1. Introduction
Currently, zeolites are widely used in the production of modern adsorbents and catalysts for the petrochemical and oil refining industries due to their successful combination of microporous structures, strong acid sites, molecular sieving effects, and high hydrothermal and thermal stability [
1,
2]. Among the various types of molecular sieves, ZSM-5 zeolite is the most widely used in catalytic systems. It belongs to the MFI structural type and has a three-dimensional porous structure consisting of rectangular and elliptical channels with dimensions of 5.3 × 5.6 Å and 5.1 × 5.5 Å, respectively [
3].
The main efforts of researchers in the field of ZSM-5 zeolite synthesis have focused on the development of methods for the preparation of nanoscale and hierarchical crystals in order to overcome the diffusion limitations in their micropores [
4,
5,
6,
7,
8,
9,
10]. It has been demonstrated that catalytic systems based on nanoscale and hierarchical ZSM-5 zeolite crystal structures exhibit high activity, selectivity, and stability in several industrially important catalytic processes [
11,
12,
13,
14,
15].
One of these important processes is the hydroconversion of n-alkanes to produce high-quality fuel and oil. This process proceeds in a hydrogen medium by a bifunctional mechanism that requires a catalyst, which is an acid support on which acid center isomerization and cracking take place, with metal nanoparticles (usually Pt or Pd) providing the hydrogenation-dehydrogenation stages [
16,
17].
The selectivity of ZSM-5 for the hydroisomerization of n-alkanes is rather low compared to that of other zeolites whose micropores consist of 10R rings, such as MCM-22 and ZSM-23 [
18,
19]; the main reaction in this case is hydrocracking. However, catalysts based on ZSM-5 zeolite have been successfully used in the industrial process of dewaxing diesel fuel to improve its low-temperature properties [
16].
It has been shown [
20] that on ZSM-5 zeolite with a developed secondary porous structure, it is possible to achieve higher activity values in the conversion of hexadecane and a slight increase in selectivity for hexadecane isomers compared to conventional microporous ZSM-5 due to the reduction of the diffusion path length.
Unfortunately, most methods for synthesizing these materials rely on the use of crystal growth modifiers and pore-forming templates. However, the main drawback of these methods for producing nanoscale and hierarchical zeolite crystals, such as ZSM-5 zeolite, is their high cost, which makes them unsuitable for large-scale industrial production.
In industrial processes, zeolite-based catalysts, such as ZSM-5, are used in granular form. These granules are produced by mixing and pelletizing powdered zeolite with boehmite, followed by drying at temperatures between 120 and 150 °C, and calcining at temperatures between 500 and 650 °C. During this process, boehmite is transformed into γ-Al
2O
3 [
21,
22]. During granulation, the pores of the zeolite crystals may be partially blocked by a binder. The amount of binder used depends on the amount of Al
2O
3 present in the mixture [
21,
22].
In [
23], a method for the preparation of granular ZSM-5 zeolite without the use of binders was proposed. This method involves crystallizing the granules at 180 °C for 24 h in a 0.01 M NaOH solution, using dried aluminosilicate gel granules prepared with sodium silicate as a temporary binder. It has been shown that this approach allows for the synthesis of granular ZSM-5 zeolite without the use of binders. However, it should be noted that this study does not provide information on the mechanical strength of the granules, which is a critical parameter for industrial catalysts.
In [
24], a method was proposed for the synthesis of granular zeolite Na-Y with a developed secondary porous structure. The method involves the crystallization of granules consisting of Y zeolite and metakaolin (SiO
2/Al
2O
3 = 2.0–2.21), which, during the crystallization process, are transformed into individual clusters of crystals, including nanoscale crystals. This approach has made it possible to obtain granular materials with a high degree of crystallinity without binders and with a developed secondary (micro-, meso-, and macroporous) porous structure. However, the use of kaolin for the preparation of zeolites with a molar SiO
2/Al
2O
3 ratio higher than 30 is not promising due to its low silicon content.
Therefore, the aim of this study was to develop a method for the synthesis of granular binder-free ZSM-5 zeolites using amorphous aluminosilicates obtained by the sol-gel process as a temporary binder.
2. Materials and Methods
2.1. Materials
The following reagents were used in this work: sodium aluminate (Na2O(Al2O3)x xH2O, 99%, No. CAS 11138-49-1) and ground silica gel (SiO2, No. CAS 112926-00-8), tetrabutylammonium bromide (TBABr, No. CAS 1643-19-2), ethanol (C2H5OH, 99%, No. CAS 64-17-5), tetraethyl orthosilicate (TEOS, 99%, No. CAS 78-10-4), aluminum nitrate (Al(NO3)3*9H2O, 98%, No. CAS 7784-27-2), ammonia (NH4OH, 30%, No. CAS 1336-21-6), polyvinyl alcohol (PVA, 99%, No. CAS 9002-89-5), ammonium nitrate (NH4NO3, 98%, No. CAS 6484-52-2), H2PtCl6 × 6H2O (99%, № CAS 26023-84-7) purchased from Sigma-Aldrich (Saint Louis, MO, USA); sodium silicate (Na2O(SiO2)x xH2O, 99%, No. CAS 6834-92-0), aluminum sulfate (Al2(SO4)3*18H2O, 99%, No. CAS 7784-31-8), n-hexadecane (H-C16H34, 99%, No. CAS 26023-84-7) purchased from Acros Organics (Geel, Belgium); boehmite (AlO(OH), 78% Al2O3, No. CAS 1318-23-6) purchased from Sasol (Hamburg, Germany); nitric acid (HNO3, 67%, No. CAS 7697-37-2) purchased from Reachem (Moscow, Russia).
2.2. Synthesis of Powdered ZSM-5 Zeolite
Highly dispersed Na-ZSM-5 zeolite with a SiO2/Al2O3 ratio of 50 was obtained by hydrothermal crystallization in Teflon-coated autoclaves at 160 °C for 48 h from an amorphous alkaline aluminosilicate with the following composition: 0.03Na2O∙0.04TBABr∙0.02Al2O3∙1.00SiO2∙16.00H2O, prepared by mixing solutions of sodium silicate and sodium aluminate, ground silica gel, and an organic template—tetrabutylammonium bromide.
The crystallization products were separated by centrifugation and washed with distilled water until the pH was neutral. They were then dried at 150 °C for 8–10 h. The resulting powdered zeolite was named the Na-ZSM-5 sample.
2.3. Synthesis of Amorphous Aluminosilicates
Amorphous aluminosilicate (molar ratio of SiO
2/Al
2O
3 = 50) was synthesized using an organic silicon source by a two-step sol-gel method following the procedure described in [
25]. In the first stage, calculated amounts of distilled water and ethanol were added to tetraethyl orthosilicate under intensive stirring. Aluminum nitrate was then added to the resulting solution. The resulting solution, with a pH of approximately 3, was then kept in a thermostat at 60 °C for 25 h until the gelation point was reached. An aqueous solution of ammonia was then added to the resulting gel with vigorous stirring until a pH of 10 was reached. The mixture was then aged at 25 °C for a further 24 h. Finally, the gel was dried at 120 °C for 5 h to obtain the final product. The resulting amorphous aluminosilicate was named the ASM-1 sample.
An amorphous aluminosilicate with a molar ratio of SiO2/Al2O3 = 50 was synthesized using an inorganic silicon source. The synthesis process involved mixing aqueous solutions of sodium silicate and aluminum sulfate to form a gel. The gel was then aged for 24 h at 25 °C, filtered, and washed with distilled water to remove impurities. After this washing step, the amorphous aluminosilicate was dried at 120 °C for 5 h. The final product of this process, called the ASM-2 sample, is an amorphous material with the desired molar ratio.
2.4. Preparation of Granules Containing Zeolite ZSM-5 and Amorphous Aluminosilicates
The initial granules were prepared by combining powdered Na-ZSM-5 zeolite and amorphous aluminosilicates in a mixer (VINCI Technologies MX 0.4). The resulting mixture was moistened with a solution of polyvinyl alcohol to form granules with a diameter of 1.4–1.6 mm and a length of 5–6 mm. The granules were then formed on an extruder (VINCI Technologies VTE1).
The contents of powdered ZSM-5 zeolite and amorphous aluminosilicate in the granules were 60% and 40% by weight, respectively. The granules obtained using amorphous aluminosilicates ASM-1 and ASM-2 were designated as Na-ZSM-5-ASM-1 and Na-ZSM-5-ASM-2 zeolites, respectively.
2.5. Preparation of Granules Containing ZSM-5 Zeolite and Boehmite
Granular ZSM-5 zeolite in the Na form, with a binder, was prepared as follows: The Na-ZSM-5 sample and boehmite were thoroughly mixed in a mixer to obtain a homogeneous mixture. The content of boehmite in the granules, expressed as Al2O3, was 30% by weight.
The resulting mixture was wetted with a 5% nitric acid solution and extruded into granules (diameter: 1.4–1.6 mm, length: 5–6 mm) using an extruder. The granules were then dried at 150 °C for 24 h and calcined at 500–600 °C for 6 h to convert boehmite to γ-Al2O3. The sample obtained by this method was designated as Na-ZSM-5-BD.
2.6. Preparation of Granular Binder-Free ZSM-5 Zeolites
Granular Na-ZSM-5-ASM-1 and Na-ZSM-5-ASM-2 zeolites were crystallized in a solution of sodium silicate. The concentrations of sodium and silicon were chosen on the basis of the composition of the reaction mixture (RM): 2.3R: 3.2Na2O: Al2O3: 60SiO2: 550H2O (R—organic template, tetrabutylammonium bromide).
The granules were stored at room temperature for 1–32 h prior to crystallization. Crystallization was performed in a Teflon-lined autoclave. The temperature for crystallization was 160 °C, and the duration was 48 h. After the crystallization process, the samples were washed to remove any residual components from the mother liquor, dried at 120 °C for 5–6 h, and finally calcined at 600 °C for 3–4 h to remove the template.
The samples obtained by crystallization of Na-ZSM-5-ASM-1 and Na-ZSM-5-ASM-2 were designated as Na-ZSM-5-WB-1 and Na-ZSM-5-WB-2, respectively.
2.7. Preparation of H-Form Zeolites
The H-form of the powder and granular samples was obtained after crystallization by ion exchange of Na+ cations with NH4+ cations in an aqueous solution of ammonium nitrate at 70 °C for 1 h with stirring. The granules were then dried at 120 °C and calcined at 550 °C for 4 h in air. The ion exchange process was performed three times. The residual sodium (Na2O) content was less than 0.003%. The H-form samples were assigned an H-index.
2.8. Preparation of a Bifunctional Catalyst
Fractions of 0.1 to 0.5 mm were obtained from H-ZSM-5-WB-1, H-ZSM-5-WB-2, and H-ZSM-5-BD samples by grinding and passing through sieves. This material was then heat-treated at 350 °C for 6 h in air. The catalyst was then impregnated with an aqueous solution of H2PtCl6 × 6H2O at a concentration of 0.5 wt. % of Pt per support weight. The catalyst was then dried at 100 °C for 24 h and calcined at 550 °C for 5 h. Prior to the reaction, the catalyst was reduced under a hydrogen atmosphere at 400 °C for 5 h. Samples containing 0.5% Pt were designated as Pt/ZSM-5-WB-1, Pt/ZSM-5-WB-2, and Pt/ZSM-5-BD.
2.9. Zeolite Research Methods
The chemical composition of the samples was determined using a Shimadzu EDX-7000P spectrometer manufactured by Shimadzu Corporation.
The phase composition and crystallinity of the samples were determined by X-ray diffraction using a Shimadzu XRD 7000 diffractometer with CuKα radiation. Scanning was performed in the range of angles 2θ from 5° to 40° with increments of 1°/min. The X-ray images were analyzed using the Shimadzu PC XRD software (version 7.04) and the PDF2 database (version 2.2201). Crystallinity was calculated using the Shimadzu Crystallinity software (version 7.04), taking into account the halo in the range of 15–30°, which is characteristic of the amorphous phase.
The morphology and crystal size of the samples were analyzed by scanning electron microscopy (SEM) using a Hitachi Regulus SU8220 microscope. Images were taken in the secondary electron mode with an acceleration voltage of 5 kV. Prior to imaging, the samples were positioned on a 25 mm diameter aluminum stage and secured with conductive carbon tape.
A target-oriented approach was used to optimize the analytical measurements [
26]. Prior to the measurements, the samples were deposited on 3 mm carbon-coated copper grids from an isopropanol suspension. Observations were carried out using a Hitachi Regulus8230 field-emission scanning electron microscope (FE-SEM). Images were taken in the transmitted electron mode at an acceleration voltage of 30 kV.
The characteristics of the porous structure, including the BET-specific surface area and the volume of micro-, meso-, and macropores, were measured using low-temperature N2 adsorption−desorption on a Quantachrome Nova 1200e sorption meter and mercury porosimetry using a Carlo Erba Porosimeter-2000.
The volume of micropores in the presence of mesopores was calculated using the t-plot method. The pore size distribution was determined using the BJH model (Halenda). Mercury porosimetry was used to determine the volume of the macropores. Mercury penetration into pores with radii ranging from 30 to 10,000 Å was carried out under pressures ranging from 0.1 to 200 MPa. During data processing, differential curves were obtained for the distribution of pores over radii, from which the contributions of pores of different sizes to the total volume of the porous space of the catalyst were determined.
The acid site types and concentrations were determined by IR spectroscopy after pyridine adsorption (IR-Py). IR spectra of the adsorbed pyridine were recorded using a Bruker Vertex-70V IR spectrometer (Bruker Optic GmbH, Ettlingen, Germany) with a resolution of 4 cm
−1. The samples were precalcined at 450 °C for 2 h in a 10
−2 Pa vacuum. Pyridine was adsorbed onto the molecular sieve samples at 150 °C for 30 min. Pyridine was desorbed at 150 °C, 250 °C, and 350 °C. The number of Brønsted and Lewis acid sites was determined by integrating the bands in the ranges 1570–1510 and 1475–1410, respectively. The average molar extinction coefficients from references [
27] were used to calculate the concentrations.
The mechanical strength of the granular samples was tested on a LinteL PC-21 machine under static conditions using the compression method. Cylindrical granules with a length of 5–6 mm and a diameter of 1.4–1.6 mm were tested according to the ASTM D6175 standard.
2.10. Methods for Testing Catalysts
The catalytic transformations of n-hexadecane were studied in a flow reactor at temperatures between 220 and 260 °C and a pressure of 3 MPa. A molar ratio of H2/H-C16H34 = 10 was used, and the mass feed rate was 2 h−1. The reaction products were analyzed by gas-liquid chromatography on a Chromatek-Crystal 5000 chromatograph with a flame ionization detector and a glass capillary column (50 m, HP-1). Chromato-mass spectrometry was also used with a Shimadzu instrument whose chromatograph was equipped with a DB-5 column (50 m).
3. Results and Discussion
The catalytic properties of ZSM-5 zeolite depended on the degree of crystallinity and the presence of impurity phases.
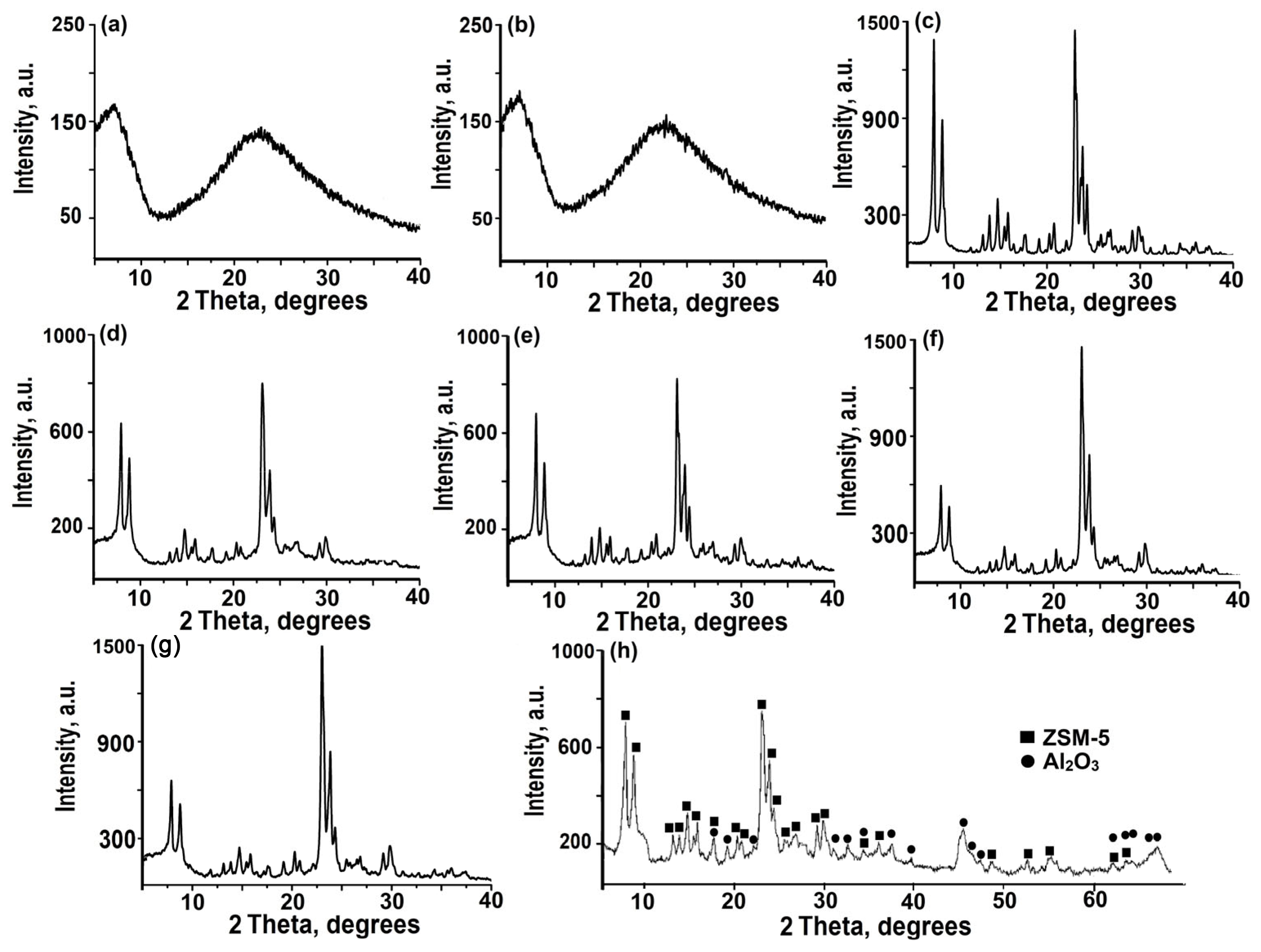
Figure 1 shows the X-ray diffraction patterns of amorphous aluminosilicates, initial powdered ZSM-5 zeolite, granules containing amorphous aluminosilicates and boehmite, and granules of ZSM-5 zeolite without a binder.
It can be seen from the X-ray diffraction pattern of the Na-ZSM-5 zeolite powder sample (
Figure 1c) that there are several intense signals at specific angles: 7.92°, 8.94°, 23.04°, 23.38°, and 23.94° degrees 2θ. These specific angles are characteristic of the MFI crystal structure with high phase purity (PDF№00-037-0390).
Amorphous aluminosilicates (
Figure 1a,b) show a strong diffraction peak in the 2θ angle range of 15–30°, regardless of the silicon source used. Granular samples containing ZSM-5 zeolite and amorphous aluminosilicates (
Figure 1d,e) show a well-defined diffraction peak in the 20–30° angle range, with a degree of crystallinity that does not exceed 70% for these granules.
After crystallization, the amorphous phase in the granules transforms into ZSM-5 zeolite with a high degree of purity and a crystallinity of at least 95% (
Figure 1f,j). These results indicate that the granular sample has a similar level of crystallinity to that of powdered ZSM-5 zeolite.
Table 1 shows the results of the study of the chemical composition of amorphous aluminosilicates and powdered and granular ZSM-5 zeolites. The SiO
2/Al
2O
3 ratio in powdered Na-ZSM-5 zeolite was found to be slightly lower than that in the reaction mixture due to incomplete aluminum incorporation during crystallization. This is in contrast to amorphous aluminosilicates, where the SiO
2/Al
2O
3 ratio was closer to the calculated value.
In granular samples, the SiO2/Al2O3 ratios are also similar before and after crystallization, indicating that the SiO2/Al2O3 ratio of amorphous aluminosilicates and zeolite ZSM-5 crystals remains constant during synthesis. However, in the granular sample of Na-ZSM-5-BD zeolite, there is a decrease in the SiO2/Al2O3 ratio due to the addition of boehmite to the granules.
The morphology and size of zeolite crystals is also one of the key factors affecting their catalytic properties [
28].
Figure 2 shows scanning transmission electron microscope (STEM) images of amorphous aluminum silicates obtained using different silicon sources. These images show that amorphous aluminosilicates are xerogels composed of spherical particles. ASM-1 aluminosilicate, obtained using TEOS, has particles with sizes between 5 and 10 nm, whereas ASM-2, obtained using an inorganic silicon source, is characterized by larger particles with sizes between 10 and 20 nm.
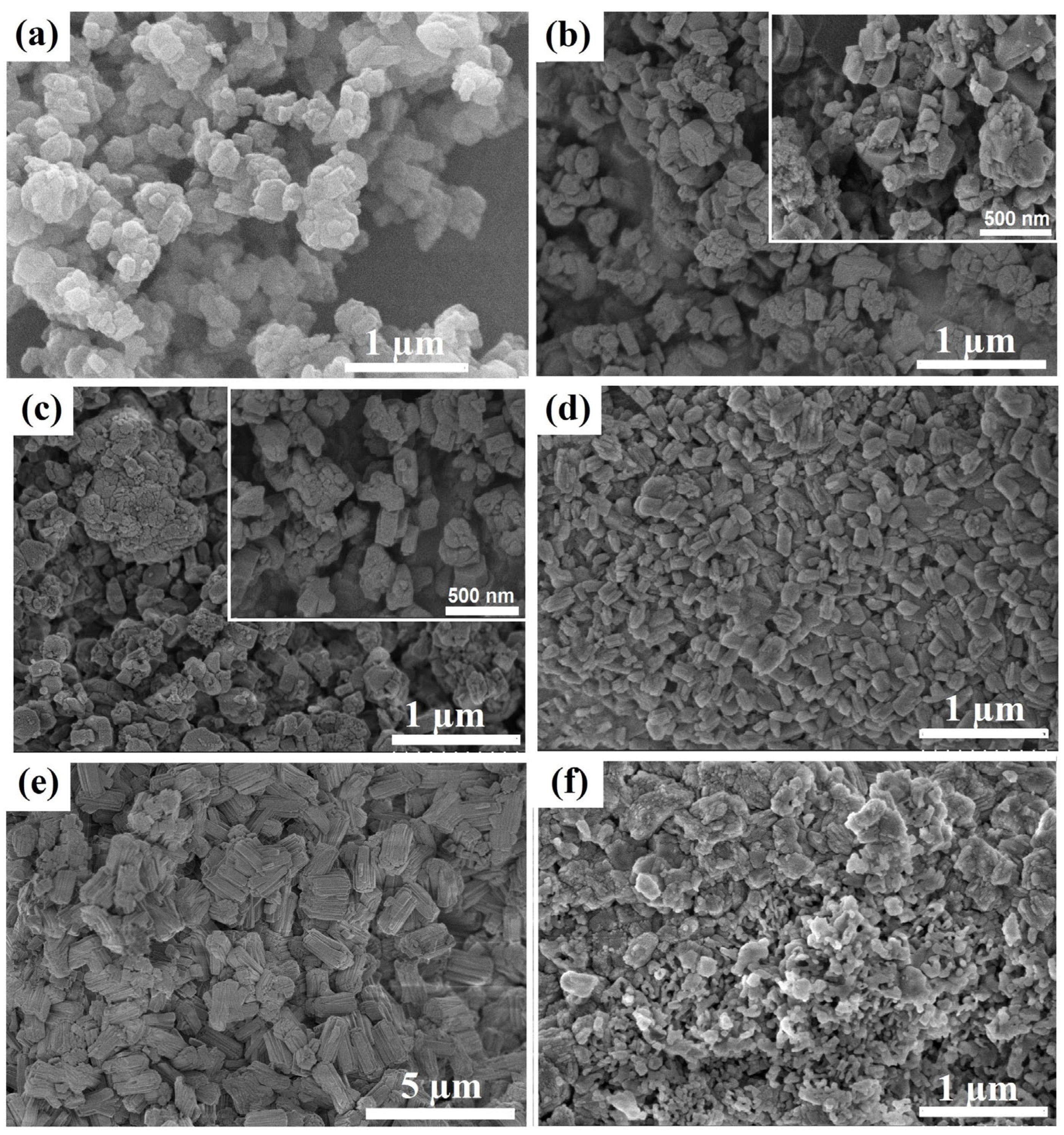
According to the SEM data (
Figure 3), the powdered Na-ZSM-5 sample consists of crystals in the form of fused prisms with sizes ranging from 300 to 500 nm. The Na-ZSM-5-ASM-1 and Na-ZSM-5-ASM-2 granules produced from the Na-ZSM-5 sample are composite materials composed of Na-ZSM-5 molecular sieve crystals with sizes ranging from 300 to 500 nm and highly dispersed particles of amorphous aluminosilicate filling the spaces between the crystals. The granules of the Na-ZSM-5-WB-1 and Na-ZSM-5-WB-2 zeolite samples are composed of clusters of crystals with different morphologies. These crystals can be cubic in shape with sizes between 50 and 200 nm or elongated prismatic in shape with sizes between 300 and 500 nm. It is worth noting that the Na-ZSM-5-WB-1 zeolite sample contains crystals that are smaller in size compared to those in the Na-ZSM-5-WB-2 zeolite sample. The results obtained can be explained by the fact that during the crystallization of granules containing ASM-1 aluminosilicate, a higher degree of supersaturation of crystal nuclei is created compared to the crystallization of granules containing ASM-2 aluminosilicate. This results in the formation of more finely dispersed crystals.
The Na-ZSM-5-BD zeolite sample consists of ZSM-5 molecular sieve crystals, with crystal sizes ranging from 300 to 500 nm, and highly dispersed aluminum oxide particles filling the spaces between the crystals.
Figure 4 shows photographs of the granules both before and after the crystallization process. It is clear that crystallization had little or no effect on the appearance or size of the granules.
Granular industrial catalysts based on ZSM-5 zeolite require high mechanical strength, as large amounts of catalyst in the reactor can cause the lower layers to collapse under the pressure of their own weight.
Table 2 presents the data on the mechanical strength of ZSM-5 zeolite granules after the crystallization process. The strength of the granules increased 2–3 times after crystallization compared to granules with amorphous aluminosilicates. This is due to the fusion of zeolite crystals from the initial granules with crystals formed during the crystallization of the amorphous component in the granules. As we mentioned previously, these granular materials form a single structure of fused crystals [
24]. The Na-ZSM-5-WB-1 sample has a higher mechanical strength than the Na-ZSM-5-WB-2 sample due to the fact that its initial granules contain aluminosilicates with a more uniform particle distribution. This leads to an increase in the contact surface area and denser crystal fusion, resulting in an increase in strength.
Comparing the mechanical strength of the Na-ZSM-5-WB and Na-ZSM-5-BD granules, it can be observed that the strength of the samples with a developed secondary porous structure is approximately 20% higher due to the unique formation process.
Figure 5 shows the nitrogen adsorption−desorption isotherms and pore size distribution for all samples. It can be seen that all isotherms are close to type IV, with a sharp increase in the low-pressure region and a hysteresis loop of type H3 between 0.8 and 1.0 pressure. This type of isotherm is typical for micro-mesoporous materials. The formation of mesopores in Na-ZSM-5 is due to the partial melting of nanoscale crystals into prism-like structures, as can be clearly seen from the SEM images (
Figure 3).
Table 3 shows the characteristics of the porous structure of the powdered zeolite and the granules made from it. It can be seen that the introduction of ~40% by weight of amorphous aluminosilicate into the granules (Na-ZSM-5-ASM-1 zeolite sample) results in a decrease of about 30% in the volume of micropores and an increase in the volume of mesopores. This decrease in micropore volume and increase in mesopore volume is due to the addition of mesoporous amorphous aluminosilicate to the composition. The Na-ZSM-5-ASM-1 sample has a higher specific surface area and mesopore volume than the Na-ZSM-5-ASM-2 sample. This is because the former contains an aluminosilicate with a smaller particle size. After crystallization, granular samples experience an increase in micropore volume due to the formation of ZSM-5 zeolite crystals.
The specific surface area and volume of mesopores in the Na-ZSM-5-WB-1 sample are higher than those in the Na-ZSM-5-WB-2 sample due to the smaller crystals formed in its granules. In a granular sample containing a boehmite-based binder, there is a decrease in the specific surface area and volume of micropores due to the presence of the binder in the granules.
A higher volume of macropores was observed in the Na-ZSM-5-WB-2 zeolite sample than that in the Na-ZSM-5-WB-1 sample. This is due to the presence of larger crystals in the granules of the Na-ZSM-5-WB2 sample. Among the granular samples, the ZSM-5 samples without binders were characterized by the highest volume of macropores. Therefore, granular samples without binders can be considered as materials with secondary porosity, in which a well-developed secondary porous structure consisting of meso- and macropores has formed.
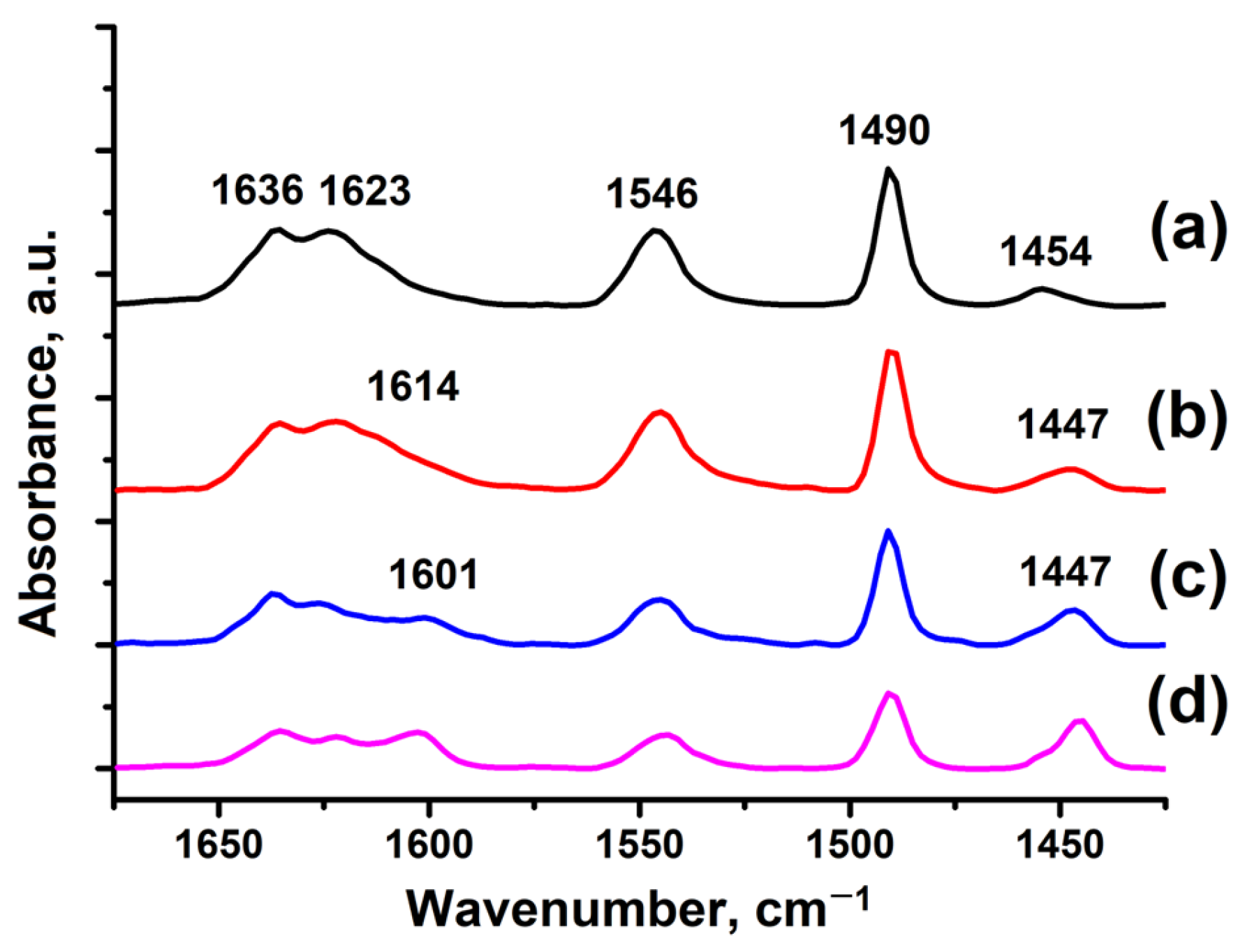
The acidic properties of the H-ZSM-5 zeolite samples were investigated by IR spectroscopy of pyridine adsorption (
Figure 6). The bands at 1636 and 1546 cm
−1 correspond to pyridine adsorbed on Brønsted acid sites (BAS). The bands at 1623 and 1454 cm
−1 in H-ZSM-5-WB-1 (
Figure 6b), H-ZSM-5-WB-2 (
Figure 6c), and H-ZSM-5-BD-1 (
Figure 6d) zeolite samples correspond to pyridine adsorbed on Lewis acid sites in zeolite (LAS). Samples H-ZSM-5-WB-1, H-ZSM-5-WB-2, and H-ZSM-5-BD show bands at 1614, 1601, and 1447 cm
−1, respectively, which are associated with adsorption on weak Lewis acid sites on extraframework aluminum. The band at 1490 cm
−1 is attributed to both BAS and LAS.
The sample based on the original powdered ZSM-5 in H-form is mainly characterized by the presence of Brønsted acidity.
Table 4 shows that the BAS content is 295 μmol/g, and the LAS content is 39 μmol/g. The total BAS concentration of the H-ZSM-5-WB-1 zeolite is higher than that of the original H-ZSM-5 powder at 318 μmol/g. However, the amount of strong BAS (desorption at 350 °C) is the same. This can be explained by the fact that the H-ZSM-5-WB-1 granular sample has a higher crystal dispersion-specific surface area and a higher concentration of acid sites.
The H-ZSM-5-WB-2 zeolite sample shows a decrease in the number of BAS to 206 μmol/g and an increase in the number of LAS to 90 μmol/g. The majority of these are weak Lewis acid sites (desorbing up to 250 °C). It is likely that there is more amorphous aluminosilicate present in the composition of H-ZSM-5-WB-2 than in H-ZSM-5-WB-1, as almost all of the amorphous component has crystallized in the latter.
Similarly, an even greater decrease in BAS concentration to 148 μmol/g in the H-ZSM-5-BD granular sample is associated with the dilution of the ZSM-5 zeolite by γ-Al2O3. Apparently, γ-Al2O3 simultaneously acts as a source of weak LAS, and therefore, an increase in the LAS concentration to 106 μmol/g is observed.
As a result of testing the catalytic properties of Pt/ZSM-5 zeolite samples in the hydroconversion of n-hexadecane, we found that the highest activity was exhibited by the Pt/ZSM-5-WB-1 sample with the highest BAS concentration (
Table 4) and specific surface area. The Pt/ZSM-5-BD sample with the lowest BAS concentration and specific surface area (
Table 3) was the least active for hexadecane hydroconversion. At 220 °C, the hexadecane conversions were 59.1%, 50.2%, and 31.5% for the Pt/ZSM-5-WB-1, Pt/ZSM-5-WB-2, and Pt/ZSM-5-BD zeolite samples, respectively.
Although the Pt/ZSM-5-WB-1 and Pt/ZSM-5-WB-2 samples have the same SiO
2/Al
2O
3 values (
Table 1), Pt/ZSM-5-WB-1 is characterized by a higher concentration of Brønsted acid sites, which play a significant role in the bifunctional mechanism of hydroconversion at the isomerization/cracking stage [
20], and a higher specific surface area and thus better accessibility of acid sites for hexadecane molecules. Therefore, the granular binder-free Pt/ZSM-5-WB-1 sample is more active in the conversion of hexadecane. The Pt/ZSM-5-BD sample showed even lower activity because the binder in its composition led to a decrease in the content of H-ZSM-5 as the active component and probably a decrease in the availability of active sites due to partial pore blocking.
It has been found that the main reaction pathway for the conversion of hexadecane over Pt/ZSM-5 catalysts is hydrocracking (
Figure 7), which produces hydrocarbons with carbon numbers ranging from 5 to 10 as the main products (
Table 5). As the reaction temperature is increased from 220 to 260 °C, the yield of gaseous C
2–C
4 hydrocarbons increases, while the content of C
11–C
13 hydrocarbons decreases. At the same time, there is a decrease in the concentration of normal-structure hydrocarbons relative to iso-structure hydrocarbons (i/n ratio).
Another direction of hexadecane conversion using Pt/ZSM-5 catalyst samples is hydroisomerization (
Figure 7). The Pt/ZSM-5-WB-1 sample, which has a higher crystal dispersion, shows the highest selectivity for hexadecane isomers. At 220 °C, the selectivities for hexadecane isomer formation on the Pt/ZSM-5-BD, Pt/ZSM-5-WB-2, and Pt/ZSM-5-WB-1 samples were 13.6%, 14.8%, and 15.7%, respectively.
The low activity in the hydroisomerization reaction compared to hydrocracking is due to the peculiarities of the structure of zeolite ZSM-5 [
18]. The three-dimensional channel microporous structure of this zeolite allows the conversion of n-paraffins into highly branched isomers. The isomers can experience stronger diffusion difficulties in the micropores formed by the 10R rings. This leads to their further destruction. The formation of transport pores leads to improved diffusion. Although, with increasing mesopore volume (
Table 3), an almost indistinguishable increase in selectivity for hexadecane isomers is observed in samples of the Pt/ZSM-5-BD, Pt/ZSM-5-WB-2, and Pt/ZSM-5-WB-1 series.
4. Conclusions
The physicochemical properties of the products of crystallization of granules (containing 60% by weight of powdered ZSM-5 and 40% by weight of a temporary binder) into granular zeolite using the “less-bind” technology were studied using XRD, XRF, SEM, N2 adsorption-desorption, mercury porosimetry, and IR spectroscopy with pyridine adsorption.
Two amorphous aluminosilicates with a SiO2/Al2O3 molar ratio of 50 were used as temporary binders. One was prepared from tetraethyl orthosilicate and aluminum nitrate by a two-step sol-gel process, while the other was prepared from aqueous solutions of sodium silicate and aluminum sulfate.
It was found that during the crystallization process, granules prepared using amorphous aluminosilicates as a temporary binder yielded granular ZSM-5 zeolites (d = 1.5 mm, l = 5 mm) with high phase purity and a developed secondary porous structure. The specific surface area of these granules was found to be between 330 and 350 m2/g, with mesopore volumes between 0.20 and 0.28 cm3/g and macropore volumes between 0.20 and 0.30 cm3/g.
It has been found that during the crystallization of granules containing amorphous aluminosilicate with a larger specific surface area and smaller particle size, nanoscale crystals of ZSM-5 zeolite are formed, which are smaller in size.
It has been shown that granular ZSM-5 zeolites with high phase purity and a developed secondary porous structure outperform granular ZSM-5 zeolites with an aluminum oxide binder in terms of mechanical strength.
It was found that a bifunctional catalyst is formed on the H-form of granular ZSM-5 zeolite with a high degree of crystallinity and a developed secondary porous structure when 0.5 wt. % Pt is added. This catalyst exhibits higher activity in the hydroconversion of n-hexadecane (particularly hydrocracking) compared to a catalyst prepared using granular ZSM-5 zeolite with a binder.