Abstract
Biosurfactants are amphipathic molecules with considerable potential for application in different industries due to their biochemical characteristics, low toxicity as well as greater biodegradability and stability compared to chemical surfactants when submitted to adverse environmental conditions. The aim of the present study was to investigate the production of a biosurfactant by Candida lipolytica UCP 0988 grown in a medium containing 4.0% molasses, 2.5% used soybean frying oil, and 2.5% corn steep liquor for 144 h at 200 rpm. The biosurfactant was characterized; its stability and toxicity were investigated, and the compound was applied in oil removal tests. In the C. lipolytica growth and biosurfactant production studies, the surface tension of the medium was reduced from 72 mN/m to 25 mN/m, the critical micellar concentration (CMC) was 0.5 g/L (w/v), and the yield was 12 g/L. Tests under extreme conditions of temperature, pH, and NaCl indicated the stability of the biosurfactant. Fourier-transform infrared and nuclear magnetic resonance spectroscopy of the chemical structure of the purified biosurfactant suggested that the biosurfactant is a glycolipid. The anionic biosurfactant exhibited no toxicity to the microcrustacean Artemia salina or vegetable seeds (Brassica oleracea). Dispersion tests in seawater demonstrated 100% efficiency of the biomolecule against motor oil. The biosurfactant was efficient at removing oil from sand in static and kinetic tests at concentrations of ½ CMC (0.25 g/L), CMC (0.5 g/L), and 2 × CMC (1.0 g/L), with removal rates of 70 to 96%, whereas the synthetic surfactants tested removed only 10 to 18% of the oil. Based on the findings, the biosurfactant analyzed has considerable potential for the remediation of contaminated coastal and marine environments due to oil spills.
1. Introduction
Oil spills exert a negative impact on ecosystems. Whether in aquatic or terrestrial environments, some harm is irreparable and mainly affects the local population [1].
Petroleum hydrocarbons are hydrophobic compounds that bind to soil particles and have little solubility in water, reducing their bioavailability to microorganisms and hampering biodegradation [2]. Transporting the pollutant to the aqueous phase increases the bioavailability of petroleum contaminants. Surfactants are used to achieve desorption and enhance the solubility of hydrocarbons, which facilitates biodegradation by microorganisms [3].
Studies have shown that microbial surfactants solubilize and effectively mobilize organic compounds adsorbed to soil particles [4]. A number of synthetic surfactants, such as Tween 80 and Triton X–100, can also increase the concentration of non-polar compounds in the aqueous phase. However, synthetic surfactants can be toxic and resistant to biodegradation [5].
Researchers have investigated the replacement of synthetic surfactants with natural compounds, known as biosurfactants. However, the production cost of these natural products is significantly higher compared to that of synthetic surfactants, which impedes the use of biosurfactants on an industrial scale [4]. Therefore, competitive processes are needed to lower the cost of production. Strategies such as the use of alternative nutrient sources can reduce the overall production cost by 10 to 80%. Moreover, biotechnological processes employed in the production of biosurfactants involve less waste of material and energy, making the process more lucrative in the long term [6].
Various renewable, low-cost waste products are used as substrates for biosurfactant production, which constitutes an effective cost-reduction strategy combined with the management of waste products [7]. The production of biosurfactants employing low-cost substrates from agro-industrial activities can ensure sustainable, economical production processes [8].
Biosurfactants produced from microorganisms cultivated in soluble (carbohydrates) and insoluble substrates (hydrocarbons, oils and residues) have a considerable diversity of chemical structures, such as lipopeptides, glycolipids, protein complexes, polysaccharides, neutral lipids, phospholipids, and fatty acids, offering a variety of properties and physiological functions among the various families of these biopolymers [9]. The diversity of structures and properties enables the use of biosurfactants in different industrial processes, including novel applications for these biomolecules. Indeed, biosurfactants have been called the “multifunctional molecules” of the new century [10].
The aim of the present study was to produce a biosurfactant from Candida lipolytica UCP0998 using agro-industrial waste products, with descriptions of its properties, isolation, characterization, and toxicity as well as the removal of oil and petroleum derivatives from terrestrial and marine environments.
2. Materials and Methods
2.1. Materials
All chemical products were reagent grade. Culture media were acquired from Difco Laboratories (USA) were purchased at Casa do Laboratório, Recife, PE, Brazil. Two industrial waste products were used as substrates for the production of the biosurfactant. Used soybean frying oil kindly donated by ASA LTDA in the city of Recife (Brazil) was the insoluble substrate. Corn steep liquor (by product of the manufacturing of corn-based products) obtained from Corn Products do Brazil in the city of Cabo de Santo Agostinho (Brazil) was the soluble substrate.
2.2. Microorganism
Candida lipolytica UCP 0988 from the Culture Bank of the Center for Research in Environmental Sciences and Biotechnology of the Catholic University of Pernambuco was tested for biosurfactant production. Cultures were kept in slanted test tubes containing yeast mold agar (YMA) at 5 °C. Sub-cultures were performed every 30 days.
2.3. Preparation of Inoculum
The yeast inoculum was standardized. The cultures were transferred to tubes with the YMA medium for the obtainment of a young culture. Next, the samples were transferred to flasks with 50 mL of YMB medium, followed by incubation with constant stirring (150 rpm) at 28 °C for 24 h. Dilutions were then performed until reaching a concentration of 108 cells/mL.
2.4. Production of Biosurfactant
Fermentation was performed in a medium containing 4.0% molasses, 2.5% used soybean frying oil, and 2.5% corn steep liquor for the production of the biosurfactant. The medium was incubated with a cellular suspension of 108 cells/mL. The flasks were kept under constant stirring (200 rpm) for 144 h at pH 5.5.
2.5. Determination of Emulsification Activity
The samples were centrifuged at 4500× g for 15 min for the determination of emulsification activity, followed by analysis using the method proposed by Cooper and Goldenberg [11]. One mL of used motor oil was added to 1.0 mL of the broth in a graduated tube, which was shaken for two minutes in a vortex. After 24 h, the water-in-oil emulsion was measured based on the height of the formed halo in centimeters.
2.6. Growth Curve
Aliquots were collected throughout fermentation (2, 4, 6, 8, 12, 24, 30, 36, 48, 60, 72, 96, 120, and 144 h). The samples were filtered and centrifuged at 4500 rpm for 15 min at 9 °C. Surface tension, pH, and biosurfactant yield were determined in the cell-free broths. The dry weight of the biomass was determined after centrifuging 50 mL of the culture at 5000 rpm for 15 min, discarding the supernatant and drying the biomass in a laboratory oven at 105 °C for 24 h [12].
2.7. Assessment of Stability of Biosurfactant (Effects of PH, NaCl and Temperature)
The effects of different pH values (2.0, 4.0, 6.0, 8.0, 10.0, and 12.0), concentrations of NaCl (2.0, 4.0, 6.0, 8.0 and 10.0%), and temperatures (5 °C, 70 °C, 100 °C and 120 °C) were tested by determining the surface tension and emulsification activity. All analyses were performed in triplicate.
2.8. Determination of Surface Tension and Critical Micelle Concentration
A tensiometer (KSV Instruments Ltd, Sigma 700, Helsinki, Finland) with a du Noüy ring was used for the determination of surface tension. The critical micelle concentration (CMC) of the biosurfactant was determined by measuring the surface tension of water to which the biosurfactant was gradually added until achieving constant tension. The CMC was expressed as g/L of extracted biosurfactant [12].
2.9. Isolation of Biosurfactant
Centrifugation was performed at 4500 rpm for 20 min at 10 °C for the removal of cells. A solution of HCl 6.0 M was used to adjust the pH of the broth to 2, followed by precipitation with methanol at a proportion of 1:2. Ethyl acetate was used to extract the broth and the process was repeated three times. After transferring the solvent to a separation funnel, the aqueous phase was discarded and the solvent phase was dried using sodium sulfate, filtered and evaporated [13].
2.10. Fourier-Transform Infrared Spectroscopy
A Fourier-transform infrared spectroscope (Spectrum 400, Perkin Elmer, Shelton, CT, USA) was used for the characterization of the biosurfactant extract recovered from the supernatant. The resolution was 4 cm−1 in the region of 400 to 4000 wave numbers (cm−1).
2.11. Nuclear Magnetic Resonance Spectroscopy
Following the redissolution of the extracted biosurfactant in deuterated chloroform, a spectrometer (Agilent 300-Mz, Agilent, Santa Clara, CA, USA) operating at 300.13 MHz at 25 °C was used to determine 1 H MNR spectra. Chemical shifts (δ) were shown on the ppm scale in relation to tetramethylsilane (TMS).
2.12. Toxicity of Biosurfactant to Brine Shrimp, Artemia Salina
Toxicity to microcrustaceans was tested with the brine shrimp (Artemia salina), which is a commonly used marine bioindicator. One liter of saline solution composed of 33 g of dissolved sea salt was used for the test. After incubation, 10 microcrustaceans were placed into solutions of the biosurfactant at concentrations of ½ CMC (0.25 g/L), CMC (0.5 g/L), and 2 × CMC (1.0 g/L) in distilled water [14].
2.13. Phytotoxicity Test
Phytotoxicity was investigated by exposing seeds of the cabbage plant (Brassica oleracea) to the biosurfactant and observing the effects on germination and growth of the roots [15]. Test solutions were prepared with different concentrations of the isolated biosurfactant (½ CMC—0.25 g/L; CMC—0.5 g/L, and 2 × CMC—1.0 g/L in distilled water), followed by incubation for 5 days in the dark. The following formulas were used for the determination of seed germination, root growth (≥5 mm) and the germination index (GI):
Relative seed germination (%) = (n° of seeds germinated in extract treatment/n° of seeds germinated in control treatment) × 100
Relative root length (%) = (average root length in extract treatment/average root length in control treatment) × 100
GI = [(percentage of seed germination) × (percentage of root growth)]/100%
2.14. Motor Oil Dispersion Test in Water
A drop of motor oil was placed in 40 mL of water in a Petri dish measuring 15 cm in diameter to simulate an oil slick in the laboratory. The effects of the crude and isolated biosurfactant were tested at concentrations of ½ CMC (0.25 g/L), CMC (0.5 g/L), and 2 × CMC (1.0 g/L) for the determination of the dispersant power of the biosurfactant. The average diameter of the clear zone formed in the oil by the biosurfactant was calculated from triplicate experiments. The dispersion index was defined as the percentage of the average diameter in relation to the diameter of the Petri dish (15 cm) [12].
2.15. Assessment of Biosurfactant and Chemical Surfactants for Removal of Motor Oil from Sand-Kinetic Test
Samples of normal sand for testing NBR 7214 cement [16] were used, the organic matter of which is expressed in terms of tannic acid and does not surpass 100 parts per million. Sand retained in sieves with mesh sizes from 0.3 mm to 0.15 mm was used.
Samples (50 g) of standard sand were contaminated with 10% motor oil, transferred to 500-mL Erlenmeyer flasks, and submitted to autoclaving. Next, 100 mL of each surfactant was added to the flasks containing the contaminated sand. The biosurfactant and synthetic surfactants (Tween 20 and Tween 80) were used at concentrations of ½ CMC (0.25 g/L), CMC (0.5 g/L), and 2 × CMC (1.0 g/L). The cell-free broth (crude biosurfactant) was also tested with the same quantity (100 mL). The control was a flask containing contaminated sand and 100 mL of water without the addition of surfactants. Agitation was performed at 150 rpm and 28 °C for 24 h. The treated sand and washing solution were then separated for analysis.
2.16. Removal of Hydrophobic Contaminant from Sand by Surfactants in Static Test
Approximately 200 g of sand contaminated with 10% used motor oil was packed into class columns measuring 55 × 6 cm. The system was flushed with 200 mL of different concentrations (½ CMC—0.25 g/L, CMC—0.5 g/L, and 2 × CMC—1.0 g/L) of the biosurfactant solution or synthetic surfactants (Tween 20 and Tween 80). The cell-free broth (crude biosurfactant) was also tested with the same quantity (200 mL). The control column contained sand and 200 mL of water without the addition of the surfactants. The percolation of the surfactant solutions was monitored at 5-min intervals for 24 h. The content of the Erlenmeyer flask was left to rest for 5 min at the end of each washing. A pipette was used to separate the washing solution. The sand remaining in the flask was washed with distilled water for the removal of the oil remaining on the walls of the flask and remnants of the biosurfactant solution from the sand [17].
2.17. Analysis of Contaminants on Sand
The samples of standard sand were submitted to gravimetric analysis for the estimation of motor oil. Residual motor oil was extracted with hexane in a pre-weighed beaker and decantation funnel three times to ensure complete oil recovery. The hexane was then evaporated and the oil removed from the sand was weighed. The percentage of oil removed was calculated as follows: motor oil removal rate (%) = [(Oinitial − Oremaining)/Oinitial] × 100, in which Ointcial is the oil on the contaminated soil and Oremaining is the oil that remained on the sand (g) after washing.
3. Results and Discussion
3.1. Growth Curve and Biosurfactant Production
As the production costs of natural surfactants remain high, there is a need to seek more cost-effective alternatives for this production, such as the use of low-cost substrates, including the use of agro-industrial waste products [18].
In the present study, a biosurfactant was produced by C. lipolytica cultivated in a medium containing 2.5% used soybean frying oil, 2.5% corn steep liquor and 4.0% molasses over a 6-day period. Based on the analysis of the growth curve (Figure 1), an exponential phase occurred between 5 and 12 h of cultivation, at which time biomass production was 9.0 g/L. Maximum biosurfactant production (12 g/L) was found in the stationary phase. At the start of the experiment, the surface tension of the medium was 55 mN/m and was reduced to 28 mN/m after 144 h. A little increase in pH occurred beginning at 24 h. This increase is related to the metabolic processes of the microbial cells involved in production.
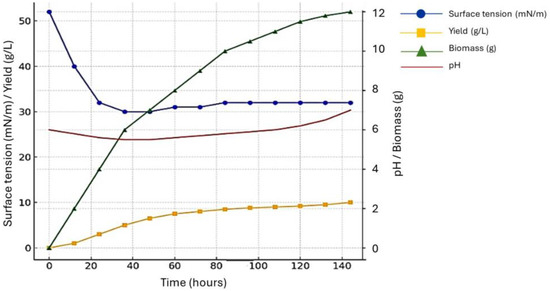
Figure 1.
Growth curve, pH, and biosurfactant production of C. lipolytica grown in distilled water supplemented with molasses (4.0%), corn steep liquor (2.5%), and waste frying oil (2.5%) for 144 h at 200 rpm and 28 °C.
The literature reports that most species of Candida produce efficient biosurfactants. Gaur et al. [19] investigated the growth of C. albicans and Candida glabrata in a synthetic defined (SD) medium comprised of glucose (2%) and a yeast nitrogen base (0.67%) in distilled water. The surface tension of the cell-free broth was reduced from 71 N/m to 42 N/m and 55 N/m, respectively, after 72 h of cultivation and the yield of biosurfactant was 1320 mg/L for C. albicans and 1600 mg/L for C. glabrata. According to Akbari et al. [20], effective biosurfactants reduce the surface tension of water from 72 to 35 mN/m.
The optimization of the biosurfactant production process exerts an influence on the total yield [21]. A variety of factors, including the accessibility of carbon and nitrogen sources, contribute substantially to biosurfactant production. Silva et al. [22] produced a commercial biosurfactant from the yeast Starmerella bombicola grown in a medium containing cotton oil (50 g/L), glucose (25 g/L), yeast extract (1 g/L), MgSO4.7H2O (0.5 g/L), KH2PO4 (0.5 g/L), and NaNO3 (0.3 g/L), reporting a reduction in surface tension to 32 mN/m and a biosurfactant yield of 32.5 g/L using ethyl acetate as the extraction solvent.
3.2. Stability of Biosurfactant Based on Emulsification Index
High tolerance to extreme environmental conditions makes microbial surfactants an excellent alternative to synthetic surfactants. Thus, the influence of pH, temperature, and salinity on the stability of a biosurfactant needs to be studied, as stability is an important parameter for the application of these molecules in different fields, especially the environmental field, in which factors of the environment are continually changing [23].
The results of the emulsification tests demonstrated high affinity of the crude biosurfactant (cell-free broth) to motor oil, which was well emulsified irrespective of changes in pH and temperature or the addition of NaCl (Table 1). Alvionita et al. [24] produced a biosurfactant from Halomonas elongata BK-AG18 to test the stability of biosurfactant emulsion under various conditions such as salinity and pH, and the index of emulsification (IE24) of the biosurfactant was obtained around 50%. These are lower values when compared to the biosurfactant achieved by C. lipolytica grown in distilled water supplemented with molasses (4.0%), corn steep liquor (2.5%), and waste frying oil (2.5%).

Table 1.
Stability of engine oil emulsification by biosurfactant from C. lipolytica grown in distilled water supplemented with molasses (4.0%), corn steep liquor (2.5%), and waste frying oil (2.5%) with variations in NaCl, temperature and pH.
3.3. Stability of Biosurfactant Related to Surface Tension
Stability was investigated in the cell-free broth after 144 h of cultivation based on surface tension as a function of temperature, pH, and concentration of NaCl (Table 2). No significant changes in surface tension were found with the change in pH. Likewise, the surface tension remained stable at the different temperatures tested and independently of the concentration of NaCl added.

Table 2.
Surface tension stability of biosurfactant from C. lipolytica grown in distilled water supplemented with molasses (4.0%), corn steep liquor (2.5%), and waste frying oil (2.5%) as substrates in the presence of pH (A), temperature (B) and NaCl (C).
The influence of salinity was assessed to determine the possibility of using the biosurfactant in marine environments. The highest salinity in oceans and seas throughout the world is 3% [25]. The biosurfactant produced by C. lipolytica was able to maintain the capacity to reduce surface tension in the presence of NaCl at concentrations up to 12%.
3.4. Critical Micelle Concentration
The biosurfactant produced by C. lipolytica reduced the surface tension of water from 72 mN/m to 28 mN/m with the increase in its concentration to 0.5% (w/v), i.e., 0.5 g/L, establishing the critical micelle concentration (CMC) (Figure 2). This CMC was lower than that reported for other biosurfactants, such as those produced by Starmerella bombicola (5.0 g/L to 10.0 g/L) [26] and Yarrowia lipolytica (12.0 g/L) [27]. Biosurfactants are applied for the reduction in surface and/or interfacial tension between the solution and surface at the air/water or oil/water interfaces [28]. The surfactant causes a reduction in surface tension to the point that micellar, vesicular, and bilayer structures are formed, which is known as the CMC.
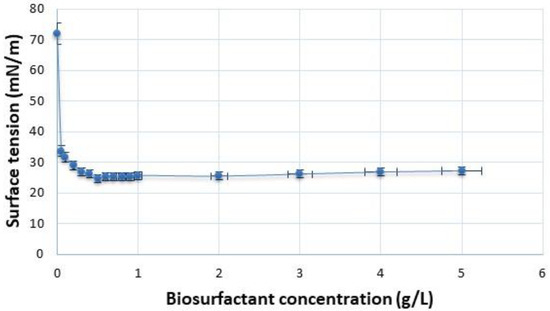
Figure 2.
Critical micelle concentration of the biosurfactant from C. lipolytica grown in distilled water supplemented with molasses (4.0%), corn steep liquor (2.5%), and waste frying oil (2.5%).
Biosurfactants with low molecular weight reduce surface tension, whereas those with high molecular weight form stable emulsions. The CMC is the minimum concentration of surfactant at the moment when micelles are formed in the solution [29].
3.5. Ionic Charge of Biosurfactant
The zeta potentiometer revealed that the biosurfactant produced by C. lipolytica was negatively charged in the hydrophilic region (−97.9 ZPmV, 282.6 µS/cm) at 22.4 °C, indicating that the surfactant was anionic. The same test also revealed the anionic nature of other biosurfactants produced by the species of Candida [13,22].
3.6. Structural Characterization of Biosurfactant
The biosurfactant isolated from C. lipolytica was characterized by FT-IR (Figure 3) and NMR (Figure 4 and Figure 5) analyses. The infrared spectrum obtained showed an elongated signal in the region 3750 cm⁻1 and 3000 cm⁻1, indicating the possible presence of hydroxyl, and peaks in the region of 2922 cm⁻1 and 2851 cm⁻1, indicating the presence of aliphatic chains. The peak at 1562 cm⁻1 suggests the presence of a carboxylic group. The bands at 1445 cm⁻1 reinforce the presence of C-H bonds in aliphatic chains, and the peak at 880 cm⁻1 may indicate the presence of double bonds between carbons in the molecular structure of the biosurfactant. This IR spectrum provides good evidence for the identification of the compound as a fatty acid, possibly unsaturated, due to the combination of characteristic bands observed.
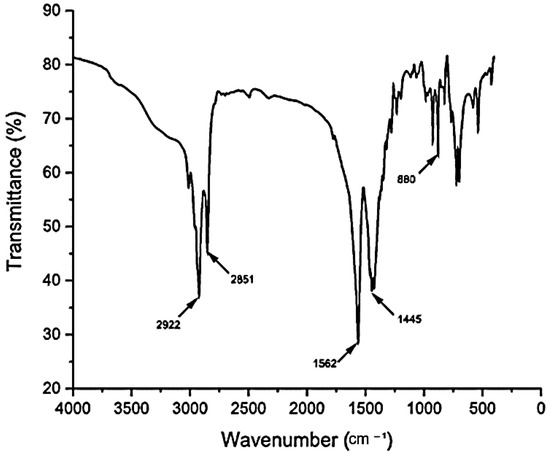
Figure 3.
FT-IR spectrum of biosurfactant produced by C. lipolytica UCP0988 grown in medium containing molasses (4.0%), waste soybean oil (2.5%), and corn steep liquor (2.5%).
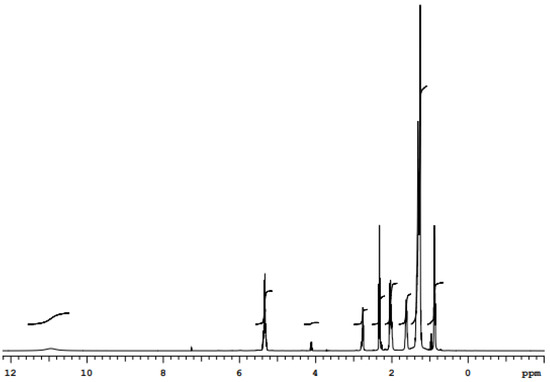
Figure 4.
1H NMR spectrum for biosurfactant extract produced by C. lipolytica UCP0988 grown in medium containing molasses (4.0%), waste soybean oil (2.5%), and corn steep liquor (2.5%).
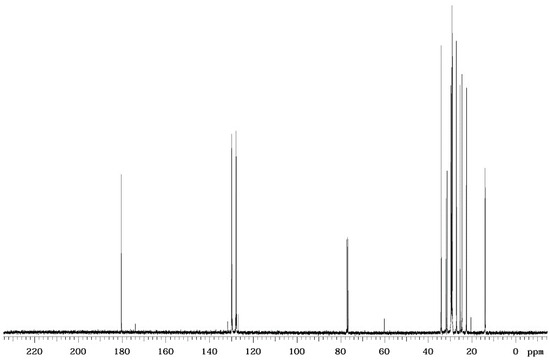
Figure 5.
13C NMR spectrum for produced biosurfactant extract by C. lipolytica UCP0988 grown in medium containing molasses (4.0%), waste soybean oil (2.5%), and corn steep liquor (2.5%).
1H NMR analysis showed a set of signals in the region between 0 and 3 ppm responsible for the aliphatic chain present in the molecular structure; this chain is responsible for the nonpolar fraction of the biosurfactant (Figure 4). The signal found close to 4 ppm suggests the presence of the hydroxyl functional group. In the region between 5 and 6 ppm, signs of possible hydrogens linked to carbons containing double bonds were detected. The elongated signal in the 11 ppm region suggests the presence of the carboxylic acid functional group.
The 13C NMR (Figure 5) showed signals between 0and 40 ppm, characteristic of molecules containing a long carbon chain. The signal found at 60 ppm may be responsible for the hydroxyl present in the molecular structure, the signal at 80 ppm is responsible for the solvent used in the analysis (deuterated chloroform), the peaks found in the region between 120 ppm and 140 ppm relate to double bonds and, finally, the signal in the 180 ppm region is characteristic of the carboxylic acid group.
The results obtained through the analysis of infrared spectra and NMR are in agreement, showing that the biosurfactant produced by C. lipolytica evaluated in this study is a possible hydroxylated fatty acid.
3.7. Phytotoxicity Test
Toxicity is the harmful effect of a substance on living organisms and the degree of toxicity is related to the concentration and properties of the substance as well as the exposure time [30]. The toxicity of the biosurfactant was tested using cabbage seeds (Brassica oleracea). The results demonstrated that the biosurfactant did not inhibit either seed germination or root growth. The germinations indices were 99%, 98%, and 95% for the solutions at ½ CMC (0.25 g/L), CMC (0.5 g/L), and 2 × CMC (1.0 g/L), respectively.
The results demonstrate that the microbial biosurfactant produced in this study has no inhibitory effect on germination at the concentrations tested (½ CMC—0.25 g/L; CMC—0.5 g/L, and 2 × CMC—1.0 g/L).
3.8. Toxicity of Biosurfactant to Brine Shrimp, Artemia Salina
Toxic substances can cause irreparable harm to local living beings in aquatic environments. Artemia salina is a marine organism used as a standard in ecotoxicology tests due to its viable maintenance on the laboratory scale, simple growth conditions, and short lifecycle [13]. The survival rate of the Artemia salina larvae exposed to the different concentrations of the biosurfactant (½ CMC—0.25 g/L; CMC—0.5 g/L and 2 × CMC—1.0 g/L) for 24 h was 100%, demonstrating the absence of toxicity under the conditions tested. Pinto et al. [13] analyzed a biosurfactant produced from C. bombicola at concentrations of 0.5% and 1% and found lethal rates of 50 and 100%, respectively, whereas the isolated biosurfactant had no significant lethal rates.
3.9. Motor Oil Dispersion Test in Water
The treatment of environments contaminated with hydrocarbons requires a surfactant with oil-dispersing capacity, which accelerates mobilization by breaking down oil droplets and increasing the surface area in contact with microorganisms capable of degrading oil [3].
The best dispersion results were obtained with the crude biosurfactant, which exhibited 100% dispersing capacity, followed by the isolated biosurfactant at a concentration of 2 × CMC (1.0 g/L), which dispersed 95% of the oil, indicating high surface activity. The results at concentrations of the CMC (0.5 g/L) and ½ CMC (0.25 g/L) were, respectively, 70% and 40%.
Biosurfactants with good washing and dispersing capacities are quite attractive for the remediation of ecosystems contaminated by hydrophobic pollutants. Moreover, the non-need for isolation and purification procedures constitutes a considerable economic advantage.
3.10. Experiments of Washing Hydrophobic Compounds Adsorbed to Rock
Coral reefs are extremely delicate and few methods are adequate for the effective cleaning of such ecosystems. Dispersants could also be an attractive method in other sensitive ecosystems threatened by hydrophobic pollutants, such as mangroves [31].
Tests were conducted with the biosurfactant in its crude and isolated forms. The results revealed oil removal rates of 100% for the crude biosurfactant (cell-free broth), 34% for the biosurfactant at ½ CMC (0.25 g/L), 67% at CMC (0.5 g/L), and 95% for the biosurfactant at 2 × CMC (1.0 g/L), demonstrating the viability of its application as a biological dispersant for the removal of hydrophobic pollutants in sensitive ecosystems, such as coral reefs.
Luna et al. [32] produced a biosurfactant from C. sphaerica that removed 70% of motor oil adsorbed to a porous surface. Another study reported a 60% removal rate of motor oil adsorbed to porous surfaces using the crude biosurfactant produced by C. sphaerica, demonstrating its potential as a dispersant [33].
3.11. Removal of Hydrophobic Contaminant on Sand by Surfactants in Kinetic Test
Table 3 displays the motor oil removal rates from sand by the biosurfactant produced from C. lipolytica in the kinetic test. The highest removal rate (80%) was achieved with the crude biosurfactant, followed by the biosurfactant at 2 × CMC (1.0 g/L) and CMC (0.5 g/L) (70% and 61%, respectively), demonstrating that the biosurfactants solubilize the contaminants.

Table 3.
Removal rates of hydrophobic contaminant by biosurfactant from C. lipolytica in kinetic assay.
The crude biosurfactant produced from C. lipolytica was also tested and achieved an oil removal rate of 50%, demonstrating adequate efficiency. This is an important finding, as purification processes account for up to 60% of the total production cost.
3.12. Removal of Hydrophobic Contaminant on Sand by Surfactants in Static Test
In this test, a glass column was filled with contaminated sand and used to study the effects of two synthetic surfactants (Tween 20 and Tween 80) and the biosurfactant produced by C. lipolytica on the solubilization of the oil. The crude and isolated biosurfactants achieved removal rates of 20, 33, and 50% with the different concentrations tested (CMC—0.5 g/L; ½ CMC—0.25 g/L, and 2 × CMC—1.0 g/L, respectively) (Table 4). The best result (60% removal rate) was achieved with the crude biosurfactant. The chemical surfactants Tween 20 and Tween 80 achieved lower removal rates (10 and 18%, respectively).

Table 4.
Removal of hydrophobic contaminant from sand in static test with glass columns.
Santos et al. [34] produced a biosurfactant from C. sphaerica, which removed motor oil from sand in the static packed column test, reporting removal rates of 30%, 48%, and 70% for the biosurfactant at concentrations of ½ CMC, CMC, and 2 × CMC, respectively. Using a biosurfactant produced by C. lipolytica, Rufino et al. [35] reported removal rates of percolated solutions in packed columns of 26% for the cell-free both (crude biosurfactant), 33% for the biosurfactant at the CMC, and 37% for the biosurfactant at three times the CMC, demonstrating the influence of the concentration, whereas the removal rate using Tween 80 was only 12%.
Biosurfactants are amphipathic compounds that form micelles that unite with the contaminant, promoting a greater removal rate. The biosurfactant produced from C. sphaerica UCP0995 demonstrated the capacity to reduce the interfacial tension between oil and sand, facilitating its mobilization, and demonstrating excellent removal capacity.
4. Conclusions
The biosurfactant produced by C. lipolytica grown in a low-cost medium supplemented with industrial waste products was non-toxic and exhibited good surface tension reduction and yield as well as stability under extreme conditions. The biosurfactant demonstrated efficiency for use in the removal of motor oil adhered to different matrices, such as soil and seawater, with potential application in the bioremediation processes, ensuring maximum efficiency in the recovery of ecosystems impacted by oily residues.
Despite the considerable potential of biosurfactants in the remediation of contaminated environments, the production cost needs to be lowered before these natural compounds can compete with their synthetic counterparts. In this respect, agro-industrial waste products can be used as substrates, enabling a substantial reduction in the overall production cost. Indeed, renewable materials and waste products are substrates of considerable potential for lowering biosurfactant production costs. Moreover, the use of agro-industrial byproducts increases the sustainability of biosurfactants by reducing the need for resources and contributing to waste management.
Author Contributions
Conceptualization, J.M.L., R.D.R. and L.A.S.; methodology, B.G.A.L., R.R.S., J.C.V.S. and H.M.M.; validation, J.M.L., R.R.S., R.D.R. and H.M.M.; formal analysis, B.G.A.L. and H.M.M.; investigation, B.G.A.L. and H.M.M.; writing—original draft preparation, J.M.L. and R.R.S.; writing—review and editing, J.M.L., R.R.S. and M.C.F.C.; visualization, J.M.L. and L.A.S.; supervision, J.M.L.; project administration, J.M.L. and L.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following Brazilian fostering agencies: Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE [State of Pernambuco Science and Technology Assistance Foundation]), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq [National Council for Scientific and Technological Development] and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES [Coordination for the Advancement of Higher Education Personnel], Finance Code 001).
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the Catholic University of Pernambuco (UNICAP) and the Advanced Institute of Technology and Innovation (IATI), Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- George, I.I.; Nawawi, M.G.M.; Mohd, Z.J.; Farah, B.S. Environmental effects from petroleum product transportation spillage in Nigeria: A critical review. Environ. Sci. Pollut. Res. 2024, 31, 1719–1747. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Singh, S.; Dhanjal, D.S.; Datta, S.; Sharma, D.; Singh, N.K.; Singh, J. Biosurfactant-based bioremediation. In Bioremediation of Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–358. [Google Scholar] [CrossRef]
- Singh, V.; Waris, Z.; Saha, S.; Singh, J.; Padmanabhan, P. Role of Biosurfactants on Microbial Degradation of Oil-Contaminated Soils. In Microbes and Microbial Biotechnology for Green Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 423–441. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C.; Romão-Dumaresq, A.S. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnol. Prog. 2021, 37, e3093. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Sharma, N.; Bhatia, S.; Verma, A.; Soni, S.; Batra, N. Insights on sustainable approaches for production and applications of value added products. Chemosphere 2022, 286, 131623. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.I.; Olaniran, A.O. Production and potential biotechnological applications of microbial surfactants: An overview. Saudi J. Biol. Sci. 2021, 28, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, Y.; Cao, M.; Wang, J.; Lu, J.R.; Xu, H. Rational design, properties, and applications of biosurfactants: A short review of recent advances. Curr. Opin. Colloid Interface Sci. 2020, 45, 57–67. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Pele, M.A.; Ribeaux, D.R.; Vieira, E.R.; Souza, A.F.; Luna, M.A.; Rodríguez, D.M.; Campos-Takaki, G.M. Conversion of renewable substrates for biosurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Electron. J. Biotechnol. 2019, 38, 40–48. [Google Scholar] [CrossRef]
- Pinto, M.I.S.; Guerra, J.M.C.; Meira, H.M.; Sarubbo, L.A.; Luna, J.M. A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions. Foods 2022, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Rodríguez, D.M.; Ferreira, I.N.S.; de Almeida, S.M.; Takaki, G.M.C.; de Lima, M.A.B. Novel production of biodispersant by Serratia marcescens UCP 1549 in solid-state fermentation and application for oil spill bioremediation. Environ. Technol. 2021, 43, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef] [PubMed]
- ABNT NBR 7214; Areia Normal Para Ensaio de Cimento—Especificação. ABNT—Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2015; p. 8.
- Ali, N.; Bilal, M.; Khan, A.; Ali, F.; Iqbal, H.M. Effective exploitation of anionic, nonionic, and nanoparticle-stabilized surfactant foams for petroleum hydrocarbon contaminated soil remediation. Sci. Total Environ. 2020, 704, 135391. [Google Scholar] [CrossRef] [PubMed]
- López-Prieto, A.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M.; Moldes, A.B. Characterization of extracellular and cell bound biosurfactants produced by Aneurinibacillus aneurinilyticus isolated from commercial corn steep liquor. Microbiol. Res. 2021, 242, 126614. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp. Appl. Food Emuls. Antibact. Agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Asgher, M.; Afzal, M.; Qamar, S.A.; Khalid, N. Optimization of biosurfactant production from chemically mutated strain of Bacillus subtilis using waste automobile oil as low-cost substrate. Environ. Sustain. 2020, 3, 405–413. [Google Scholar] [CrossRef]
- Silva, A.F.; Banat, I.M.; Giachini, A.J.; Robl, D. Fungal biosurfactants, from nature to biotechnological product: Bioprospection, production and potential applications. Bioprocess. Biosyst. Eng. 2021, 44, 2003–2034. [Google Scholar] [CrossRef] [PubMed]
- Somoza-Coutiño, G.; Wong-Villarreal, A.; Blanco-González, C.; Pérez-Sariñana, B.; Mora-Herrera, M.; Mora-Herrera, S.I.; Rivas-Caceres, R.R.; Portilla-López, N.; Lugo, J.; Vaca-Paulín, R.; et al. A Bacterial Strain of Pseudomonas aeruginosa B0406 pathogen opportunistic, produce a biosurfactant with tolerance to changes of pH, salinity and temperature. Microb. Pathog. 2020, 139, 103869. [Google Scholar] [CrossRef] [PubMed]
- Alvionita, M.; Hertadi, R.; Fazli, R.R.; Dewi, A.A.R.F.; Rose, T.O. The Study of Biosurfactant Stability and The Effect on Lipase Activity. Biol. Med. Nat. Prod. Chem. 2024, 13, 235–238. Available online: https://10.14421/biomedich.2024.131.235-238 (accessed on 20 May 2024). [CrossRef]
- Millero, F.J.; Feistel, R.; Wright, D.G.; Mcdougall, T.J. A composição da água do mar padrão e a definição da escala de salinidade da composição de referência. Deep Sea Res. Parte I Doc. Pesqui. Ocean ográfica 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Ayachit, T.; Parate, M.; Sahasrabuddhe, S.; Sansarode, D. Comparative study between surfactant and biosurfactant (sophorolipid) with characterization. World J. Pharm. Res. 2020, 9, 926. [Google Scholar]
- Radha, P.; Suhazsini, P.; Prabhu, K.; Jayakumar, A.; Kandasamy, R. Chicken tallow, a renewable source for the production of biosurfactant by Yarrowia lipolytica MTCC9520, and its application in silver nanoparticle synthesis. J. Surfactants Deterg. 2020, 23, 119–135. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.K.; Kant, C.; Verma, H.; Kumar, D.; Singh, P.P.; Modi, A.; Droby, S.; Kesawat, M.S.; Alavilli, H.; et al. Microbial biosurfactant: A new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 2021, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Oladi, M.; Shokri, M.R. Multiple Benthic Indicators are Efficient for Health Assessment of Coral Reefs Subjected to Petroleum Hydrocarbons Contamination: A Case Study in the Persian Gulf. J. Hazard. Mater. 2021, 409, 124993. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties. Process Saf. Environ. Prot. 2016, 102, 558–566. [Google Scholar] [CrossRef]
- Sobrinho, H.B.S.; Luna, J.M.; Rufino, R.D.; Porto, A.L.F.; Sarubbo, L.A. Assessment of toxicity of a biosurfactant from Candida sphaerica UCP 0995 cultivated with industrial residues in a bioreactor. Electron. J. Biotechnol. 2013, 16, 4. [Google Scholar] [CrossRef]
- Santos, E.F.; Teixeira, M.F.S.; Converti, A.; Porto, A.L.F.; Sarubbo, L.A. Production of a new lipoprotein biosurfactant by Streptomyces sp. DPUA1566 isolated from lichens collected in the Brazilian Amazon using agroindustry wastes. Biocatal. Agric. Biotechnol. 2019, 17, 142–150. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Marinho, P.H.C.; Farias, C.B.B.; Ferreira, S.R.M.; Sarubbo, L.A. Removal of petroleum derivative adsorbed to soil by biosurfactant Rufisan produced by Candida lipolytica. J. Pet. Sci. Eng. 2013, 109, 117–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).