Fabrication of Electroplated Nickel Composite Films Using Cellulose Nanofibers Introduced with Carboxy Groups as Co-Deposited Materials
Abstract
1. Introduction
2. Experimental Procedure
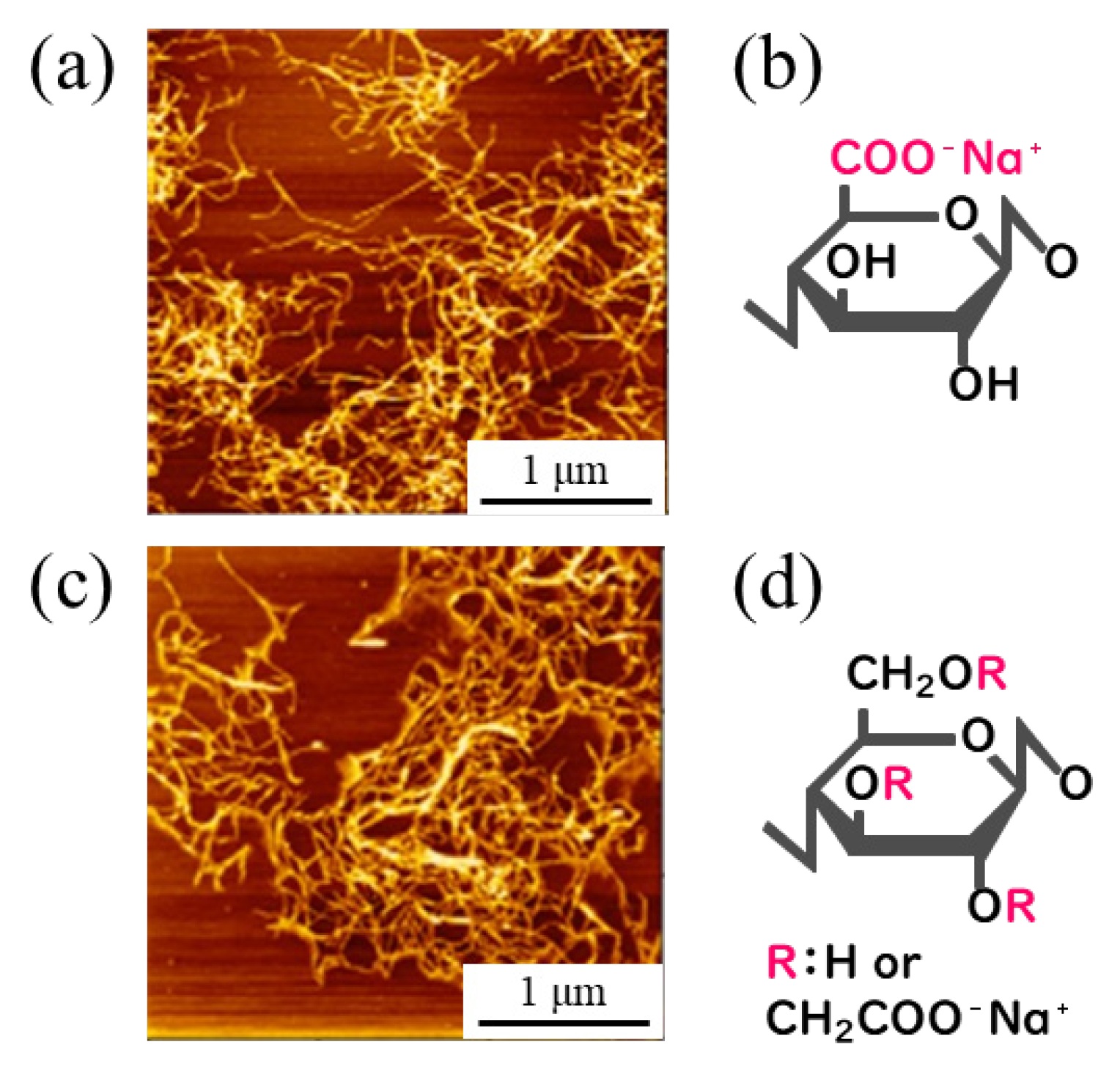
2.1. Preparing Sample Materials
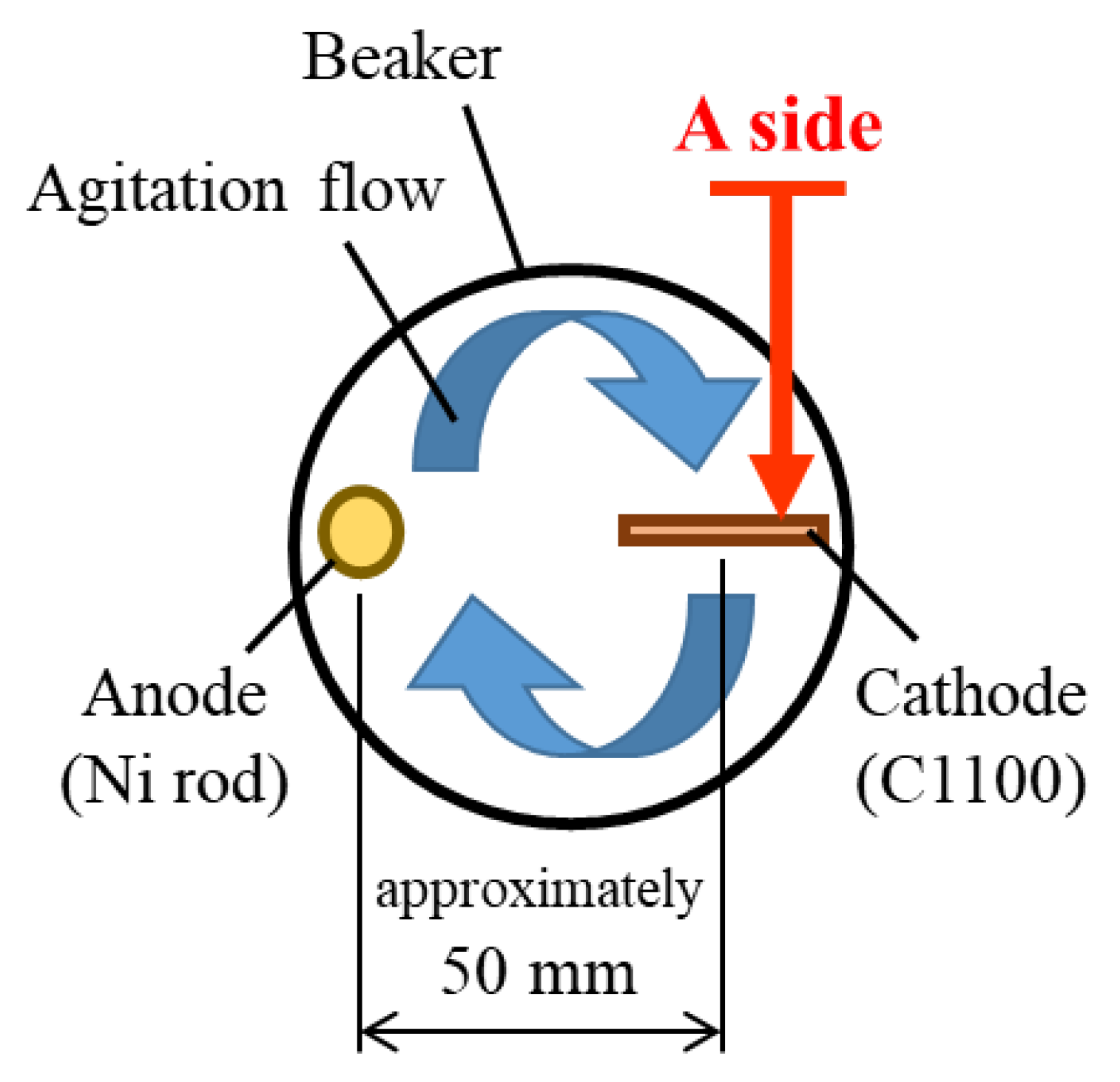
2.2. Plating Process
2.3. Analyzing Process
2.3.1. Observation of Plated Film
2.3.2. Surface Roughness Measurement
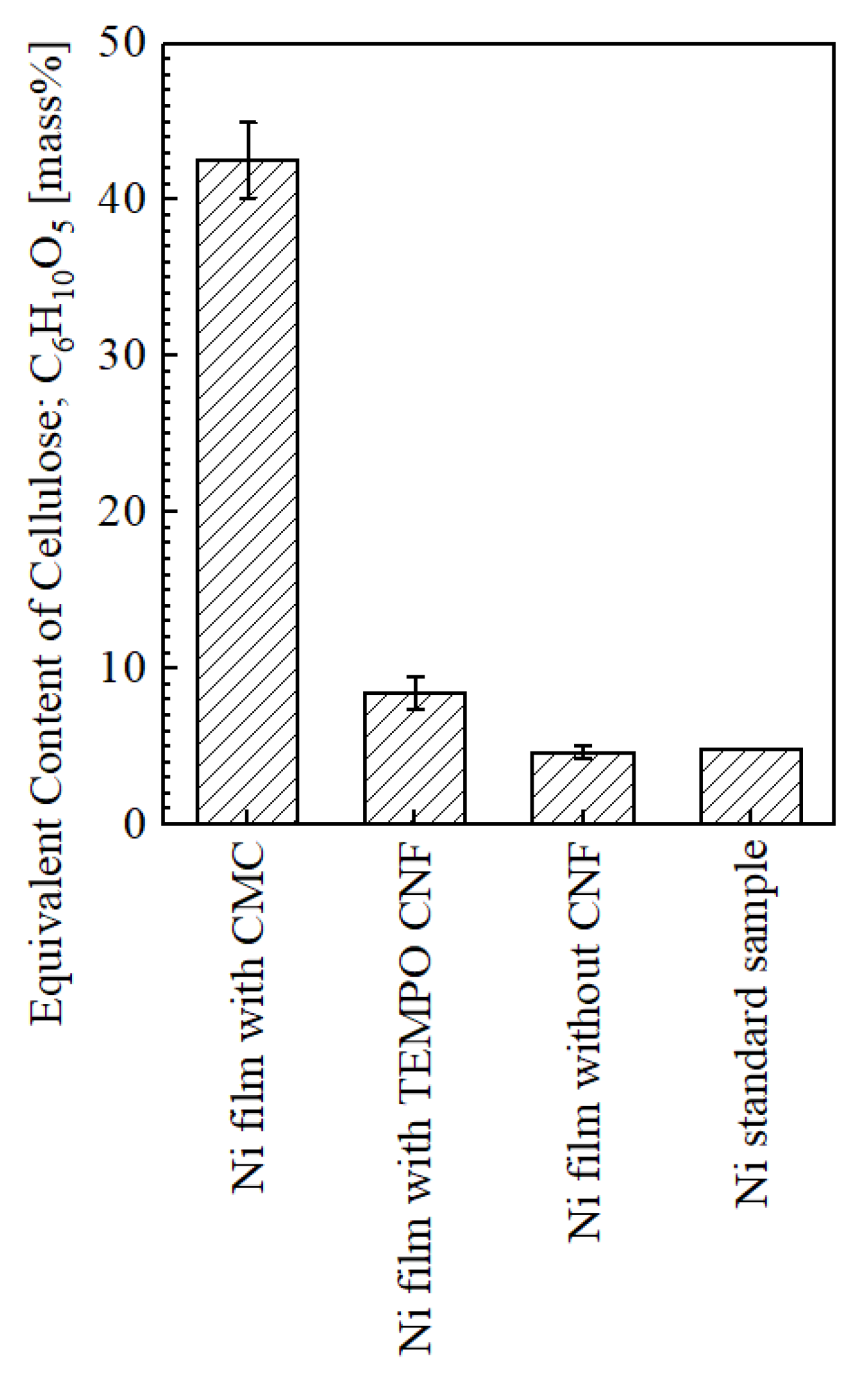
2.3.3. Quantification Analysis
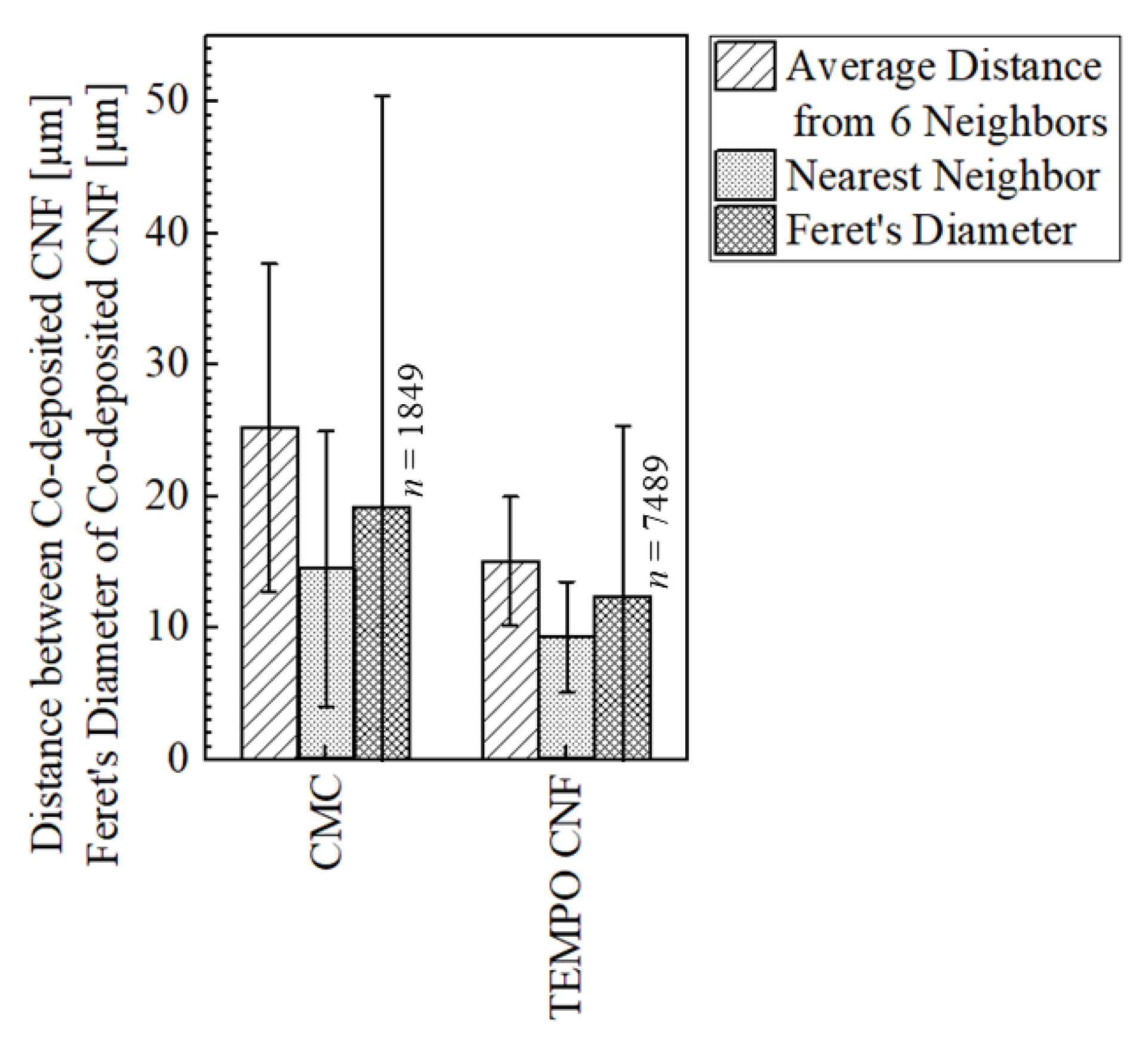
2.3.4. Evaluation of Dispersion of CNF in Plated Film
2.3.5. Vickers Hardness Test
3. Results and Discussions
3.1. Morphology and Microstructure of Plated Films
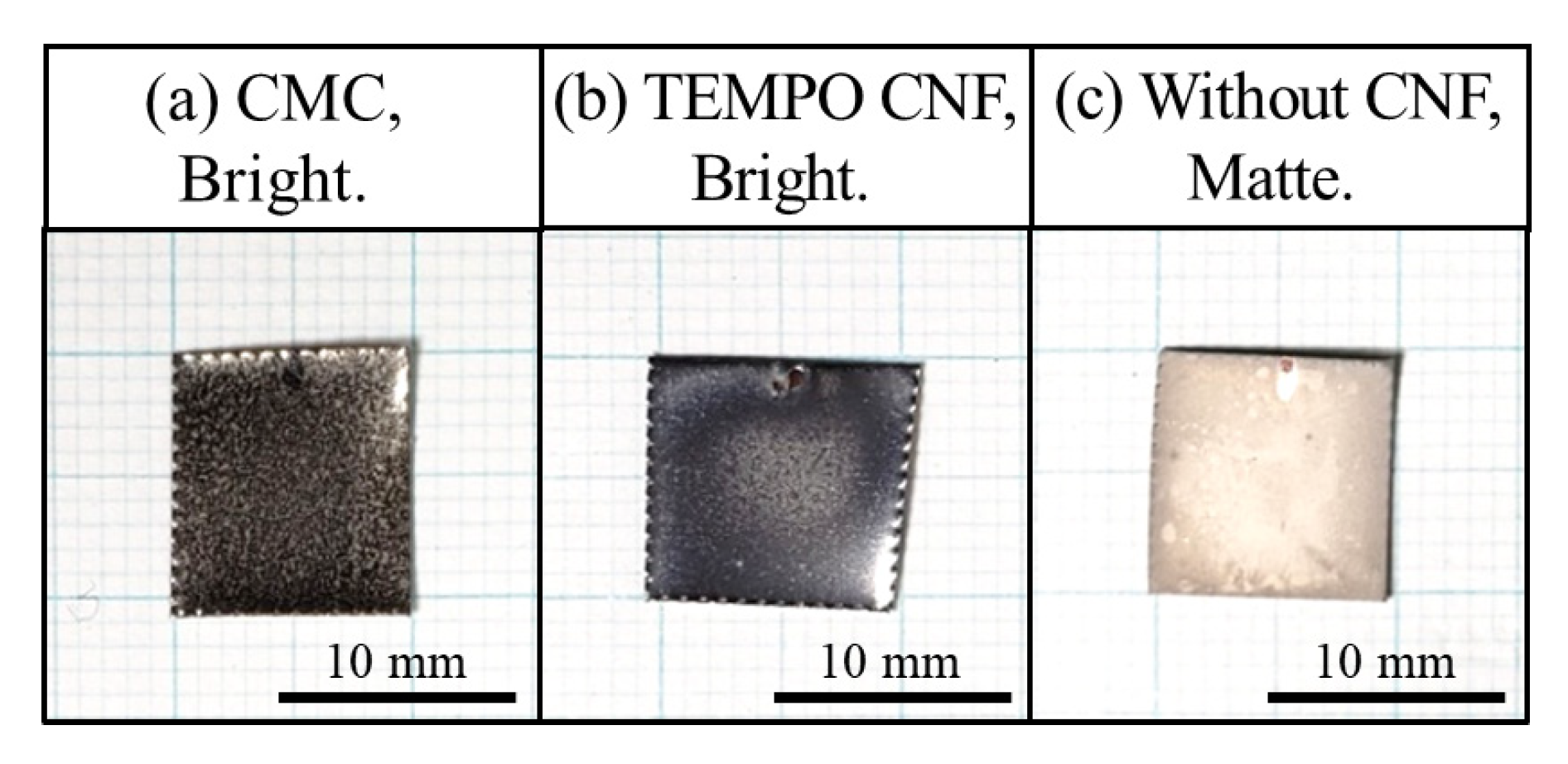
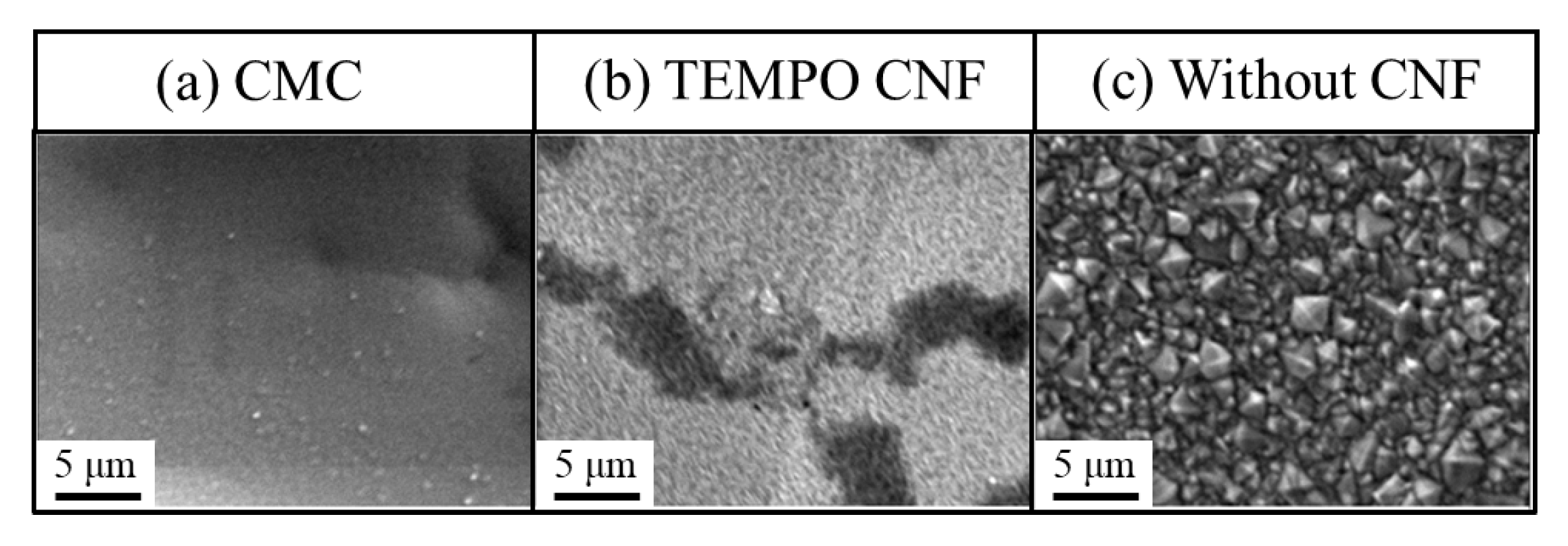
3.1.1. Morphology
3.1.2. Microstructure of Surface
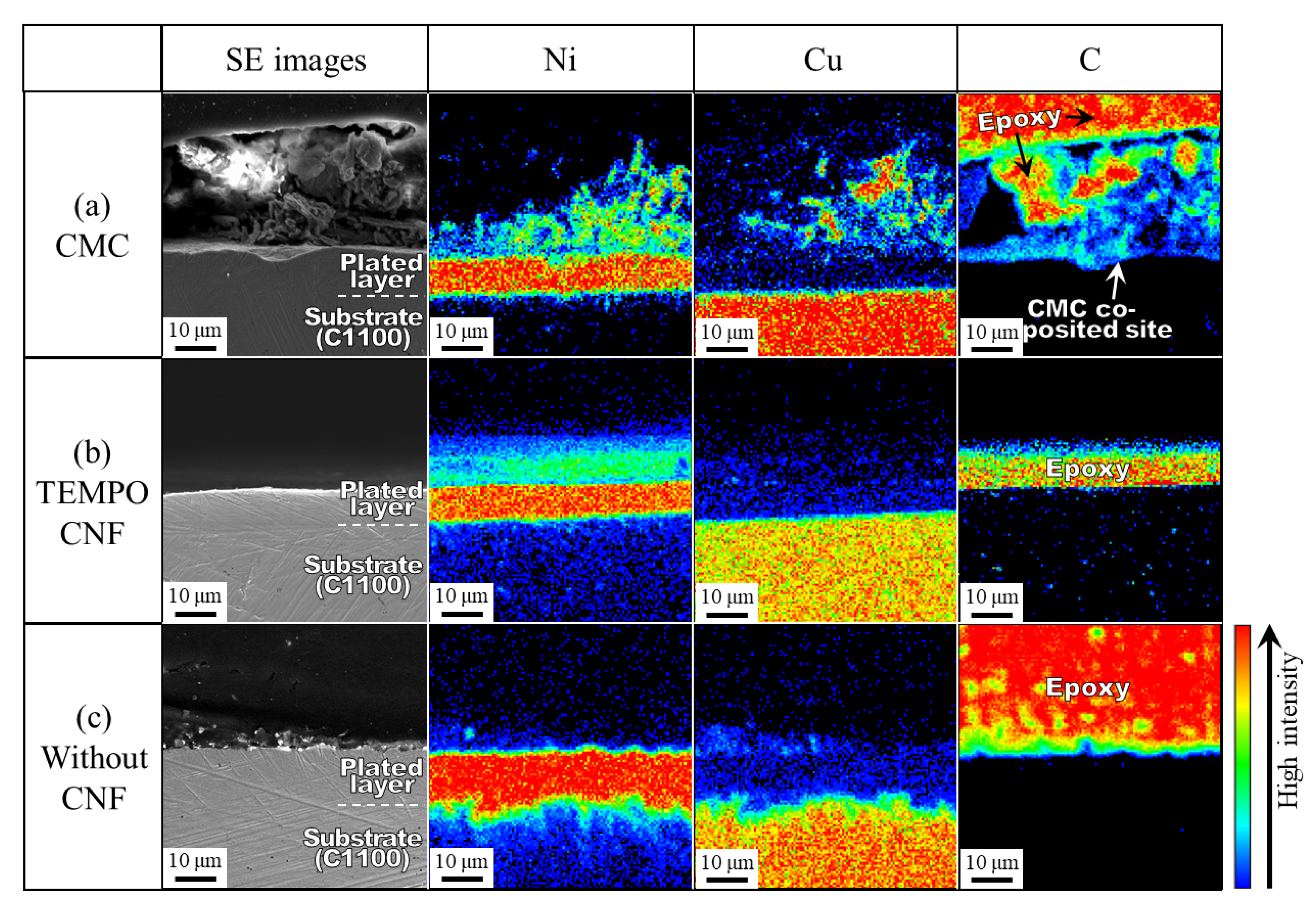
3.1.3. Microstructure of Cross-Section
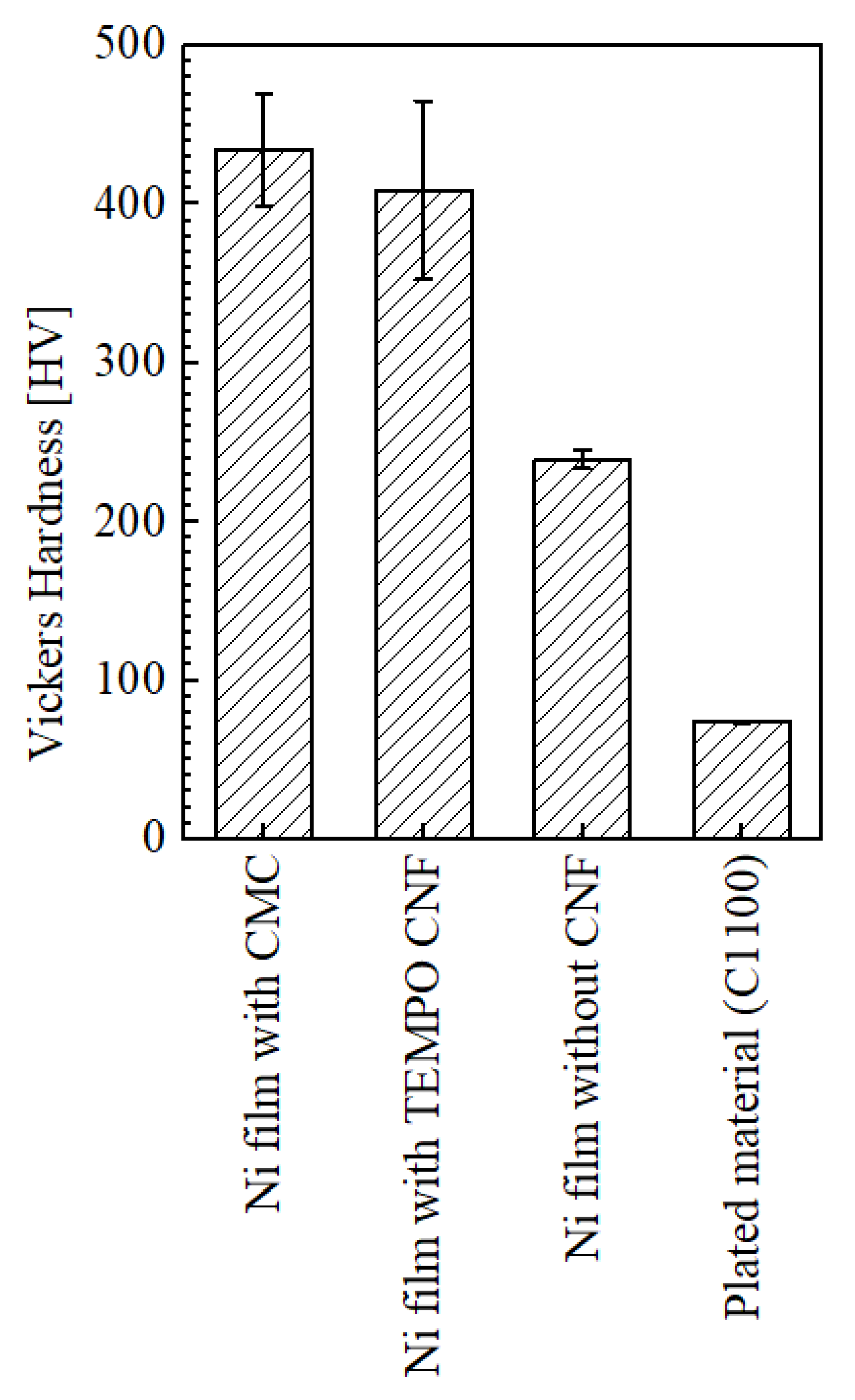
3.2. Vickers Hardness of Plated Films
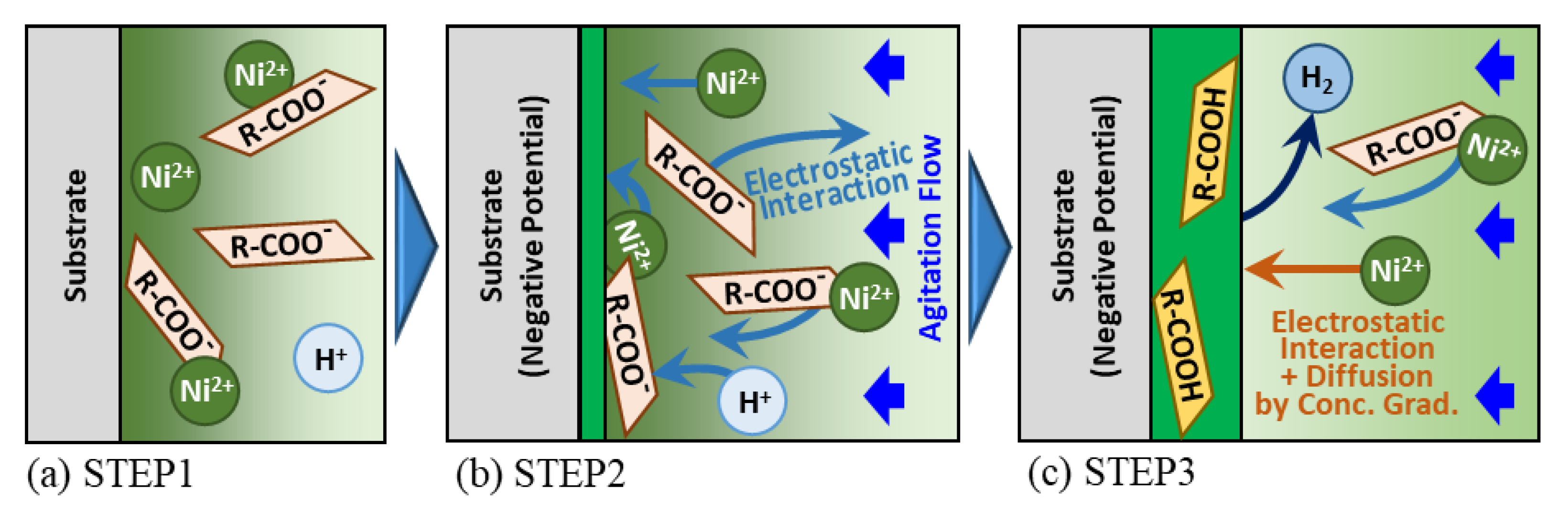
3.3. Co-Deposition Mechanism
4. Conclusions
- Co-deposition was observed with both CMC and TEMPO CNF.
- The surface Vickers hardness of the specimen with CMC was higher than that of TEMPO CNF, and the hardness improvement was approximately 82% compared to the conventional Ni-plated film without CNF. Even though the hardness of the specimen with TEMPO CNF was slightly inferior, it was improved by approximately 71%.
- The image analysis showed that the average distance between the co-deposited TEMPO CNF was shorter than that of CMC by approximately 40%, indicating a fine and dense co-deposited morphology.
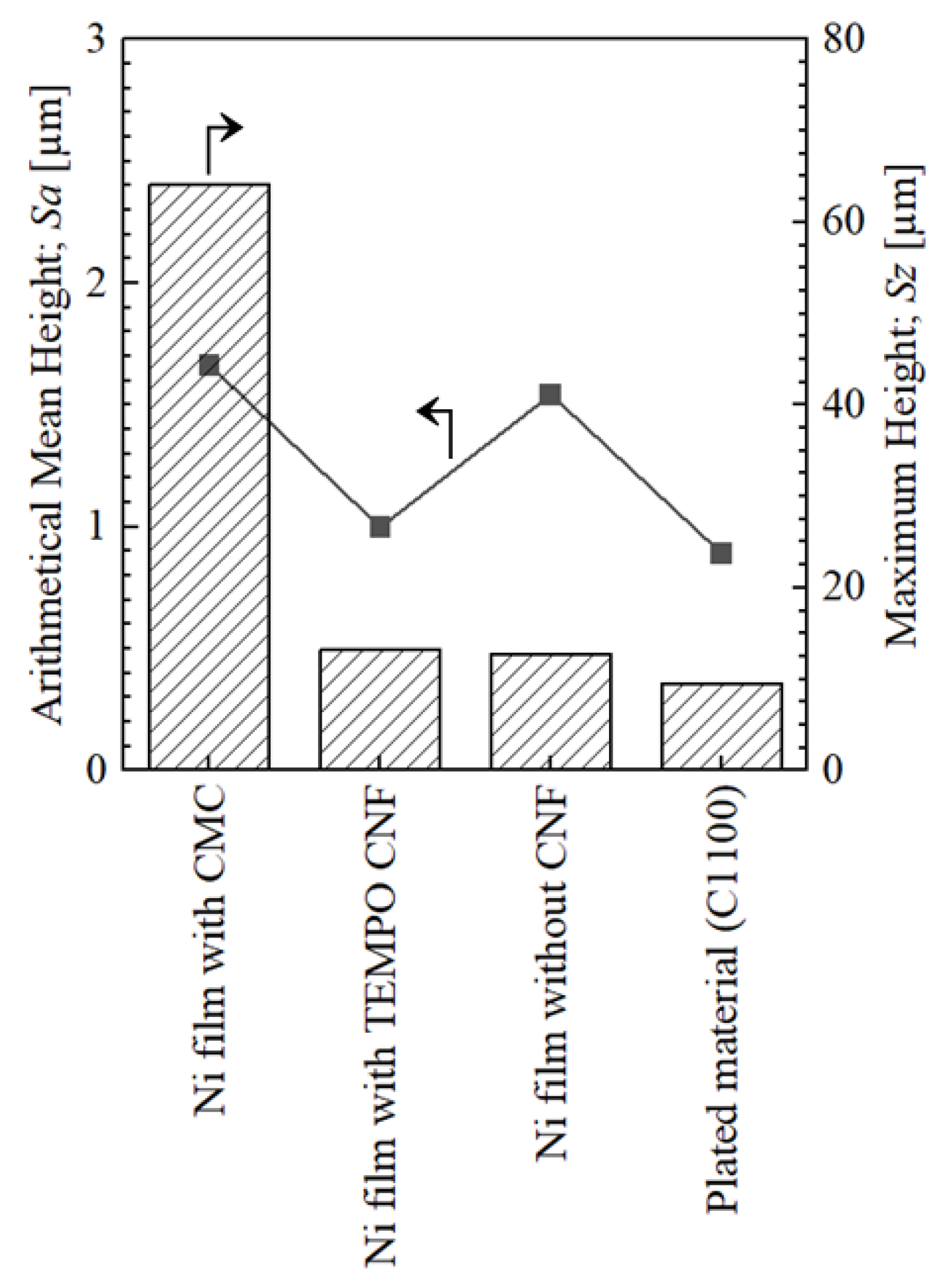
- The surface roughness of the specimen with TEMPO CNF was significantly smooth and that was superior compared to the conventional Ni-plated film, whereas that with CMC had a noticeably rougher surface.
- Models for the co-deposition mechanism of Ni-CNF composite electroplating were proposed in the case of CNF with introduced carboxy groups on the surface. In this model, nickel is deposited from the chelate complex formed between the carboxylate of CNF and Ni ion. It was considered that this causes CNF to be incorporated into the Ni-plated film and improves hardness through grain refinement and dispersion strengthening effects simultaneously.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janowiak, M.; Webster, C. Promoting ecological sustainability in woody biomass harvesting. J. For. 2010, 108, 16–23. [Google Scholar]
- Spîrchez, C.; Lunguleasa, A.; Croitoru, C. The importance of the wood biomass in environment protection. AIP Conf. Proc. 2017, 1918, 02007. [Google Scholar]
- Gosalvez, E. Biomass: A Sustainable Energy Source for the Future? College of Natural Resources News, North Carolina State University: Raleigh, NC, USA, 15 January 2021. Available online: https://cnr.ncsu.edu/news/2021/01/biomass-a-sustainable-energy-source-for-the-future/ (accessed on 11 May 2023).
- Yano, H. Cellulose nanofibers and their utilization. J. Imaging Soc. Jpn. 2016, 55, 356–360. [Google Scholar]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Moon, D.; Tsukahara, K.; Sagisaka, M.; Tahara, K. Effect of cellulose nanofibers composites in automotive components on greenhouse gas emissions. J. Jpn. Inst. Energy 2016, 95, 648–652. [Google Scholar] [CrossRef]
- Nogi, M.; Yano, H. Transparent nanocomposites based on cellulose produced by bacteria offer potential innovation in the electronics device industry. Adv. Mater. 2008, 20, 1849–1852. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Halim, N.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; Wan Yunus, W.M.Z.; Farid, M.A.A.; et al. Performance evaluation of cellulose nanofiber reinforced polymer composites. Funct. Compos. Struct. 2021, 3, 024001. [Google Scholar] [CrossRef]
- Phuong, H.T.; Thoa, N.K.; Tuyet, P.T.A.; Van, Q.N.; Hai, Y.D. Cellulose nanomaterials as a future, sustainable and renewable material. Crystals 2022, 12, 106. [Google Scholar] [CrossRef]
- Iioka, M.; Kawanabe, W.; Shohji, I.; Kobayashi, T. An experimental study of fabrication of cellulose nano-fiber composited Ni film by electroplating. Mater. Trans. 2022, 63, 821–828. [Google Scholar] [CrossRef]
- Shohji, I.; Watanabe, H.; Okashita, T.; Osawa, T. Impact properties of lead-free Sn-Ag-Cu-Ni-Ge solder joint with Cu electrode. Mater. Trans. 2008, 49, 1513–1517. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L. Properties and microstructures of Sn-Ag-Cu-X lead-free solder joints in electronic packaging. Adv. Mater. Sci. Eng. 2015, 2015, 639028. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical characteristics and wear-resistant mechanism of nano coatings: A review. Coatings 2020, 10, 233. [Google Scholar] [CrossRef]
- Zaki, E.G.; Selim, M.S.; Hao, Z.; Elsaeed, S.M.; El-Saeed, A.M. Special issue: Recent trends in wear and erosion resistance of alloys. Coatings 2023, 13, 64. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent development of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. Ion-exchange behavior of carboxylate groups in fibrous cellulose oxidized by the TEMPO-mediated system. Carbohydr. Polym. 2005, 61, 183–190. [Google Scholar] [CrossRef]
- Saito, T.; Okita, Y.; Nge, T.T.; Sugiyama, J.; Isogai, A. TEMPO-mediated oxidation of native cellulose. Microscopic analysis of fibrous fractions in the oxidized products. Carbohydr. Polym. 2006, 65, 435–440. [Google Scholar] [CrossRef]
- Kwon, G.; Lee, K.; Kim, D.; Jeon, Y.; Kim, U.-J.; You, J. Cellulose nanocrystal-coated TEMPO-oxidized cellulose nanofiber films for high performance all-cellulose nanocomposites. J. Hazard. Mater. 2020, 398, 123100. [Google Scholar] [CrossRef]
- Kaffashsaie, E.; Yousefi, H.; Nishino, T.; Matsumoto, T.; Mashkour, M.; Madhoushi, M.; Kawaguchi, H. Direct conversion of raw wood to TEMPO-oxidized cellulose nanofibers. Carbohydr. Polym. 2021, 262, 117938. [Google Scholar] [CrossRef]
- Isogai, A. Emerging nanocellulose technologies: Recent developments. Adv. Mater. 2020, 33, 2000630. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A. Cellulose nanofibers. J. Surf. Finish. Soc. Jpn. 2020, 71, 389–395. [Google Scholar] [CrossRef]
- By courtesy of Nippon Paper Industries, Chiyoda-ku, Japan, 16 May 2023.
- Bari, G.A.D. Electrodeposition of nickel. In Modern Electroplating, 5th ed.; Schlesinger, M., Paunovic, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 79–114. [Google Scholar]
- Rasband, W.S. ImageJ: U.S. National Institutes of Health, Bethesda, MD, USA, 1997–2020. Available online: https://imagej.nih.gov/ij/ (accessed on 3 April 2023).
- Haeri, M.; Haeri, M. ImageJ plugin for analysis of porous scaffolds used in tissue engineering. J. Open Res. Softw. 2015, 3, e1. [Google Scholar] [CrossRef]
- Matsuda, K. The hardness of electro plated coatings. J. Jpn. Soc. Tribol. 1995, 40, 234–239. [Google Scholar]
- Rose, I.; Whittington, C. Chemistry of nickel electroplating solutions. In Nickel Plating Handbook 2014, 1st ed.; Nickel Institute: Brussels, Belgium, 2014; pp. 13–16. [Google Scholar]
- Doi, T.; Mizumoto, K.; Aonuma, M.; Tanaka, S. Properties of the film from nickel electroplating bath using citric acid. J. Surf. Finish. Soc. Jpn. 2002, 53, 335–340. [Google Scholar] [CrossRef]
- Doi, T. Acting mechanism of citric acid on the properties of nickel citrate electroplated film. Bull. Tokyo Metrop. Ind. Technol. Res. Inst. 2015, 10, 18–21. [Google Scholar]
- Nakahara, S. Incorporation of impurities in deposited film. J. Surf. Finish. Soc. Jpn. 2012, 63, 200–208. [Google Scholar] [CrossRef]
- Watanabe, T. Structure control theory of plated film—Part of surface morphology. J. Jpn. Inst. Metals 2002, 66, 339–349. [Google Scholar] [CrossRef]
- Watanabe, T. Structure control theory of plated film—Part of crystal size. J. Jpn. Inst. Metals 2002, 66, 350–361. [Google Scholar] [CrossRef]
- Fras, L.; Stana-Kleinschek, K.; Ribitsch, V.; Sfiligoj-Smole, M.; Kreze, T. Quantitative determination of carboxyl groups in cellulose by complexometric titration. Lenzing. Ber. 2002, 81, 80–88. [Google Scholar]
- Yamamoto, M.; Noguchi, H.; Kubota, H.; Ogiwara, Y. Generation of wet strength in paper sheet made of fibrous carboxymethyl cellulose. Jpn. TAPPI J. 1975, 29, 247–253. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Mori, B. Dietary fiber: Determination methods and definition. J. Jpn. Assoc. Diet. Fiber Res. 1999, 3, 1–11. [Google Scholar]
- Iioka, M.; Shohji, I.; Kobayashi, T. Accuracy assessment of quantification method of cellulose nano-fiber in nickel plating film using image analysis. In Proceedings of the 2021 International Conference on Electronics Packaging, Online, 13 May 2021; pp. 177–178. [Google Scholar]
- Iioka, M.; Shohji, I.; Kobayashi, T. Fundamental research of forming conditions of cellulose nano-fiber composited nickel plating film. In Proceedings of the 27th Symposium on Microjoining and Assembly Technology in Electronics, Online, 2–15 February 2021; pp. 290–291. [Google Scholar]



| Distilled Water | NiSO4·6H2O | NiCl2 | H3BO3 | CNF | |
|---|---|---|---|---|---|
| [g/L] | Bal. | 240 | 30 | 30 | 1 or 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iioka, M.; Kawanabe, W.; Kobayashi, T.; Shohji, I.; Sakamoto, K. Fabrication of Electroplated Nickel Composite Films Using Cellulose Nanofibers Introduced with Carboxy Groups as Co-Deposited Materials. Surfaces 2023, 6, 164-178. https://doi.org/10.3390/surfaces6020012
Iioka M, Kawanabe W, Kobayashi T, Shohji I, Sakamoto K. Fabrication of Electroplated Nickel Composite Films Using Cellulose Nanofibers Introduced with Carboxy Groups as Co-Deposited Materials. Surfaces. 2023; 6(2):164-178. https://doi.org/10.3390/surfaces6020012
Chicago/Turabian StyleIioka, Makoto, Wataru Kawanabe, Tatsuya Kobayashi, Ikuo Shohji, and Kota Sakamoto. 2023. "Fabrication of Electroplated Nickel Composite Films Using Cellulose Nanofibers Introduced with Carboxy Groups as Co-Deposited Materials" Surfaces 6, no. 2: 164-178. https://doi.org/10.3390/surfaces6020012
APA StyleIioka, M., Kawanabe, W., Kobayashi, T., Shohji, I., & Sakamoto, K. (2023). Fabrication of Electroplated Nickel Composite Films Using Cellulose Nanofibers Introduced with Carboxy Groups as Co-Deposited Materials. Surfaces, 6(2), 164-178. https://doi.org/10.3390/surfaces6020012






