1. Introduction
The widespread use of concrete as a construction material worldwide is due to its versatility and durability [
1]. The degradation of reinforced concrete is generally not attributed to a single cause, but rather to a combination of various factors. Concrete acts as a physical barrier that protects reinforcement steel from environmental elements that could induce corrosion. Once corrosion of the reinforcement bars begins, particularly in high-salinity marine environments where chloride ions are present, the propagation rate increases, especially in the presence of elevated oxygen levels [
2].
In urban areas, unlike in rural areas, a more aggressive environment is present for reinforced concrete structures [
3]. In coastal zones, the most aggressive factors affecting these constructions are the high humidity and salinity characteristics of coastal regions, which attack the concrete and eventually reach the steel reinforcement, initiating the corrosion process. This occurs when the reinforcement steel encounters environmental moisture that penetrates the concrete, carrying elements that are naturally present in the atmosphere [
4]. These elements, in aqueous solution, react with the steel, triggering the corrosion of the internal structures of reinforced concrete, which deteriorate at early ages, leading to the consumption of resources to repair the damage. For this reason, in recent years, the use of natural materials has gained greater relevance.
The following study will analyze the effect of nopal mucilage on the corrosion rate of reinforcement steel in reinforced concrete structures.
One of the factors causing the deterioration of reinforced concrete structures is the corrosion of the steel bars used as reinforcement. This occurs due to environmental factors, poor construction processes, and imperfections generated during the concrete setting process [
5].
Mexico is a country with a high-humidity and high-salinity climate in its coastal zones, which extend for approximately 10,000 km [
6]. Generally, environments with high temperatures and relative humidity above 80% promote the corrosion of iron and other metals. Atmospheric pollutants accelerate metal corrosion. Among these pollutants, SO
2 and NaCl are particularly significant, and their effects have been the subject of multiple studies [
7].
Among the methods to mitigate this problem is the use of industrial products, which present certain drawbacks: they are typically high-cost, require specialized labor for application, and are not environmentally friendly. An alternative to these materials is the use of organic compounds derived from certain plants as additives for mortars used in construction [
8].
Currently, efforts are being made to develop new technology aimed at preventing negative environmental impacts [
9], promoting the use of sustainable and efficient materials and techniques, avoiding the use of costly and inaccessible technology, and reducing the need for transportation and storage of additional materials and resources.
The incorporation of natural polymers as a sustainable alternative to enhance the properties of concrete [
9] and their influence on the degradation process of structures is a relatively unexplored phenomenon. In this study, concrete mixtures enriched with different percentages of nopal mucilage were analyzed with regard to parameters such as open-circuit potential, linear polarization resistance, and SEM.
These organic materials are also referred to as natural polymers because they enhance the properties of concrete, such as its moisture resistance. Additionally, they can be sourced from locations near construction sites and, as part of the natural environment, they are low-cost [
10].
Cactus extract modifies certain properties of mortar, providing special characteristics that help to protect embedded reinforcement bars in construction structures. This results in a delay or reduction in the corrosion rate of these bars, ultimately extending the service life of the structures [
11].
In this work, the influence of nopal mucilage on the corrosion rate of reinforcing steel under natural atmospheric conditions and high-humidity conditions is investigated, using the electrochemical technique of open-circuit potential. This research contributes to the development of sustainable construction practices by offering a natural, cost-effective alternative to industrial additives. By improving the durability of concrete structures, particularly in challenging environments, it helps to reduce maintenance costs and extends the lifespan of infrastructure, ultimately minimizing environmental impact.
2. Materials and Methods
Cladodes were collected in the municipality of Tampico, located in the southern region of the state of Tamaulipas. Although there are various methods for the extraction of mucilage [
12], the selected method avoids the use of specialized equipment and materials. The collected cladodes were cut into approximately 2 cm squares, until 1 kg was obtained. They were then macerated in water at a 1:9 ratio for 24 h at room temperature. After this period, the mixture was blended and left to rest for another 24 h. Finally, it was filtered using a household sieve [
13].
Figure 1 illustrates the mucilage extraction process.
The mix design was carried out based on data obtained during the characterization of fine and coarse aggregates. According to the methodology specified in the Mexican standard NMX-C-155-ONNCCE-2014, the required amounts of cement, water, coarse aggregate, and fine aggregate were determined to obtain concrete with a compressive strength of 19.6 MPa [
14].
Using this design as the base or reference mix, concrete mixtures with different mucilage contents (0, 5, 10, and 15%), replacing mixing water, were prepared. These mixtures were designated as M0, M1, M2, and M3, respectively, and the test specimens were fabricated. Compressive strength tests were conducted, and it was observed that at early ages of 10 and 14 days, the mixes containing mucilage exhibited lower values compared to the reference mix, M0. However, after 60 days of age, the mixes with 5 and 10% mucilage achieved values exceeding the reference by more than 25%.
To analyze the topographical characteristics and pore size at the surface level of the specimens, a GMLQ HTS-5-1144 digital microscope (X4D-1600X Handheld Electron Microscope; Brand: GMLQ, Model: HTS-5-1144, Made in China) was used [
15]. The mucilage concentrations (0, 5, 10, and 15%) were chosen to evaluate the effect of varying mucilage levels on concrete’s corrosion resistance, providing insights into optimal concentrations. Some studies have shown that efficiencies ranging from 40 to 70% can be achieved by using mucilage concentrations of 1.5 to 8% in mix design [
16]. Higher concentrations may lead to a reduction in the mechanical strength of concrete [
17].
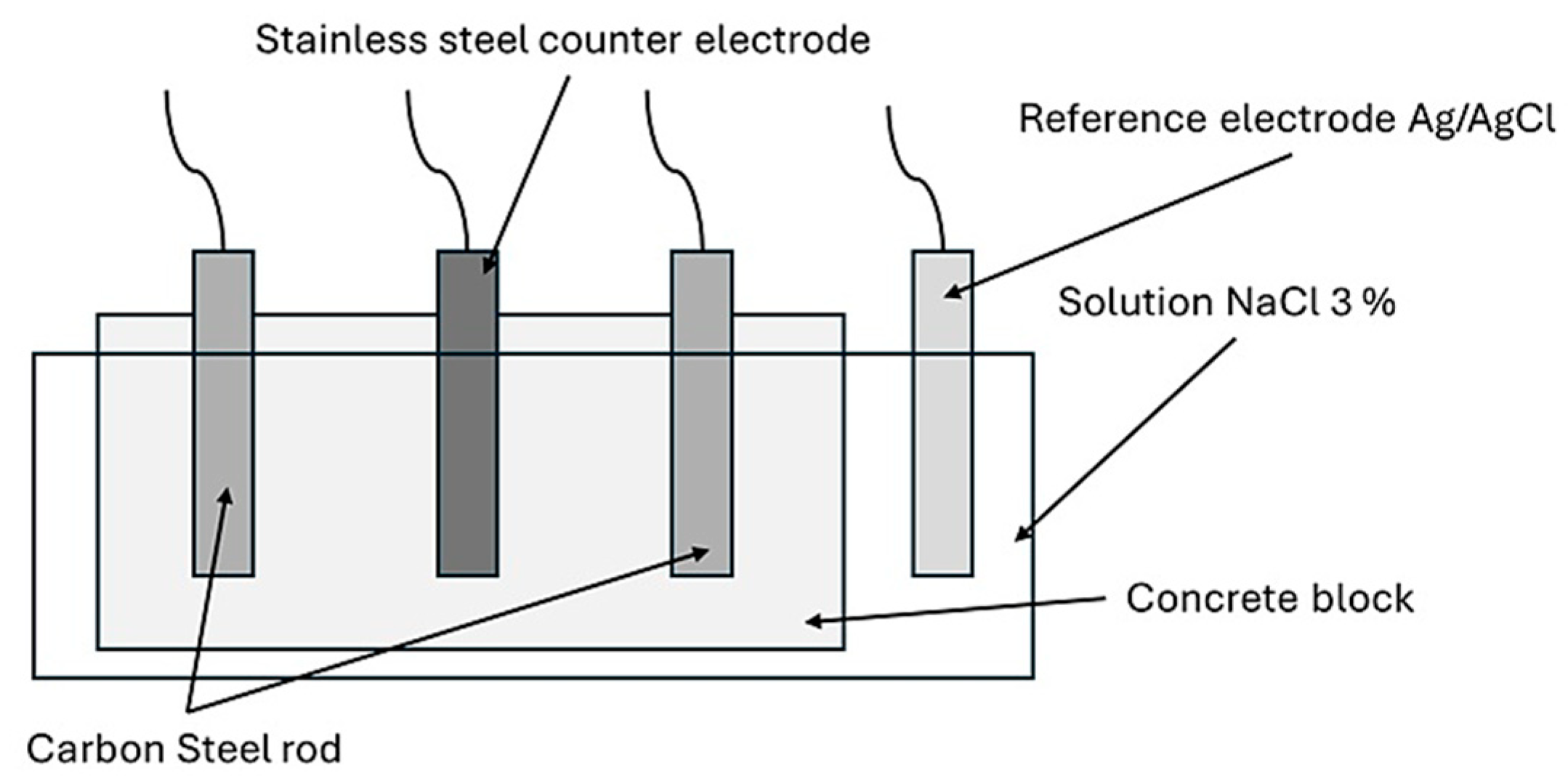
Two sets of prismatic specimens were fabricated, each with base dimensions of 9 cm × 12 cm and a height of 12 cm, incorporating embedded stainless steel electrodes as counter-electrodes and carbon steel electrodes as working electrodes for open-circuit potential and corrosion rate tests.
Figure 2 illustrates the electrode arrangement for electrochemical testing.
The test specimens were simultaneously exposed to two different environments throughout the testing period: outdoor exposure and high-humidity conditions, designated as A and B, respectively. The recorded outdoor atmospheric conditions during the testing period were a temperature of 23.4 ± 5 °C, and a precipitation of 568 mm during the rainy season and 4 mm during the dry season, with an annual relative humidity of 70–90% [
18]. For the electrochemical tests, the specimens in environment B were kept in a container with potable water, submerged until the water surface was 0.5 cm below the top level of the concrete specimen [
19]. Meanwhile, the specimens in environment A were placed in a bucket with potable water for 48 h before each measurement, to ensure complete concrete saturation.
The characteristics of the potable water used in this experiment are regulated by the Mexican Official Standard NOM-127-SSA1-2021, which establishes the permissible quality limits that water intended for human consumption must meet.
Table 1 presents the most relevant physicochemical parameters and their acceptable ranges [
19].
The working electrode (carbon steel) measured the corrosion potential, while the counter-electrode (stainless steel) helped to close the circuit. The reference electrode (Ag/AgCl) ensured accurate potential measurements, allowing the corrosion rate to be determined through polarization resistance analysis.
To perform the open-circuit potential (OCP) test, the electrochemical cell was connected to a Metrohm AUTOLAB PGSTAT 302N potentiostat/galvanostat (Manufacturer: Metrohm AG, City: Herisau, Country: Switzerland). The concrete specimen was connected, considering the carbon steel rebar as the working electrode, with a surface area of 24 cm
2, and the stainless steel rebar as the counter-electrode, both embedded in the concrete. The OCP method (open-circuit potential) was employed, and the equipment determined the open-circuit potential of the cell [
20].
Figure 3 illustrates the working cell arrangement for electrochemical testing.
To determine the corrosion rate, the stainless steel rebar was connected as the counter-electrode, the carbon steel as the working electrode, and an Ag/AgCl electrode as the reference electrode. A working potential was applied from the open-circuit potential ±20 mV in the cathodic direction, with a scan rate of 0.05 mV/min [
20]. The equipment determined the corrosion rate using the Stern–Geary equation, based on the polarization resistance and corrosion current values obtained during the analysis.
3. Results and Discussion
Through micrographic analysis, the size of the pores formed in the mixtures throughout the test period was determined.
Table 2 presents the average size of the formed pores.
The data exhibit an interval of ±0.01 mm. This value represents the standard deviation determined through basic statistical analysis, based on the reproducibility of the various experimental studies conducted. Mucilage is a gel capable of absorbing significant amounts of water [
21]. Over time, the water contained in the mucilage migrates towards the concrete, integrating during the curing stage, leaving void spaces. The higher the amount of mucilage, the larger these voids. A reduction in pore size corresponds to the incorporation of water into the concrete [
17].
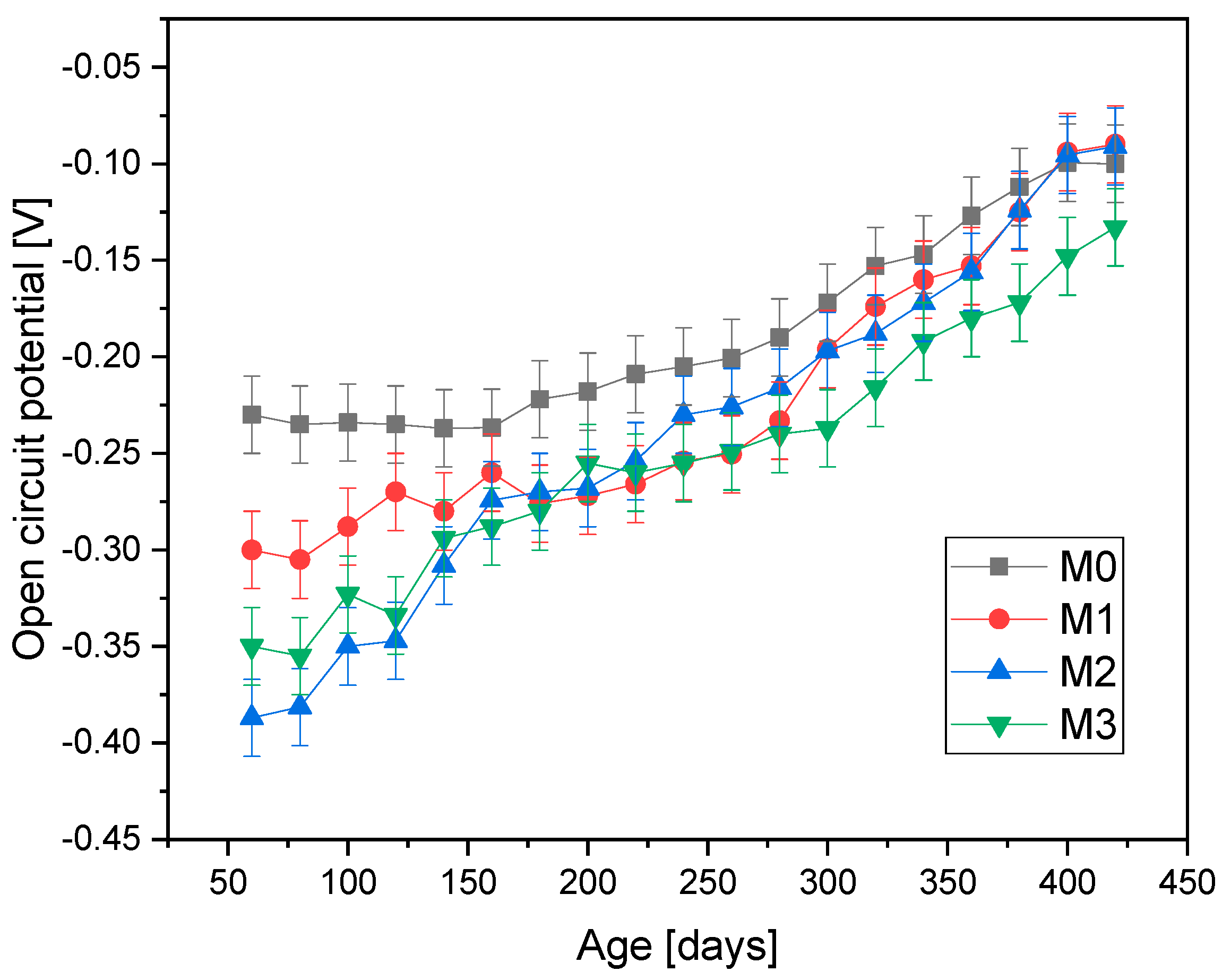
Figure 4 presents the open-circuit potential values for the samples at different ages exposed to environment A, that is, outdoor conditions. It is observed that at the beginning of the test, during the first 200 days, the reference sample maintains values of −0.33 V, while M1’s values range from −0.35 V to −0.43 V, M2’s values from −0.38 V to −0.44 V, and M3’s values from −0.45 V to −0.52 V. This variation corresponds to a decrease of 0.08 V for each mixture, with each showing similar slopes. Each electrochemical measurement was performed five times, yielding a repeatability of 92 ± 7%.
The data indicate that as the mucilage content in the concrete increases, the reported open-circuit potential value decreases. This suggests that the mucilage gradually releases water, maintaining concrete hydration and optimizing the curing stage [
11]. As time progresses, the graph values for mixtures M1 and M2, with low mucilage contents, stabilize, remaining higher compared to the those of the reference sample, M0. The reference sample exhibits stable behavior during the first 200 days of the process, during which most of the available water in the mixture is consumed. After this period, a potential decrease is observed due to the breakdown of the oxide layer protecting the steel, exposing it to contaminants present in the environment [
2]. From day 350 onwards, stability in the values is observed for all mixtures.
Several studies have shown that wetting and drying cycles lead to an increase in the corrosion rate of reinforcing steel, due to the fluctuating moisture and oxygen conditions within the system [
22]. Specifically, it has been observed that during the drying phase, the corrosion rate increases, likely due to the accumulation of oxygen within the concrete pores [
22]. This phenomenon is exacerbated when the concrete lacks mucilage, as it exhibits higher effective porosity, facilitating the ingress of aggressive agents such as oxygen and chlorides [
23]. In contrast, the addition of mucilage gel partially blocks the pores, thereby reducing the diffusion of contaminants toward the steel [
24]. However, when the concrete is exposed to acidic attacks, such as those caused by certain environmental pollutants, a drop in pH occurs, which weakens the passive layer that protects the steel, further promoting the corrosion process [
3].
The above is the result of capillary formation during the consolidation stage of concrete in the M0 mixture. These capillaries create a network of channels through which contaminants flow, connecting the material’s surface with the steel bars, thereby generating low open-circuit potential values at the end of the test period [
16,
25]. The same phenomenon occurs in the M3 mixture, where an excess of water generates larger pores, and consequently facilitates the formation of capillary channels that allow for the transport of water and contaminants, maintaining open-circuit potential values at lower levels compared to the other mixtures.
The high values reported for M1 and M2 at the end of the test period indicate that the water contained in the mucilage is transferred to the concrete mixture, allowing chemical reactions to continue, and producing a more stable mixture from an electrochemical perspective by the end of the test period [
11]. As the water present in the mucilage gradually integrates into concrete, it leaves voids or pores that are not interconnected, resulting in a final mixture with higher open-circuit potential values compared to the reference sample, M0.
Figure 5 presents the open-circuit potential values obtained for the specimens exposed to environment B, characterized by high humidity. It is observed that the initial values are higher than those obtained for the specimens exposed to the natural atmospheric conditions of environment A. This indicates that the nopal mucilage obstructs the pores formed during the concrete curing process, preventing the transport of contaminants into the depth of the reinforcing steel [
11].
As the test period progresses, the open-circuit potential value gradually increases, reaching values of −0.1 V. This represents an improvement in the protective properties of mucilage-modified concrete for all samples, with mixtures M1 and M2 achieving the best performance at the end of the test period, reaching values of −0.08 V.
The values obtained for environments A and B, to which the four mixtures were subjected, demonstrate that the addition of mucilage alters the properties of the concrete, resulting in a material that enhances the corrosion protection for carbon steel reinforcement structures embedded in the test material.
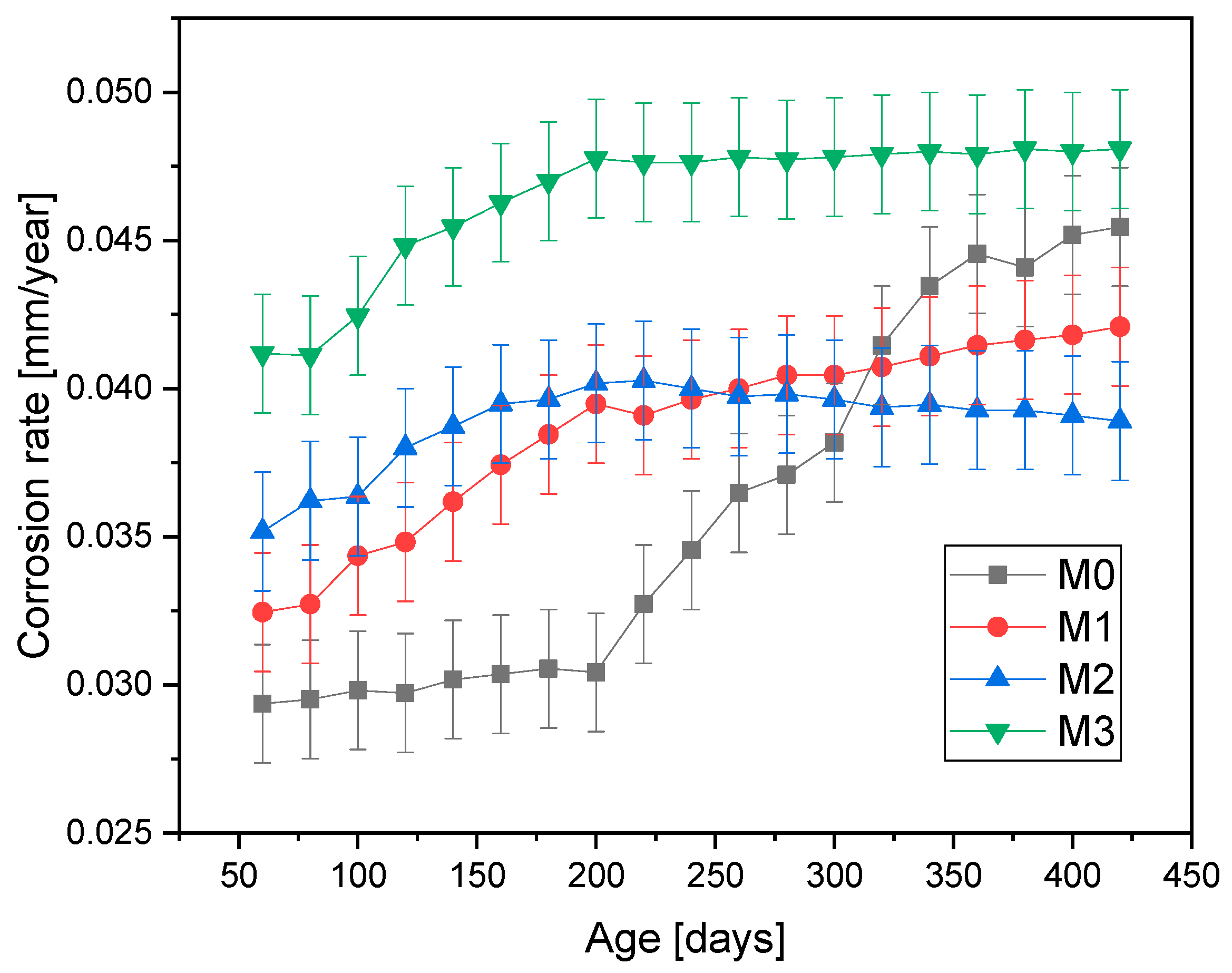
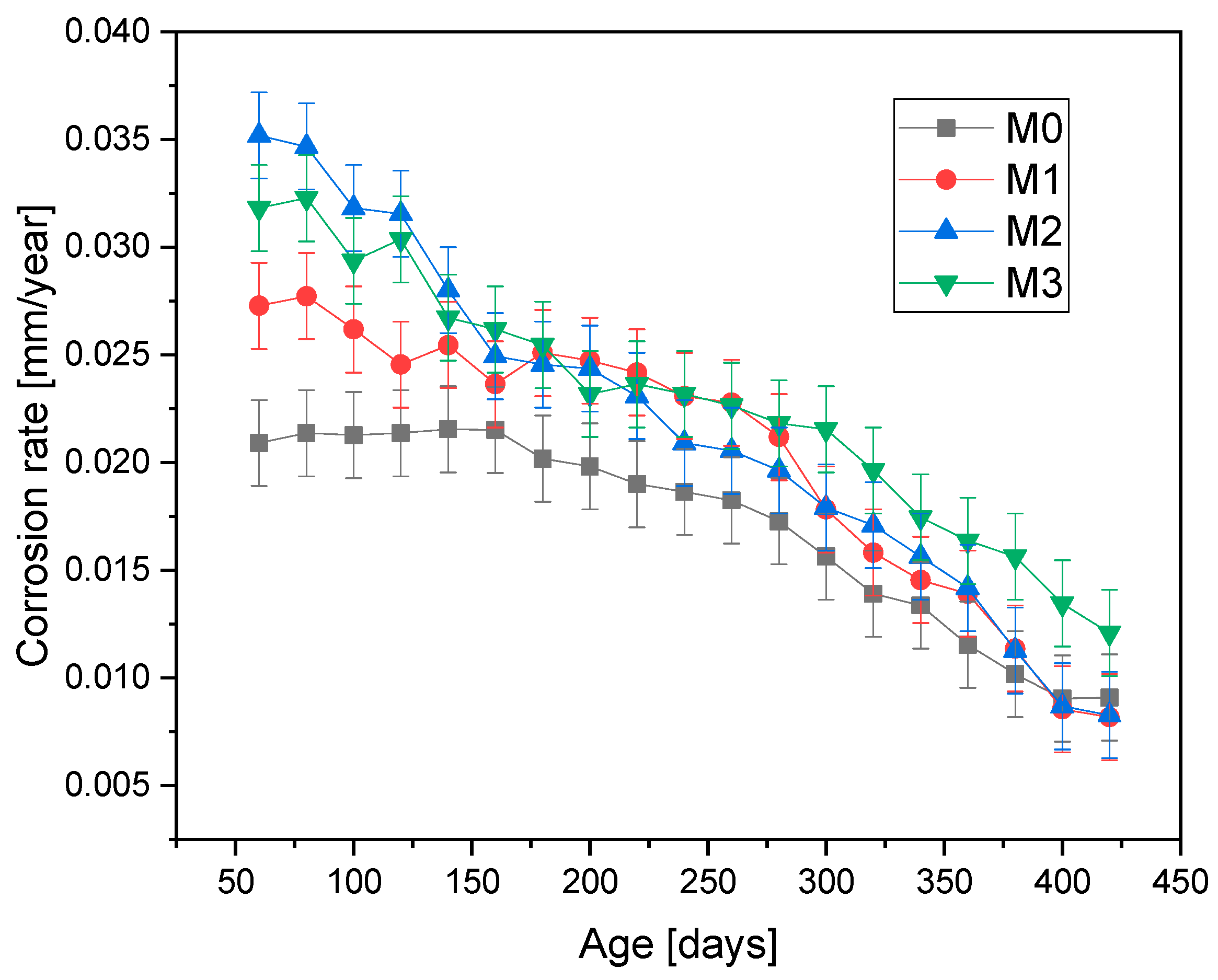
Figure 6 and
Figure 7 present the corrosion rate results for the four mixtures and the two environments to which the test specimens were subjected. It is observed that the corrosion rate is higher for the specimens exposed to environment A (natural atmospheric conditions) compared to the values obtained in environment B (high humidity).
The mixtures with 5% and 10% mucilage perform better, likely because these concentrations provide an optimal balance between hydration and pore structure refinement, enhancing corrosion resistance without creating excessive voids. The mucilage’s water-regulating properties help to maintain concrete hydration, improving the overall matrix. Unexpectedly, the 15% mucilage mixture exhibits higher corrosion rates, likely due to larger pores, which may facilitate the transport of contaminants to the steel reinforcement. This suggests that too much mucilage disrupts the concrete’s structural integrity, reducing its protective properties.
The presence of water in the mixtures serves two functions: on the one hand, it hydrates the mixture and promotes the chemical reactions of the concrete; on the other hand, it acts as a medium in which contaminants dissolve and are transported until they reach the reinforcing steel. In the case of environment A (outdoor exposure), salts were deposited on the surface of the specimens, and when exposed to moisture—such as rain, or, in this experiment, the pre-hydration before electrochemical testing—dissolution and transport of the salts occurred, allowing them to migrate into the specimen and reach the reinforcing steel [
26]. For the specimens kept in environment B (high humidity), water acted as a barrier against the dissolution of environmental salts, thereby minimizing the transport of salts to the reinforcing steel. Additionally, the constant presence of water facilitated the continuation of the concrete’s curing process, and the presence of mucilage reduced the number of pores generated in the concrete [
24].
The average corrosion rate over the last 70 days of the test period was calculated, showing a trend toward data stability. In environment A, mixtures M0 and M3 exhibited higher corrosion rates, with values of 0.046 mm/year and 0.049 mm/year, respectively, compared to mixtures M1 and M2, which contained lower mucilage additions, with values of 0.041 mm/year and 0.038 mm/year, respectively. The high corrosion rate in M0 is attributed to the presence of capillaries formed during the concrete consolidation process, which facilitated the free flow of water and contaminants throughout the porous matrix. In the case of M3, the presence of large pores in areas where the mucilage was initially present increased the likelihood of pore interconnection, forming capillaries that allowed the transport of contaminants to the reinforcing steel.
The corrosion rates of the mixtures in environment B were 0.010 mm/year for M0, 0.009 mm/year for M1 and M2, and 0.014 mm/year for M3. The similarity of these values is attributed to the saturation conditions to which the specimens were exposed, regardless of their mucilage content. In the initial stage of the process, M1 and M2 exhibited higher corrosion rates than M0; however, over time, the difference between these rates decreased. By the end of the test period, M1 and M2 showed lower values than M0. These differences are attributed to the presence of prickly pear mucilage, which may have acted as a water regulator in the concrete mixtures [
11].
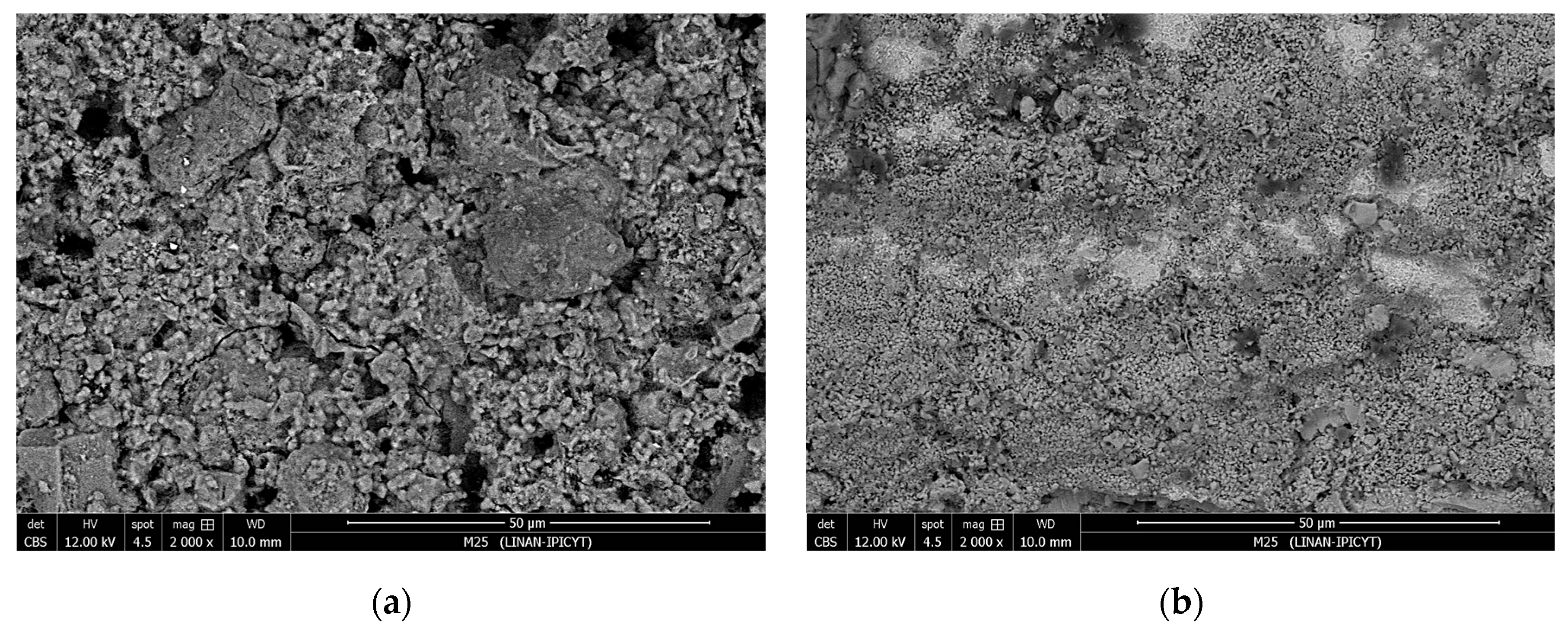
The SEM analysis of the specimens at the end of the test period revealed morphological differences resulting from the environmental conditions to which they were exposed.
Figure 8 displays the steel surface of specimens from M0 and M2, which were subjected to outdoor exposure. In the first image, corrosion of the steel embedded in the control mixture is observed. This corrosion product layer is approximately 40 µm thick, covering the entire surface of the steel. In the second image, bright areas are visible, indicating sections of the protective corrosion product film, and a lower level of corrosion products, with a layer about 15 µm thick on the steel surface [
27].
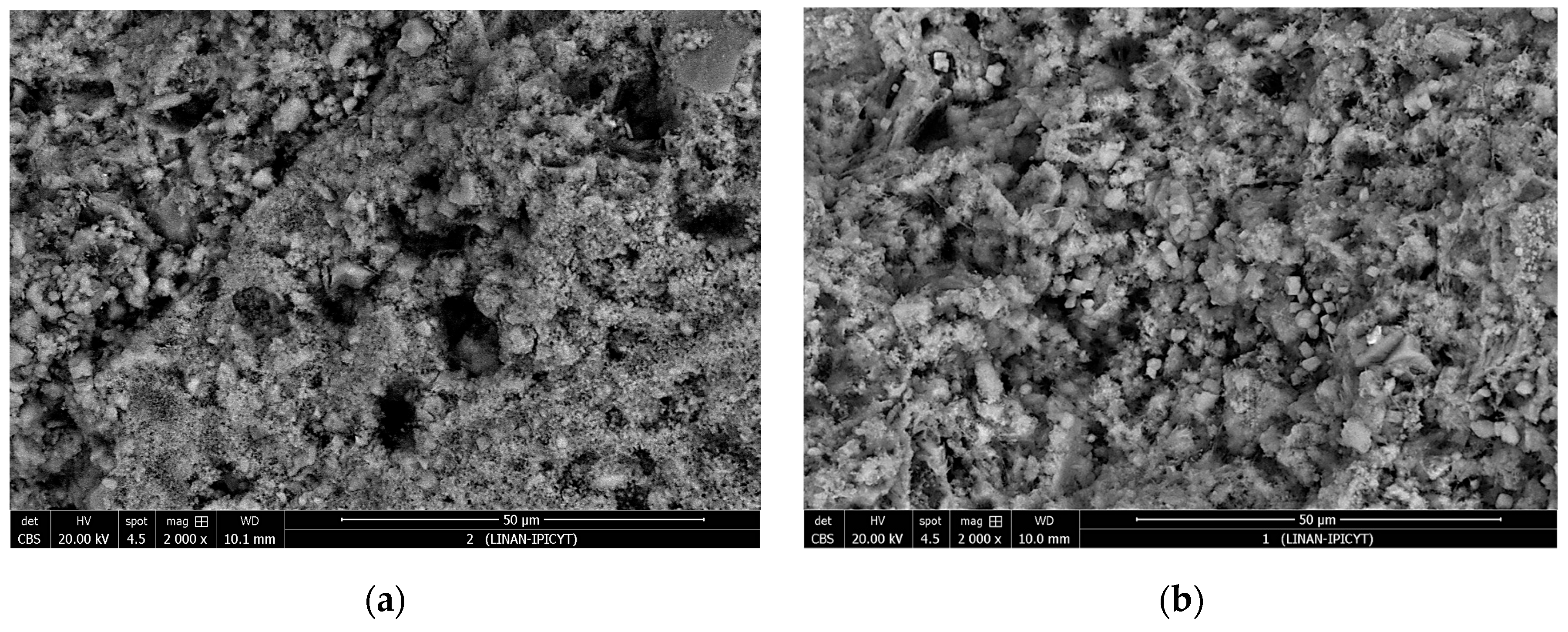
In
Figure 9, the microstructure of the concrete at the depth of the reinforcing steel is shown for specimens exposed to outdoor conditions with M0 and M2 mixtures at the end of the test period. In the first image, pores in the concrete without additives are observed. In the second image, pores left by mucilage droplets after releasing water to the concrete are visible, along with a more uniform matrix compared to the reference [
27]. Different shades of gray are observed, indicating the compounds found: light gray indicates the presence of Ca(OH)
2, dark gray represents C-S-H, and black corresponds to voids in the material [
17]. In the second image, a better distribution of particles with interlaced crystals of Ca(OH) and C-S-H is observed, along with smaller-sized voids [
24].
Mucilage acts as a natural biopolymer that modifies the microstructure of concrete by reducing the size of its pores. This modification results in lower permeability of the material, hindering the passage of water and other substances into the cementitious matrix [
16]. Therefore, the capillaries in the concrete are partially obstructed, which limits the diffusion of aggressive agents, such as oxygen and chloride ions, responsible for initiating the corrosion process in the reinforcing steel. This reduction in the concentration and mobility of contaminants within the concrete significantly contributes to slowing down the corrosion rate of the embedded steel [
24].
According to the data obtained for the samples in environment A, the mixtures that exhibited the best performance were M1 and M2, which contained 5% and 10% mucilage, respectively, as a replacement for mixing water. These two mixtures achieved efficiency values of 11% and 17%, respectively. For environment B, M1 and M2 showed efficiency values of 5% compared to M0.
4. Conclusions
Based on the above findings, it can be concluded that concrete mixtures with low mucilage concentrations (within the range of 5% to 10%) have a positive impact by reducing the corrosion rate, even in high-humidity environments.
Mucilage should be used as a replacement for a percentage of mixing water, and never as an additional component, as this would introduce an excess of water, potentially leading to undesirable results, such as a decrease in reinforced concrete quality.
The addition of mucilage modifies the properties of concrete, and it should be used in moderate percentages to achieve significant improvements, since higher proportions can lead to a reduction in concrete quality.
The use of nopal mucilage as a concrete additive provides additional, low-cost, and immediately applicable protection, which is particularly relevant for rural areas where specialized laboratory materials or reagents are not available.
The reduction in construction costs will be directly proportional to the cost of industrial products used to protect reinforcing steel, given that cactus cladodes are readily available in rural areas of México.
This study has social, economic, and environmental implications, as it focuses on service life processes and provides a better understanding of the mechanisms involved.
These findings suggest that nopal mucilage could be a viable, sustainable solution for enhancing the durability of concrete in coastal or high-humidity regions.
Fieldwork is currently underway to assess the scalability and applicability of the technique described in this study.
In further work, it would be useful to optimize the concentration of mucilage and investigate its impact on other concrete properties, such as mechanical strength, to ensure its practical application in the construction industry.
This research opens the door to developing more environmentally friendly materials, potentially reducing the need for expensive industrial additives, while improving the lifespan of reinforced concrete structures.