Abstract
In this study, we investigated the surface-confined coupling reactions of 1,8-dibromobiphenylene (BPBr2) on Cu(111) to elucidate the details of the organometallic intermediates via Ullmann reactions. We used scanning tunneling microscopy (STM) to characterize the resulting organometallic intermediates. Moreover, submolecular resolution of the non-contact atomic force microscopy (nc-AFM) qPlus technique enables the bond-resolving within the organometallic dimer product. Our findings reveal the debromination of BPBr2 on Cu(111), leading to the formation of an organometallic dimer intermediate at room temperature. Through nc-AFM measurements, we confirm and visualize the formation of the C-Cu-C bond. These insights enhance our understanding of Ullmann reaction and hold potential implications for the design of novel two-dimensional electronic devices.
1. Introduction
In recent years, surface-confined coupling reactions have gained significant attention for constructing two-dimensional nanostructures under controlled conditions [1,2,3]. These reactions are fundamental to the “bottom-up” synthesis of conjugated nanostructures, which are significant for developing future electronic devices [4,5,6,7]. A notable example of an on-surface coupling reaction is the aryl–aryl coupling of halogenated aromatic compounds on metal surfaces [8]. From previous studies, it is clear that the surfaces play multiple roles in these reactions [1]. For example, the surfaces can confine the reaction space, restricting the conformational flexibility of molecules by adsorption. Additionally, they can catalyze the reaction by providing adatoms that participate in intermediate steps and can lower the activation barrier, such as in the dehalogenation of aryl halides [1,8]. The coupling of halogenated aryl compounds on coinage metal surfaces is often referred to as an Ullmann-type reaction [1].
The Ullmann coupling reaction, a classical metal-catalyzed synthesis of C-C bonds, involves coupling two aryl halides on a copper catalyst to form a diaryl molecule [9]. This reaction on metal substrates has been extensively studied. Typically, an Ullmann-like reaction involves two steps: (1) the dehalogenation process and (2) the ejection of metal atoms to form covalent bond coupling (mainly C-C coupling) [10,11,12]. Both conventional solution-phase and surface-phase Ullmann coupling are known to involve organometallic intermediates. Organometallic intermediates play a crucial role in surface-coupling reactions, influencing both reactivity and selectivity. However, the precise properties of these intermediates still remain a subject of debate and require further investigation [13].
Wang et al. demonstrated the existence of organometallic intermediates through a combination of scanning tunneling microscopy (STM) and density functional theory (DFT) calculations, unveiling radical dimers interconnected by a C-Cu-C bridge [14]. Similarly, Chung et al. observed the emergence of Ag-C bonds during the reaction of dibromo-p-terphenyl molecules on Ag(111) [15]. These findings elucidate the formation of organometallic intermediates, attributing their occurrence to the presence of surface-bound atoms. Moreover, recent studies on polymerizations at the adsorbate phase also highlight the importance of organometallic intermediates in surface reactions [16,17].
Biphenylene has a unique structure, featuring two benzene rings joined together without sharing a bond [18,19,20,21,22,23,24]. It is antiaromatic because of the 4n π-electrons (n is a positive integer) in a planar ring system but is considerably stable in comparison to other antiaromatic compounds [23]. Therefore, the chemical properties of biphenylene have gained much attention in the field of aromatic hydrocarbons. On the other hand, the bond dissociation energy (BDE) of the C–C σ-bond on the central four-membered ring is much smaller (2.84 eV) than that of the C–C σ-bond on biphenyl (4.96 eV) [20,25]. Hence, many researchers have studied the reaction involving biphenylene to explore its potential as a precursor to an aromatic network [26,27,28,29]. Biphenylene-based two-dimensional carbon networks may find applications in photonics, optoelectronics, quantum information technology, energy storage, and molecular filters due to their excellent mechanical, electronic, and transport properties [19,30].
While organometallic intermediates have been frequently reported in many surface Ullmann coupling reactions, detailed bond information with bond-resolving high resolution has been less studied [31,32,33,34]. In this study, we used scanning tunneling microscopy (STM) to characterize the 1,8-dibromobiphenylene (BPBr2) molecules deposited on Cu(111), confirming the formation of an organometallic intermediate dimer. The results were further compared with the covalent biphenylene dimer fabricated on hBN/Rh(111) [35]. In addition, we used non-contact atomic force microscope (nc-AFM) equipped with a qPlus technique to clearly characterize the chemical structure of BPBr2 molecules. With a CO functionalized tip, the submolecular resolution of nc-AFM clearly resolves the six-membered rings, four-membered rings and bond length information of the organometallic intermediate dimer.
2. Materials and Methods
BPBr2 was synthesized via the same route as our previous study [24,35]. The Cu(111) sample was cleaned by cycles of Ar+ sputtering and annealing. The cleanness of Cu(111) is checked by STM measurement, as shown in Figure 1. The molecule was deposited onto the Cu(111) surface through physical vapor deposition from a Kentax evaporator under an Ultra High Vacuum (UHV). During deposition, the quartz crucible was heated to 20 °C, and the pressure was maintained at 7 × 10−8 mbar in the deposition chamber. The Cu(111) substrate was kept at room temperature (RT). The deposition of the BPBr2 film was stopped after 60 s, resulting in very low coverage, which is optimal for AFM measurement.
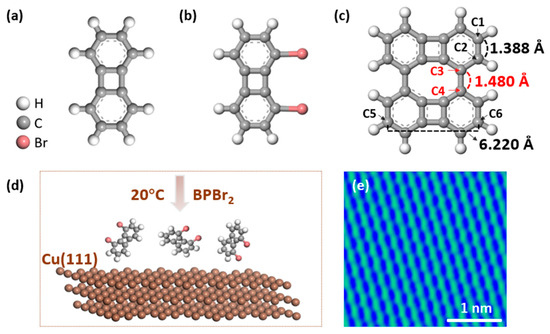
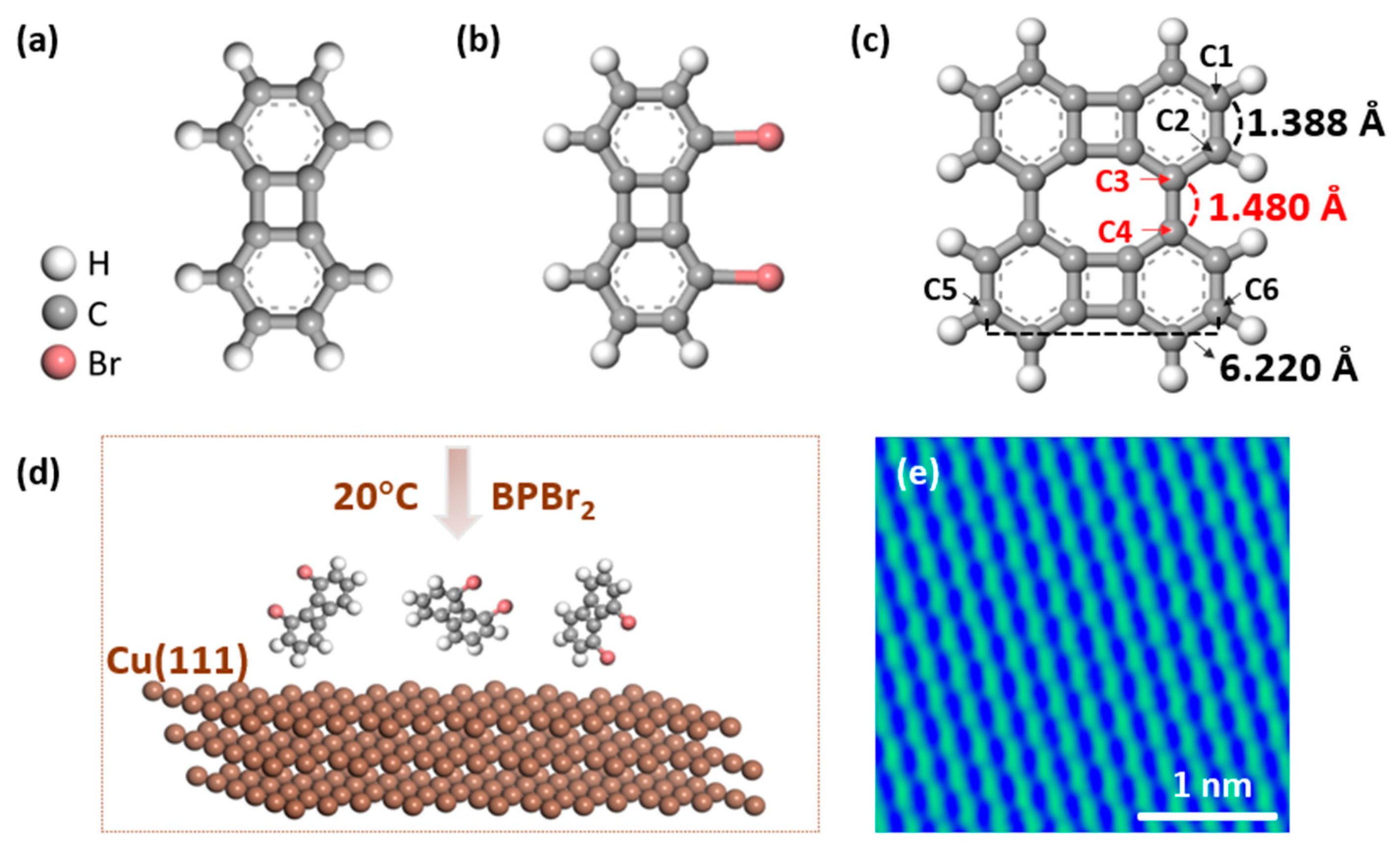
Figure 1.
DFT-optimized structure of (a) biphenylene, (b) BPBr2 (1,8-dibromobiphenylene) and (c) biphenylene dimer. The hydrogen, carbon and bromine atoms are represented by white, gray and red balls, respectively. BPBr2 is the molecular precursor we used in the study. (c) From the optimized dimer structure, the length of the C1–C2, C3–C4 and C5–C6 are 1.388 Å, 1.480 Å, and 6.220 Å, respectively. (d) Schematic illustration of the deposition process of BPBr2 molecules onto the Cu(111) surface. The evaporation temperature of BPBr2 is 20 °C; the Cu(111) substrate is kept at room temperature. (e) Atomic-resolution STM image of Cu(111) surface. (Bias = −1 V, It = 100 pA). The Cu(111) sample was cleaned by cycles of Ar+ sputtering and annealing.
All STM and nc-AFM experiments were performed on a Createc LT-STM/AFM system (CreaTec Fischer & Co. GmbH in Erligheim, Germany) in a UHV environment (base pressure as low as 10−10 mbar) operated at 5 K. All STM/nc-AFM results in the study were performed by an qPlus AFM sensor (also from CreaTec) with a PtIr tip cut using a focused ion beam before being transferred into the LT-STM/nc-AFM system. In the LT-STM/nc-AFM chamber, the tip was further shaped by controlled indentations into the Cu substrate yielding a copper coated tip.
For AFM measurements, the qPlus sensor tip was functionalized by picking up CO from Cu(111), following the routine described by Bartels et al. [36,37]. The used sensor had a resonance frequency f0 = 29 kHz and a quality factor of Q ≈ 29,000 at 5 K. We used oscillation amplitudes of 100 pm. All AFM images were obtained in constant height mode. In the experiment, the tip height was set with respect to a reference height given by the STM set point (parameters: bias = −40 mV, It = 0.5 nA) above the uncovered Cu(111) substrate in the vicinity of the molecule. The calibration of the scanner was carried out by Au(111) with atomic resolution. STM and AFM data were analyzed using WSxM software (version 5.0) [38].
The density functional theory (DFT) calculations were performed for single biphenylene, BPBr2 and biphenylene dimer molecules using the B3LYP-d3 functional [39] in combination with the def2-TZVP basis set [40], as implemented in the ORCA quantum chemistry package (version 5.0.4) [41,42].
3. Results and Discussion
As mentioned in the introduction, biphenylene has a unique structure with two benzene rings connected without sharing a bond, making it antiaromatic with 4n π-electrons [20]. Despite this, it is relatively stable, attracting interest in aromatic hydrocarbons [43]. Its central C–C σ-bond has a lower bond dissociation energy than in biphenyl, leading researchers to explore its reactions, particularly as a candidate for the novel 2D biphenylene carbon structure [19,44].
Figure 1a shows the structure of biphenylene in its gas phase as calculated by DFT. Biphenylene is a relatively simple molecule and is known to be volatile. When heated on a metallic substrate, the relatively stable structure of biphenylene does not lead to covalent bond formation [43]. In our study, we halogenated biphenylene to obtain BPBr2 (Figure 1b), where two bromine atoms are in the same plane and attached to the benzene rings. This molecule is flat, making it easy to visualize using scanning tunneling microscopy (STM). Bromination of biphenylene facilitates the formation of covalent biphenylene dimer, whose calculated gas-phase structure is shown in Figure 1c.
Based on previous knowledge, we know that heating BPBr2 on a Cu(111) substrate results in a covalently bonded carbon structure [24]. However, the behavior of BPBr2 on Cu(111) without heating remains to be studied. In this study, we are particularly interested in the initial adsorption step of the BPBr2 precursor on a Cu(111) substrate, as illustrated in Figure 1d. We investigated the initial step of BPBr2 on Cu(111) at room temperature and revealed the bond-resolved structure of the reaction product, an organometallic dimer, using STM and nc-AFM. Figure 1e displays the clean STM image of the Cu(111) surface with atomic resolution measured at 5 K before depositing a BPBr2 molecule. The image shows the characteristic hexagonal lattice pattern of the Cu(111) surface.
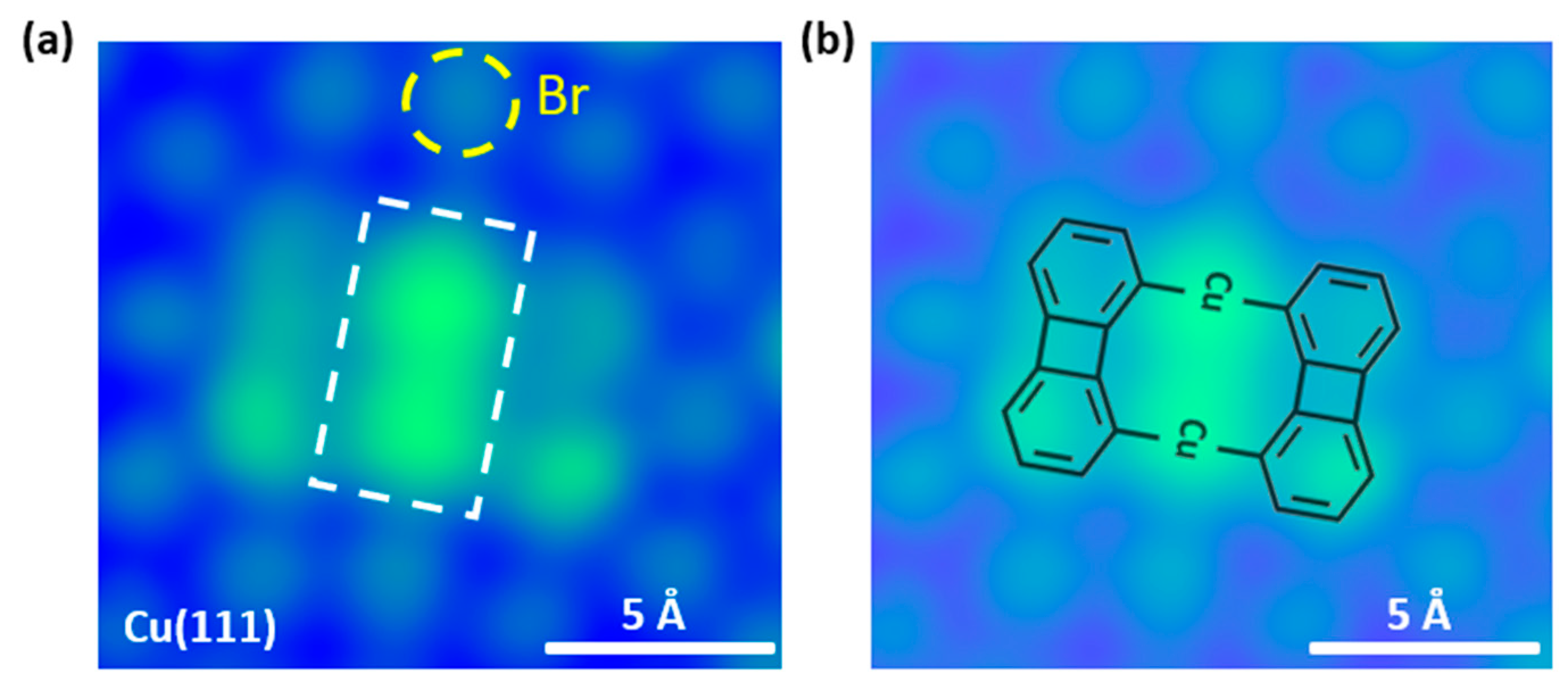
Figure 2a shows the experimental results of adsorption of BPBr2 on Cu (111) at room temperature, measured by STM at 5 K. The STM image clearly reveals that the BPBr2 molecules have debrominated and then converted into organometallic dimers on Cu(111) at room temperature. The two brightest dots in the middle of one organometallic dimer are assigned to two Cu adatoms [45]. The dark sides of this organometallic dimer are biphenylenes. The corresponding structure of the organometallic dimer is shown in Figure 2b. On the other hand, the dots surrounding organometallic dimer, marked by yellow circles in STM image, are assigned to the Br atoms [45].
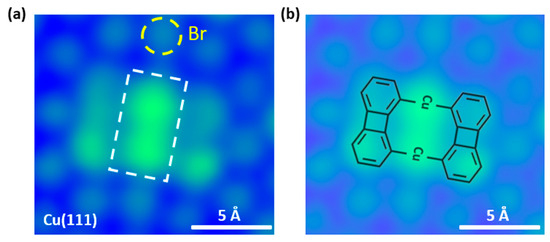
Figure 2.
An organometallic intermediate dimer is formed on Cu(111). (a) STM image of the organometallic intermediate dimer on Cu(111) (Bias = −500 mV, It = 100 pA). The bright spots around the organometallic intermediate are Br atoms. We mark one Br atom with a yellow circle for clarity. Notably, there are two bright spots (marked with a white rectangle) in the middle of the product, which is assigned to the Cu atoms. The corresponding structure is shown in (b).
nc-AFM with the qPlus technique has become essential in on-surface chemistry due to its exceptionally high resolution in revealing bond information [46]. This direct observation has been used to investigate molecules [47] as well as on-surface chemical reactions [48,49]. Furthermore, together with local probe-induced dehydrogenation or dehalogenation, high-resolution AFM has been used for identifying the structures of arynes [50] and triangulene [51]. Therefore, to further investigate the details of this organometallic intermediate, we used nc-AFM with the qPlus technique for detailed observations.
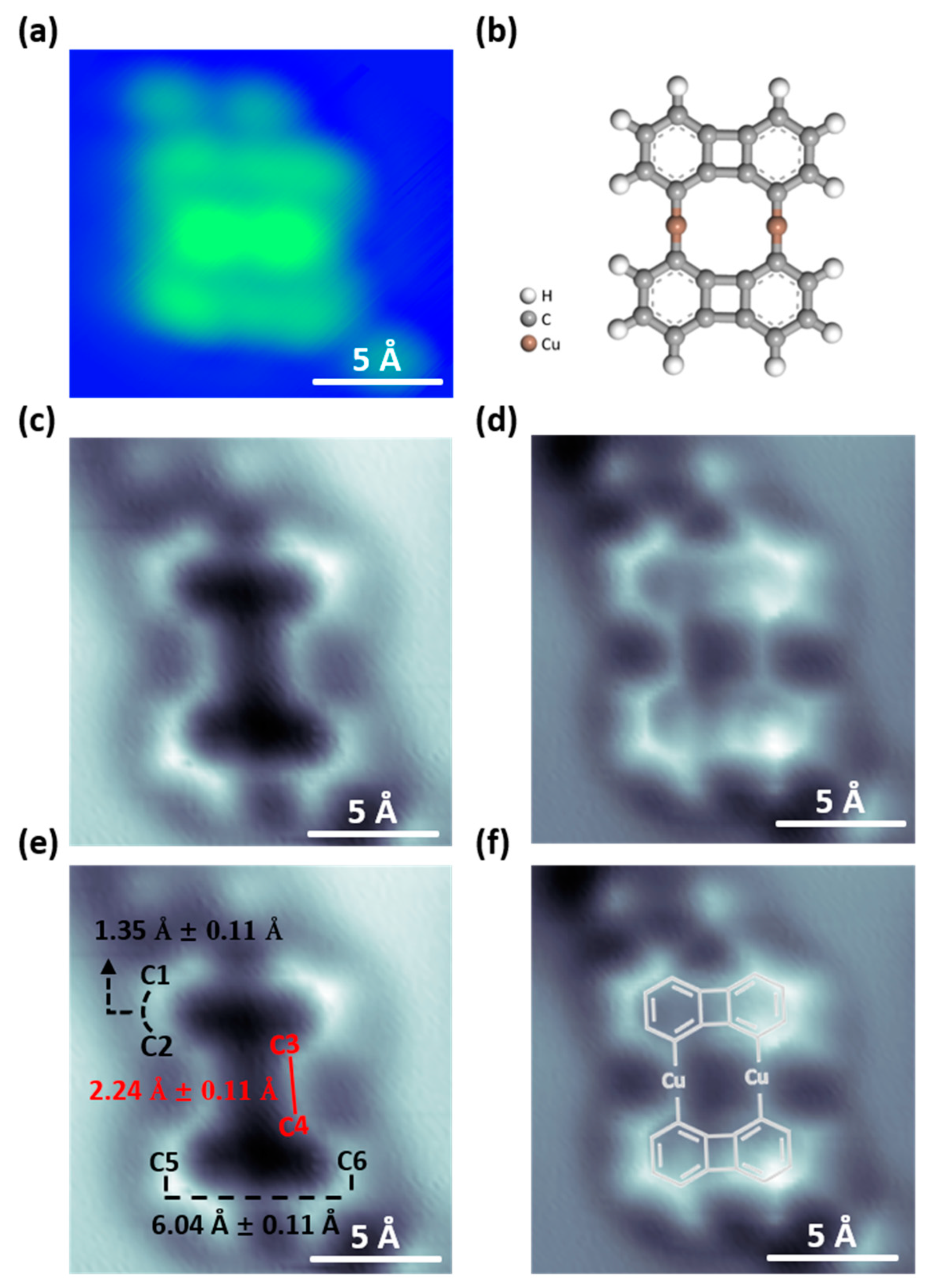
Figure 3a shows the STM topography of organometallic intermediates on Cu(111). Figure 3b presents a model of the organometallic intermediate to aid in understanding the molecular structure. To investigate the organometallic intermediates in greater detail in regard to chemical structure, we used nc-AFM with a CO-functionalized tip at a constant height mode, as shown in Figure 3c–f. Figure 3c,d show the results measured at different tip heights, with the tip being 20 pm closer to the sample in (d) compared to (c). We note that, although Figure 3d resembles the chemical structure, it may not represent truly existing bonds [52]. These artifact bonds are usually caused by enhanced interactions between the probe and the sample. If the interaction force between the probe and the sample is too strong, it may cause displacement or abnormal vibrations of the probe, resulting in artifacts in the images.
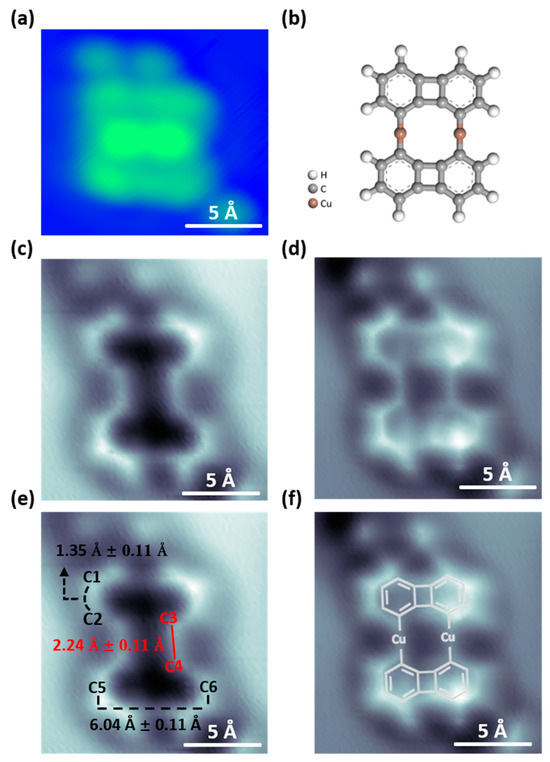
Figure 3.
STM images and AFM images of organometallic intermediate structures. (a) STM image of the organometallic intermediate dimer on Cu(111). (Bias = −40 mV, It = 50 pA). (b) The corresponding ball-and-stick model of organometallic intermediates. (c,d) AFM images of organometallic intermediates, taken at the same area as in (a) with different tip-sample distances. (c) The tip is 20 pm closer to the sample compared to the STM set point (parameters: bias = −40 mV, It = 0.5 nA) above the uncovered Cu(111) substrate in the vicinity of the molecule. (d) The tip is 20 pm closer to the sample in (d) compared to (c). The AFM images with corresponding measurements and model structures are shown in (e,f). C1–C2 bond: 1.35 Å ± 0.11 Å, C3–C4 bond: 2.24 Å ± 0.11 Å, C5–C6: 6.04 Å ± 0.11 Å.
On the other hand, Figure 3c presents a structure that accurately reflects the true bonding information. Therefore, we use Figure 3c to measure the bond lengths, and the results are shown in Figure 3e. We obtain the following bonding length information: C1–C2 bond length: 1.35 Å ± 0.11 Å, C3–C4 bond length: 2.24 Å ± 0.11 Å, and C5–C6 length is 6.04 Å ± 0.11 Å. Figure 3f additionally shows the molecular structure for clarity. The calculated bond length of biphenylene dimer in Figure 1c shows that C1–C2 is 1.388 Å and the length between C5 and C6 is 6.220 Å. By comparing the bond length between biphenylene dimer and organometallic dimer, it can be observed that the C1–C2 and C5–C6 bond lengths in the covalent biphenylene dimer are equal to those in the organometallic dimer. However, the C3–C4 carbon-carbon bond length in the covalent biphenylene dimer is 1.480 Å. Whereas, in the organometallic dimer, the C3–C4 bond length is 2.245 Å. The C3–C4 length in the organometallic intermediate is significantly longer than that in the covalent biphenylene dimer, which confirms again the formation of the C-Cu-C bonding in the organometallic intermediate dimer.
Therefore, by examining the organometallic intermediate dimer at the sub-nanometer scale using nc-AFM, we confirm that the organometallic intermediate dimer forms directly at room temperature when BPBr2 is deposited on Cu(111).
4. Conclusions
In summary, we have studied the adsorption behavior of BPBr2 molecules on Cu(111). Using high-resolution nc-AFM with a CO-functionalized tip, we clearly discerned the structure of the organometallic intermediate dimer. By comparing the organometallic intermediates on Cu(111) detected by STM and AFM, along with DFT calculations, the C-Cu-C bond was clearly observed. We confirm that the debromination of BPBr2 on Cu(111) at room temperature results in the formation of an organometallic intermediate dimer. This conclusion provides new insights into the understanding and design of organometallic intermediates and offers new ideas for the development of two-dimensional electronic devices.
Author Contributions
Conceptualization, T.Z. and Y.W.; methodology, X.H., Y.L. and T.Z.; software, H.J.; validation, Q.Z., H.Y. and L.L.; formal analysis, X.H.; investigation, Y.L.; data curation, T.W. and H.F.; writing—original draft preparation, X.H. and Y.L.; writing—review and editing, X.H., Y.L. and T.Z.; visualization, X.H.; supervision, T.Z. and Y.W.; project administration, T.Z.; funding acquisition, T.Z. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 62271048, 62101037, 12304205, 62371041, 92163206) and National Key Research and Development Program of China (2019YFA0308000, 2021YFA1400100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dong, L.; Liu, P.N.; Lin, N. Surface-activated coupling reactions confined on a surface. Acc. Chem. Res. 2015, 48, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.; Hanke, F. Towards design rules for covalent nanostructures on metal surfaces. Chem. A Eur. J. 2014, 20, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Hla, S.-W.; Bartels, L.; Meyer, G.; Rieder, K.-H. Inducing all steps of a chemical reaction with the scanning tunneling microscope tip: Towards single molecule engineering. Phys. Rev. Lett. 2000, 85, 2777. [Google Scholar] [CrossRef] [PubMed]
- Franc, G.; Gourdon, A. Covalent networks through on-surface chemistry in ultra-high vacuum: State-of-the-art and recent developments. Phys. Chem. Chem. Phys. 2011, 13, 14283–14292. [Google Scholar] [CrossRef] [PubMed]
- Klappenberger, F.; Zhang, Y.-Q.; Björk, J.; Klyatskaya, S.; Ruben, M.; Barth, J.V. On-surface synthesis of carbon-based scaffolds and nanomaterials using terminal alkynes. Acc. Chem. Res. 2015, 48, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, T.; Chen, C.; Pedramrazi, Z.; Haberer, D.; de Oteyza, D.G.; Fischer, F.R.; Louie, S.G.; Crommie, M.F. Molecular bandgap engineering of bottom-up synthesized graphene nanoribbon heterojunctions. Nat. Nanotechnol 2015, 10, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Song, F.; Nguyen, M.T.; Li, Z.; Studener, F.; Stöhr, M. Comparing Ullmann coupling on noble metal surfaces: On-surface polymerization of 1, 3, 6, 8-tetrabromopyrene on Cu (111) and Au (111). Chem. A Eur. J. 2016, 22, 5937–5944. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.; Hanke, F.; Stafstrom, S. Mechanisms of halogen-based covalent self-assembly on metal surfaces. J. Am. Chem. Soc. 2013, 135, 5768–5775. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Recent advancement of Ullmann-type coupling reactions in the formation of C–C bond. ChemTexts 2016, 2, 17. [Google Scholar] [CrossRef]
- Judd, C.J.; Haddow, S.L.; Champness, N.R.; Saywell, A. Ullmann coupling reactions on Ag (111) and Ag (110); substrate influence on the formation of covalently coupled products and intermediate metal-organic structures. Sci. Rep. 2017, 7, 14541. [Google Scholar] [CrossRef]
- Sperotto, E.; van Klink, G.P.; van Koten, G.; de Vries, J.G. The mechanism of the modified Ullmann reaction. Dalton Trans. 2010, 39, 10338–10351. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl− aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Cristea, I. Kinetics and mechanism of the copper (I)-induced homogeneous Ullmann coupling of o-bromonitrobenzene. J. Am. Chem. Soc. 1976, 98, 748–753. [Google Scholar] [CrossRef]
- Wang, W.; Shi, X.; Wang, S.; Van Hove, M.A.; Lin, N. Single-molecule resolution of an organometallic intermediate in a surface-supported Ullmann coupling reaction. J. Am. Chem. Soc. 2011, 133, 13264–13267. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-H.; Koo, B.-G.; Kim, H.; Yoon, J.K.; Kim, J.-H.; Kwon, Y.-K.; Kahng, S.-J. Electronic structures of one-dimensional metal–molecule hybrid chains studied using scanning tunneling microscopy and density functional theory. Phys. Chem. Chem. Phys. 2012, 14, 7304–7308. [Google Scholar] [CrossRef] [PubMed]
- Lipton-Duffin, J.A.; Ivasenko, O.; Perepichka, D.F.; Rosei, F. Synthesis of polyphenylene molecular wires by surface-confined polymerization. Small 2009, 5, 592–597. [Google Scholar] [CrossRef]
- Eichhorn, J.; Strunskus, T.; Rastgoo-Lahrood, A.; Samanta, D.; Schmittel, M.; Lackinger, M. On-surface Ullmann polymerization via intermediate organometallic networks on Ag (111). Chem. Commun. 2014, 50, 7680–7682. [Google Scholar] [CrossRef]
- Lothrop, W.C. Biphenylene. J. Am. Chem. Soc. 1941, 63, 1187–1191. [Google Scholar] [CrossRef]
- Fan, Q.; Yan, L.; Tripp, M.W.; Krejčí, O.; Dimosthenous, S.; Kachel, S.R.; Chen, M.; Foster, A.S.; Koert, U.; Liljeroth, P. Biphenylene network: A nonbenzenoid carbon allotrope. Science 2021, 372, 852–856. [Google Scholar] [CrossRef]
- Takano, H.; Ito, T.; Kanyiva, K.S.; Shibata, T. Recent advances of biphenylene: Synthesis, reactions and uses. Eur. J. Org. Chem. 2019, 2019, 2871–2883. [Google Scholar] [CrossRef]
- Toda, F.; Garratt, P. Four-membered ring compounds containing bis (methylene) cyclobutene or tetrakis (methylene) cyclobutane moieties. Benzocyclobutadiene, benzodicyclobutadiene, biphenylene, and related compounds. Chem. Rev. 1992, 92, 1685–1707. [Google Scholar] [CrossRef]
- Perthuisot, C.; Edelbach, B.L.; Zubris, D.L.; Simhai, N.; Iverson, C.N.; Müller, C.; Satoh, T.; Jones, W.D. Cleavage of the carbon–carbon bond in biphenylene using transition metals. J. Mol. Catal. A Chem. 2002, 189, 157–168. [Google Scholar] [CrossRef]
- Miljanić, O.Š.; Vollhardt, K.P.C. [n] phenylenes: A novel class of cyclohexatrienoid hydrocarbon. In Carbon-Rich Compounds: From Molecules to Materials; Wiley: Hoboken, NJ, USA, 2006; pp. 140–197. [Google Scholar]
- Zhang, T.; Grazioli, C.; Yang, H.; Jiang, K.; Brumboiu, I.E.; Jia, L.; Liu, L.; Puglia, C.; Zhuang, X.; Wang, Y. Spectroscopic evidence of new low-dimensional planar carbon allotropes based on biphenylene via on-surface Ullmann coupling. Chemistry 2021, 3, 1057–1062. [Google Scholar] [CrossRef]
- Jones, W.D. Mechanistic studies of transition metal-mediated C–C bond activation. CC Bond Act. 2014, 346, 1–31. [Google Scholar]
- Greulich, T.W.; Suzuki, N.; Daniliuc, C.G.; Fukazawa, A.; Yamaguchi, E.; Studer, A.; Yamaguchi, S. A biphenyl containing two electron-donating and two electron-accepting moieties: A rigid and small donor–acceptor–donor ladder system. Chem. Commun. 2016, 52, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Rong, H.-T.; Heister, K.; Yang, Y.-J.; Buck, M.; Zharnikov, M. Response of biphenyl-substituted alkanethiol self-assembled monolayers to electron irradiation: Damage suppression and odd− even effects. Langmuir 2002, 18, 3142–3150. [Google Scholar] [CrossRef]
- Seth, S.; Savitha, G.; Moorthy, J.N. Metal-Mediated Self-Assembly of a Twisted Biphenyl-Tetraacid Linker with Semi-rigid Core and Peripheral Flexibility: Concomitant Formation of Compositionally Distinct MOFs. Cryst. Growth Des. 2018, 18, 2129–2137. [Google Scholar] [CrossRef]
- Seth, S.; Jhulki, S. Porous flexible frameworks: Origins of flexibility and applications. Mater. Horiz. 2021, 8, 700–727. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, T.; Dong, X.; Liu, G.; Li, H.; Yang, N.; Liu, D.; Xiao, X. A comparative study of the electronic transport and gas-sensitive properties of Graphene+, T-graphene, Net-graphene, and biphenylene-based two-dimensional devices. ACS Sens. 2023, 8, 3510–3519. [Google Scholar] [CrossRef]
- Houtsma, R.K.; van Zuilen, J.; Stöhr, M. On-Surface Ullmann-Type Coupling: Reaction Intermediates and Organometallic Polymer Growth. Adv. Mater. Interfaces 2024, 11, 2300728. [Google Scholar] [CrossRef]
- Barton, D.; Gao, H.Y.; Held, P.A.; Studer, A.; Fuchs, H.; Doltsinis, N.L.; Neugebauer, J. Formation of Organometallic Intermediate States in On-Surface Ullmann Couplings. Chem. –A Eur. J. 2017, 23, 6190–6197. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, G.; Di Giovannantonio, M.; Cupo, A.; Xing, S.; Lipton-Duffin, J.; Ebrahimi, M.; Vasseur, G.; Kierren, B.; Fagot-Revurat, Y.; Tristant, D. An unexpected organometallic intermediate in surface-confined Ullmann coupling. Nanoscale 2019, 11, 7682–7689. [Google Scholar] [CrossRef]
- Fan, Q.; Zhu, J.; Gottfried, J. Organometallic Structures and Intermediates in Surface Ullmann Coupling. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–353. [Google Scholar]
- Zhang, T.; Li, R.; Hao, X.; Zhang, Q.; Yang, H.; Hou, Y.; Hou, B.; Jia, L.; Jiang, K.; Zhang, Y. Ullmann-Like Covalent Bond Coupling without Participation of Metal Atoms. ACS Nano 2023, 17, 4387–4395. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.; Mohn, F.; Moll, N.; Schuler, B.; Criado, A.; Guitián, E.; Peña, D.; Gourdon, A.; Meyer, G. Bond-Order Discrimination by Atomic Force Microscopy. Science 2012, 337, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Bartels, L.; Meyer, G.; Rieder, K.H. Controlled vertical manipulation of single CO molecules with the scanning tunneling microscope: A route to chemical contrast. Appl. Phys. Lett. 1997, 71, 213–215. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Totani, R.; Grazioli, C.; Zhang, T.; Bidermane, I.; Lüder, J.; De Simone, M.; Coreno, M.; Brena, B.; Lozzi, L.; Puglia, C. Electronic structure investigation of biphenylene films. J. Chem. Phys. 2017, 146, 054705. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, B.; Deng, K.; Feng, X.; Wagner, M.; Gale, J.D.; Müllen, K.; Zhi, L. Graphenylene, a unique two-dimensional carbon network with nondelocalized cyclohexatriene units. J. Mater. Chem. C 2013, 1, 38–41. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, D.; Wang, T.; Chen, Q.; Matèj, A.; Huang, J.; Han, D.; Xu, Q.; Zhao, A.; Jelínek, P. Chemisorption-induced formation of biphenylene dimer on Ag (111). J. Am. Chem. Soc. 2021, 144, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.; Mohn, F.; Moll, N.; Liljeroth, P.; Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 2009, 325, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.; Mohn, F.; Moll, N.; Meyer, G.; Ebel, R.; Abdel-Mageed, W.M.; Jaspars, M. Organic structure determination using atomic-resolution scanning probe microscopy. Nat. Chem. 2010, 2, 821–825. [Google Scholar] [CrossRef] [PubMed]
- de Oteyza, D.G.; Gorman, P.; Chen, Y.-C.; Wickenburg, S.; Riss, A.; Mowbray, D.J.; Etkin, G.; Pedramrazi, Z.; Tsai, H.-Z.; Rubio, A. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 2013, 340, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Haapasilta, V.; Lindner, B.D.; Tahara, K.; Spijker, P.; Buitendijk, J.A.; Pawlak, R.; Meier, T.; Tobe, Y.; Foster, A.S. Thermal control of sequential on-surface transformation of a hydrocarbon molecule on a copper surface. Nat. Commun. 2016, 7, 12711. [Google Scholar] [CrossRef] [PubMed]
- Pavliček, N.; Schuler, B.; Collazos, S.; Moll, N.; Pérez, D.; Guitián, E.; Meyer, G.; Peña, D.; Gross, L. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 2015, 7, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Pavliček, N.; Mistry, A.; Majzik, Z.; Moll, N.; Meyer, G.; Fox, D.J.; Gross, L. Synthesis and characterization of triangulene. Nat. Nanotechnol. 2017, 12, 308–311. [Google Scholar] [CrossRef]
- Jarvis, S.P.; Rashid, M.A.; Sweetman, A.; Leaf, J.; Taylor, S.; Moriarty, P.; Dunn, J. Intermolecular artifacts in probe microscope images of c 60 assemblies. Phys. Rev. B 2015, 92, 241405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).