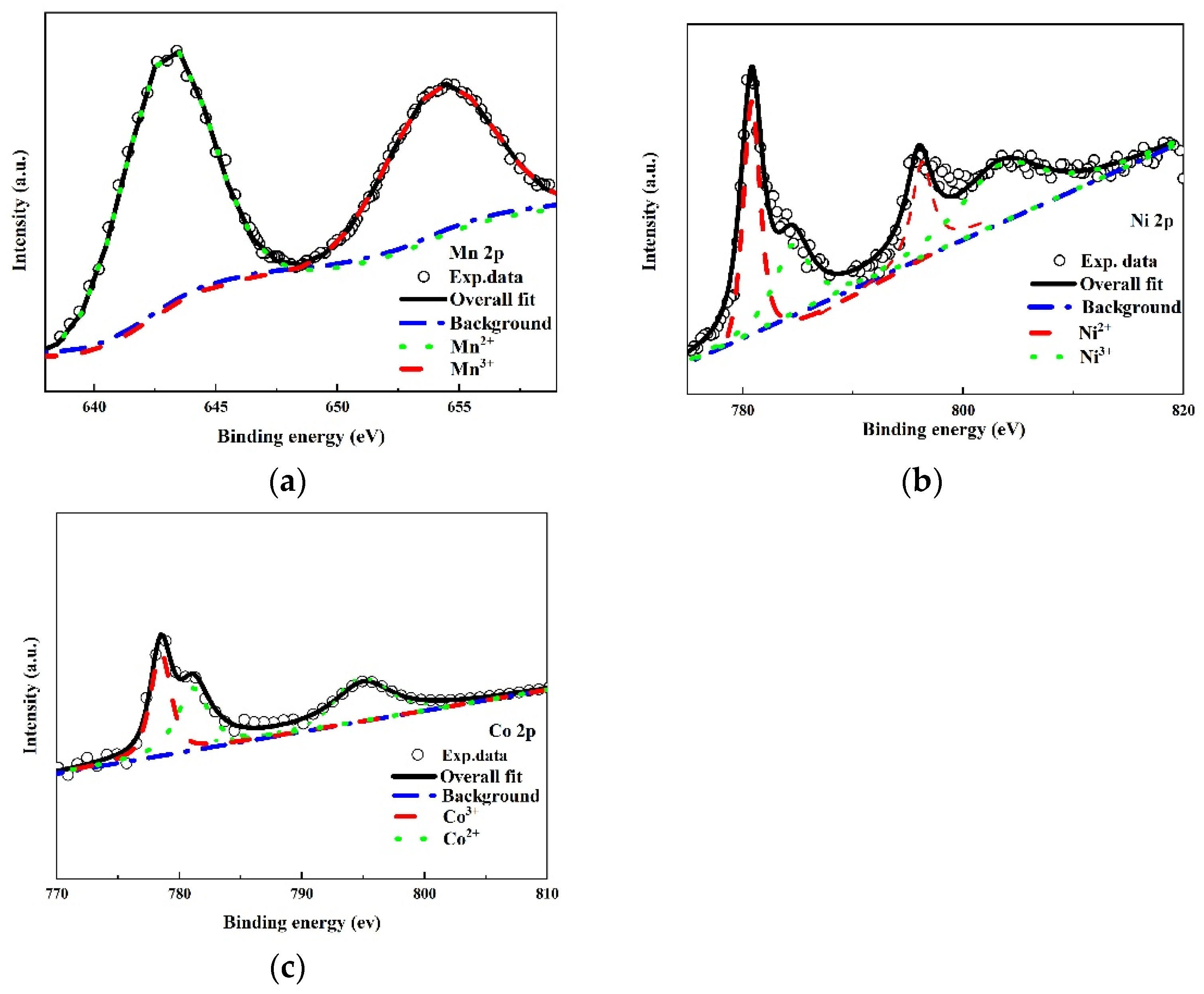
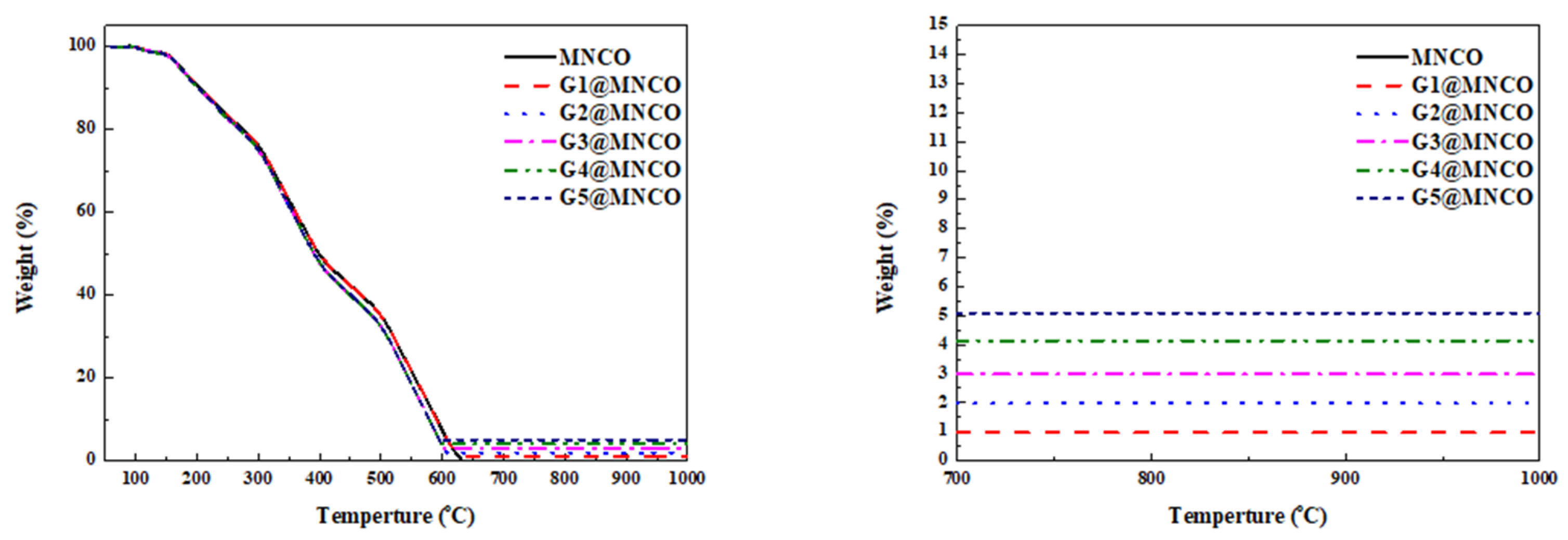
3.2. Characterization of Textural Properties
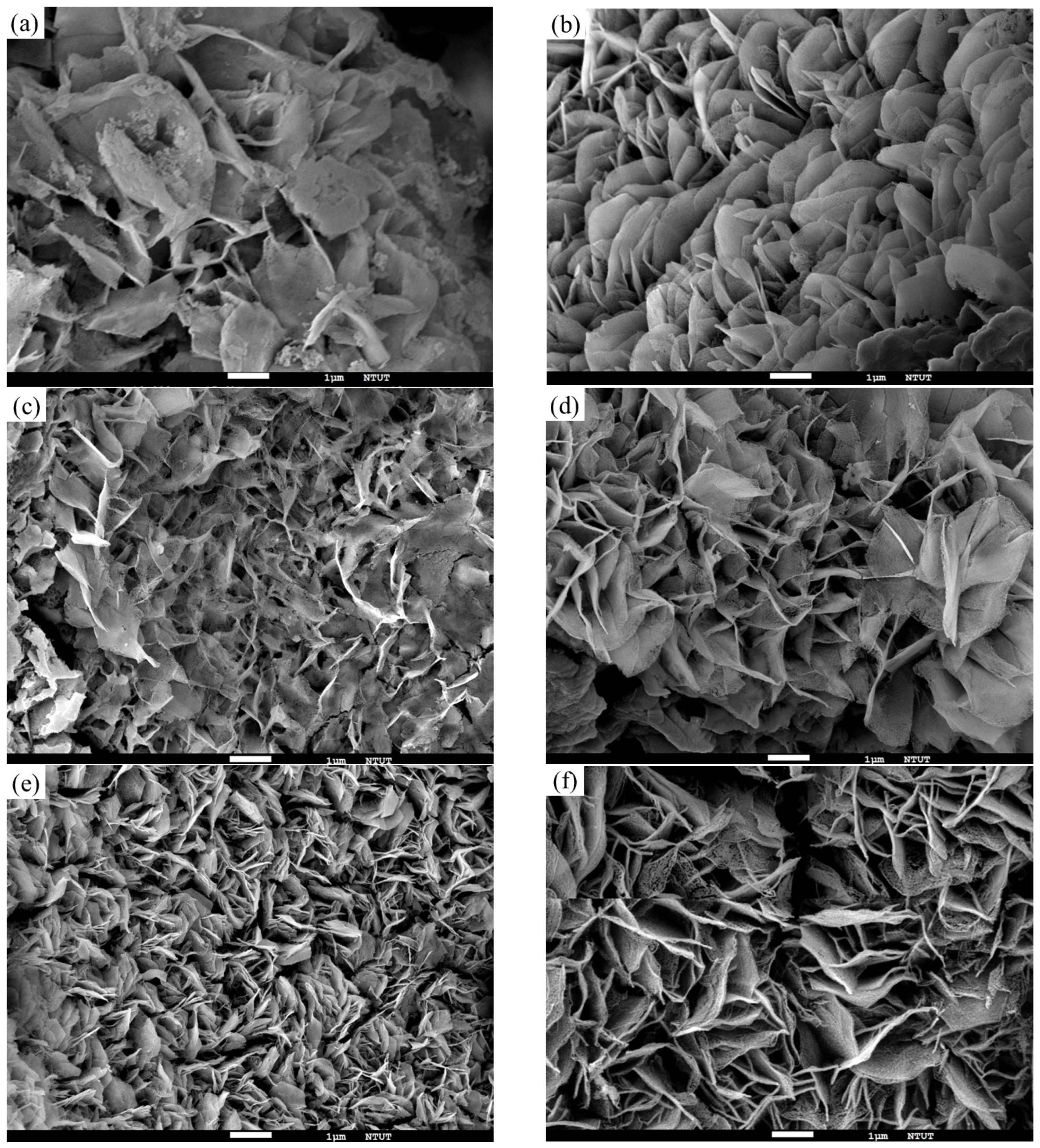
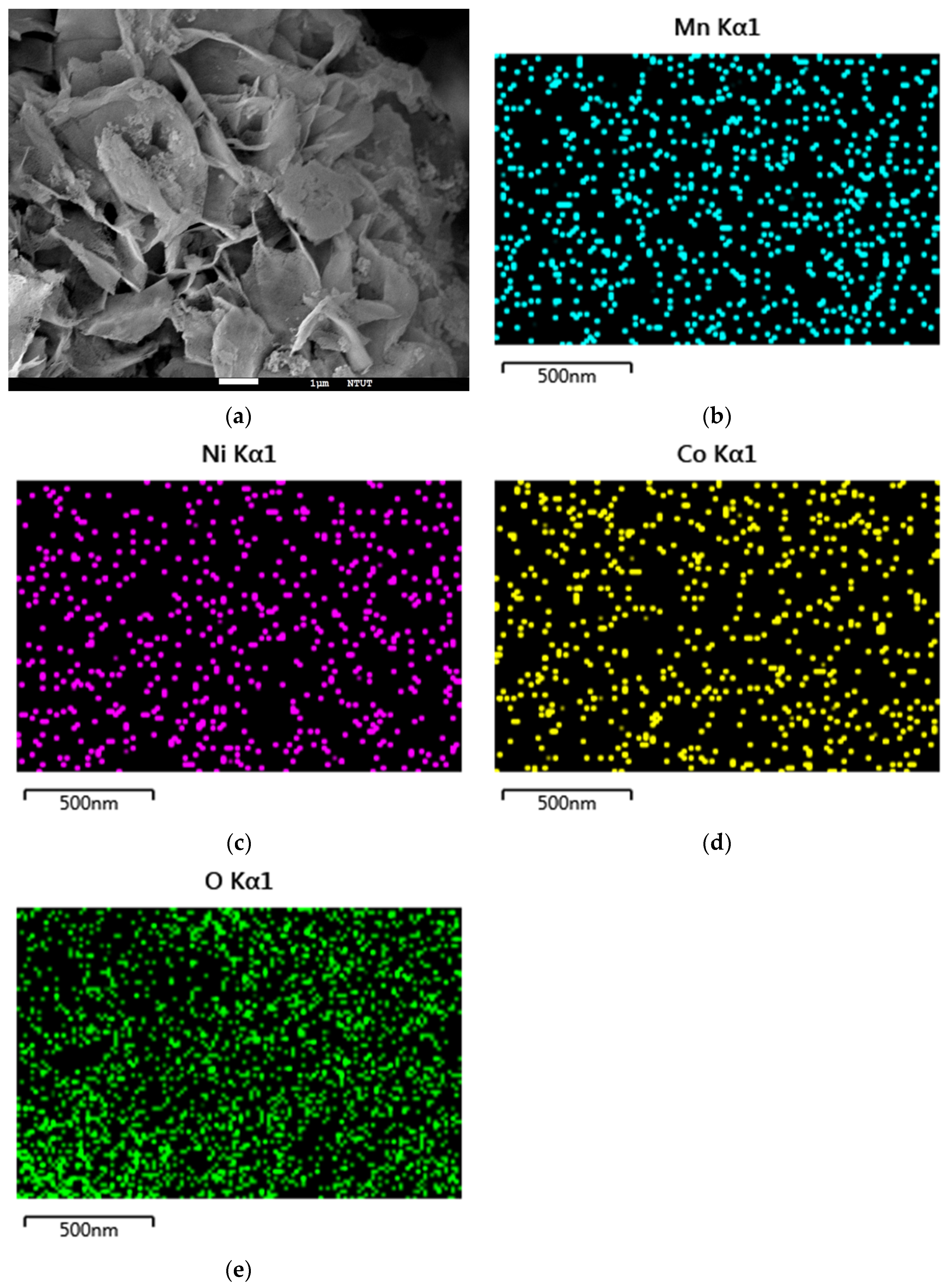
3.3. Morphology Analyses
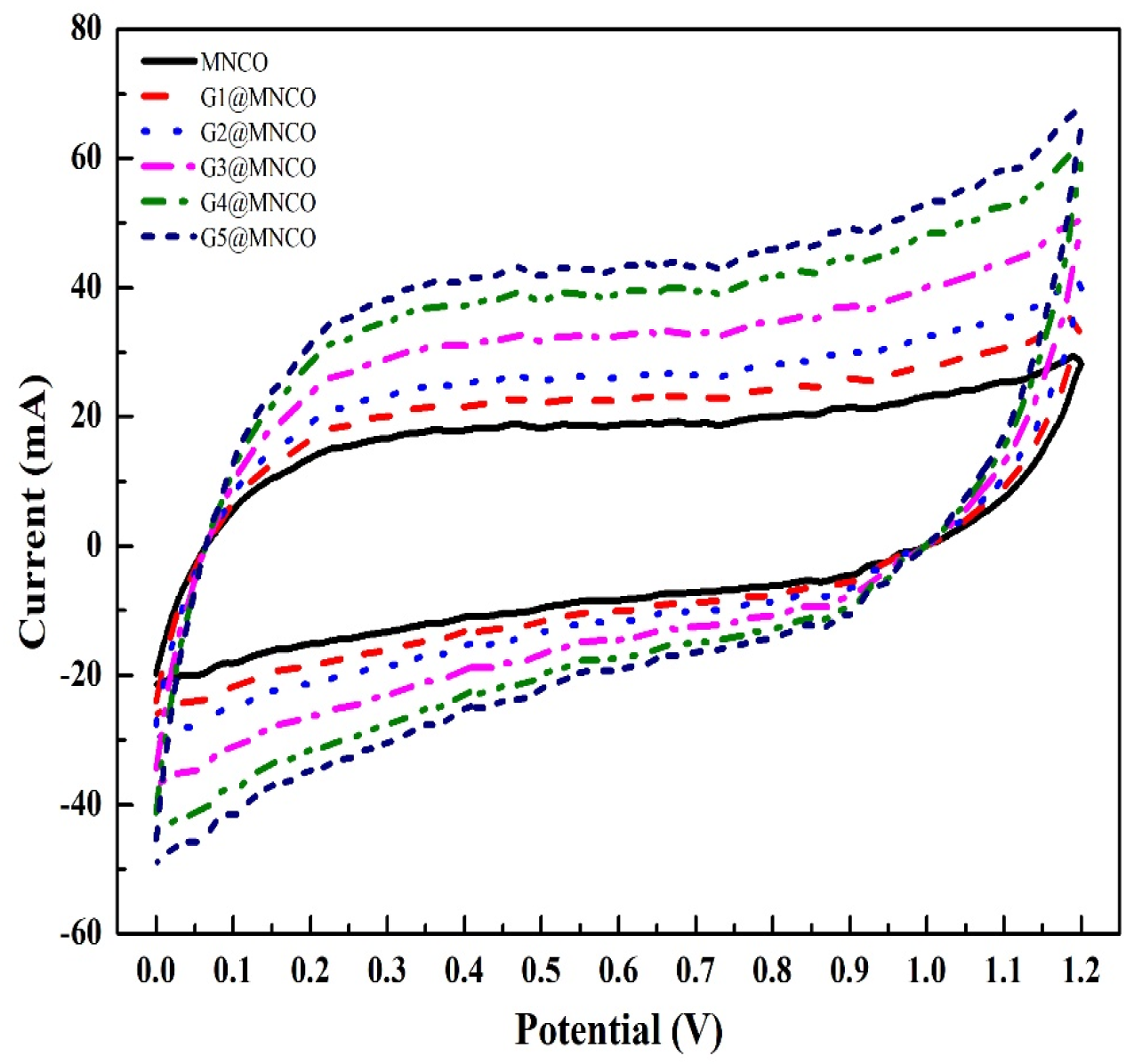
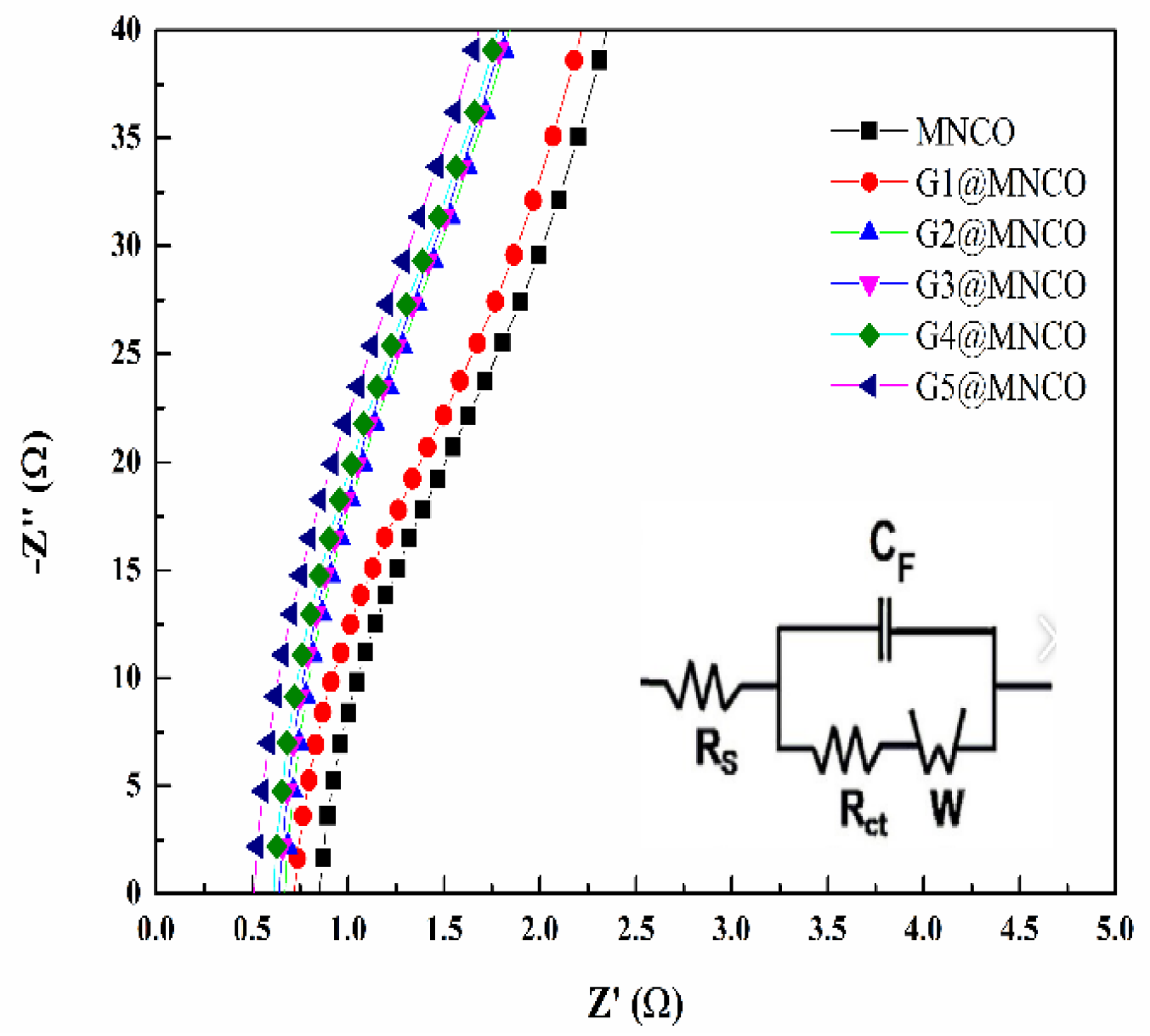
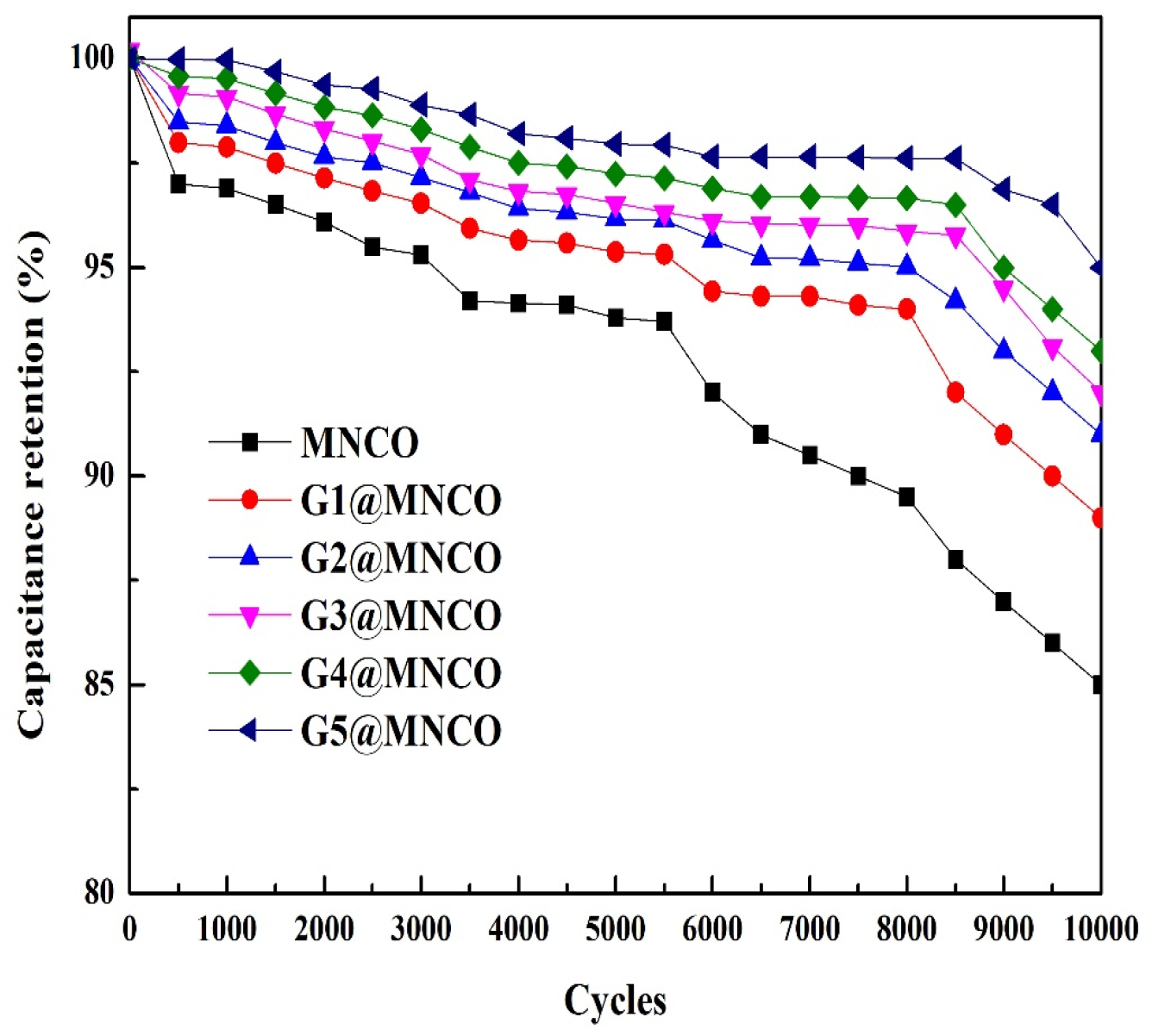
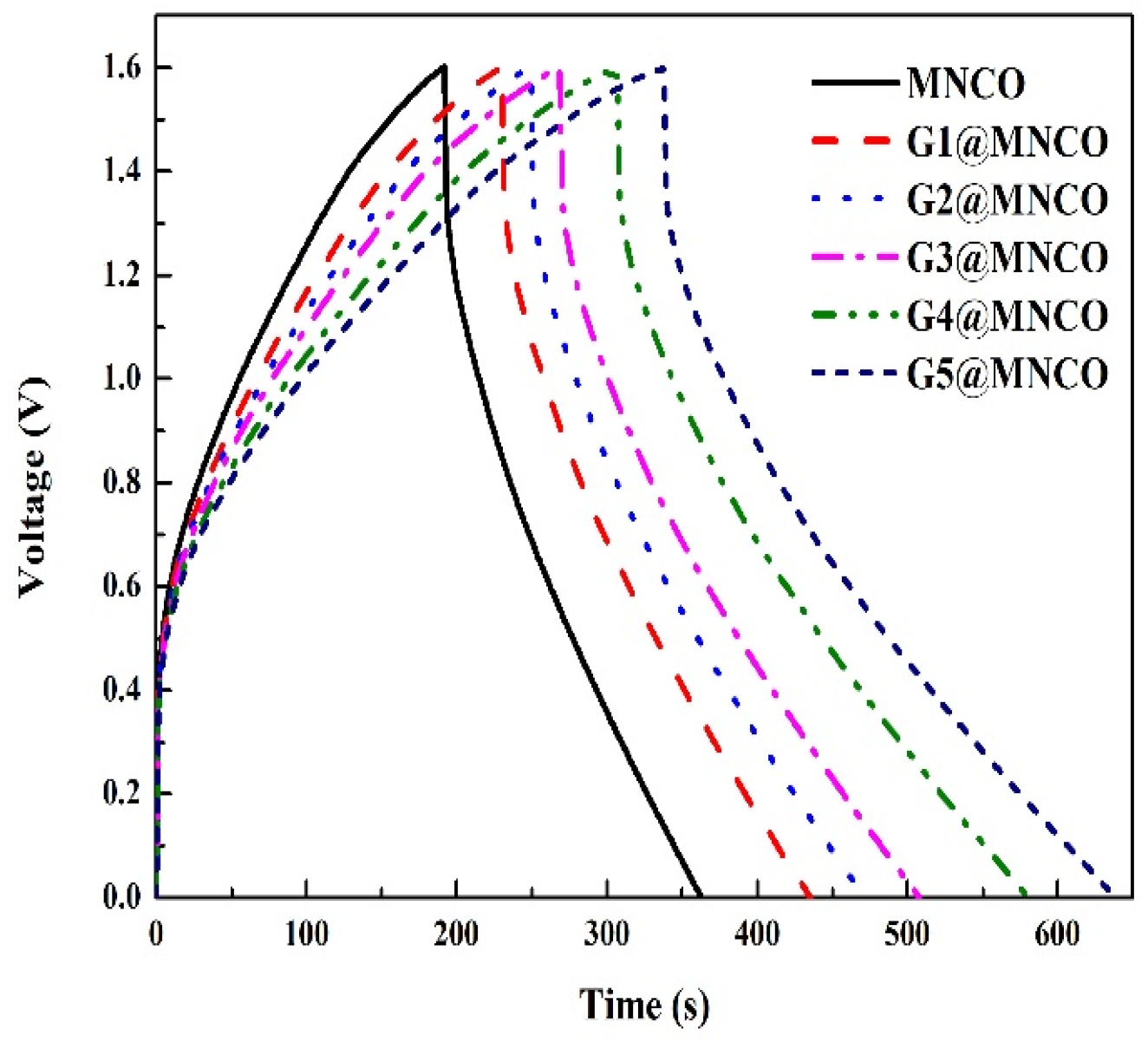
3.4. Electrochemical Performance of G@MNCO Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yun, Y.S.; Park, H.H.; Jin, H.-J. Pseudocapacitive effects of N-doped carbon nanotube electrodes in supercapacitors. Materials 2012, 5, 1258–1266. [Google Scholar] [CrossRef]
- Lee, H.-M.; Lee, K.; Kim, C.-K. Electrodeposition of manganese-nickel oxide films on a graphite sheet for electrochemical capacitor applications. Materials 2014, 7, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Chabot, V.; Yu, A. Carbon nanomaterials supported Ni(OH)2/NiO hybrid flower structure for supercapacitor. Electrochim. Acta 2013, 109, 370–380. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef]
- Shinde, S.; Jalak, M.; Ghodake, G.; Maile, N.; Kumbhar, V.; Lee, D.; Fulari, V.; Kim, D.-Y. Chemically synthesized nanoflakes-like NiCo2S4 electrodes for high-performance supercapacitor application. Appl. Surf. Sci. 2019, 466, 822–829. [Google Scholar] [CrossRef]
- Deng, B.; Luong, D.X.; Wang, Z.; Kittrell, C.; McHugh, E.A.; Tour, J.M. Urban mining by flash Joule heating. Nat. Commun. 2021, 12, 5794. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Zhang, S.; Luo, W.; Zhang, G. Controllable synthesis of CuS hollow microflowers hierarchical structures for asymmetric supercapacitors. Appl. Surf. Sci. 2018, 442, 711–719. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Shin, J.C. Core-shell structure of Co3O4@ CdS for high performance electrochemical supercapacitor. Chem. Eng. J. 2018, 335, 693–702. [Google Scholar] [CrossRef]
- He, Y.-L.; Xu, R.-D.; He, S.-W.; Chen, H.-S.; Li, K.; Zhu, Y.; Shen, Q.-F. Effect of NaNO3 concentration on anodic electrochemical behavior on the Sb surface in NaOH solution. Int. J. Miner. Metall. Mater. 2018, 25, 288–299. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.-W. Mesoporous transition metal oxides for supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Chen, S.; Wang, H.; He, Y.; Ma, C. Ni–SiC composite coatings with improved wear and corrosion resistance synthesized via ultrasonic electrodeposition. Ceram. Int. 2021, 47, 9437–9446. [Google Scholar] [CrossRef]
- Devendra, B.K.; Praveen, B.; Tripathi, V.; Nagaraju, G.; Prasanna, B.; Shashank, M. Development of rhodium coatings by electrodeposition for photocatalytic dye degradation. Vacuum 2022, 205, 111460. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Zhong, Y.; Wang, M.; Guo, Z. Ultrasound-Assisted Electrodeposition of Fe-Ni-Co Ternary Alloy Film: Microstructure, Corrosion Resistance, and Soft Magnetic Property. JOM 2024, 76, 4987–4997. [Google Scholar] [CrossRef]
- Costa, J.M.; De Almeida Neto, A.F.D. Ultrasound-assisted electrodeposition and synthesis of alloys and composite materials: A review. Ultrason. Sonochemistry 2020, 68, 105193–105204. [Google Scholar] [CrossRef]
- Liu, B.; Mei, T.; Chu, H.; Wang, J.; Du, S.; Miao, Y.; Zhang, W. Ultrasonic-assisted electrodeposition of Ni/diamond composite coatings and its structure and electrochemical properties. Ultrason. Sonochemistry 2021, 73, 105475–705485. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Feng, Y.; Yang, L.; Shi, X.; Xu, P.; Zhang, J.; Wang, P.; Wang, M.; Che, R. A flexible film toward high-performance lithium storage: Designing nanosheet-assembled hollow single-hole Ni–Co–Mn–O spheres with oxygen vacancy embedded in 3D carbon nanotube/graphene network. Small 2019, 15, 1901343. [Google Scholar] [CrossRef]
- Ahmad, F.; Zahid, M.; Jamil, H.; Khan, M.A.; Atiq, S.; Bibi, M.; Shahbaz, K.; Adnan, M.; Danish, M.; Rasheed, F. Advances in graphene-based electrode materials for high-performance supercapacitors: A review. J. Energy Storage 2023, 72, 108731. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Mineral. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Biju, V. Ni 2p X-ray photoelectron spectroscopy study of nanostructured nickel oxide. Mater. Res. Bull. 2007, 42, 791–796. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Yu, P.; Tian, C.; Feng, H.; Diao, Z.; Fu, H. Hierarchical porous NiCo2O4 nanosheet arrays directly grown on carbon cloth with superior lithium storage performance. Dalton Trans. 2017, 46, 4717–4723. [Google Scholar] [CrossRef]
- Chiku, M.; Abdollahifar, M.; Crosnier, O.; Dunn, B.; Brousse, T.; Fic, K.; Maćkowiak, A.; Okubo, M.; Naoi, K.; Ogihara, N.; et al. Redox Materials for Electrochemical Capacitors. Electrochemistry 2024, 92, 074002–074021. [Google Scholar] [CrossRef]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 Nanoplates Grown on Graphene as AdvancedElectrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 2010, 132, 7231–7558. [Google Scholar]
- Guan, J.; Liu, M.; Zhu, L.; Wang, J.; Han, Q.; Yang, X.; Hua, W.; Xie, L.; Cao, X. Ni/Co bimetallic organic frameworks nanospheres for high-performance electrochemical energy storage. Nano Res. 2024, 17, 5122–5130. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Liang, R.; Tjandra, R.; Yu, A. Graphene quantum dot induced tunable growth of nanostructured MnCo2O4.5 composites for high-performance supercapacitors. Sustain. Energy Fuels 2019, 3, 2499–2508. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Pan, G.-T.; Hong, Z.-Y.; Hsu, C.-T.; Chong, S.; Yang, T.C.-K.; Huang, C.-M. Biomass-derived porous carbons derived from soybean residues for high performance solid state supercapacitors. Molecules 2020, 25, 4050. [Google Scholar] [CrossRef]
- Chen, G.; Yang, G.; He, C.; Lan, T.; He, S.; Yang, H.; Liu, L.; Yang, W.; Jian, S.; Zhang, Q. High Capacitive Performance of N, O-Codoped Carbon Aerogels Synthesized via a One-Step Chemical Blowing and In Situ Activation Process. Langmuir 2024, 40, 19944–19953. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, X.; Xia, B.Y.; You, B. Efficient Noble-Metal-Free Integration Electrolysis for Solar H2 and Supercapacitor Electrode Coproduction in Acidic Water. ChemSusChem 2024, 17, e202301213. [Google Scholar] [CrossRef]
- Su, M.; Wang, Z.; Guo, H.; Li, X.; Huang, S.; Xiao, W.; Gan, L. Enhancement of the Cyclability of a Si/Graphite@ Graphene composite as anode for Lithium-ion batteries. Electrochim. Acta 2014, 116, 230–236. [Google Scholar] [CrossRef]
- Pacchioni, G. Oxygen Vacancy: The Invisible Agent on Oxide Surfaces. ChemPhyChem 2003, 4, 1041–1047. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761–104778. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Gao, G.; Fan, Y.; Wang, R.; Feng, J.; Yang, L.; Meng, A.; Zhao, J.; Li, Z. Effectively modulating oxygen vacancies in flower-like δ-MnO2 nanostructures for large capacity and high-rate zinc-ion storage. Nano-Micro Lett. 2023, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Amer, A.; Sayed, D.M.; Allam, N.K. A facile electrosynthesis approach of Mn-Ni-Co ternary phosphides as binder-free active electrode materials for high-performance electrochemical supercapacitors. Electrochim. Acta 2021, 380, 138197. [Google Scholar] [CrossRef]
- Du, C.; Shi, S.; Chen, G.; Zhang, Y.; Wei, Q.; Li, L.; Wan, G.; Deng, Z.; Wu, Y.; Su, Y. Structurally stable Fe–Ni–Co phosphide/graphene and nitrogen-doped carbon/graphene constructed by atomic layer deposition for high-performance hybrid supercapacitor. Mater. Today Energy 2023, 34, 101287. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Wang, C.; Wang, H.; Feng, S.; Li, T.; Ma, Y. Hierarchical graphene oxide/manganese dioxide/cobalt-nickel layered double hydroxide electrodes for high energy density asymmetric flexible supercapacitor. Chem. Eng. J. 2024, 484, 149430. [Google Scholar] [CrossRef]

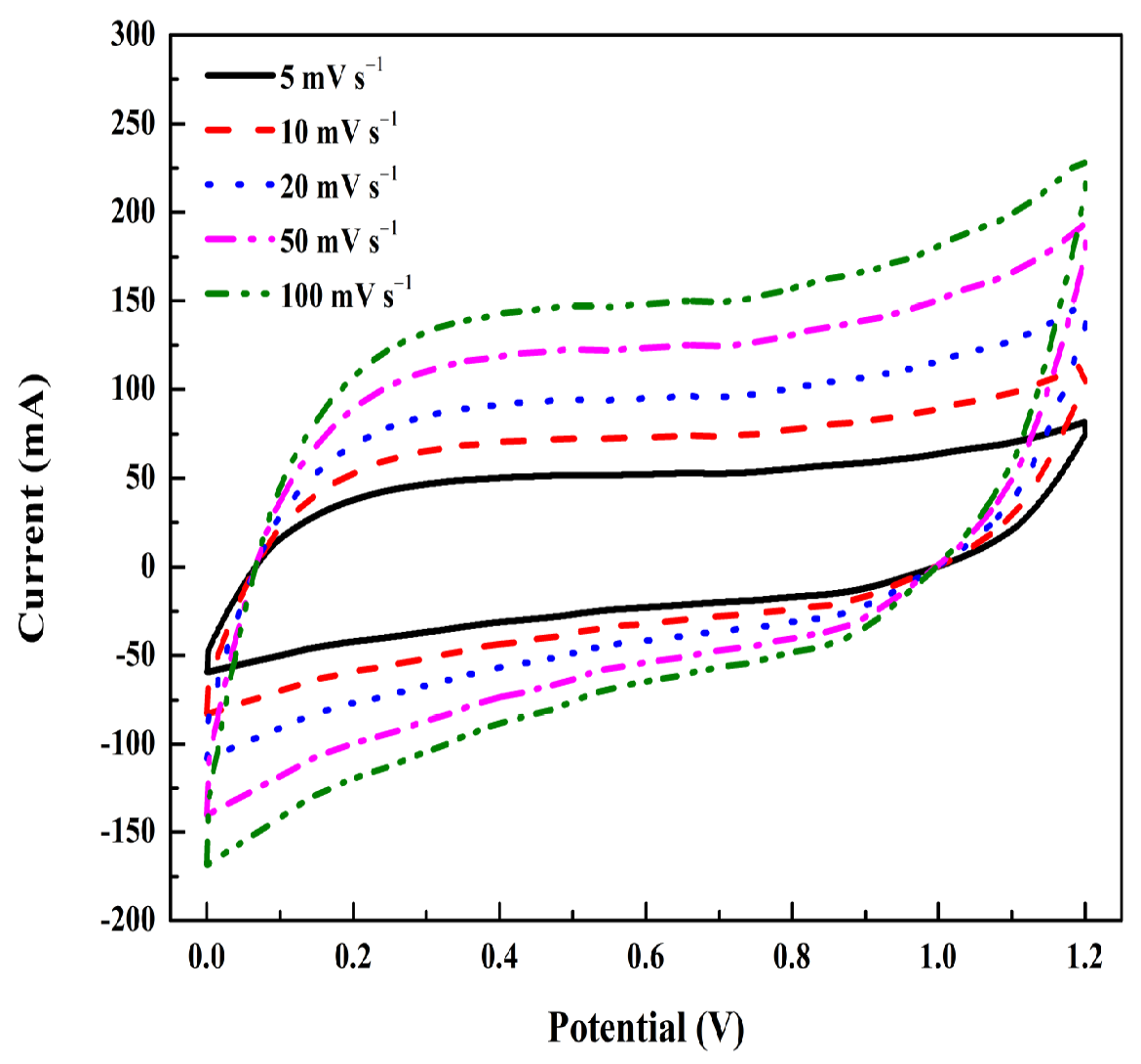
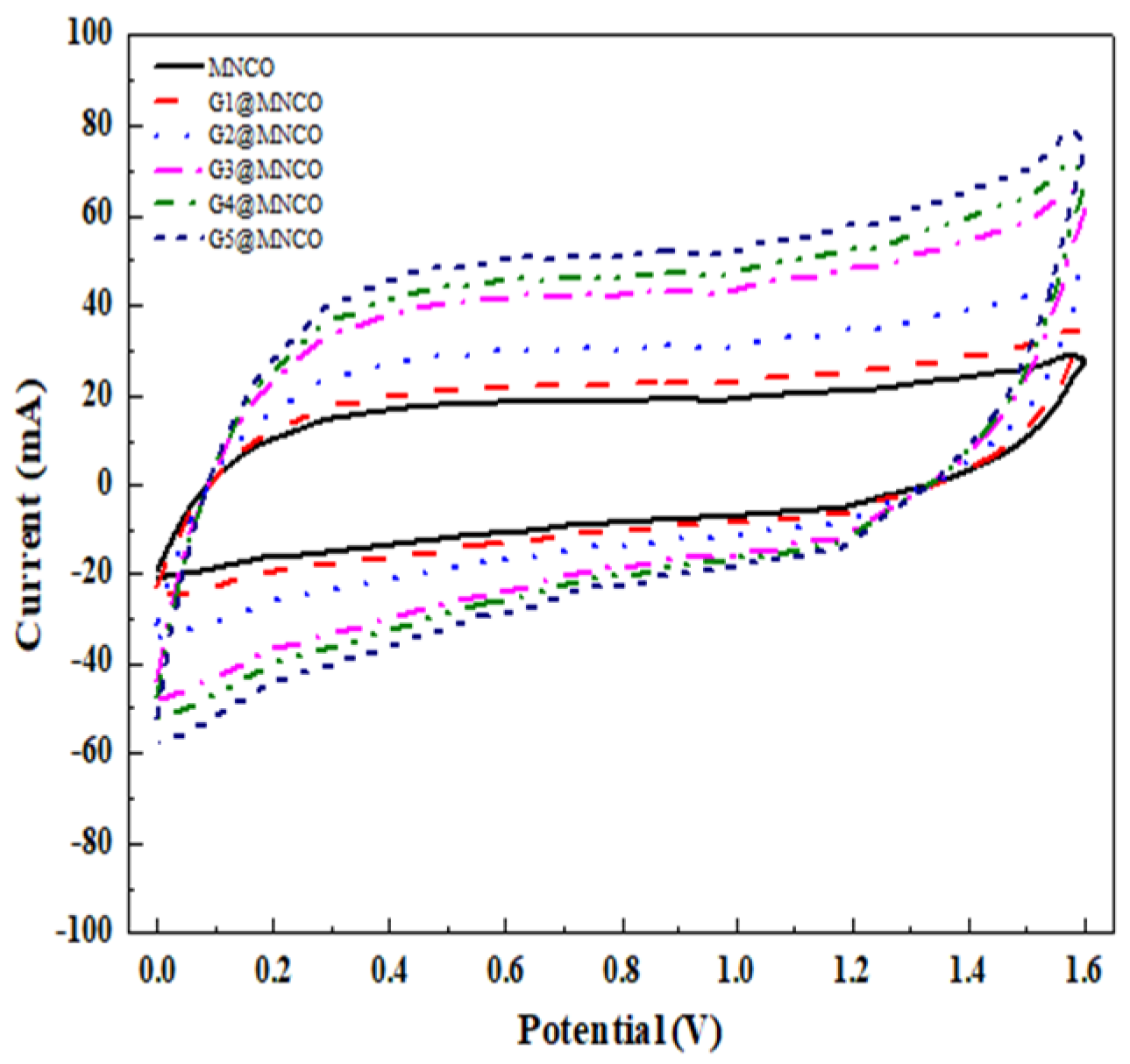
| Samples | Mole Ratios of Ni:Co:Mn | pH Value | Graphene (wt%) | Annealed Temperature (°C) |
|---|---|---|---|---|
| MNCO | 1:1:1 | 7 | 0 | 500 |
| G1@MNCO | 1:1:1 | 7 | 1 | 500 |
| G2@MNCO | 1:1:1 | 7 | 2 | 500 |
| G3@MNCO | 1:1:1 | 7 | 3 | 500 |
| G4@MNCO | 1:1:1 | 7 | 4 | 500 |
| G5@MNCO | 1:1:1 | 7 | 5 | 500 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) |
|---|---|---|---|
| Graphene | 513.28 ± 2.5 | 1.45 ± 0.03 | 6.76 ± 2 |
| MNCO | 112.45 ± 2.1 | 0.82 ± 0.02 | 157 ± 3 |
| G1@MNCO | 119.19 ± 2.1 | 0.84 ± 0.02 | 160 ± 3 |
| G2@MNCO | 126.34 ± 2.2 | 0.86 ± 0.02 | 163 ± 3 |
| G3@MNCO | 133.92 ± 2.3 | 0.89 ± 0.02 | 166 ± 3 |
| G4@MNCO | 141.96 ± 2.4 | 0.92 ± 0.03 | 169 ± 3 |
| G5@MNCO | 150.48 ± 2.5 | 0.95 ± 0.03 | 173 ± 4 |
| Sample | Mole Ratios of Ni:Co:Mn | Atomic Ratios of [Ni]/[Co]/[O]/[Mn] |
|---|---|---|
| MNCO | 1:1:1 | 1:4.16:4.9:0.019 |
| G1@MNCO | 1:1:1 | 1:4.12:3.8:0.019 |
| G2@MNCO | 1:1:1 | 1:4.31:3.1:0.016 |
| G3@MNCO | 1:1:1 | 1:4.14:2.8:0.017 |
| G4@MNCO | 1:1:1 | 1:4.11:2.5:0.016 |
| G5@MNCO | 1:1:1 | 1:4.23:2.1:0.021 |
| Sample | Atomic Ratios of [Ni]/[Co]/[O]/[Mn] |
|---|---|
| MNCO | 1:2.3:5.1:0.038 |
| G1@MNCO | 1:2.8:4.9:0.041 |
| G2@MNCO | 1:2.1:4.1:0.046 |
| G3@MNCO | 1:2.4:3.9:0.042 |
| G4@MNCO | 1:3.1:4.2:0.043 |
| G5@MNCO | 1:2.7:4.1:0.041 |
| Samples | Specific Capacitance (F/g) | Intrinsic Resistance Electrode (Ω) | Capacitance Retention After 10,000-Cycle Test |
|---|---|---|---|
| MNCO | 103 | 0.82 | 85% |
| G1@MNCO | 132 | 0.72 | 89% |
| G2@MNCO | 151 | 0.69 | 91% |
| G3@MNCO | 168 | 0.64 | 92% |
| G4@MNCO | 182 | 0.61 | 93% |
| G5@MNCO | 202 | 0.55 | 95% |
| Material Used | Synthesis Method | Specific Capacitance F/g | Scan Rate/Current Density | Ref. |
|---|---|---|---|---|
| MnNiCo/graphene | Electrochemical deposition | 531 | 5 mV/s | This work |
| Ternary metallic nickel–cobalt–manganese phosphides | Electrochemical deposition | 177 | 1 A/g | [32] |
| FeNiCoP/rGO | Atomic layer deposition | 240 | 1 A/g | [33] |
| Hierarchical graphene oxide/manganese dioxide/cobalt–nickel | Drip coating method | 163 | 1 A/g | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-C.; Pan, G.-T.; Yang, T.C.-K.; Shen, P.-C.; Pan, K.L.; Tiong, T.J.; Nikoloski, A.N.; Huang, C.-M. Hierarchical Manganese-Doped Nickel–Cobalt Oxide Electrodes with Graphene for Use as High-Energy-Density Supercapacitors. Surfaces 2025, 8, 43. https://doi.org/10.3390/surfaces8030043
Lee K-C, Pan G-T, Yang TC-K, Shen P-C, Pan KL, Tiong TJ, Nikoloski AN, Huang C-M. Hierarchical Manganese-Doped Nickel–Cobalt Oxide Electrodes with Graphene for Use as High-Energy-Density Supercapacitors. Surfaces. 2025; 8(3):43. https://doi.org/10.3390/surfaces8030043
Chicago/Turabian StyleLee, Kuan-Ching, Guan-Ting Pan, Thomas Chung-Kuang Yang, Po-Cheng Shen, Kuan Lun Pan, Timm Joyce Tiong, Aleksandar N. Nikoloski, and Chao-Ming Huang. 2025. "Hierarchical Manganese-Doped Nickel–Cobalt Oxide Electrodes with Graphene for Use as High-Energy-Density Supercapacitors" Surfaces 8, no. 3: 43. https://doi.org/10.3390/surfaces8030043
APA StyleLee, K.-C., Pan, G.-T., Yang, T. C.-K., Shen, P.-C., Pan, K. L., Tiong, T. J., Nikoloski, A. N., & Huang, C.-M. (2025). Hierarchical Manganese-Doped Nickel–Cobalt Oxide Electrodes with Graphene for Use as High-Energy-Density Supercapacitors. Surfaces, 8(3), 43. https://doi.org/10.3390/surfaces8030043










