Applicability of Fluorine Gas Surface Treatment to Control Liquid Sodium Wettability
Abstract
1. Introduction
2. Experiments
2.1. Surface Fluorination
2.2. Surface Morphology Analysis
2.3. Liquid Na Wettability Test
3. Results
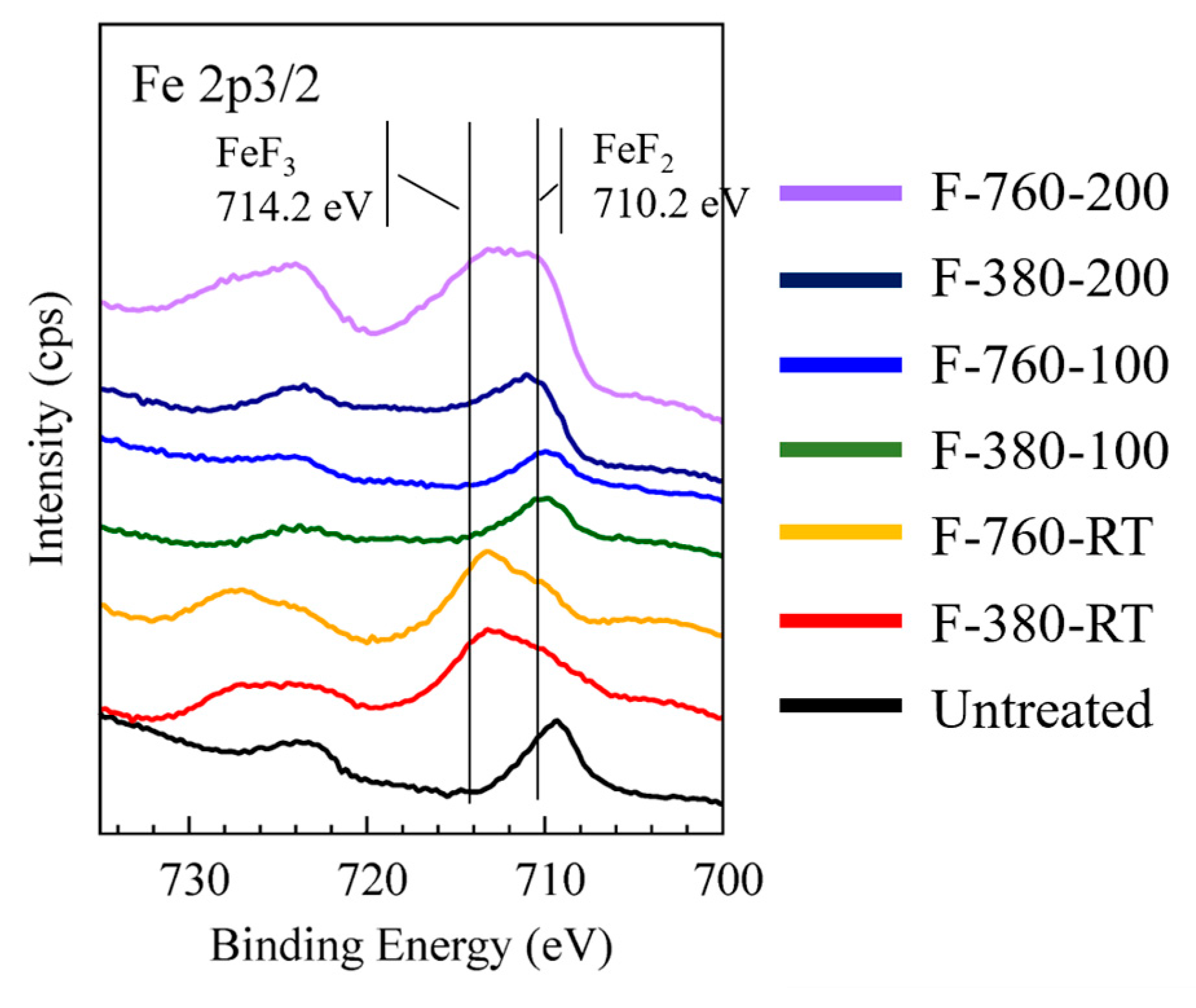
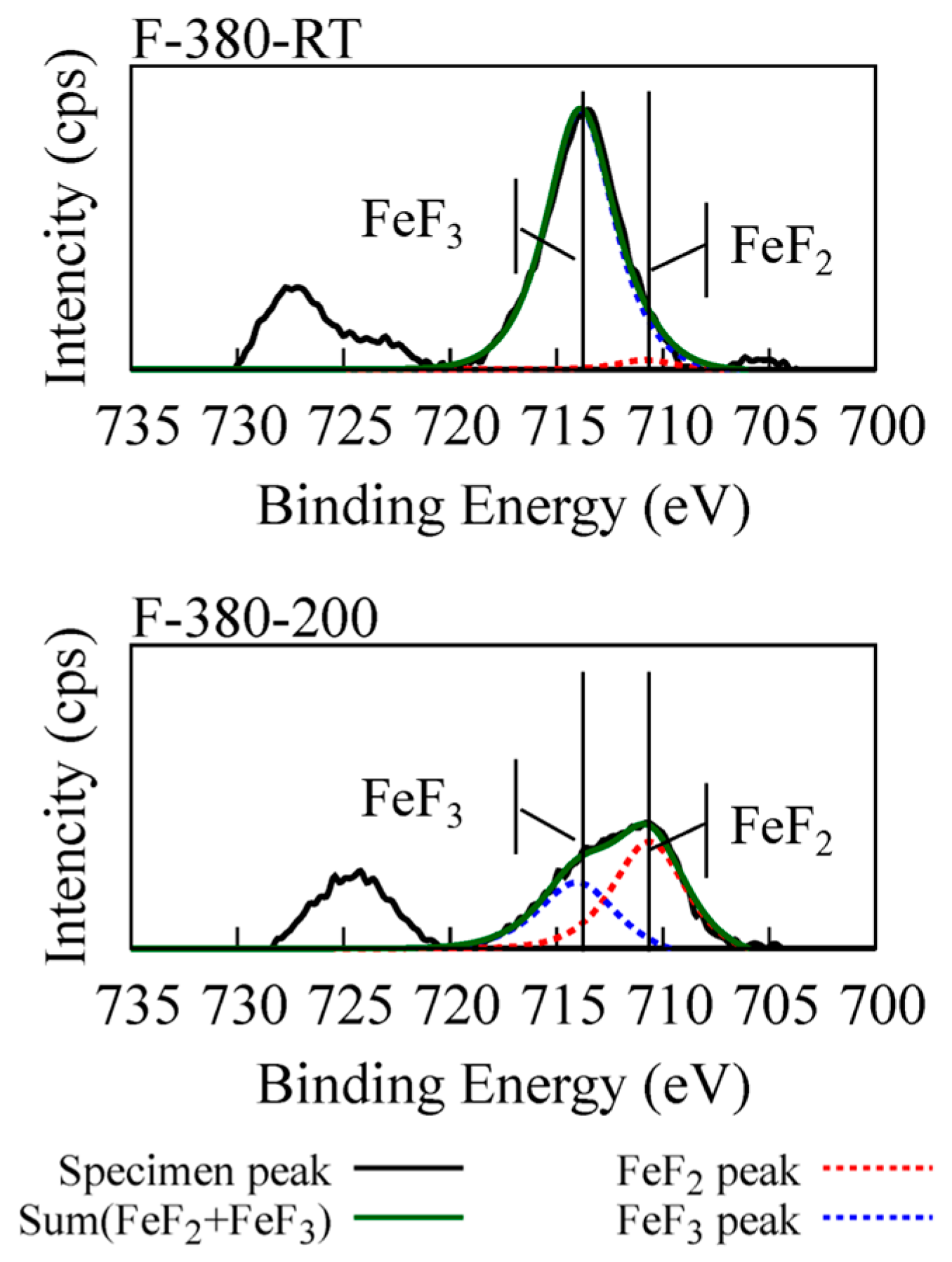
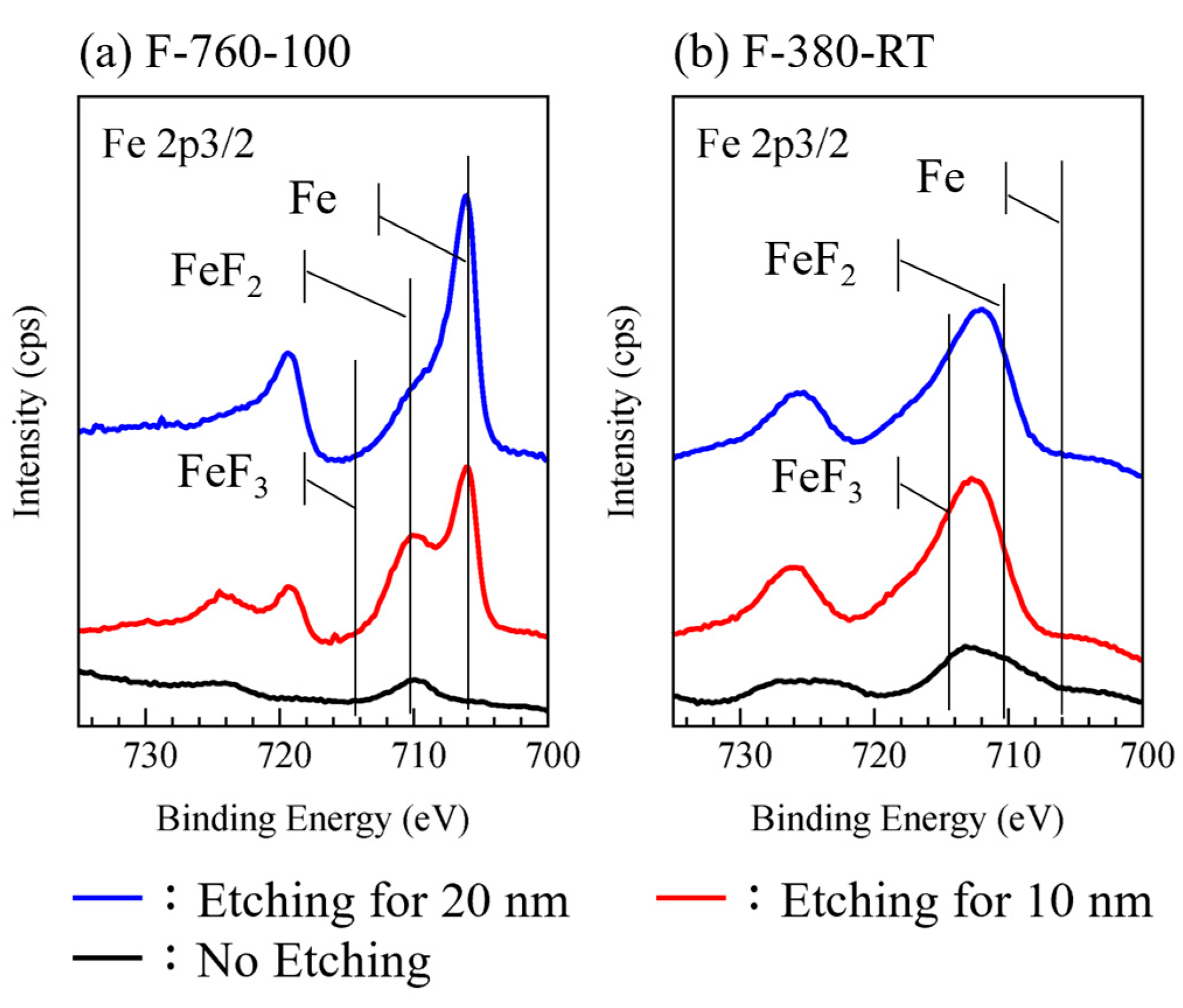
3.1. Surface Binding State
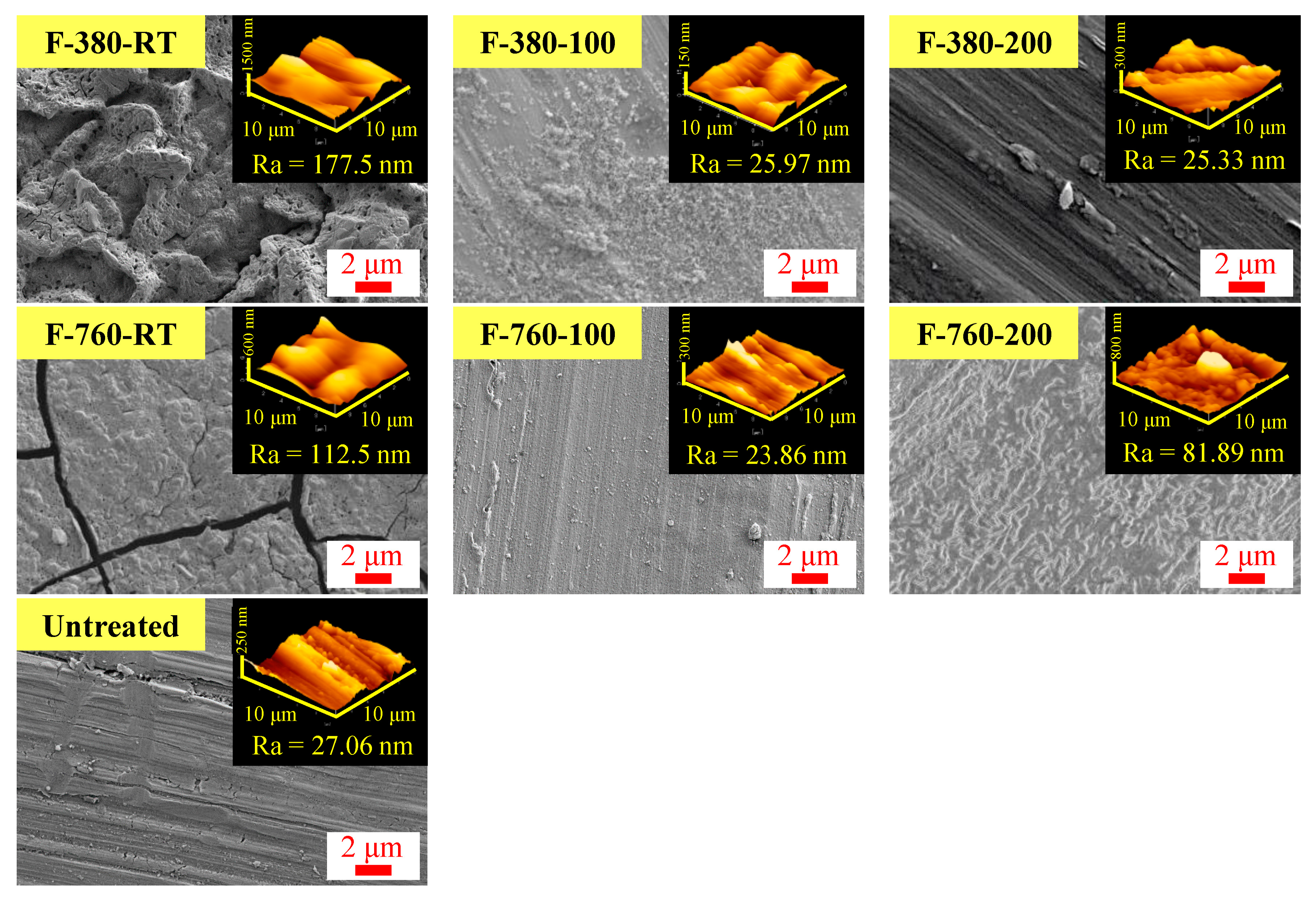
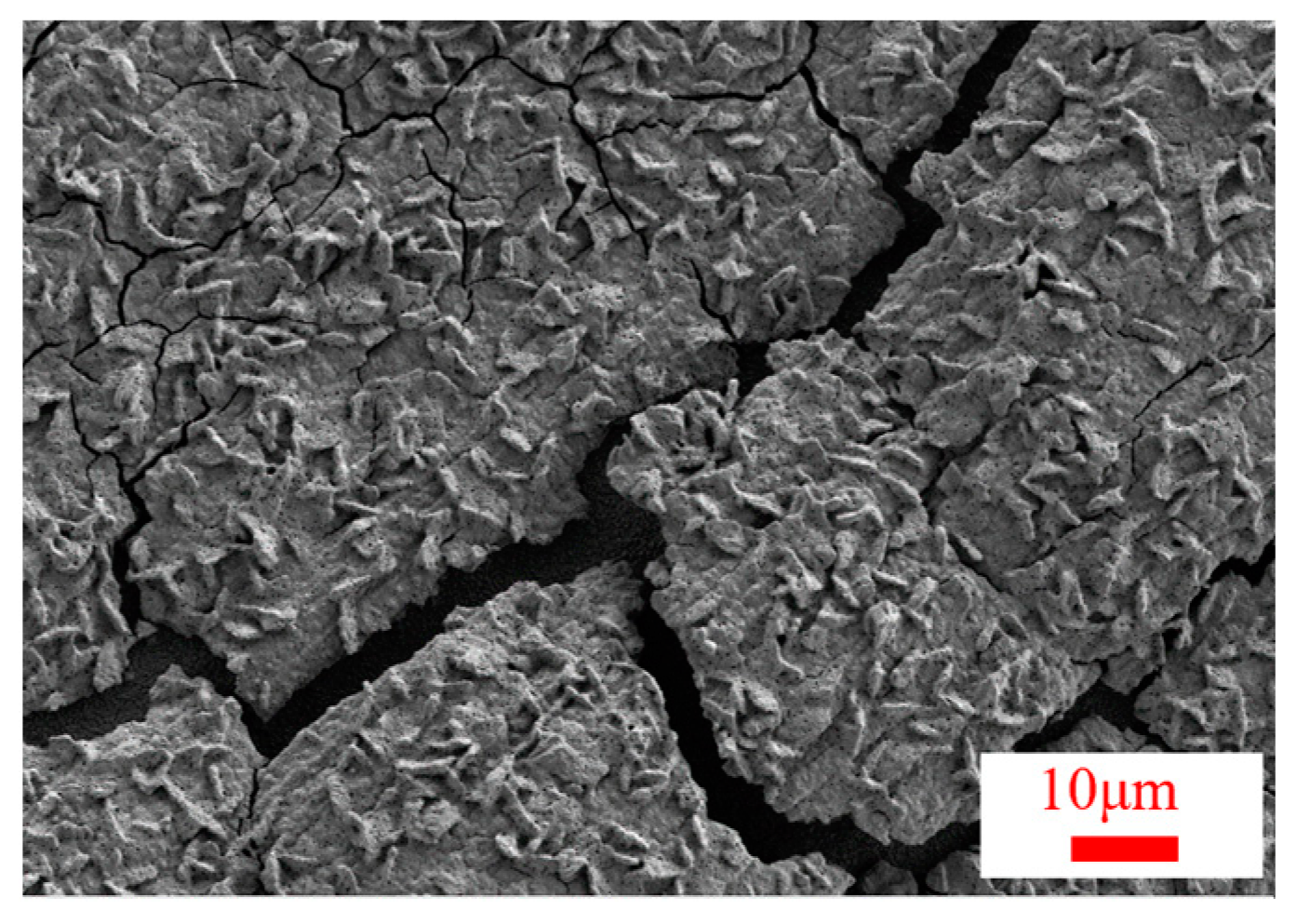
3.2. Surface Morphology
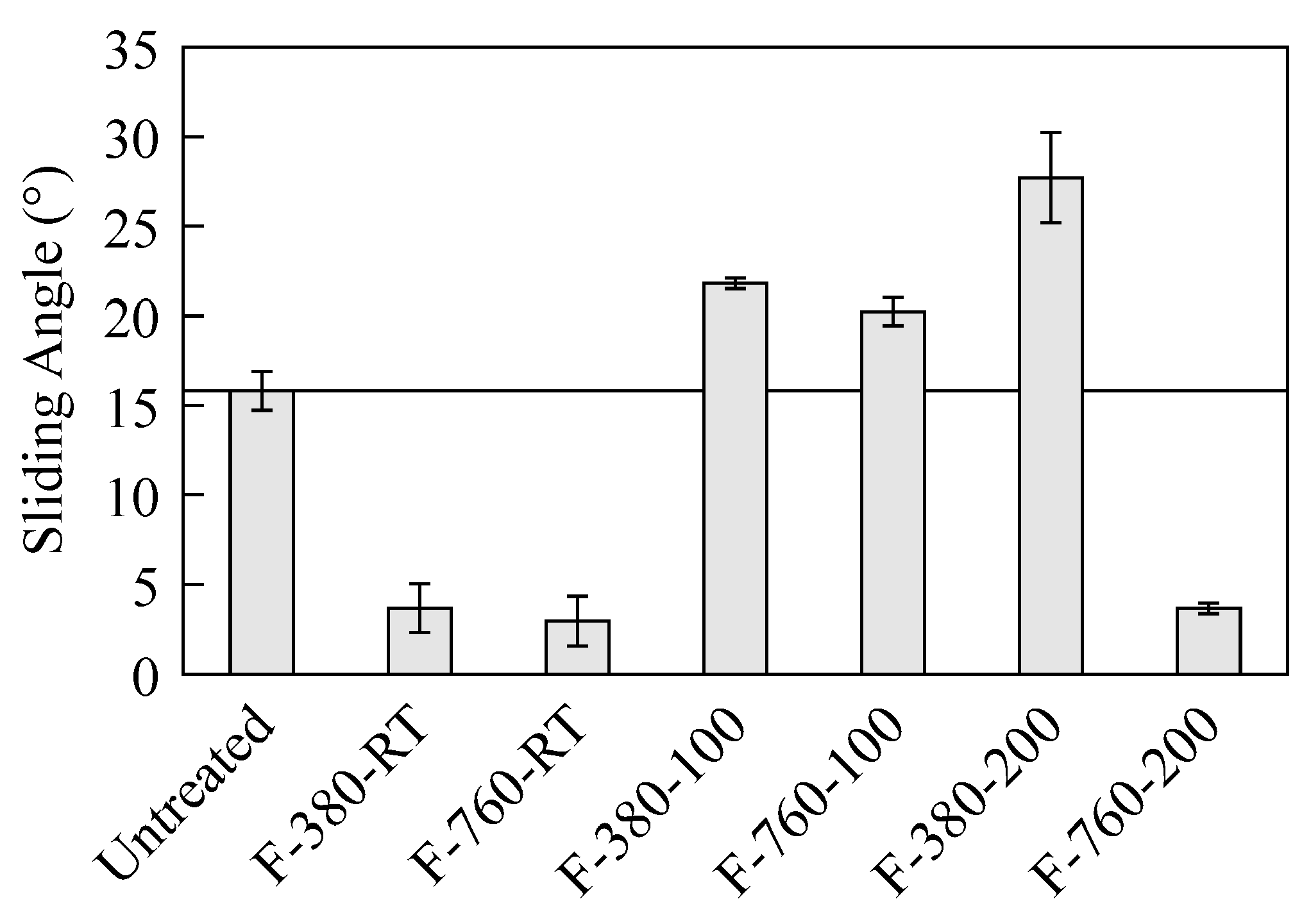
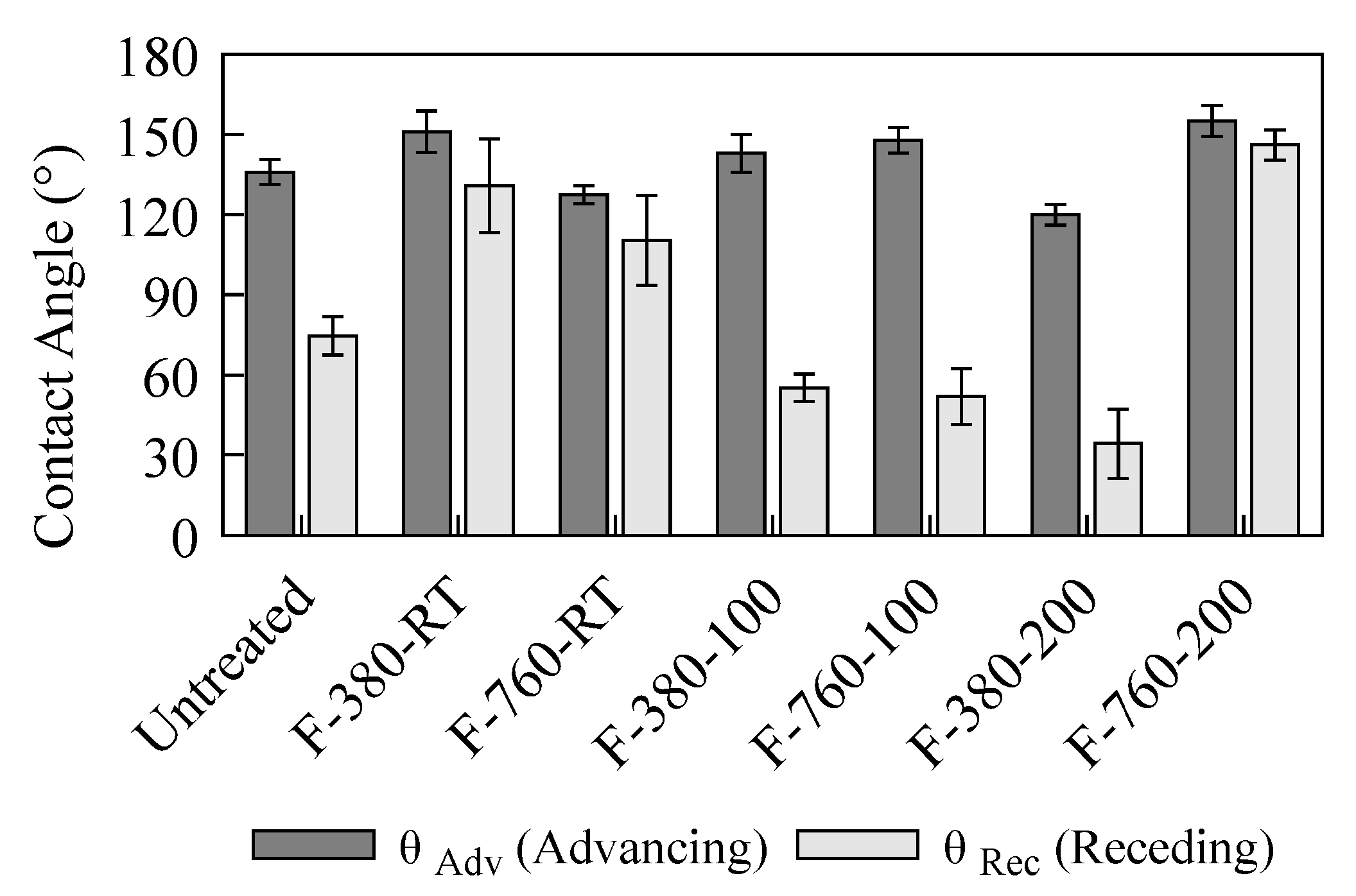
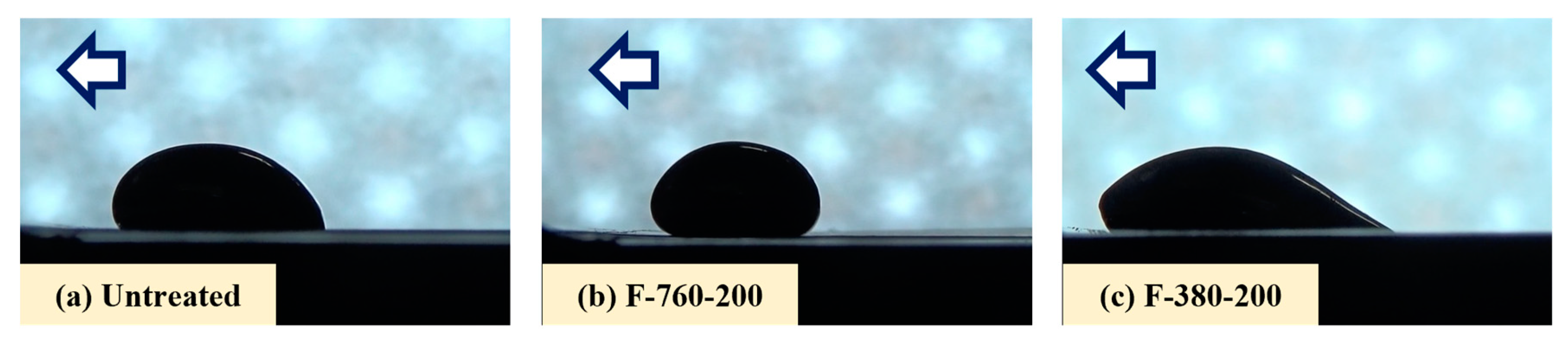
3.3. Liquid Na Wettability
4. Discussions
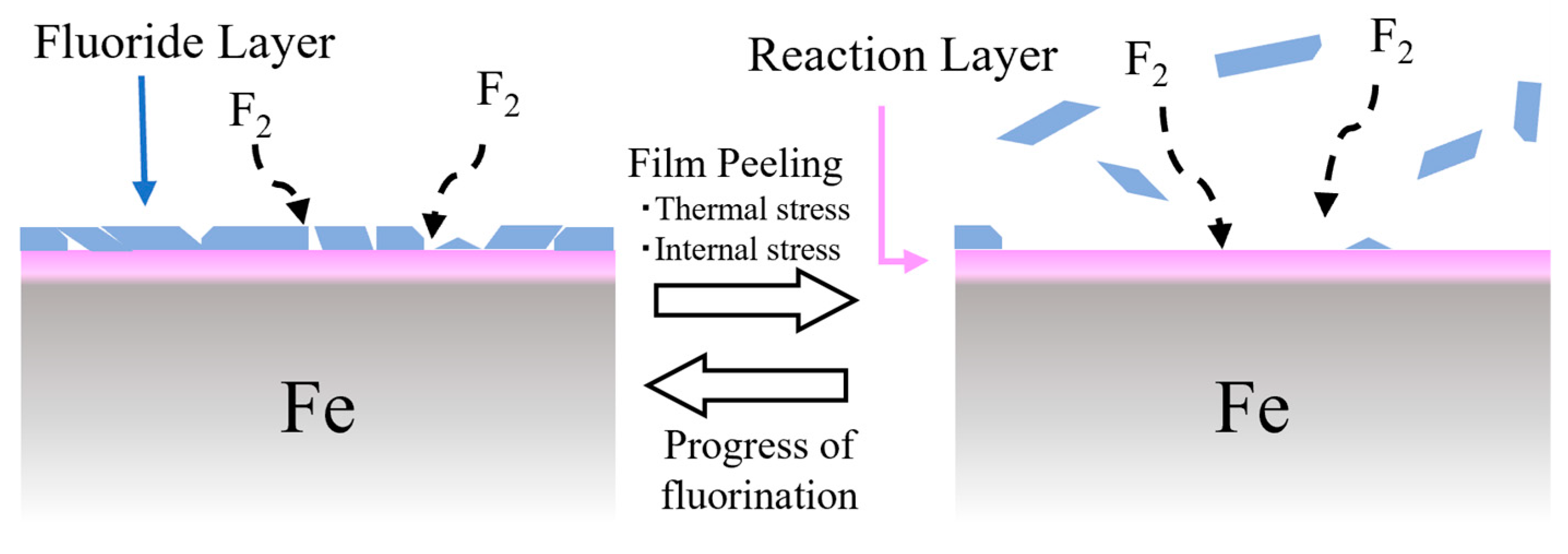
4.1. Formation Process of Fluoride Layer by Surface Fluorination
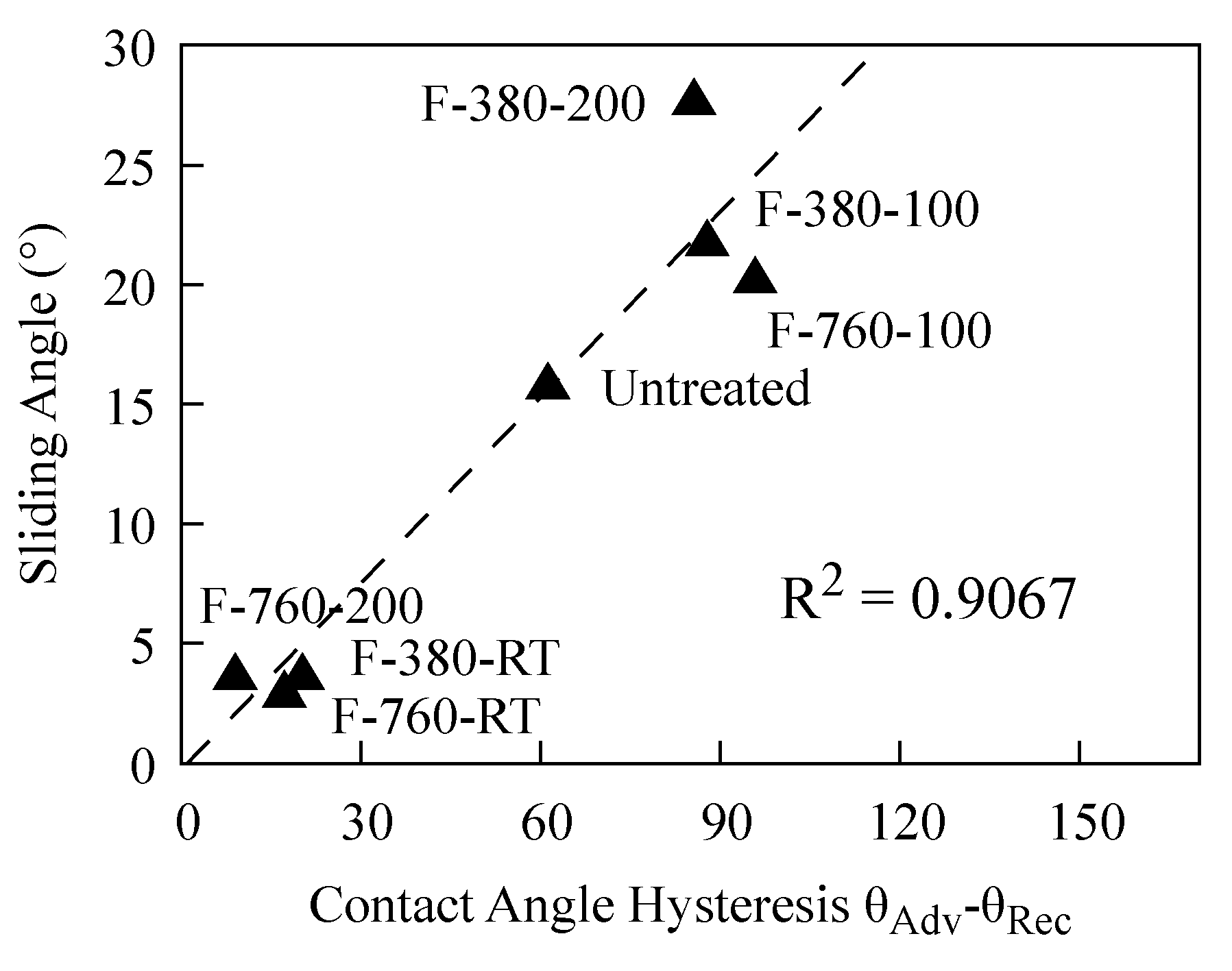
4.2. Relationship between Liquid Na Wettability and the Surface State of the Fluorinated Layer
5. Conclusions
- (1)
- F-380-RT, F-760-RT, and F-760-200 specimens had large surface roughness, approximately 3–6.5 times that (27 nm) of the untreated specimen. The XPS measurement results show that a thick layer of reaction products, primarily comprising FeF3 bonds, can be formed on the surfaces of these specimens.
- (2)
- F-380-RT, F-760-RT, and F-760-200 specimens had small sliding angles of Na droplets at measurement and a small deformation in the Na droplet shape.
- (3)
- The surface roughness of F-380-100, F-760-100, and F-380-200 specimens was similar to those of the untreated specimen. The XPS measurement results indicate that the surfaces of these specimens contain a large amount of FeF2 bonds. Together with the detection of metallic iron peaks due to the etching of the outermost surface layer, a thin reaction layer primarily comprising FeF2 bonds can be formed on their surfaces.
- (4)
- F-380-100, F-760-100, and F-380-200 specimens had large sliding angles of Na droplets at measurement and a large deformation in the Na droplet shape.
- (5)
- Fluorination changed the wettability between the specimen and the liquid Na to lyophilic and lyophobic states. We considered that the bond formed on the specimen surface determines whether it is easier or harder to be wetted with liquid Na.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foust, O.J. Sodium-NaK Engineering Handbook. Volume 1, Sodium Chemistry and Physical Properties; Gordon and Breach Science Publishers: New York, NY, USA, 1972. [Google Scholar]
- Chander, S.; Meikandamurthy, C.; Kalc, R.D. Experimental study of self-welding of materials in high temperature liquid sodium. Wear 1993, 162, 458–465. [Google Scholar] [CrossRef]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Gancarz, T. Potential cooling agents for fast nuclear reactors: Sodium influence on the thermophysical properties of liquid Ga-Sn-Zn eutectic alloys. J. Mol. Liq. 2019, 296, 112024. [Google Scholar] [CrossRef]
- Hejzlar, P.; Todreas, N.E.; Shwageraus, E.; Nikiforova, A.; Petroski, R.; Driscoll, M.J. Cross-comparison of fast reactor concepts with various coolants. Nucl. Eng. Des. 2009, 239, 2672–2691. [Google Scholar] [CrossRef]
- Hémery, S.; Auger, T.; Courouau, J.L.; Balbaud-Célérier, F. Liquid metal embrittlement of an austenitic stainless steel in liquid sodium. Corr. Sci. 2014, 83, 1–5. [Google Scholar] [CrossRef]
- Hémery, S.; Auger, T.; Courouau, J.L.; Balbaud-Célérier, F. Effect of oxygen on liquid sodium embrittlement of T91 martensitic steel. Corr. Sci. 2013, 76, 441–452. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, M.; Feng, Y.; Liu, H.; Yang, X.; Li, Y.; Chang, C. Experimental study on the effects of sodium and potassium proportions on the heat transfer performance of liquid metal high-temperature oscillating heat pipes. Int. J. Heat Mass Transf. 2022, 194, 123116. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Tagawa, A.; Miyahara, S. Reactive Wetting of Metallic Plated Steels by Liquid Sodium. J. Nucl. Sci. Technol. 2011, 48, 499–503. [Google Scholar] [CrossRef]
- Griffin, J.W.; Peters, T.J.; Posakony, G.J.; Chien, H.-T.; Bond, L.J.; Denslow, K.M.; Sheen, S.-H.; Raptis, P. Under-Sodium Viewing: A Review of Ultrasonic Imaging Technology for Liquid Metal Fast Reactors; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2009. [CrossRef]
- Kim, H.W.; Joo, Y.S.; Park, C.G.; Kim, J.B.; Bae, J.H. Ultrasonic Imaging in Hot Liquid Sodium Using a Plate-Type Ultrasonic Waveguide Sensor. J. Nondestruct. Eval. 2014, 33, 676–683. [Google Scholar] [CrossRef]
- Aizawa, K.; Sasaki, K.; Chikazawa, Y.; Fukuie, M.; Jinbo, N. Demonstration of Under Sodium Viewer in Monju. Nucl. Technol. 2018, 204, 74–82. [Google Scholar] [CrossRef]
- Bader, M.; Busse, C.A. Wetting by sodium at high temperatures in pure vapour atmosphere. J. Nucl. Mater. 1977, 67, 295–300. [Google Scholar] [CrossRef]
- Liang, N.; Fu, X.; Zhang, J.; Ruan, Z.; Qin, B.; Ma, T.; Long, B. Evaluation of Wetting Behaviors of Liquid Sodium on Transition Metals: An Experimental and Molecular Dynamics Simulation Study. Materials 2024, 17, 691. [Google Scholar] [CrossRef]
- Saito, J.; Kobayashi, Y.; Shibutani, H. Wettability of Pure Metals with Liquid Sodium and Liquid Tin. Mater. Trans. 2021, 62, 1524–1532. [Google Scholar] [CrossRef]
- Saito, J.; Monbernier, M. Relationship between the contact angle of pure Cu and its alloys owing to liquid Na and electronic states at the interface. Surf. Interfaces 2023, 41, 103248. [Google Scholar] [CrossRef]
- Pauling, L. The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- The 155 Committee of Fluorine Chemistry. “Fusso Kagaku Nyumon” (Introduction to Fluorochemistry); Sankyoshuppan: Tokyo, Japan, 2004. [Google Scholar]
- Tressaud, A.; Durand, E.; Labrugère, C. Surface modification of several carbon-based materials: Comparison between CF4 rf plasma and direct F2-gas fluorination routes. J. Fluor. Chem. 2004, 125, 1639–1648. [Google Scholar] [CrossRef]
- Kim, J.H.; Mishina, T.; Namie, M.; Nishimura, F.; Yonezawa, S. Effects of surface fluorination on the dyeing of polycarbonate (PC) resin. J. Coat. Technol. Res. 2021, 19, 617–624. [Google Scholar] [CrossRef]
- Miki, N.; Maeno, M.; Maruhashi, K.; Nakagawa, Y.; Ohmi, T. Fluorine passivation of stainless steel. Corros. Sci. 1990, 31, 69–74. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nishimura, F.; Kim, J.H.; Yonezawa, S. Dyeable hydrophilic surface modification for PTFE substrates by surface fluorination. Membranes 2023, 13, 57. [Google Scholar] [CrossRef]
- Namie, M.; Kim, J.H.; Yonezawa, S.; Saito, J. Wettability control of stainless steel surfaces to liquid sodium by surface fluorination treatment. In Proceedings of the 45th Fluorine Conference of Japan, Kyoto, Japan, 1–2 November 2022. [Google Scholar]
- Pouzet, M.; Dubois, M.; Charlet, K.; Béakou, A. From hydrophilic to hydrophobic wood using direct fluorination: A localized treatment. C. R. Chim. 2018, 21, 800–807. [Google Scholar] [CrossRef]
- Namie, M.; Saito, J. Atomic interactions at the interface between iron or iron fluoride, and sodium by the first-principles calculation. Comput. Mater. Sci. 2024, 239, 112963. [Google Scholar] [CrossRef]
- Kim, J.H.; Umeda, H.; Ohe, M.; Yonezawa, S.; Takashima, M. Preparation of pure LiPF6 using fluorine gas at room temperature. Chem. Lett. 2011, 40, 360–361. [Google Scholar] [CrossRef]
- Kasrai, M.; Urch, D.S. Electronic Structure of Iron (II) and (III) Fluorides using X-ray Emission and X-ray Photoelectron Spectroscopies. J. Chem. Soc. Faraday Trans. 1979, 75, 1522–1531. [Google Scholar] [CrossRef]


| Specimen Names | Material | Fluorination Conditions | ||
|---|---|---|---|---|
| Fluorine Gas Pressure | Temp. | Time | ||
| /Torr (kPa) | /°C | /hour | ||
| Untreated | Fe | — | — | — |
| F-380-RT | 380 (50.7) | RT (25) | 24 | |
| F-760-RT | 760 (101.3) | RT (25) | 24 | |
| F-380-100 | 380 (50.7) | 100 | 24 | |
| F-380-200 | 760 (101.3) | 100 | 24 | |
| F-760-100 | 380 (50.7) | 200 | 24 | |
| F-760-200 | 760 (101.3) | 200 | 24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namie, M.; Saito, J.-i.; Ikeda, A.; Oka, R.; Kim, J.-H. Applicability of Fluorine Gas Surface Treatment to Control Liquid Sodium Wettability. Surfaces 2024, 7, 550-559. https://doi.org/10.3390/surfaces7030037
Namie M, Saito J-i, Ikeda A, Oka R, Kim J-H. Applicability of Fluorine Gas Surface Treatment to Control Liquid Sodium Wettability. Surfaces. 2024; 7(3):550-559. https://doi.org/10.3390/surfaces7030037
Chicago/Turabian StyleNamie, Masanari, Jun-ichi Saito, Asuka Ikeda, Ryotaro Oka, and Jae-Ho Kim. 2024. "Applicability of Fluorine Gas Surface Treatment to Control Liquid Sodium Wettability" Surfaces 7, no. 3: 550-559. https://doi.org/10.3390/surfaces7030037
APA StyleNamie, M., Saito, J.-i., Ikeda, A., Oka, R., & Kim, J.-H. (2024). Applicability of Fluorine Gas Surface Treatment to Control Liquid Sodium Wettability. Surfaces, 7(3), 550-559. https://doi.org/10.3390/surfaces7030037







