Abstract
The fabrication of metal oxide semiconductor heterostructures is a major way to enhance their properties in photocatalytic and antibacterial applications. In the present work, ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 are chosen to create the heterostructure of thin films using the spray pyrolysis method. This paper compares the experimental results of the structural and morphological properties of the prepared thin layers using XRD, Raman and SEM. The X-ray diffraction shows that the obtained thin film heterostructures crystallize in a hexagonal phase of ZnO, a cubic phase of In2O3 and a tetragonal structure of SnO2, with all of the preceding phases positioned on the rhombohedral phase of the hematite α-Fe2O3. In addition, the SEM study provided the morphology and surface structure and confirmed the presence of a highly folded, rough, uneven surface with imperfections of 20 and 65 nm for In2O3/α-Fe2O3 and SnO2/α-Fe2O3. The photoactivity of the prepared materials was tested via the photocatalytic degradation of methylene blue (MB) dye. Consequently, our findings demonstrate that the cracked surface improves the rapid absorption of contaminants and allows water to easily pass through the surface of the thin layers. Finally, the antibacterial abilities of ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films were investigated by using the agar well-diffusion technique, comparing the results to the Gram-negative of Pseudomonas aeruginosa and Gram-positive of Bacillus subtilis, and these thin films were found to have high antibacterial activity.
1. Introduction
Recent years have seen a significant increase in interest in thin film technology because of its many uses, including in gas sensors [,], energy storage [,,], photovoltaic solar cells [,,], heterogeneous photocatalysis for water splitting processes [,,], and optoelectronic devices [,,]. The amount of wastewater polluted with undesirable and dangerous dye has remarkably increased. The colored dyes that are frequently employed in the textile sector are one of the primary causes of pollution. Water’s transparency and gas solubility can be impacted by even minute amounts of dye, which can also give the water an incredibly vibrant color [,].
As a result, it is now crucial to explore the possibility of functionalizing various surface types without altering their optical characteristics. There exist numerous techniques for obtaining thin films, such as electroplating [], anodic treatment [], chemical vapor deposition (CVD) [,], atomic layer deposition (ALD) [,], and spin coating []. The spray pyrolysis process is the most commonly used because it is a simple way to make films with any material in any amount by adding the film to the spray precursor solution.
Owing to its low cost, small bandgap, environmental friendliness, and thermodynamic stability, hematite (Fe2O3) is a promising semiconductor material for use as an efficient photocatalyst under visible light irradiation. To increase the photoinduced electron–hole separation and, consequently, the photocatalytic activity of Fe2O3, heterojunction construction is used. Because it is an environmentally beneficial technology, the photocatalytic reduction of methylene blue has been applied extensively in a variety of semiconductor materials, including Fe2O3, In2O3, SnO2, and ZnO, achieved via exposing them to UV and visible light. In this context, Alofi et al. [] describe the mechanism of photogenerating radical species through a particulate film, such as OH• (instead of h+) and super oxide O2–• (instead of e−) radicals. However, it is unclear why these species would not react quickly with one another as they pass through the film from the bulk to the surface. Different kinds of dyes are also investigated as different types of pollution; Singh et al. [] used doped ZnO as a photocatalyst to decompose methyl orange (MO), methylene blue (MB), and congo red (CR), respectively. The insertion of another material into the oxide semiconductor lattice can alter its optical, morphological, and structural characteristics, among other physical and chemical modifications. These modifications might alter the semiconductor’s photocatalytic activities and shift them toward a visible area of the spectrum. Semiconductor heterojunctions, which comprise two or more semiconductors arranged in delayed energy bands, enable the separation of photogenerated electron–holes and the retention of redox capacity. R. M. Mohamed et al. [] studied the effect of increasing the solar light-driven photocatalytic hydrogen evolution of different semiconductor photocatalysts; this technique might be employed when using organic and non-metal-based new-age materials, especially if combined with numerous modification procedures. The present paper aims to provide a well-founded direct comparison of three thin film heterostructures (ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3) prepared using a simple chemical technique. The photodegradation of methylene bule dye MB and the antibacterial application of the prepared materials were tested and compared. We need to point out that no previous research has been conducted to date using this method to create these thin film heterostructures.
2. Experimental Section
2.1. Film Preparation
2.1.1. ZnO Thin Film Preparation
Zinc acetate (C4H6O4Zn, 2H2O) was chosen as the primary precursor and was dissolved in isopropyl alcohol at a concentration of 10−2 mol/l to create the precursor solution.
Using the chemical spray approach, ZnO thin films were applied to a glass substrate at 460 °C [].
2.1.2. In2O3 Thin Film Preparation
An aqueous solution of indium chloride (InCl3) with a concentration of 0.01 M was sprayed onto glass substrates and heated to 350 °C using a N2 gas pressure of 0.5 bar to create an In2O3 thin layer.
2.1.3. SnO2 Thin Film Preparation
To create SnO2 thin films, a precursor was obtained in a 0.05 M solution by dissolving tin chloride pentahydrate (SnCl4.5H2O) in pure ethanol (C2H5OH). This solution was sprayed at 0.5 bar of N2 gas pressure onto glass substrates that were heated to 450 °C on a hot plate.
2.1.4. ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 Thin Film Preparation
To prepare ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin film heterostructures, an aqueous solution containing iron (III) and chloride dehydrate (FeCl3.2H2O) was utilized. After preparing this solution, we sprayed it at 350 °C onto the ZnO, In2O3, and SnO2 substrates for use as a precursor. Using a nozzle with a 0.5 mm diameter, 20 mL of the resultant solution was sprayed at a rate of 4 mL/min. There was a 30 cm distance between the substrate and the spray gun.
2.2. Characterization Techniques
Initially, to study the structural properties using Cu Kα radiation (λ = 0.15418 nm) and 2θ varying from 10° to 70°, the X-ray diffraction spectra of the produced thin films were examined using a copper-source diffractometer (Analytical X Pert PROMP D, Sakaka, Jouf). In addition, at room temperature, Raman scattering studies were recorded using the Jobin Yvon Horibra LABRAM-HR micro-Raman system (Sakaka, Jouf), which was detectable within the 200–1200 cm−1 range. Subsequently, a scanning electron microscope (SEM) of the JEOL-JSM 5400 type (Sakaka, Jouf) was used to examine the films’ morphology. An energy-dispersive X-ray (EDX) spectrometer (Sakaka, Jouf) connected to a thermal field emission scanning electron microscope (FESEM, Sakaka, Jouf) was used to analyze the elements with an electron gun. Electrical tests were carried out, and resistivity, hall mobility, and carrier concentration were determined at room temperature in a light magnetic field of around 0.554 T, using a hall measurement device that employed the van der Pauw method.
Ultimately, the rate at which the methylene blue (MB) aqueous solution degraded in the presence of sunlight was used to evaluate the photocatalytic activity of the structures based on the obtained thin film heterostructures. The cylindrical batch reactor used in the research was opened to the air. For the photocatalytic tests, methylene blue MB (Aldrich) was selected as the model molecule. A steady stream of water flowing through the reactor maintained the MB solution at room temperature. The starting MB concentration was 4.5 mg/L. To achieve adsorption equilibrium, the MB solution was agitated at a magnetic stirrer for one hour in the dark. Under continuous stirring, the aqueous suspension comprising MB and the photocatalysts of ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films was exposed to solar radiation. Four analytical samples were taken from the MB solution every 30 min in order to determine the impact of sunlight irradiation. Each sample’s MB concentration was determined using a UV–Vis spectrophotometer.
Since the absorbance, A, and concentration, C, of MB are proportionate according to the Beer–Lambert law, the efficiency of MB degradation was determined using the following equation:
where A0, A and C0, C represent the MB absorbance and concentration, respectively, corresponding to the start time and the variable time. Lastly, counting forming unity (CFU) was used to assess the bactericidal qualities of ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin film heterostructures. The experimental strains of the Gram-negative bacteria Pseudomonas aeruginosa and Gram-positive bacteria Bacillus subtilis were employed. In Tryptic Lauria Bertani (LB) medium, the bacterial cells were cultivated at 37 °C until they reached an optical density (OD) of 0.2 at 600 nm. During the exponential growth phase, the culture was inoculated with 106 CFU in a fresh LB medium, and 10 μL was placed onto the ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films. It was then incubated at 37 °C for 24 h in a dark environment. An identical process was applied to inert glasses devoid of thin coatings, which served as the control. Following incubation, bacterial suspensions were serially diluted and plated for CFU counting on Plate Count Agar (PCA) plates measuring 9 cm in diameter. The effect of thin films on bacterial growth was determined by looking at the decrease in CFU/mL. The residual bacterial viability was determined as follows:
3. Results and Discussions
3.1. Structural Properties
XRD was used to evaluate the crystal phase of the prepared ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin film heterostructures. Firstly, all peaks observed in the crystal structure of ZnO/α-Fe2O3 (Figure 1a) were located at 2 values of 24.3°, 31.4°, 33.4°, 34.9°, 40.6°, 44.5°, 54.2°, and 57.7°, which are related to the (012), (100), (104), (110), (113), (024), (110), and (122) planes, respectively. These peaks indicate the hexagonal phase of ZnO and the rhombohedral phase of the hematite α-Fe2O3 according to the JCPDS card numbers 036-1451 and 01-1053, respectively. These findings are consistent with those of Noukelag et al., who synthesized zincite ZnO and hematite α-Fe2O3 for the first time from an aqueous extract of rosemary [].
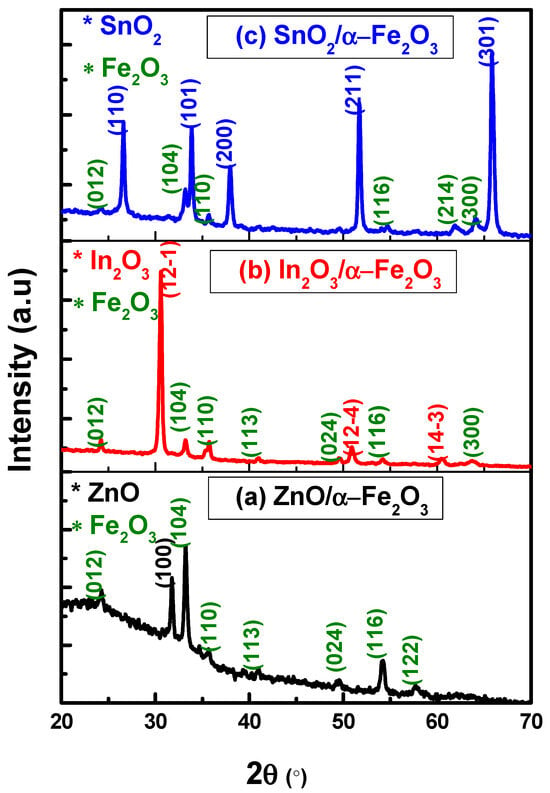
Figure 1.
XRD results of (a) ZnO/α-Fe2O3, (b) In2O3/α-Fe2O3, and (c) SnO2/α-Fe2O3 thin film heterostructures.
The XRD of In2O3/α-Fe2O3 is shown in Figure 1b, with the primary diffraction peaks corresponding to the cubic phase of In2O3 (JCPDS No: 06-0416) and the rhombohedral phase of α-Fe2O3 (JCPDS No: 01-1053). The absence of further impurity peaks or mixed oxides suggests that the different metal oxides did not chemically interact with each other [].
The XRD pattern of the SnO2/α-Fe2O3 thin film is shown in Figure 1c. The patterns reveal many peaks at (110), (101), (200), (211), and (301), which match the lattice planes of the tetragonal structure JCPDS (no. 41–1445). In addition, weak peaks are revealed at (012), (104), (110), (116), (214), and (300) that are compatible with the JCPDS (no. 01-1053) associated with rhombohedral hematite α-Fe2O3 [].
3.2. Raman Measurements
Raman spectroscopy is a dependable method for characterizing materials. It distinguishes various material phases using discrete vibrational modes. The phase type and purity of the material are confirmed using the numerous unique phonon modes revealed in the Raman spectra. Figure 2 shows the Raman spectrum analysis of the ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin film heterostructures. The Eg modes of α-Fe2O3 are represented by the peaks at 243, 292, 404, and 604 cm−1, whereas the A1g modes are associated with the peak at 497 cm−1. These peaks appeared in all the different thin films. These results enhance the production of pure hematite α-Fe2O3 [,,].
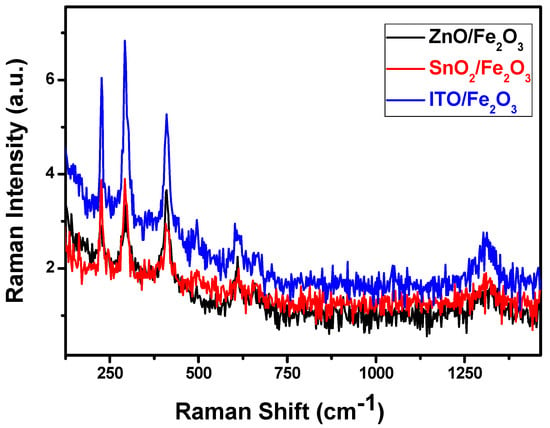
Figure 2.
SEM micrographs of SnO2/α-Fe2O3 thin films, In2O3/α-Fe2O3 thin films, and ZnO/α-Fe2O3 thin films.
3.3. Morphological Properties
SEM analysis was used to evaluate the morphology of ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin film heterostructures presented in Figure 3.
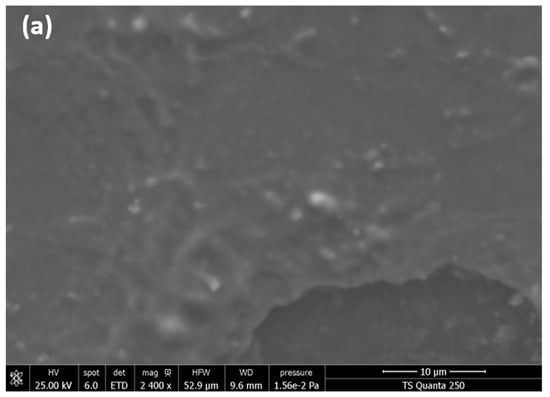
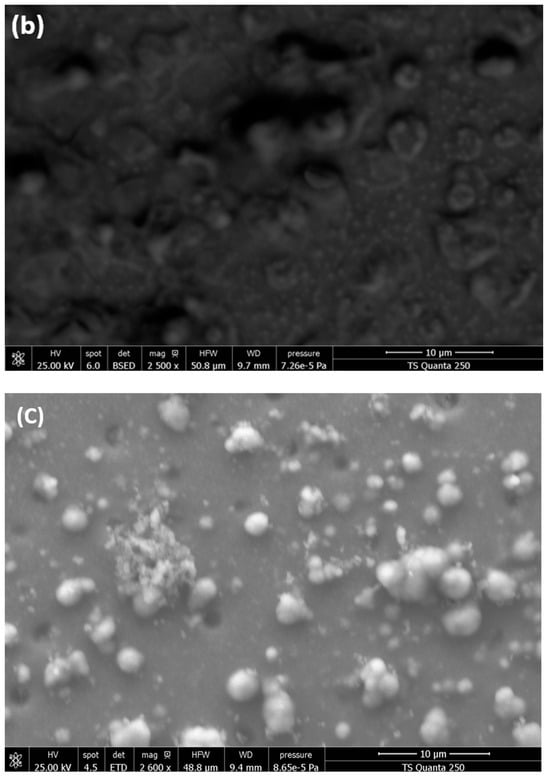
Figure 3.
SEM micrographs of (a) ZnO/α-Fe2O3 thin films, (b) In2O3/α-Fe2O3 thin films, and (c) SnO2/α-Fe2O3 thin films.
ZnO/Fe2O3 has a homogeneous and smooth surface with a very small particle size. However, the thin film SEM pictures of SnO2/Fe2O3 and In2O3/Fe2O3 have a completely different shape compared to ZnO/Fe2O3. Figure 3b,c show a highly folded, rough, uneven surface with imperfections confined between 20 and 65 nm. The elaboration process and the formation of thin layers in the heterostructure may cause these irregularities. This cracked surface improves the rapid absorption of contaminants and allows water to easily pass through the surface of our thin layers.
3.4. EDX Measurments
EDX was used to support the aforementioned findings; the atomic percentages (at.%) of Zn (from ZnO), Sn (from SnO2), In (from In2O3), and Fe (from the Fe2O3) found on thin films are presented in Figure 4a–c, which display the corresponding mapping structure of the obtained thin films.
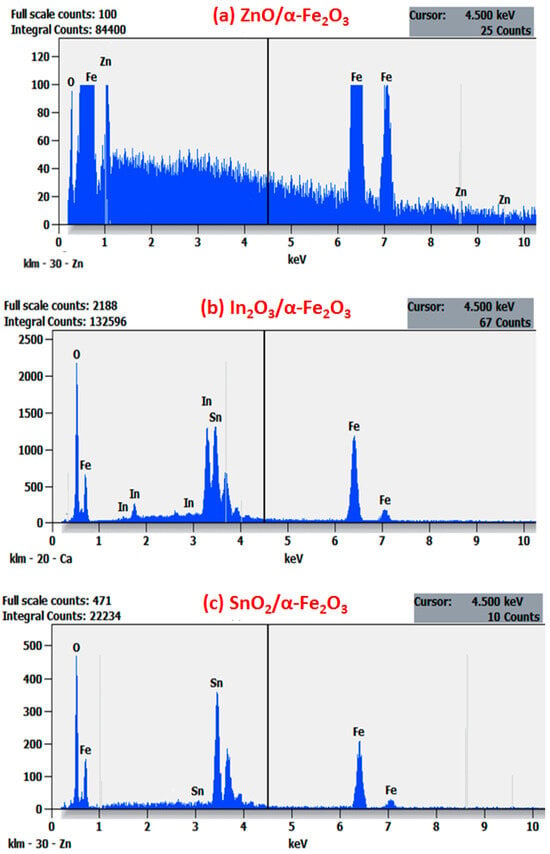
Figure 4.
EDXS spectra of (a) ZnO/α-Fe2O3 thin films, (b) In2O3/α-Fe2O3 thin films, and (c) SnO2/α-Fe2O3 thin films.
Only three components were found in the spectrum for these films: O, Fe, and Zn for ZnO/α-Fe2O3; O, Fe, In for In2O3/α-Fe2O3; and O, Fe, and Sn for SnO2/α-Fe2O3. No other elements were discovered within the apparatus’ sensitivity range, which shows the great purity of the thin films.
On the other hand, we noted that the distribution of Fe and O signals was uniform throughout the entire structure. Seldom were the Zn, In, and Sn signals found outside of the core region; they were mostly found inside. The unique characteristics of the ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 heterostructure nanocomposites were validated by these results, which align with the findings documented in the previous literature [,].
3.5. Hall Effect Study
The Hall effect measurements provide important information, such as the type of material (n-type or p-type), carrier concentration, electrical resistivity, and Hall mobility. These findings are detailed in Table 1.

Table 1.
Hall effect results of In2O3/α-Fe2O3 and SnO2/α-Fe2O3 thin films.
Firstly, the results indicate that the conductivity of the ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films was n-type in nature and all the information shows notable changes between the obtained thin film heterostructures. The increase in the carrier concentration and the mobility of SnO2/α-Fe2O3 can be attributed to the increase in the number of the grain boundary on the surface of layer and the corresponding increase in the dislocation density across the boundaries.
On the other hand, the heterostructure of the ZnO/α-Fe2O3 thin film has a high resistivity. Consequently, the results showed no changes.
3.6. Photocatalytic Test
Adsorption is a crucial step in every catalytic reaction process, regardless of the type of photodegradation employed. More effective adsorption enables improved pollutant–catalyst interactions. In this work, MB was adsorbed on the synthesized thin film heterostructures at room temperature, as plotted in Figure 5.
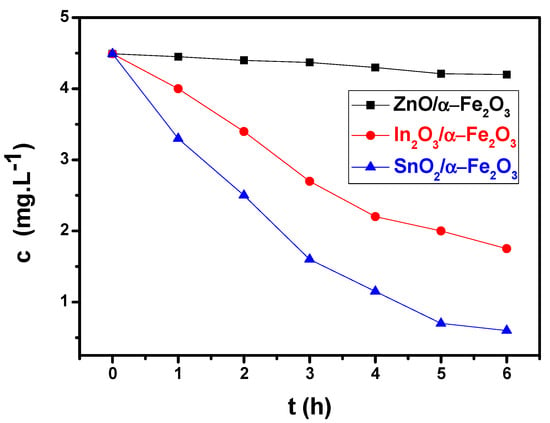
Figure 5.
Photocatalysis of methylene blue: normalized concentration of MB as a function of time for ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films under solar radiation.
During photoelectrochemical water-splitting, the observed photocurrent is adversely affected by the consumption of photoexcited electrons. Under sun irradiation, ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 are excited. The photogenerated electrons quickly move from ZnO, In2O3, and SnO2 CB to α-Fe2O3’s VB, allowing for holes to collect in ZnO, In2O3, and SnO2 VB, and for electrons to gather in high-potential α-Fe2O3 CB, where they can reduce H+ in H2.
Morphology becomes important at this point. The photocatalyst’s precise engineering and design may be able to solve the issues with charge carrier transit and recombination. Indeed, there are numerous indications of the existence of a relationship between reduced-dimension structures and enhanced photocurrent density, which is linked to the occurrence of defects in nanostructures. Under visible light irradiation, we discovered that the concentration of MB decreases and the peak almost vanishes after six hours, but only for SnO2/α-Fe2O3. This good response is due to the presence of a cracked surface, which increases the rapid absorption of contaminants and allows water to easily pass through the surface of our thin layers. Because of its improved nanoarchitecture, which facilitates dye molecule diffusion and oxygen species transport during the photoactivity process, the porous sample exhibits a higher photocatalytic performance than the pure one. Electron–hole pairs (e−, h+) are produced when photon energy (hv) is absorbed by the thin film heterojunctions during radiation.
After photogeneration occurs close to the surface, the electrons combine with oxygen to make superoxide (⋅O2−) (Equation (3)), which then reacts with water to form hydroxyl radicals (⋅OH). In parallel with this, the water and photogenerated holes react to produce ⋅OH radicles.
Intermediate reactions also result in the formation of
⋅OH radicals and the powerful oxidants finally convert MB into CO2 and H2O.
O2 + e− → ⋅O2‾
⋅O2‾ + H2O → OH‾ + ⋅HO2
H2O + h+ → ⋅OH + H+
h+ + OH‾ → ⋅OH
⋅HO2 + H2O → H2O2 + ⋅OH
H2O2 + e− → ⋅OH + OH‾
MB + (⋅OH + ⋅O2 ‾) → CO2 + H2O
3.7. Antibacterial Activity
The antibacterial effectiveness against Gram-negative Pseudomonas aeruginosa (Pa) and Gram-positive Bacillus subtilis (Bs) bacteria was also assessed using ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films. The In2O3/α-Fe2O3 thin film demonstrated a residual viability equal to 0.5% and 0.187% of bacterial cells against of Pa and of Bs, respectively. In addition, the SnO2/α-Fe2O3 showed a viability of 0.65% and 0.62%. However, the results of the bacteria test in the presence of ZnO/α-Fe2O3 revealed higher values, as illustrated in Table 2. The generation of reactive oxygen species (ROS) and the electrostatic interaction between In2O3/α-Fe2O3 and the absorbed OH- and H2O may be the cause of the antibacterial action. One possible explanation for the increased antibacterial properties of In2O3/α-Fe2O3 thin films could be their greater root mean square roughness (Rms) in relation to that of the other thin films.

Table 2.
Antibacterial activity of In2O3/α-Fe2O3, SnO2/α-Fe2O3, and ZnO/α-Fe2O3 thin films against Pseudomonas aeruginosa and Bacillus subtilis bacteria.
The combined benefits of the photocatalytic activity and the antibacterial impact are crucial for a variety of applications (medical, food storage, etc.). To the best of our knowledge, thin films have never been used to deteriorate Pa and Bs.
4. Conclusions
This study presents the preparation of heterostructure ZnO/α-Fe2O3, In2O3/α-Fe2O3, and SnO2/α-Fe2O3 thin films using the spray pyrolysis method. The XRD and Raman spectroscopy confirm the presence of a rhombohedral structure of α-Fe2O3 and hexagonal phase of ZnO, a cubic phase of In2O3, and a tetragonal structure of SnO2. The surface morphology of the obtained thin films was analyzed via scanning electron microscopy (SEM). The results show a homogeneous and smooth surface with a very smaller particle size for ZnO/Fe2O3. Contrarily, the thin film SEM pictures of SnO2/Fe2O3 and In2O3/Fe2O3 show a highly folded, rough, uneven surface with imperfections at 20 and 65 nm. The Hall effect indicates an increase in the carrier concentration and the mobility of SnO2/α-Fe2O3, which can be attributed to the increase in the number of grain boundaries on the surface of the layer and corresponding increase in the dislocation density across the boundaries. When the photocatalytic activity of the sprayed thin films was evaluated, it was found that the paired In2O3/α-Fe2O3 photoatalysts had an impact on the photocatalytic activity and degradation mechanism of the MB dye. This fractured surface enhances the speed at which impurities are absorbed and facilitates water’s easy penetration into the surface of the thin layers. Additionally, when a thin coating of In2O3/α-Fe2O3 was used, only 5% of Gram-negative Pseudomonas aeruginosa (Pa) and 0.187% of Gram-positive Bacillus subtilis (Bs) bacterial cells were still viable. As this heterostructure can be produced using an easy spray pyrolysis approach, these results are very interesting. This study offers a path for additional research in the healthcare industry. The In2O3/α-Fe2O3 and SnO2/α-Fe2O3 thin films yielded the most effective combined efficiency.
Author Contributions
This paper was edited equally by A.A. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Graduate Studies and Scientific Research at Jouf University under grant No (DGSSR-2023-02-02337).
Data Availability Statement
We declare that all data are available in the manuscript file.
Acknowledgments
We declare that this manuscript is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rzaij, J.M. A novel room-temperature nitrogen dioxide gas sensor based on silver-doped cerium oxide thin film. Sens. Actuatots A Phys. 2023, 363, 114748. [Google Scholar] [CrossRef]
- Rathod, A.P.S.; Mishra, P.K.; Mishra, A. Fast-response/recovery In2O3 thin-film transistor-type NO2 gas sensor with floating-gate at low temperature. Sens. Actuators B Chem. 2023, 394, 134477. [Google Scholar]
- Zhao, T.; Ye, Y.; Guo, K.; Cui, R.; Zhang, M.; Wang, X.; Zhang, B.; Zhang, J.; Deng, C. High energy storage properties of calcium-doped barium titanate thin films with high breakdown field strength. J. Alloys Compd. 2024, 970, 172487. [Google Scholar] [CrossRef]
- Song, H.; Son, J.Y. Energy storage and mutiferroic properties of La-doped epitaxial BiFeO3 thin films according to La doping concentration. J. Energy Storage 2023, 68, 107729. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Hu, S.; Yu, K.; Yang, F.; Shi, Y.; Li, J.; Hou, M.; Liu, A.; Zheng, M.; et al. Enhanced energy storage properties in PbZrO3 thin films via the incorporation of NiO. Curr. Appl. Phys. 2023, 52, 24. [Google Scholar] [CrossRef]
- Cantos, A.; Gundogan, S.H.; Turkoglu, F.; Koseoglu, H.; Aygun, G.; Ozyuzer, L. Photovoltaic performance of magnetron sputtered antimony selenide thin film solar cells buffered by cadmium sulfide and cadmium sulfide/zinc sulfide. Thin Solid Films 2023, 784, 140070. [Google Scholar] [CrossRef]
- Franco, M.A.M.; Castaneda, C.A.R.; Romero, P.M.M.; Barragan, J.J.P.; Quintero, O.A.J.; Hu, H. A direct correlation between structural and morphological defects of TiO2 thin films on FTO substrates and photovoltaic performance of planar perovskite solar cells. Mater. Sci. Semicond. Process. 2023, 161, 107452. [Google Scholar] [CrossRef]
- Akcay, N.; Gremenok, V.F.; Ozen, Y.; Buskis, K.P.; Zaretskaya, E.P.; Ozcelik, S. Investigation on photovoltaic performance of Cu2SnS3 thin films solar cells fabricated by PF-sputtered In2S3 buffer layer. J. Alloys Compd. 2023, 949, 169874. [Google Scholar] [CrossRef]
- Yang, F.; Wang, P.; Hao, J.; Qu, J.; Cai, Y.; Yang, X.; Li, C.M.; Hu, J. Ultrasound-assisted piezoelectric photocatalysis: An effective strategy for enhancing hydrogen evolution from water splitting. Nano Energy 2023, 118, 108993. [Google Scholar] [CrossRef]
- Sarma, M.; Jaiswal, M.K.; Podder, S.; Bora, J.; Karmakar, S.; Choudhury, B.; Pal, A.R. A study on the applicability of thin film over powder for visible light photocatalysis. Phys. B Condens. Matter 2023, 670, 415354. [Google Scholar] [CrossRef]
- Li, A.; Zhang, P.; Kan, E.; Gong, J. Wettability adjustment to enhance mass transfer for heterogeneous electrocatalysis and photocatlysis. eScience 2023, 4, 100157. [Google Scholar] [CrossRef]
- Muffer, H.J.; Fischer, C.H.; Diesner, K.; Steiner, M.C.L. ILGAR- A novel film technology for sulfides. Sol. Energy Mater. Sol. Cells 2001, 67, 121–127. [Google Scholar] [CrossRef]
- Sati, D.C.; Dahshan, A.; Hegazy, H.H.; Aly, K.A.; Sharma, P. Optical and optoelectronic properties of (Ge2S8)100-xTex thin films for IR optical device fabrication. Surf. Interfaces 2023, 39, 102995. [Google Scholar] [CrossRef]
- Timoumi, A.; Alameer, O.; Alamri, S. Intensive study of coating multilayer TiO2 nanoparticles thin films used for optoelectronics devices. Results Mater. 2023, 18, 100390. [Google Scholar] [CrossRef]
- Ahmed, M.; Bakry, A.; Shaaban, E.R. Optical characteristics with high accuracy of diluted Cr doped In2O3 thin films using spectroscopic ellipsometry for optoelectronic devices. Opt. Mater. 2022, 133, 113039. [Google Scholar] [CrossRef]
- Prakash, P.; Janarthanan, B. Enhancement of sensitization and electron transfer by kumkum dye in dye in dye sensitized solar cell applications. Optik 2023, 287, 171093. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Ono, T. Magnetostriction of electroplated TbFeCo thin films. J. Magn. Magn. Mater. 2023, 577, 170799. [Google Scholar] [CrossRef]
- Semnani, S.Z.M.; Youselpour, M.; Zareidoost, A. Enhancing the biocompatibility of ZrO2 thin film on Zr-2.5Nb alloy by anodizing treatment using an electrolyte containing biofunctional groups. Thin Solid Films 2022, 753, 139279. [Google Scholar] [CrossRef]
- Nishii, H.; Iida, S.; Yamasaki, A.; Ikenoue, T.; Miyake, M.; Doi, T.; Hirato, T. Fabrication of epitaxial V2O3 thin films on Al2O3 substrates via mist chemical vapor deposition. J. Cryst. Growth 2024, 626, 127484. [Google Scholar] [CrossRef]
- Lin, B.; Cai, Y.; Wang, Y.; Zou, Y.; Gao, C.; Liu, Y.; Liu, W.; Guo, S.; Sun, C. Effects of growth temperature and reactor pressure on AlN thin film grown by metal-organic chemical vapor deposition. Thin Solid Films 2023, 783, 140037. [Google Scholar] [CrossRef]
- Khan, R.; Ali-Löytty, H.; Tukiainen, A.; Tkachenko, N.V. Comparison of the heat-treatment effect on carrier dynamics in TiO2 thin films deposited by different methods. Phys. Chem. Chem. Phys. 2021, 23, 17672. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.; Ali-Loytty, H.; Kauppinen, M.M.; Hannula, M.; Khan, R.; Lahtonen, K.; Palmolahti, L.; Tukiainen, A.; Gronbeck, H.; Tkachenko, N.V.; et al. Tunable Ti3+-Mediated Charge Carrier Dynamics of Atomic Layer Deposition-Grown Amorphous TiO2. J. Phys. Chem. C 2022, 126, 4542–4554. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Sun, Q.; Gao, W.; Yang, X.; Wang, X. Optical, electrical and thermal stability properties of Al and F co-doped ZnO thin films prepared by sol-gel spin-coating. Thin Solid Films 2023, 776, 139889. [Google Scholar] [CrossRef]
- Alofi, S.; O’Rourke, C.; Mills, A. Photocatalytic destruction of stearic acid by TiO2 films: Evidence of highly efficient transport of photogenerated electrons and holes. J. Photochem. Photobiol. A Chem. 2023, 435, 114273. [Google Scholar] [CrossRef]
- Singh, J.; Rathi, A.; Rawat, M.; Kumar, V.; Kim, K.H. The effect of manganese doping on structural, optical, and photocatalytic activity of zinc oxide nanoparticles. Compos. Parts B Eng. 2019, 166, 361–370. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, A.; Dhiman, P.; Sharma, G.; Amirian, J.; Stadler, F.J. Progress in graphdiyne and phosphorene based composites and heterostructures as new age materials for photocatalytic hydrogen evolution. Fuel 2024, 356, 129630. [Google Scholar] [CrossRef]
- Mhamdi, A.; Alkhalifah, M.S.; Rajeh, S.; Labidi, A.; Amlouk, M.; Belgacem, S. Electrical and gas sensing investigations on the sprayed ZnO:Cu thin films. Phys. B 2017, 521, 178. [Google Scholar] [CrossRef]
- Noukelag, S.K.; Gummings, F.; Arendse, C.J.; Maaza, M. Physical and magnetic properties of biosynthesized ZnO/Fe2O3, ZnO/ZnFe2O4 and ZnFe2O4 nanoparticles. Results Surf. Interfaces 2023, 10, 100092. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Kotsikau, D.; Faglia, G.; Nelli, P. Influence of chemical composition factors of Fe2O3/In2O3 sensors on their selectivity and sensitivity to ethanol. Sens. Actuators B 2003, 96, 498–503. [Google Scholar] [CrossRef]
- Yuan, T.; Jiang, Y.; Li, Y.; Zhang, D.; Yan, M. Enhanced lithium storage performance in three-dimensional porous SnO2-Fe2O3 composite anode films. Electrochimica Acta 2014, 136, 27–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).