Ultrafast Growth of h-MoO3 Microrods and Its Acetone Sensing Performance
Abstract
1. Introduction
2. Materials and Methods
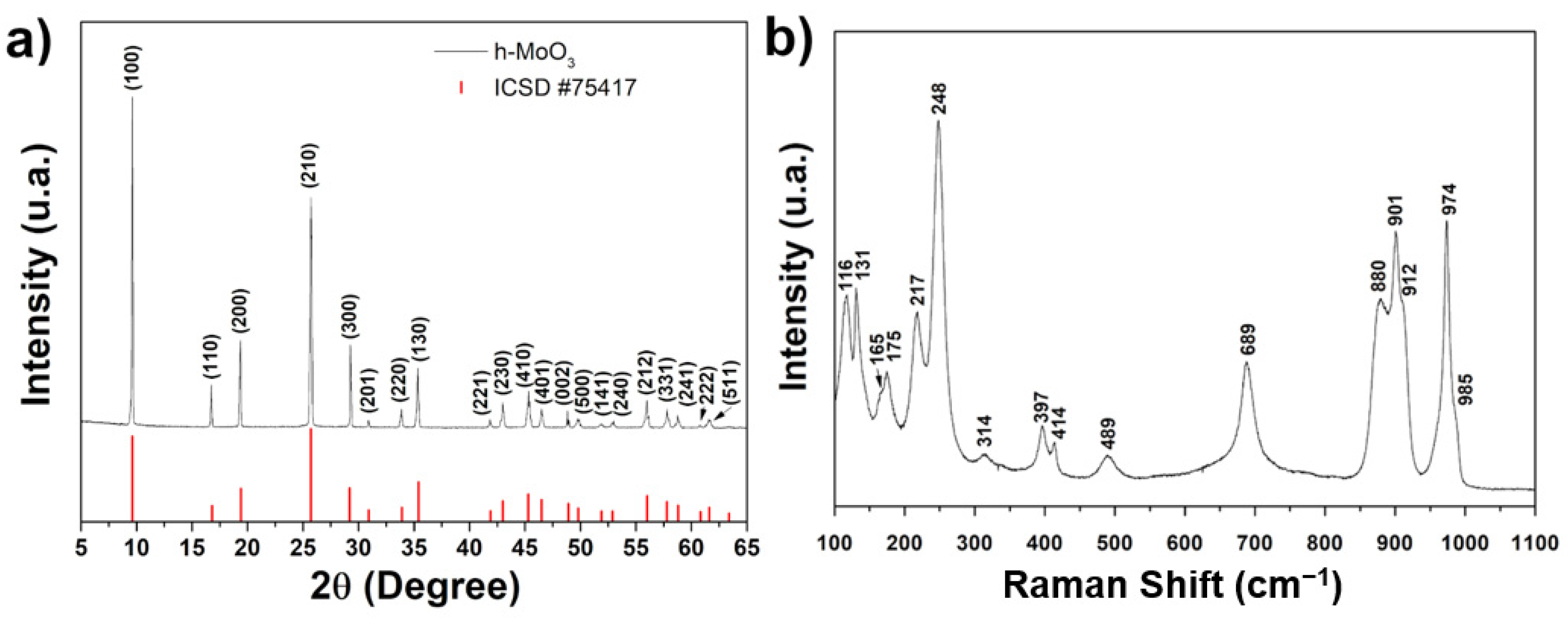
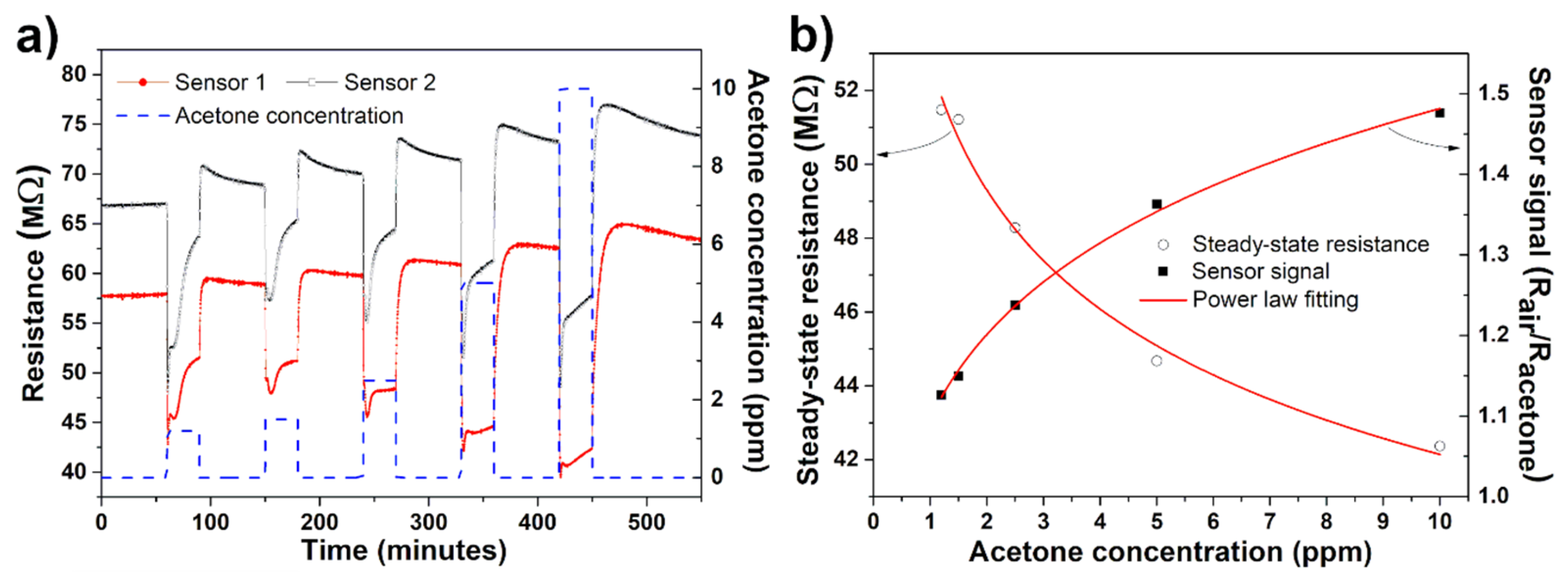
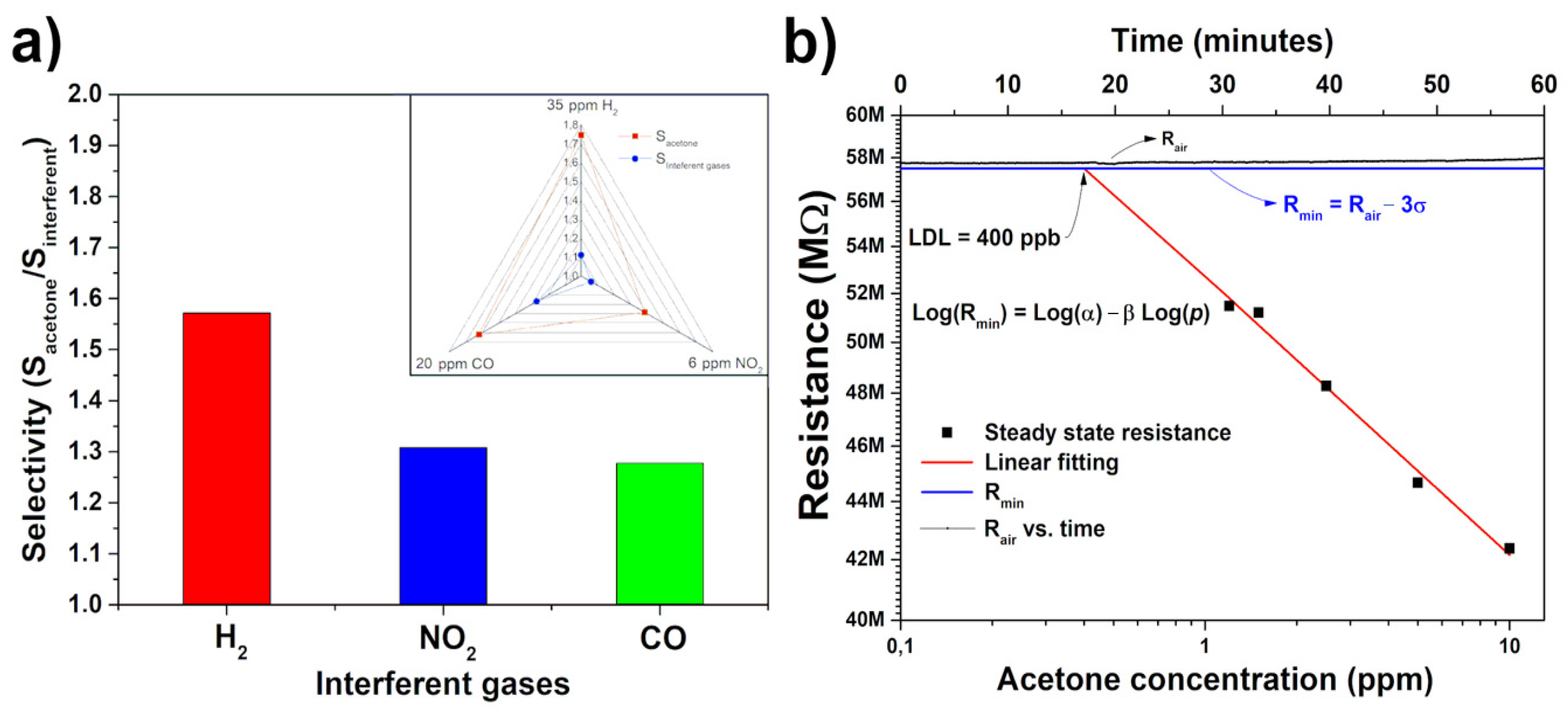
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Review—Metal Oxides: Application in Exhaled Breath Acetone Chemiresistive Sensors. J. Electrochem. Soc. 2020, 167, 037537. [Google Scholar] [CrossRef]
- Choi, S.-J.; Lee, I.; Jang, B.-H.; Youn, D.-Y.; Ryu, W.-H.; Park, C.O.; Kim, I.-D. Selective Diagnosis of Diabetes Using Pt-Functionalized WO3 Hemitube Networks As a Sensing Layer of Acetone in Exhaled Breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 Sensors for Highly Selective Detection of Acetone for Easy Diagnosis of Diabetes by Breath Analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Cao, Y.; Zhang, H.; Hu, J. Acetone Sensing Properties and Mechanism of SnO2 Thick-Films. Sensors 2018, 18, 3425. [Google Scholar] [CrossRef]
- Navale, S.T.; Yang, Z.B.; Liu, C.; Cao, P.J.; Patil, V.B.; Ramgir, N.S.; Mane, R.S.; Stadler, F.J. Enhanced acetone sensing properties of titanium dioxide nanoparticles with a sub-ppm detection limit. Sens. Actuators B Chem. 2018, 255, 1701–1710. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Hou, L.; Chen, W. Fabrication of Co3O4 nanowires assembled on the surface of hollow carbon spheres for acetone gas sensing. Sens. Actuators B Chem. 2019, 291, 130–140. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef]
- de Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum Oxides—From Fundamentals to Functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Gouma, P.; Prasad, A.; Stanacevic, S. A selective nanosensor device for exhaled breath analysis. J. Breath Res. 2011, 5, 037110. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.A.; Barbosa, M.S.; Bueno, P.R.; Orlandi, M.O. Real-Time Monitoring of Electrochromic Memory Loss of Layered α-MoO3 Nanoplates. J. Electrochem. Soc. 2020, 167, 166509. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, C.; Xu, L.; Ma, Y.; Hou, W.; Zhu, J.-J. Single-crystalline orthorhombic molybdenum oxide nanobelts: Synthesis and photocatalytic properties. CrystEngComm 2010, 12, 3740. [Google Scholar] [CrossRef]
- Gong, J.; Zeng, W.; Zhang, H. Hydrothermal synthesis of controlled morphologies of MoO3 nanobelts and hierarchical structures. Mater. Lett. 2015, 154, 170–172. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Ma, Y.; Yang, M.; Qi, Y. Rapid microwave-assisted hydrothermal synthesis of one-dimensional MoO 3 nanobelts. Mater. Lett. 2016, 164, 623–626. [Google Scholar] [CrossRef]
- Kumar, V.; Wang, X.; Lee, P.S. Formation of hexagonal-molybdenum trioxide (h-MoO3) nanostructures and their pseudocapacitive behavior. Nanoscale 2015, 7, 11777–11786. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Jin, D.; Xie, Y. Novel Metastable Hexagonal MoO 3 Nanobelts: Synthesis, Photochromic, and Electrochromic Properties. Chem. Mater. 2009, 21, 5681–5690. [Google Scholar] [CrossRef]
- Yao, D.D.; Ou, J.Z.; Latham, K.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-zadeh, K. Electrodeposited α- and β-Phase MoO3 Films and Investigation of Their Gasochromic Properties. Cryst. Growth Des. 2012, 12, 1865–1870. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Rajeswari Yogamalar, N.; Bose, A.C. Hydrothermally Synthesized h-MoO3 and α-MoO3 Nanocrystals: New Findings on Crystal-Structure-Dependent Charge Transport. Cryst. Growth Des. 2016, 16, 1984–1995. [Google Scholar] [CrossRef]
- Ramana, C.V.; Atuchin, V.V.; Troitskaia, I.B.; Gromilov, S.A.; Kostrovsky, V.G.; Saupe, G.B. Low-temperature synthesis of morphology controlled metastable hexagonal molybdenum trioxide (MoO3). Solid State Commun. 2009, 149, 6–9. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Chandra Bose, A. Role of synthesis variables on controlled nucleation and growth of hexagonal molybdenum oxide nanocrystals: Investigation on thermal and optical properties. CrystEngComm 2014, 16, 6175–6186. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Lu, Y.; Podval’naya, N.V.; Chen, W.; Zakharova, G.S. Hydrothermal synthesis of h-MoO3 microrods and their gas sensing properties to ethanol. Appl. Surf. Sci. 2015, 359, 114–119. [Google Scholar] [CrossRef]
- Song, J.; Ni, X.; Gao, L.; Zheng, H. Synthesis of metastable h-MoO3 by simple chemical precipitation. Mater. Chem. Phys. 2007, 102, 245–248. [Google Scholar] [CrossRef]
- Felix, A.A.; Longo, E.; Varela, J.A.; Orlandi, M.O. Gas sensing and conductivity relationship on nanoporous thin films: A CaCu3Ti4O12 case study. Thin Solid Films 2016, 604, 69–73. [Google Scholar] [CrossRef]
- Felix, A.A.; Silva, R.A.; Orlandi, M.O. Layered α-MoO3 nanoplates for gas sensing applications. CrystEngComm 2020, 22, 4640–4649. [Google Scholar] [CrossRef]
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.-J.; Longo, E.; Tuller, H.L.; Varela, J.A. The Role of Hierarchical Morphologies in the Superior Gas Sensing Performance of CuO-Based Chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Gurlo, A.; Barsan, N.; Weimar, U. Gas sensors based on semiconducting metal oxides. In Metal Oxides: Chemistry and Application; Fierro, J.L.G., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 683–738. [Google Scholar]
- Guo, J.D.; Zavalij, P.; Whittingham, M.S. Preparation and Characterization of a MoO3 With Hexagonal Structure. Eur. J. Solid State Inorg. Chem. 1994, 31, 833. [Google Scholar]
- Moura, J.V.B.; Silveira, J.V.; da Silva Filho, J.G.; Souza Filho, A.G.; Luz-Lima, C.; Freire, P.T.C. Temperature-induced phase transition in h-MoO3: Stability loss mechanism uncovered by Raman spectroscopy and DFT calculations. Vib. Spectrosc. 2018, 98, 98–104. [Google Scholar] [CrossRef]
- Felix, A.A.; Santos, G.T.; Orlandi, M.O.; São Paulo State University (UNESP), Araraquara, Brazil. Personal Communication, 2020.
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B Chem. 2015, 208, 122–127. [Google Scholar] [CrossRef]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Giant Chemo-Resistance of SnO disk-like structures. Sens. Actuators B Chem. 2013, 186, 103–108. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Kim, N.-H.; Hakim, M.; Tuller, H.L.; Kim, I.-D. Mesoporous WO3 Nanofibers with Protein-Templated Nanoscale Catalysts for Detection of Trace Biomarkers in Exhaled Breath. ACS Nano 2016, 10, 5891–5899. [Google Scholar] [CrossRef] [PubMed]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Thupthimchun, W.; Samransuksamer, B.; Choopun, S. Acetone gas sensors based on ZnO nanostructures decorated with Pt and Nb. Ceram. Int. 2017, 43, S557–S566. [Google Scholar] [CrossRef]
- Han, X.; Jin, M.; Xie, S.; Kuang, Q.; Jiang, Z.; Jiang, Y.; Xie, Z.; Zheng, L. Synthesis of Tin Dioxide Octahedral Nanoparticles with Exposed High-Energy {221} Facets and Enhanced Gas-Sensing Properties. Angew. Chem. Int. Ed. 2009, 48, 9180–9183. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, T.; Chuai, M.; Xiao, B.; Zhang, M. Synthesis of ZnO nanosheet arrays with exposed (100) facets for gas sensing applications. Phys. Chem. Chem. Phys. 2016, 18, 325–330. [Google Scholar] [CrossRef]

| Crystalline Phase | Acetone Concentration (ppm) | Operating Temperature (°C) | Sensor Signal | Response/ Recovery Time (s) | Reference |
|---|---|---|---|---|---|
| WO3 | 2 | 300 | 4.1 | - | [2] |
| WO3 | 10 | 280 | ~2.0 | 16/129 | [5] |
| SnO2 | 10 | 280 | ~2.0 | 7/72 | [5] |
| SnO2 | 5 | 180 | 5.0 | 70/64 | [6] |
| TiO2 | 10 | 270 | ~1.5 | - | [7] |
| Co3O4 | 10 | 150 | ~1.7 | - | [8] |
| h-MoO3 | 1.2 | 200 | 1.12 | 10/60 | This work |
| h-MoO3 | 10 | 200 | 1.48 | 60/500 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, G.T.; Felix, A.A.; Orlandi, M.O. Ultrafast Growth of h-MoO3 Microrods and Its Acetone Sensing Performance. Surfaces 2021, 4, 9-16. https://doi.org/10.3390/surfaces4010002
Santos GT, Felix AA, Orlandi MO. Ultrafast Growth of h-MoO3 Microrods and Its Acetone Sensing Performance. Surfaces. 2021; 4(1):9-16. https://doi.org/10.3390/surfaces4010002
Chicago/Turabian StyleSantos, Giovana T., Anderson A. Felix, and Marcelo O. Orlandi. 2021. "Ultrafast Growth of h-MoO3 Microrods and Its Acetone Sensing Performance" Surfaces 4, no. 1: 9-16. https://doi.org/10.3390/surfaces4010002
APA StyleSantos, G. T., Felix, A. A., & Orlandi, M. O. (2021). Ultrafast Growth of h-MoO3 Microrods and Its Acetone Sensing Performance. Surfaces, 4(1), 9-16. https://doi.org/10.3390/surfaces4010002






