Effect of High-Current Pulsed Electron Beam on Microstructure and Surface Properties of Ag-10La0.7Sr0.3CoO3 Composites
Abstract
1. Introduction
2. Materials and Methods
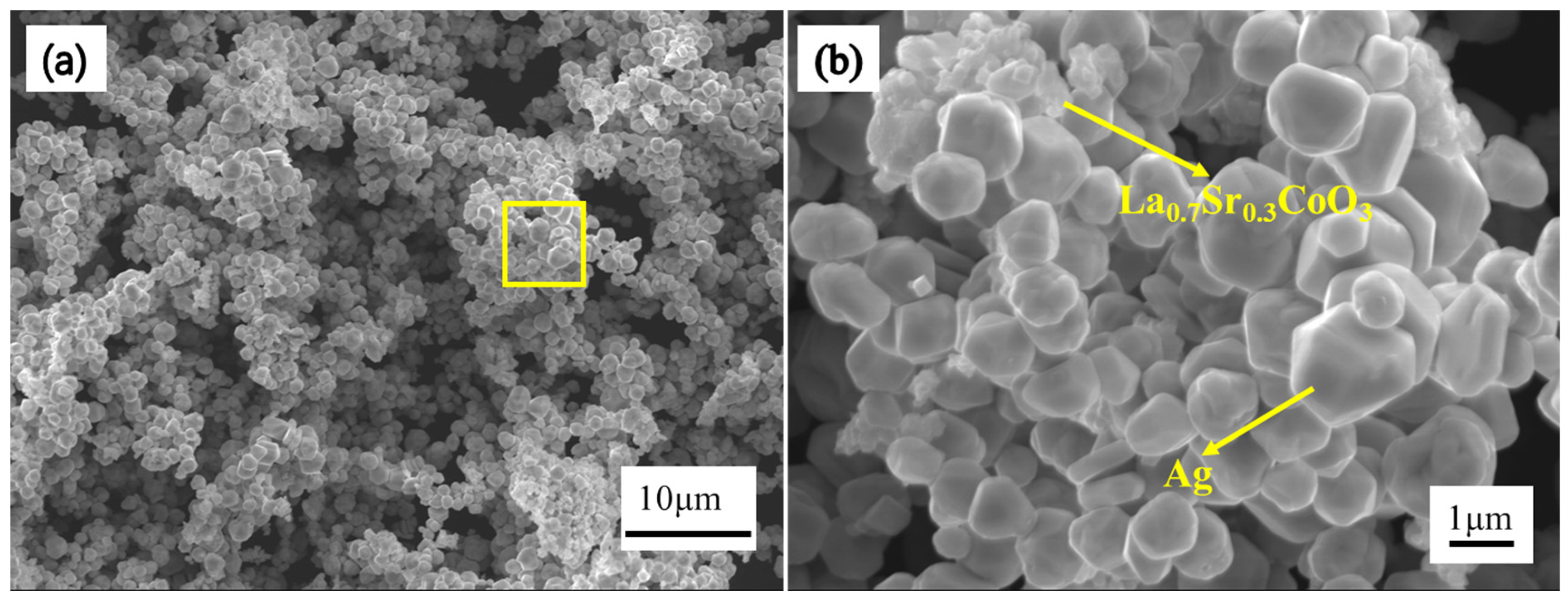
2.1. Material Preparation
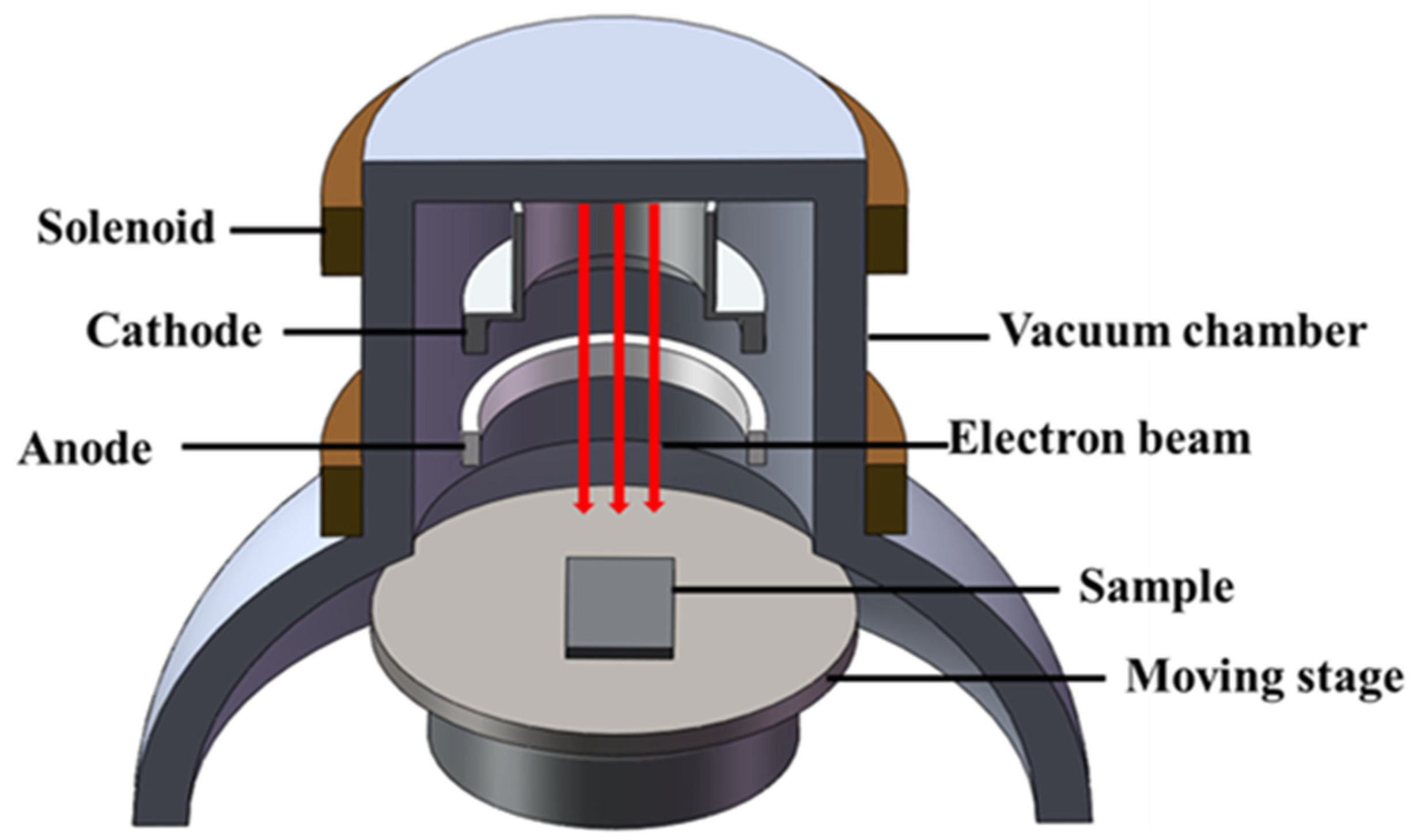
2.2. HCPEB Treatment
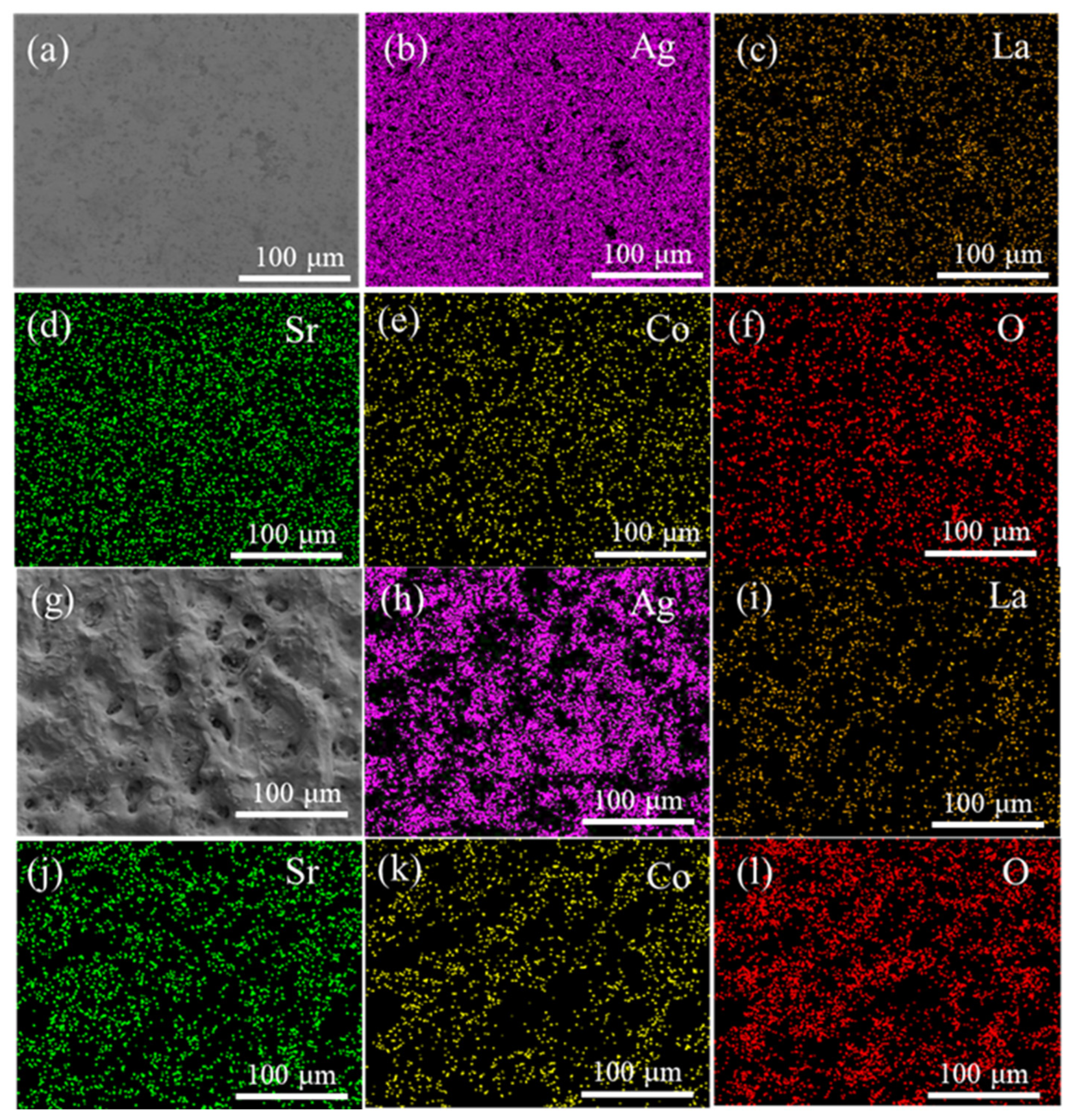
2.3. Microstructure Characterization and Performance Analysis
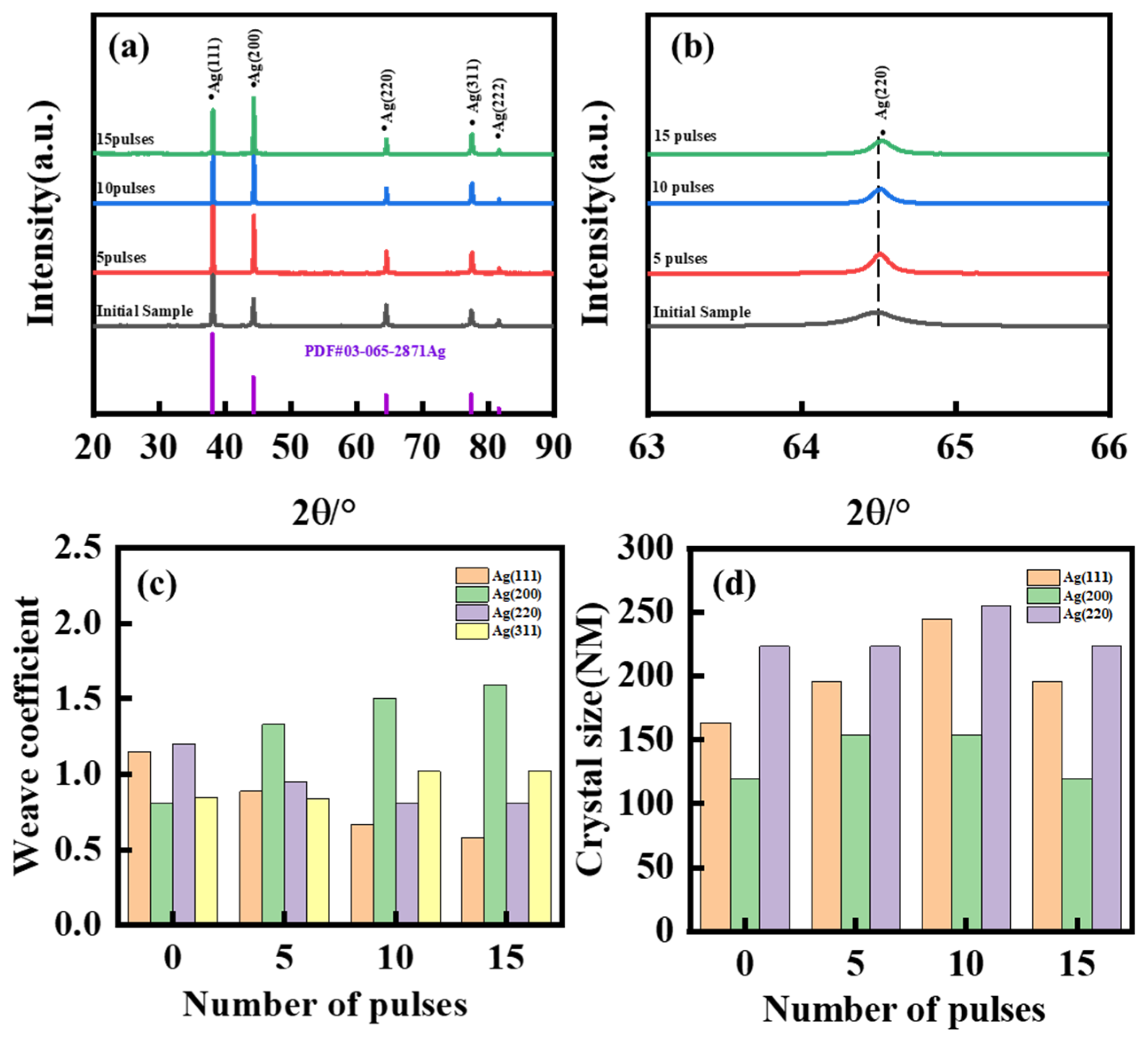
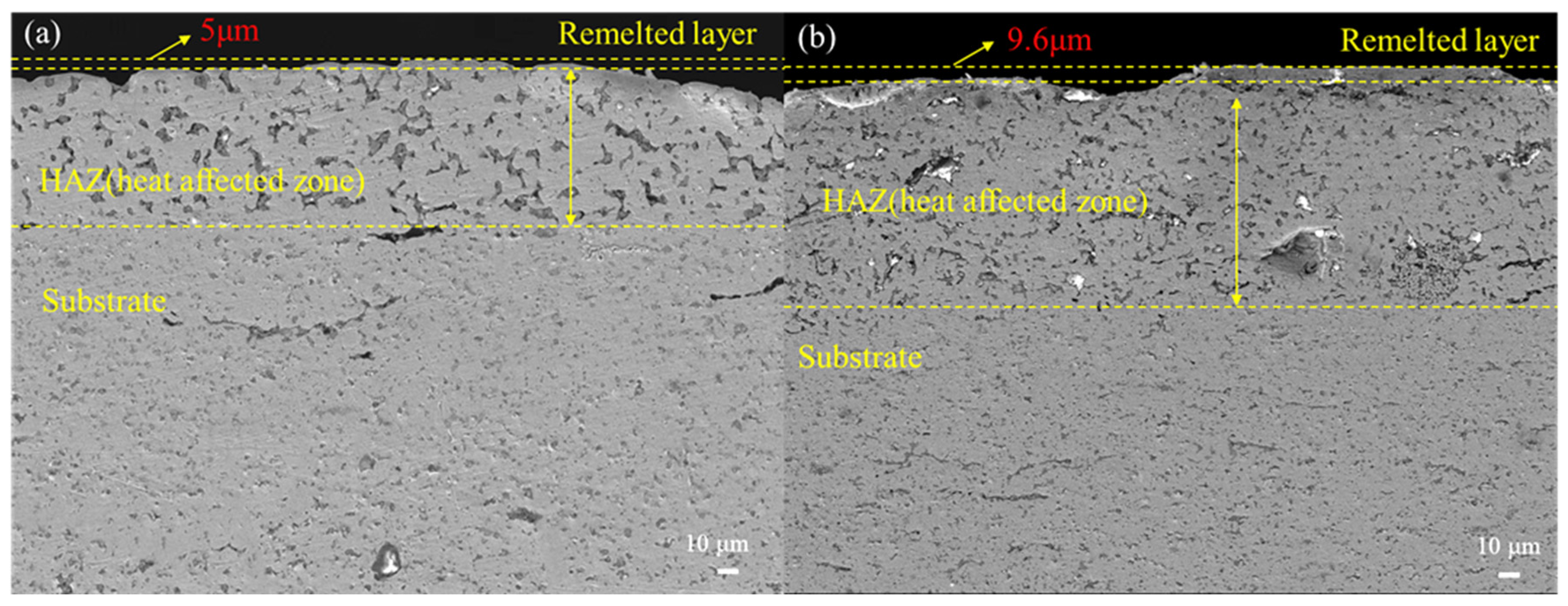
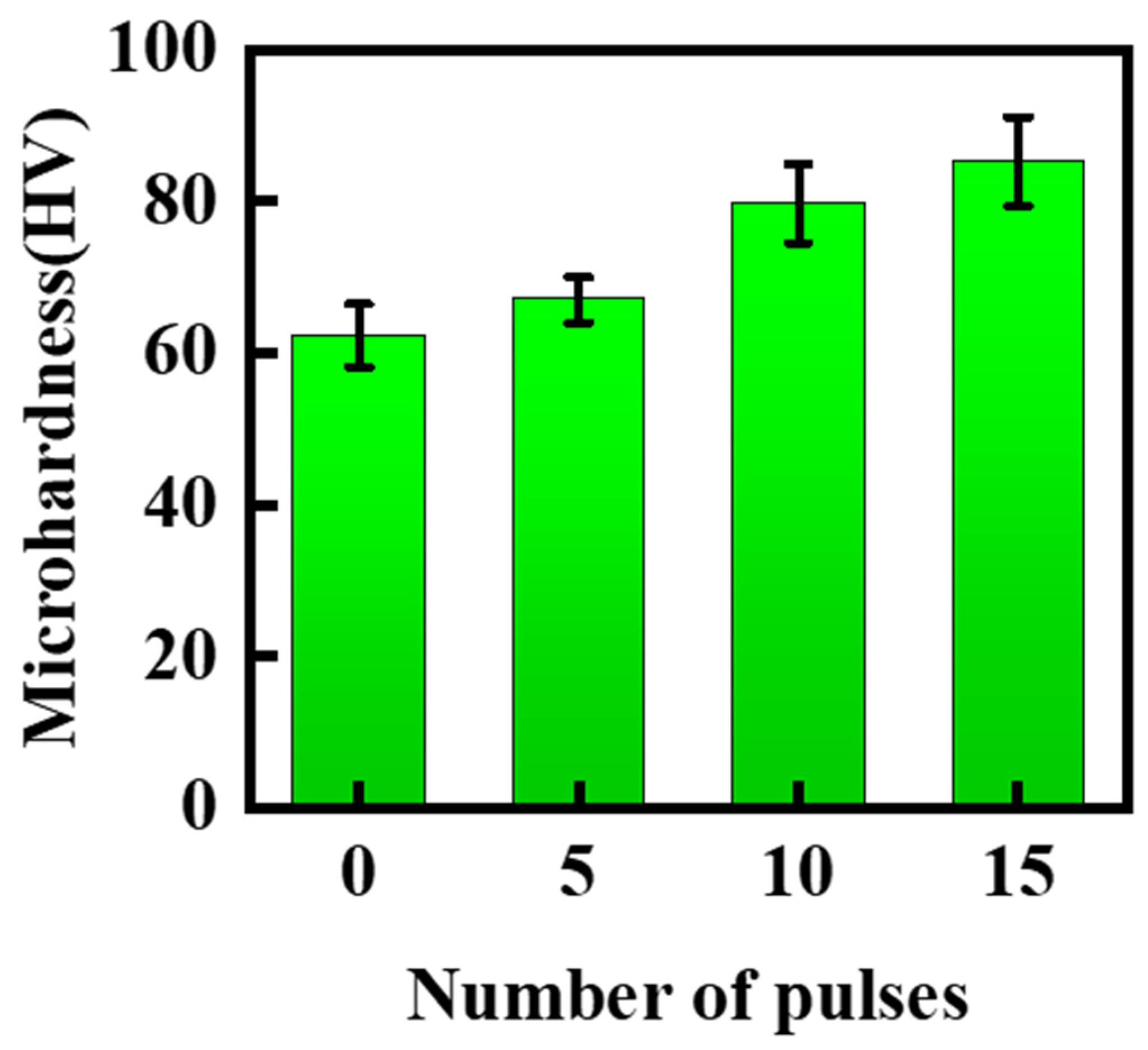
3. Results and Discussion
4. Conclusions
- (1)
- After five HCPEB treatments, local melting and rapid solidification occurred on the sample surface, resulting in the formation of many volcanic pits. After 15 treatments, the number of pits decreased, and there was obvious recrystallization and the formation of new grains. A greater number of treatments may lead to deeper melting and solidification and extensive surface modification.
- (2)
- The hardness of the material surface increased with an increasing number of treatments. The increase in hardness was attributed to the reinforcement of a uniform phase distribution, surface remelting and recrystallization, and a reduction in the number of pores.
- (3)
- The improvement in corrosion resistance was mainly due to the refinement of the surface’s microstructure and the introduction of residual compressive stress.
- (4)
- The observed improvement in electrical conductivity was attributed to the grain refinement, defect formation, and surface healing of the samples after the HCPEB treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ćosović, V.; Ćosović, A.; Talijan, N.; Živković, D.; Manasijević, D.; Minić, D. Improving dispersion of SnO2 nanoparticles in Ag–SnO2 electrical contact materials using template method. J. Alloys Compd. 2013, 567, 33–39. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, L.; Shen, T.; Qiao, Z.; Yang, H.; Fan, X.; Chen, L. Effects of Oxide-Modified Spherical ZnO on Electrical Properties of Ag/ZnO Electrical Contact Material. J. Mater. Eng. Perform. 2016, 25, 3662–3671. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Yuan, Z.; Wang, Z.; Du, D. Microstructure and arc erosion behaviors of Ag-CuO contact material prepared by selective laser melting. J. Alloys Compd. 2021, 860, 158494. [Google Scholar] [CrossRef]
- Buyanova, E.S.; Shafigina, R.R.; Morozova, M.V.; Emel’yanova, Y.V.; Khisametdinova, V.V.; Zhukovskii, V.M.; Petrova, S.A.; Tarakina, N.V. Electrochemical characteristics, thermal and chemical compatibility in the La0.7Sr0.3CoO3 electrode-γ-BIFEVOX electrolyte system. Russ. J. Inorg. Chem. 2013, 58, 554–558. [Google Scholar] [CrossRef][Green Version]
- Zhao, D.; Gao, Z.; Xian, H.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Jiang, Z.; Zhang, J.; Zheng, L.; et al. Addition of Pd on La0.7Sr0.3CoO3 Perovskite To Enhance Catalytic Removal of NOx. Ind. Eng. Chem. Res. 2018, 57, 521–531. [Google Scholar] [CrossRef]
- Li, R.; Kumar, P.; Mahendiran, R. Critical behavior in polycrystalline La0.7Sr0.3CoO3 from bulk magnetization study. J. Alloys Compd. 2016, 659, 203–209. [Google Scholar] [CrossRef]
- Liu, N.; Yan, G.; Guo, H.; Mao, Q.; Cai, Z. Effect of Dy Doping on Magnetism of La0.7Sr0.3CoO3 System. Rare Met. Mater. Eng. 2010, 39, 874–877. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Cheng, S.; Cai, W.; Yu, F.; Zhang, Y.; Wu, P.; Liu, M. A high-performance electrode for supercapacitors: Silver nanoparticles grown on a porous perovskite-type material La0.7Sr0.3CoO3 substrate. Chem. Eng. J. 2017, 328, 1–10. [Google Scholar] [CrossRef]
- Mira, J.; Rivas, J.; Vázquez, M.; Ibarra, M.R.; Caciuffo, R.; Rodríguez, M.A.S. Field-induced magnetic anisotropy in La0.7Sr0.3CoO3. Europhys. Lett. 2003, 62, 433–438. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Yuan, L.; Li, S.; Ma, W.; Liu, Z.; Feng, S. Molten Salt Flux Synthesis, Crystal Facet Design, Characterization, Electronic Structure, and Catalytic Properties of Perovskite Cobaltite. ACS Appl. Mater. Interfaces 2018, 10, 28219–28231. [Google Scholar] [CrossRef]
- Sun, H.; Lordi, V.; Takamura, Y.; Samanta, A. Unraveling the Correlation between the Interface Structures and Tunable Magnetic Properties of La1-xSrxCoO3-δ/La1-xSrxMnO3-δ Bilayers Using Deep Learning Models. ACS Appl. Mater. Interfaces 2024, 16, 30166–30175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, D.; Shen, T.; Shao, Z.; Yang, H.; Yang, F. Improving Interfacial Wettability, Physical, and Mechanical Properties of Ag/La1-xSrxCoO3 Electrical Contact Materials by In Situ Cu-Doping. J. Mater. Eng. Perform. 2023, 33, 7892–7903. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Hong, L. Fabrication and characterization of (Pd/Ag)-La0.2Sr0.8CoO3-δ composite membrane on porous asymmetric substrates. J. Membr. Sci. 2003, 224, 137–150. [Google Scholar] [CrossRef]
- Li, G.; Fang, X.; Feng, W.; Liu, J. In situ formation and doping of Ag/SnO2 electrical contact materials. J. Alloys Compd. 2017, 716, 106–111. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of monodisperse iron oxide nanocrystal formation by "heating-up" process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, H.; Wu, C.; Li, N.; Jiang, Y.; Yi, D. Preferential oxidation of intermetallic compounds in Ag-2Sn-4La alloy. Corros. Sci. 2018, 143, 177–186. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Song, K.; Shaolin, L.; Jiang, F.; Wang, X. Arc erosion resistance of hybrid copper matrix composites reinforced with CNTs and micro-TiB2 particles. J. Mater. Res. Technol. 2021, 11, 1469–1479. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Abdul Aziz, S.; Mamat, M.S. Effect of milling time on the microstructure, physical and mechanical properties of Al-Al2O3 nanocomposite synthesized by ball milling and powder metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef]
- Karabacak, A.H.; Canakci, A.; Ozkaya, S.; Tunc, S.A.; Cevik, Z.A.; Yalcin, E.D. The effects of different types and ratios of reinforcement, and machining processes on the machinability of Al2024 alloy nanocomposites. J. Compos. Mater. 2023, 57, 2811–2827. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Bhaskar, S.; Kumar, A. Parametric Optimization and Ranking Analysis of AA2024-Al2O3/AlN Alloy Composites Fabricated Via Stir Casting Route Under Dry Sliding Wear Investigation. Int. J. Metalcast. 2024, 18, 667–687. [Google Scholar] [CrossRef]
- Liang, X.; Meng, X.; Ni, P.; Zhao, Z.; Deng, X.; Chen, G.; Chen, Y.; Li, S.; Wu, S.; Liu, J.; et al. Effect of feedstock bimodal powder mixture and infiltration process on mechanical behaviour of binder jetting processed 316L stainless steel. Powder Metall. 2023, 66, 387–402. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, B.; Zhao, P.; Fu, H.; Kamali, A.R. Magnetic luffa/graphene/CuFe2O4 sponge for efficient oil/water separation. Sep. Purif. Technol. 2024, 337, 126347. [Google Scholar] [CrossRef]
- Anikeev, S.G.; Shabalina, A.V.; Kulinich, S.A.; Artyukhova, N.V.; Korsakova, D.R.; Yakovlev, E.V.; Vlasov, V.A.; Kokorev, O.V.; Hodorenko, V.N. Preparation and Electron-Beam Surface Modification of Novel TiNi Material for Medical Applications. Appl. Sci. 2021, 11, 4372. [Google Scholar] [CrossRef]
- Wang, L.; Gao, B.; Sun, Y.; Zhang, Y.; Hu, L. Effect of High Current Pulsed Electron Beam (HCPEB) on the Organization and Wear Resistance of CeO2-Modified Al-20SiC Composites. Materials 2023, 16, 4656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, G.L.; Gao, B.; Wang, L.; Li, Z.; Hu, L.; Shi, Z.; Yin, Q. Effect of High-Current Pulsed Electron Beam on Properties of Graphene-Modified Aluminum Titanium Carbide Composites. Materials 2022, 15, 7879. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, B.; Tu, G.F.; Cao, H.; Hao, S.Z.; Dong, C. Surface modification of Al–12.6Si alloy by high current pulsed electron beam. Appl. Surf. Sci. 2012, 258, 2052–2056. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, C.; Cai, J.; Lyu, P.; Jin, Y.; Guan, Q. Properties of a rapidly solidified Ni–Nb layer prepared using a high-current pulsed electron beam. Vacuum 2020, 177, 109362. [Google Scholar] [CrossRef]
- Han, Z.; Li, X.; Lu, B.; Yan, H. Influence of HCPEB Bombardment on the Microstructure and Corrosion Resistance of CMAS of Thermal Barrier Coating. Rare Met. Mater. Eng. 2023, 52, 2147–2153. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Zhang, X.; Wang, Q.; Wang, Q.; Wang, H.; Jiang, W.; Liu, S.; Liu, C.; Wang, N.; et al. The formation mechanism of (001) preferred orientation for anatase TiO2 film prepared by DC pulsed magnetron sputtering. Vacuum 2021, 190, 110287. [Google Scholar] [CrossRef]
- Elmkhah, H.; Zhang, T.F.; Abdollah-zadeh, A.; Kim, K.H.; Mahboubi, F. Surface characteristics for the TiAlN coatings deposited by high power impulse magnetron sputtering technique at the different bias voltages. J. Alloys Compd. 2016, 688, 820–827. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.X.; Du, J.W.; Chen, L.; Kim, K.H.; Wang, Q. Influence of preliminary metal-ion etching on the topography and mechanical behavior of TiAlN coatings on cemented carbides. Surf. Coat. Technol. 2022, 432, 128040. [Google Scholar] [CrossRef]
- Staia, M.H.; Puchi, E.S.; Lewis, D.B.; Cawley, J.; Morel, D. Microstructural characterization of chemically vapor deposited TiN coating. Surf. Coat. Technol. 1996, 86–87, 432–437. [Google Scholar] [CrossRef]
- Sahadat Hossain, M.; Ahmed, S. Easy and green synthesis of TiO2 (Anatase and Rutile): Estimation of crystallite size using Scherrer equation, Williamson-Hall plot, Monshi-Scherrer Model, size-strain plot, Halder-Wagner Model. Results Mater. 2023, 20, 100492. [Google Scholar] [CrossRef]
- Garg, A.; Parmar, L.K.; Garg, T.; Dager, H.S.; Bhardwaj, P.; Yadav, A. Structural analysis and dielectric behavior of low-temperature synthesized nickel cobaltite. Chem. Phys. Impact 2024, 8, 100457. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Hu, L.; Li, K.; Zhang, Y. Effect of CeO2 on Corrosion Resistance of High-Current Pulsed Electron Beam Treated Pressureless Sintering Al-20SiC Composites. Coatings 2021, 11, 707. [Google Scholar] [CrossRef]
- Mackowski, K.N.; Hargather, C.Z. A first-principles study of self-diffusion and dilute solute diffusion of Au in FCC Ag. Comput. Mater. Sci 2021, 193, 110414. [Google Scholar] [CrossRef]
- Chai, L.; Chen, B.; Wang, S.; Zhang, Z.; Murty, K.L. Microstructural, textural and hardness evolution of commercially pure Zr surface-treated by high current pulsed electron beam. Appl. Surf. Sci. 2016, 390, 430–434. [Google Scholar] [CrossRef]
- Guan, R.-G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Metall. Sin. 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Lyu, P.; Chen, Y.; Liu, Z.; Cai, J.; Zhang, C.; Jin, Y.; Guan, Q.; Zhao, N. Surface modification of CrFeCoNiMo high entropy alloy induced by high-current pulsed electron beam. Appl. Surf. Sci. 2020, 504, 144453. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Qiu, S.; Zhai, W.; Li, Q.; Hao, S. Evolution of Microstructure at the Surface of 40CrNiMo7 Steel Treated by High-Current Pulsed Electron Beam. Coatings 2020, 10, 311. [Google Scholar] [CrossRef]
- Guan, Q.; Han, J.; Zhou, S.; Guan, J.; Zhang, C.; Cao, F.; Chen, X. Improved mechanical and tribological properties of TiAlN coatings by high current pulsed electron beam irradiation. Int. J. Refract. Met. Hard Mater 2024, 118, 106435. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, Y.; Dai, H.; Wang, E. Enhanced surface quality and properties of cold spray Al coating by high current pulsed electron beam treatment. Nucl. Instrum. Methods Phys. Res. Sect. B 2023, 542, 7–13. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, J.; Zhang, F.; Liu, J.; Hu, J.; Chai, L.; Song, B.; Guo, N.; Guo, S.; Yao, Z. Effect of high current pulsed electron beam on surface microstructure and properties of cold-rolled austenitic stainless steel. J. Mater. Res. Technol. 2024, 29, 1183–1193. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, X.; Wang, J.; Lu, L.; Yang, Z.; Lyu, P.; Guan, Q.; Cai, J. Amorphization and Nano-Crystallization of Ni-Nb Coating on GH3039 Alloys by High Current Pulsed Electron Beam. J. Nanomater. 2021, 11, 347. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Guo, X.P.; Zhao, G.Y.; Dong, Z.H.; Chen, G.Z. Determination of tafel slopes from coulostatically induced transients and its integral alogrithm. Acta Phys. Chim. Sin. 2005, 21, 93–97. [Google Scholar] [CrossRef]
- Lindbergh, G.; Zhu, B.H. Corrosion behaviour of high aluminium steels in molten carbonate in an anode gas environment. Electrochim. Acta 2001, 46, 1131–1140. [Google Scholar] [CrossRef]
- Lyu, P.; Peng, T.; Miao, Y.; Liu, Z.; Gao, Q.; Zhang, C.; Jin, Y.; Guan, Q.; Cai, J. Microstructure and properties of CoCrFeNiMo0.2 high-entropy alloy enhanced by high-current pulsed electron beam. Surf. Coat. Technol. 2021, 410, 126911. [Google Scholar] [CrossRef]
- Cai, J.; Zu, Z.; Li, C.; Lyu, P.; Guan, Q.; Li, Y. Hot Corrosion Behavior of Arc Ion Plating NiCoCrAlYSiHf Coating Via High-Current Pulsed Electron Beam. Oxid. Met. 2020, 94, 569–586. [Google Scholar] [CrossRef]



| Powder | Purity/wt.% | Particle Size/µm |
|---|---|---|
| Ag | 99.9 | 3 |
| La0.7Sr0.3CoO3 | 99.9 | 0.5–2 |
| Acceleration Voltage (kV) | Energy Density (J/cm2) | Pulse Time (μs) | Pulse Frequency (Hz) | Peak Current Density (A/cm2) | Electron Beam Cross-Sectional Area(cm2) | Vacuum Level (Pa) |
|---|---|---|---|---|---|---|
| 30 | 4 | 3 | 0.1 | 500 | 40 | 6 × 10−3 |
| Samples | Icorr/A·cm−2 | Ecorr/V |
|---|---|---|
| Original | 6.614 × 10−5 | −0.6862 |
| Treated 5 times | 5.051 × 10−5 | −0.7955 |
| Treated 10 times | 5.521 × 10−5 | −0.5963 |
| Treated 15 times | 2.754 × 10−5 | −0.1221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Gao, B.; Wang, L.; Shen, W.; Lin, P.; Lan, X.; Liu, H. Effect of High-Current Pulsed Electron Beam on Microstructure and Surface Properties of Ag-10La0.7Sr0.3CoO3 Composites. Surfaces 2024, 7, 739-751. https://doi.org/10.3390/surfaces7030048
Zhang H, Gao B, Wang L, Shen W, Lin P, Lan X, Liu H. Effect of High-Current Pulsed Electron Beam on Microstructure and Surface Properties of Ag-10La0.7Sr0.3CoO3 Composites. Surfaces. 2024; 7(3):739-751. https://doi.org/10.3390/surfaces7030048
Chicago/Turabian StyleZhang, Huanfeng, Bo Gao, Lei Wang, Wenhuan Shen, Pengshan Lin, Xin Lan, and He Liu. 2024. "Effect of High-Current Pulsed Electron Beam on Microstructure and Surface Properties of Ag-10La0.7Sr0.3CoO3 Composites" Surfaces 7, no. 3: 739-751. https://doi.org/10.3390/surfaces7030048
APA StyleZhang, H., Gao, B., Wang, L., Shen, W., Lin, P., Lan, X., & Liu, H. (2024). Effect of High-Current Pulsed Electron Beam on Microstructure and Surface Properties of Ag-10La0.7Sr0.3CoO3 Composites. Surfaces, 7(3), 739-751. https://doi.org/10.3390/surfaces7030048






