Ni-Doped Titanium Dioxide Films Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ni-Doped TiO2 Films
2.2. Characterization of TiO2 Photoanodes
3. Results and Discussion
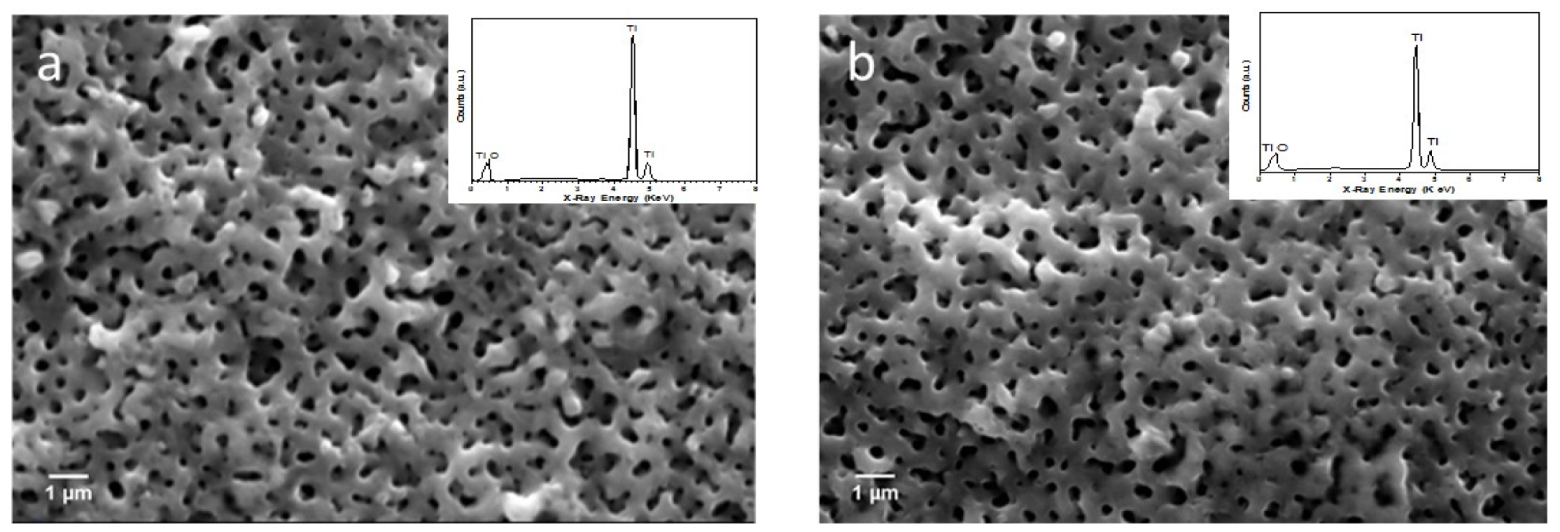
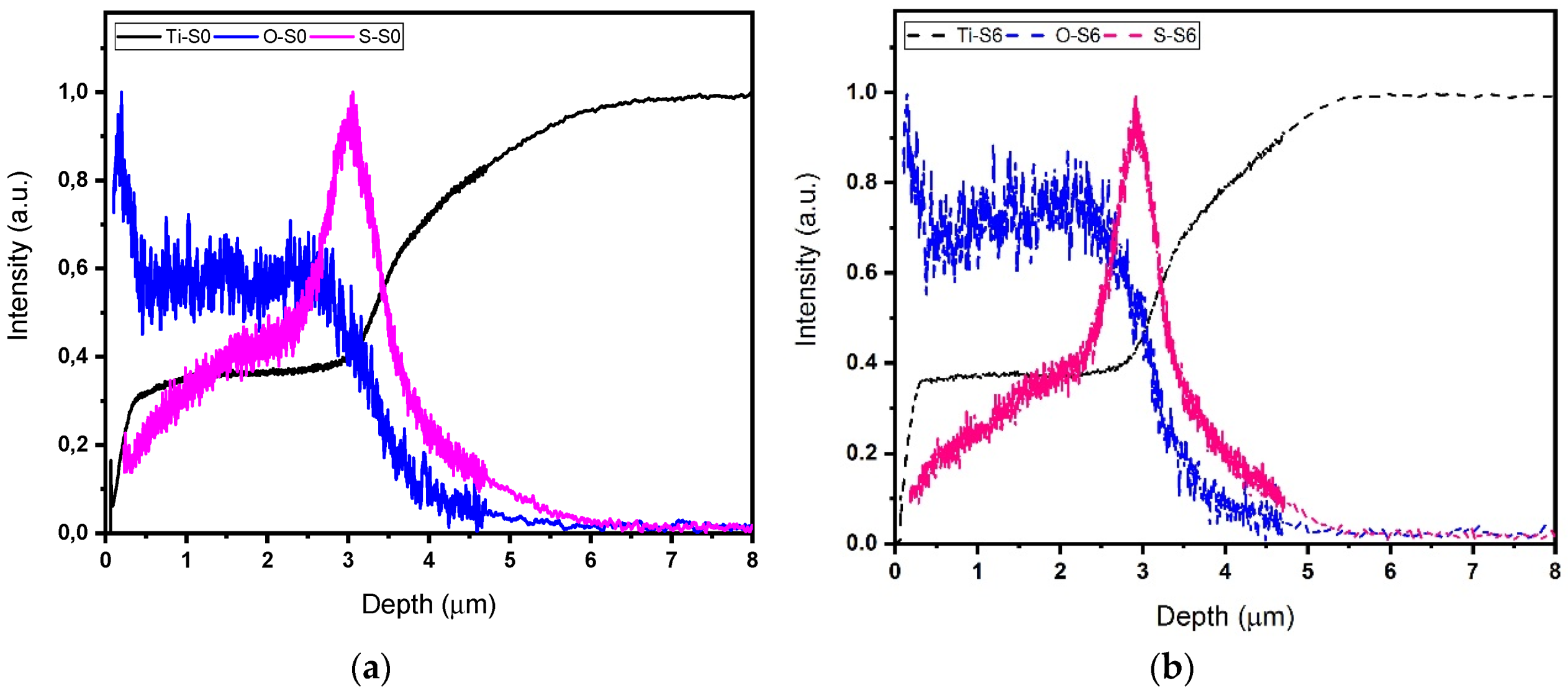
3.1. Morphology and Composition
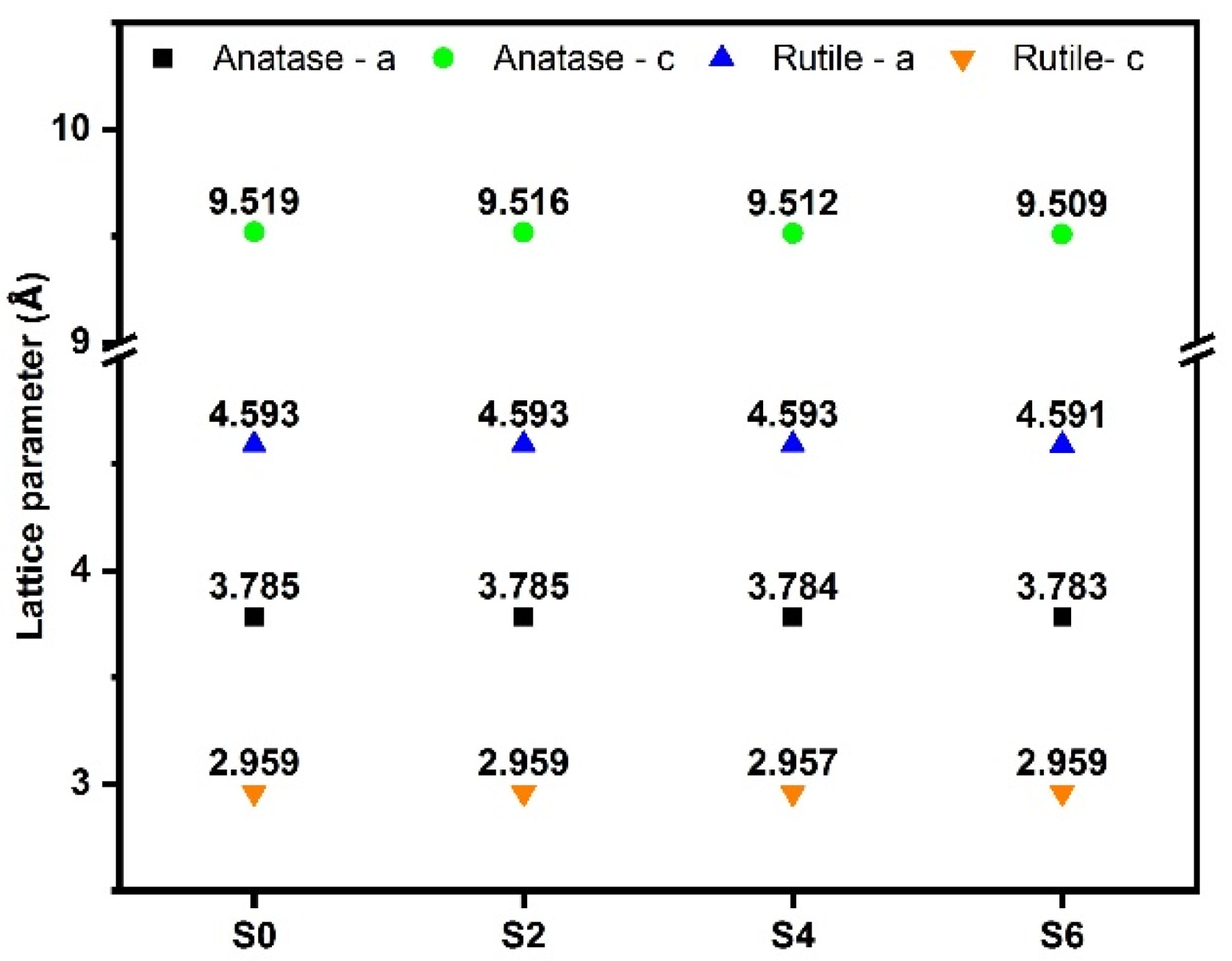
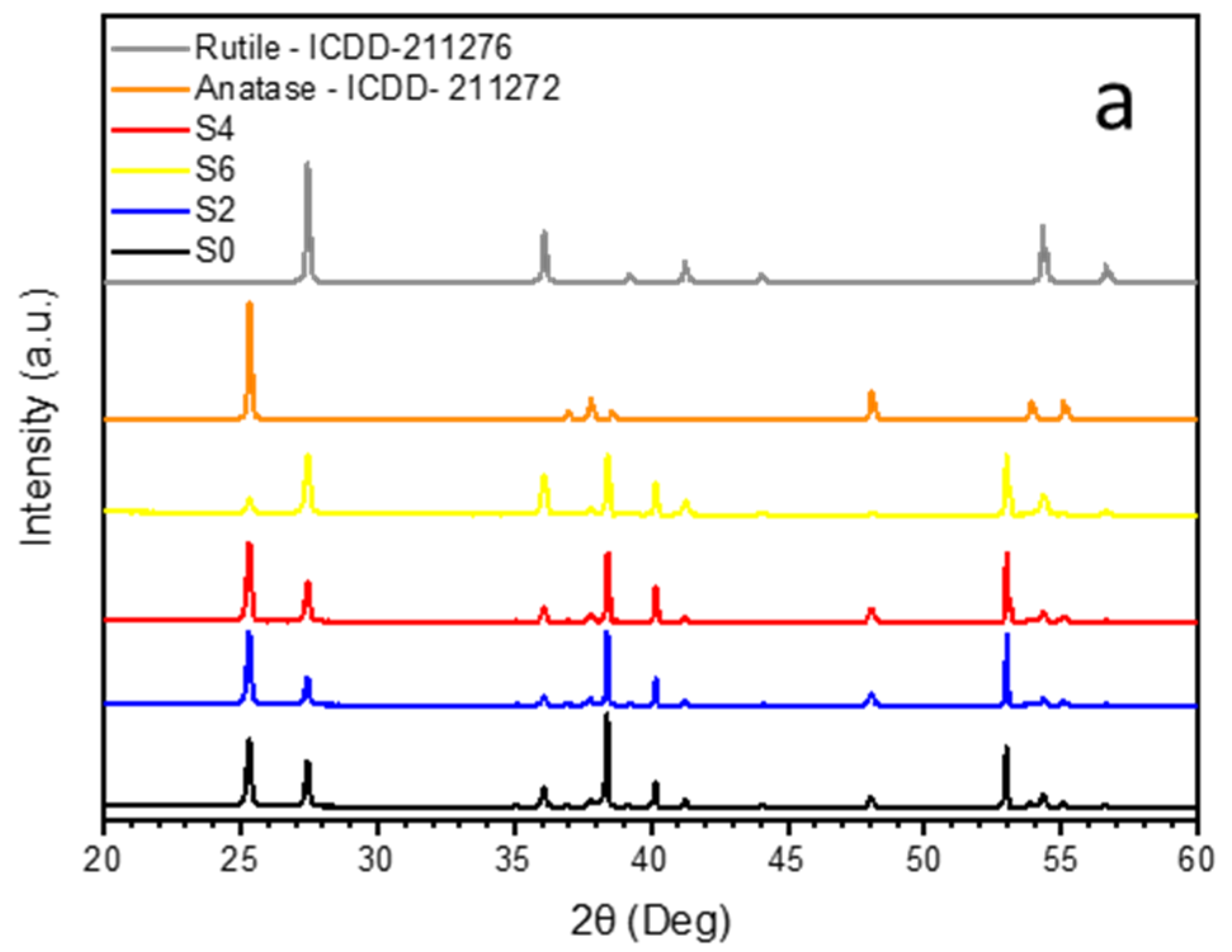
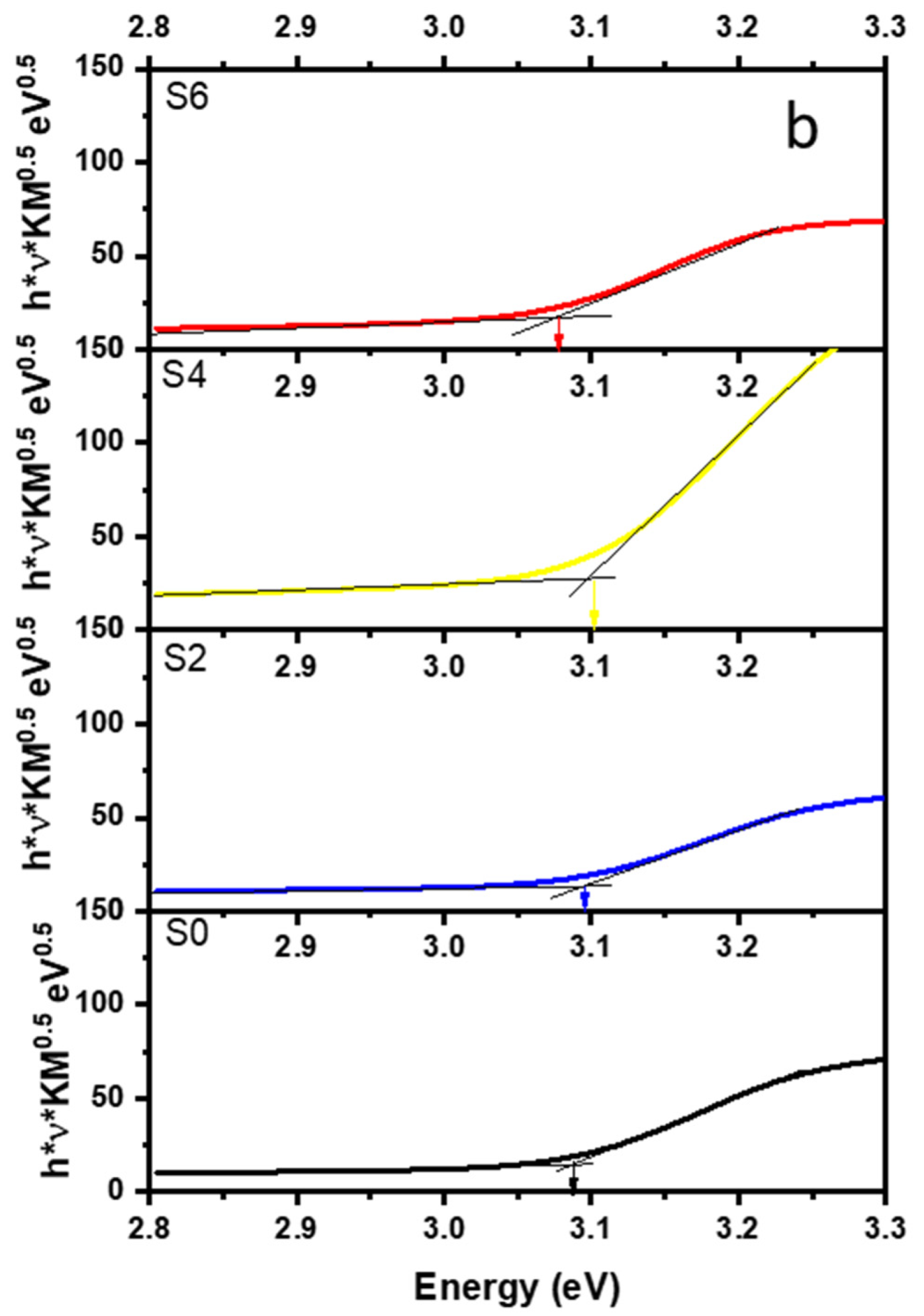
3.2. Crystal Structure and Band-Gap Determination
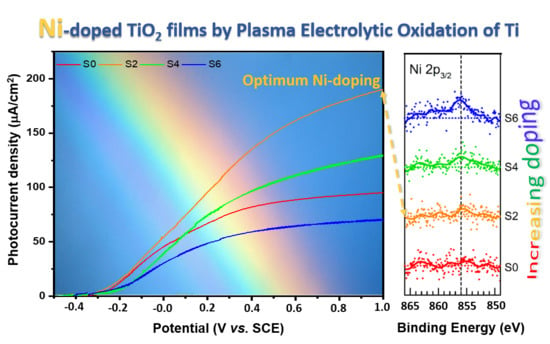
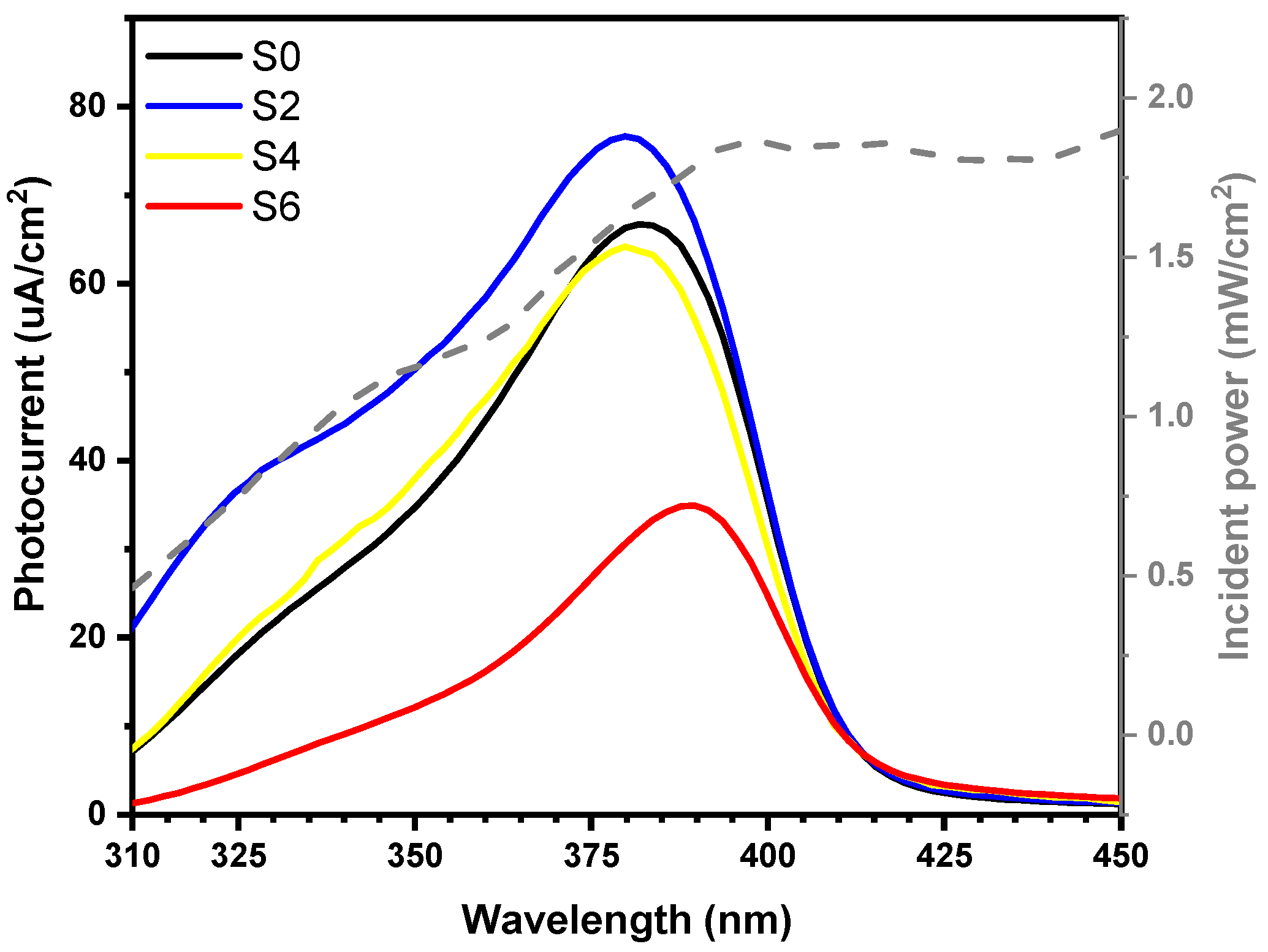
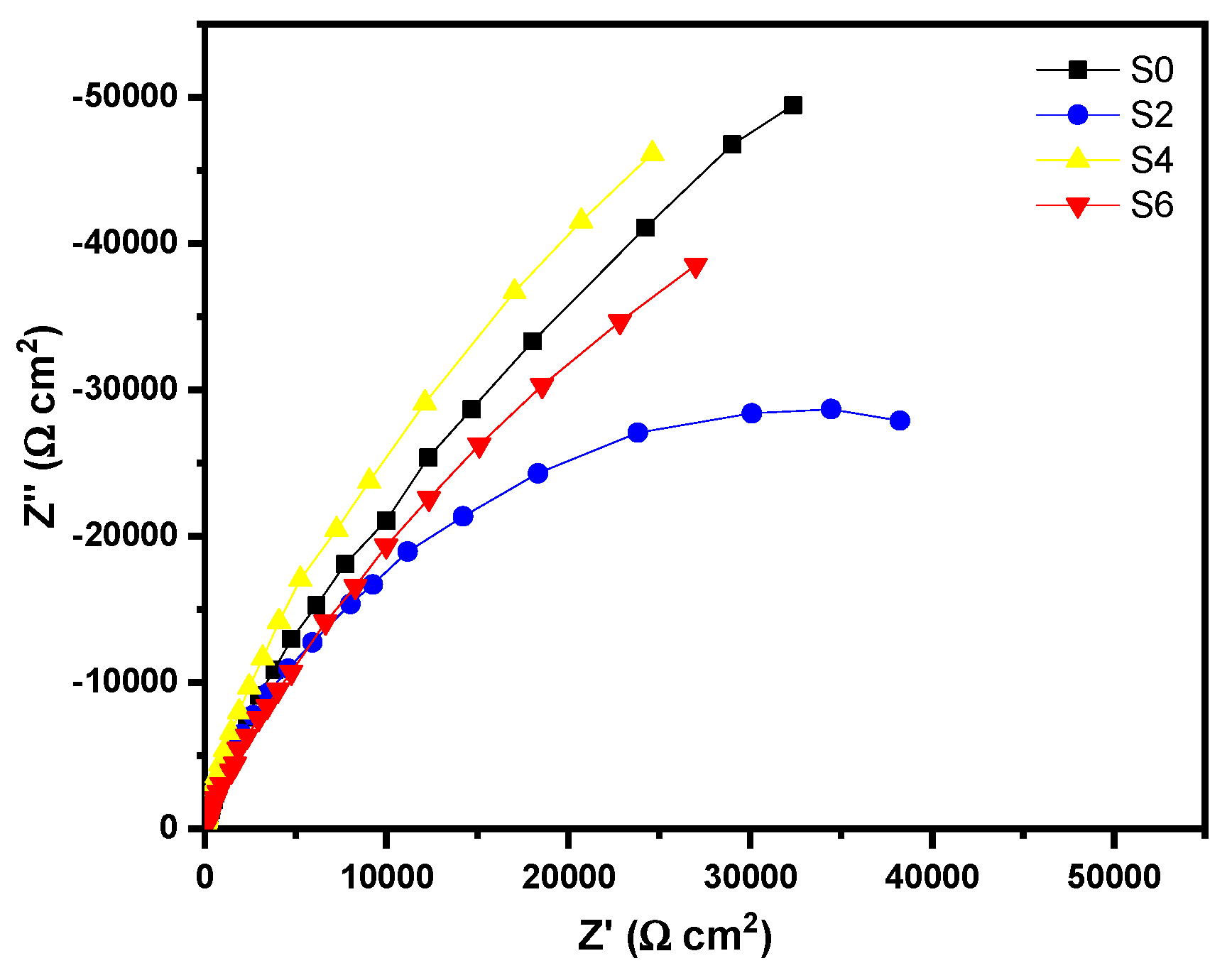
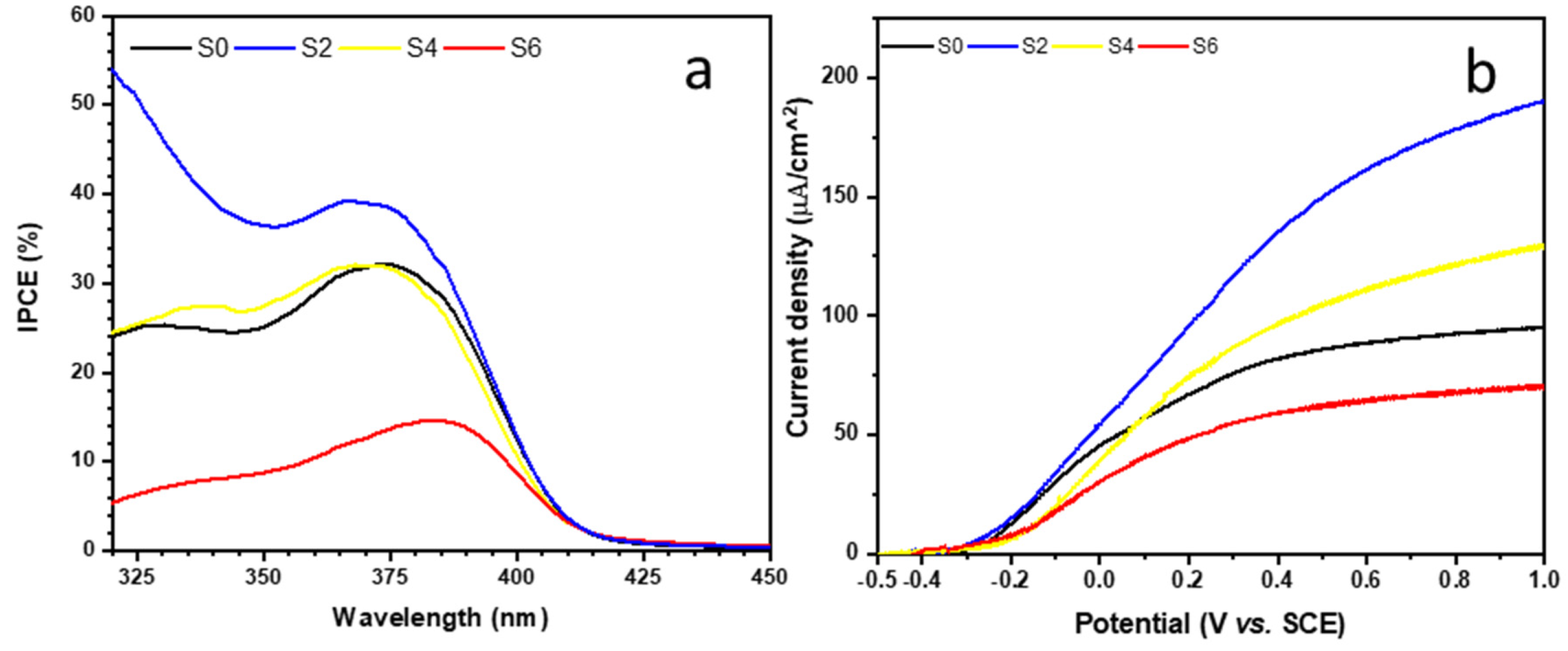
3.3. Photocurrent and IPCE
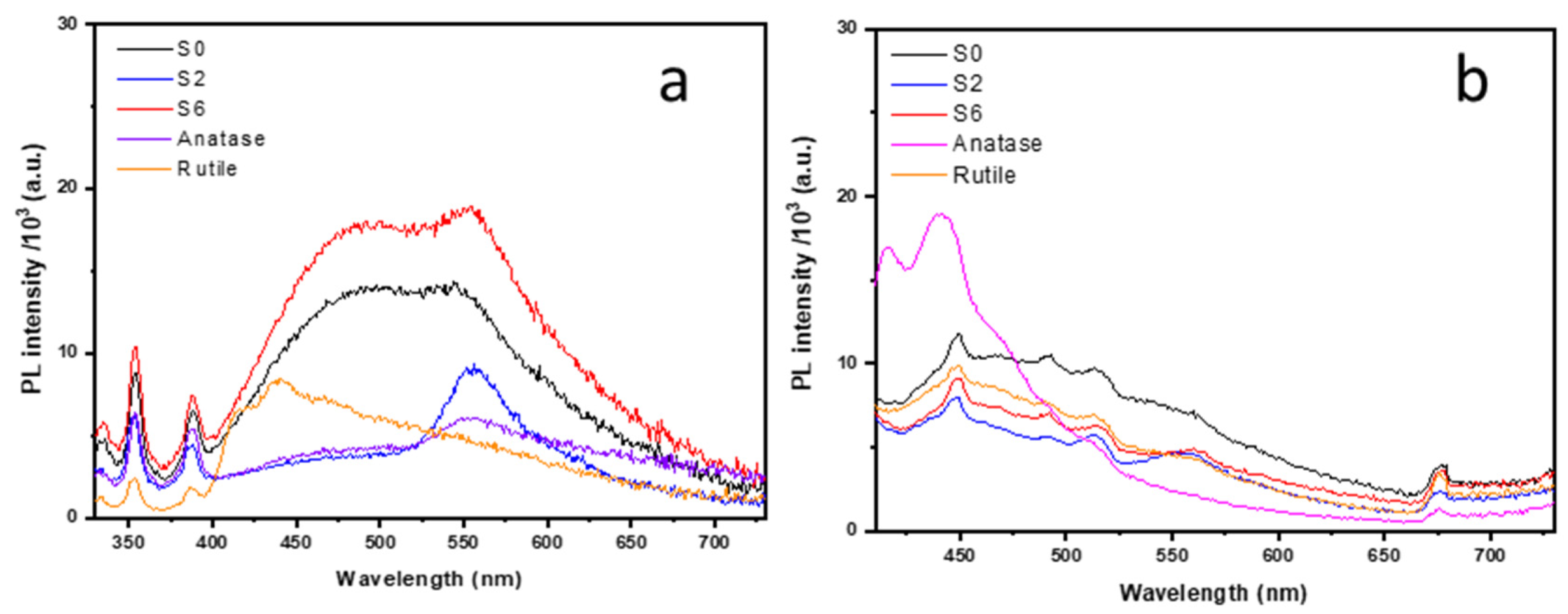
3.4. Photoluminescence Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Brady, M.D.; Sampaio, R.N.; Wang, D.; Meyer, T.J.; Meyer, G.J. Dye-Sensitized Hydrobromic Acid Splitting for Hydrogen Solar Fuel Production. J. Am. Chem. Soc. 2017, 139, 15612–15615. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, S.N.H.; Minggu, L.J.; Arifin, K.; Kassim, M.B.; Wan, W.R.D. Natural dyes as TIO2 sensitizers with membranes for photoelectrochemical water splitting: An overview. Renew. Sustain. Energy Rev. 2017, 78, 698–709. [Google Scholar] [CrossRef]
- Lang, Q.; Chen, Y.; Huang, T.; Yang, L.; Zhong, S.; Wu, L.; Chen, J.; Bai, S. Graphene “bridge” in transferring hot electrons from plasmonic Ag nanocubes to TiO2 nanosheets for enhanced visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 220, 182–190. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, B.; Yang, Y.; Huang, E.; Ge, Z.; Zhang, T.; Zhang, S.; Li, L.; Guan, N.; Ma, Y.; et al. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017, 10, 1662–1672. [Google Scholar] [CrossRef]
- Mulewa, W.; Tahir, M.; Amin, N.A.S. MMT-supported Ni/TiO2 nanocomposite for low temperature ethanol steam reforming toward hydrogen production. Chem. Eng. J. 2017, 326, 956–969. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, M.; Hou, L.; Xiao, L.; Li, Q.; Yang, J. Z-scheme BCN-TiO2 nanocomposites with oxygen vacancy for high efficiency visible light driven hydrogen production. Int. J. Hydrog. Energy 2017, 42, 28434–28444. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Hyun Kim, D.; Sub Lee, K.; Kim, Y.-S.; Chung, Y.-C.; Kim, S.-J. Photocatalytic Activity of Ni 8 wt%-Doped TiO2 Photocatalyst Synthesized by Mechanical Alloying Under Visible Light. J. Am. Ceram. Soc. 2006, 89, 515–518. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Ren, J.; Wang, S.; Wang, R.; Fu, G.; Hu, Y. Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: Realizing efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Niishiro, R.; Kato, H.; Kudo, A. Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions. Phys. Chem. Chem. Phys. 2005, 7, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Shehata, N.; Betiha, M.A.; Rabie, A.M. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2019, 555, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Rafiq, A.; Ikram, M.; Nafees, M.; Ali, S. Structural, optical, and magnetic study of Ni-doped TiO2 nanoparticles synthesized by sol-gel method. Int. Nano Lett. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Macovei, D.; Tiron, V.; Adomnitei, C.; Luca, D.; Dobromir, M.; Antohe, S.; Mardare, D. On the hydrophilicity of Ni-doped TiO2 thin films. A study by X-ray absorption spectroscopy. Thin Solid Films 2018, 657, 42–49. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Yao, Z.; Jia, F.; Tian, S.; Li, C.; Jiang, Z.; Bai, X. Microporous Ni-Doped TiO2 film photocatalyst by plasma electrolytic oxidation. ACS Appl. Mater. Interfaces 2010, 2, 2617–2622. [Google Scholar] [CrossRef]
- Xiang, N.; Zhuang, J.J.; Song, R.G.; Xiang, B.; Xiong, Y.; Su, X.P. Fabrication and photocatalytic activity of MAO–TiO2 films formed on titanium doped with cations. Mater. Technol. 2016, 31, 332–336. [Google Scholar] [CrossRef]
- Soejima, T.; Yagyu, H.; Ito, S. One-pot synthesis and photocatalytic activity of Fe-doped TiO2 films with anatase-rutile nanojunction prepared by plasma electrolytic oxidation. J. Mater. Sci. 2011, 46, 5378–5384. [Google Scholar] [CrossRef]
- Yao, Z.; Jia, F.; Jiang, Y.; Li, C.; Jiang, Z.; Bai, X. Photocatalytic reduction of potassium chromate by Zn-doped TiO2/Ti film catalyst. Appl. Surf. Sci. 2010, 256, 1793–1797. [Google Scholar] [CrossRef]
- Stojadinović, S.; Tadić, N.; Radić, N.; Grbić, B.; Vasilić, R. Effect of Tb3+ doping on the photocatalytic activity of TiO2 coatings formed by plasma electrolytic oxidation of titanium. Surf. Coatings Technol. 2018, 337, 279–289. [Google Scholar] [CrossRef]
- Bayati, M.R.; Molaei, R.; Golestani-Fard, F. Enhancing photoinduced hydrophilicity of micro arc oxidized TiO2 nanostructured porous layers by V-doping. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 51–60. [Google Scholar] [CrossRef]
- Vasilić, R.; Stojadinović, S.; Radić, N.; Stefanov, P.; Dohčević-Mitrović, Z.; Grbić, B. One-step preparation and photocatalytic performance of vanadium doped TiO2 coatings. Mater. Chem. Phys. 2015, 151, 337–344. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma Electrolysis for Surface Engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Bestetti, M.; Franz, S.; Cuzzolin, M.; Arosio, P.; Cavallotti, P.L. Structure of nanotubular titanium oxide templates prepared by electrochemical anodization in H2SO4/HF solutions. Thin Solid Films 2007, 515, 5253–5258. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Hashempour, M.; Vicenzo, A.; Franz, S.; Badiei, A.; Behnajady, M.A.; Bestetti, M. High-temperature stable anatase-type TiO2 nanotube arrays: A study of the structure-activity relationship. Appl. Catal. B Environ. 2016, 185, 119–132. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Lucotti, A.; Bestetti, M. Photoactive TiO2 coatings obtained by Plasma Electrolytic Oxidation in refrigerated electrolytes. Appl. Surf. Sci. 2016, 385, 498–505. [Google Scholar] [CrossRef]
- Franz, S.; Arab, H.; Lucotti, A.; Castiglioni, C.; Vicenzo, A.; Morini, F.; Bestetti, M. Exploiting Direct Current Plasma Electrolytic Oxidation to Boost Photoelectrocatalysis. Catalysts 2020, 10, 325. [Google Scholar] [CrossRef]
- Mirelman, L.K.; Curran, J.A.; Clyne, T.W. The production of anatase-rich photoactive coatings by plasma electrolytic oxidation. Surf. Coat. Technol. 2012, 207, 66–71. [Google Scholar] [CrossRef]
- Sundararajan, G.; Rama Krishna, L. Mechanisms underlying the formation of thick alumina coatings through the MAO coating technology. Surf. Coat. Technol. 2003, 167, 269–277. [Google Scholar] [CrossRef]
- Bayati, M.R.; Moshfegh, A.Z.; Golestani-Fard, F. In situ growth of vanadia-titania nano/micro-porous layers with enhanced photocatalytic performance by micro-arc oxidation. Electrochim. Acta 2010, 55, 3093–3102. [Google Scholar] [CrossRef]
- He, J.; Cai, Q.Z.; Ji, Y.G.; Luo, H.H.; Li, D.J.; Yu, B. Influence of fluorine on the structure and photocatalytic activity of TiO2 film prepared in tungstate-electrolyte via micro-arc oxidation. J. Alloys Compd. 2009, 482, 476–481. [Google Scholar] [CrossRef]
- Li, J.F.; Wan, L.; Feng, J.Y. Study on the preparation of titania films for photocatalytic application by micro-arc oxidation. Sol. Energy Mater. Sol. Cells 2006, 90, 2449–2455. [Google Scholar] [CrossRef]
- Wu, X.; Ding, X.; Qin, W.; He, W.; Jiang, Z. Enhanced photo-catalytic activity of TiO2 films with doped La prepared by micro-plasma oxidation method. J. Hazard. Mater. 2006, 137, 192–197. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Q.; Zhaohua, J. Influence of Fe3+ ions on the photocatalytic activity of TiO2 films prepared by micro-plasma oxidation method. Thin Solid Films 2006, 496, 288–292. [Google Scholar] [CrossRef]
- Franz, S.; Perego, D.; Marchese, O.; Bestetti, M. Photoelectrochemical advanced oxidation processes on nanostructured TiO2 catalysts: Decolorization of a textile azo-dye. J. Water Chem. Technol. 2015, 37, 108–115. [Google Scholar] [CrossRef]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef]
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of Carbamazepine by Photo(electro)catalysis on Nanostructured TiO2 Meshes: Transformation Products and Reaction Pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef]
- Shard, A.G. Detection limits in XPS for more than 6000 binary systems using Al and Mg Kα X-rays. Surf. Interface Anal. 2014, 46, 175–185. [Google Scholar] [CrossRef]
- Energy Dispersive X-ray Spectroscopy tutorial EAG Laboratories. Available online: https://www.eag.com/techniques/spectroscopy/energy-dispersive-x-ray-spectroscopy-eds/ (accessed on 20 March 2020).
- Berti, G.; Calloni, A.; Brambilla, A.; Bussetti, G.; Duò, L.; Ciccacci, F. Direct observation of spin-resolved full and empty electron states in ferromagnetic surfaces. Rev. Sci. Instrum. 2014, 85, 073901. [Google Scholar] [CrossRef] [PubMed]
- Galenda, A.; Visentin, F.; Gerbasi, R.; Battiston, S.; Habra, N. El Effective and Low-Cost Synthesis of Sulphur-Modified TiO2 Nanopowder with Improved Photocatalytic Performances in Water Treatment Applications. Water Air Soil Pollut. 2017, 228, 416. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An x-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Topalian, Z.; Niklasson, G.A.; Granqvist, C.G.; Österlund, L. Spectroscopic Study of the Photofixation of SO2 on Anatase TiO2 Thin Films and Their Oleophobic Properties. ACS Appl. Mater. Interfaces 2012, 4, 672–679. [Google Scholar]
- Randeniya, L.K.; Murphy, A.A.B.; Plumb, A.I.C. A study of S-doped TiO2 for photoelectrochemical hydrogen generation from water. J. Mater. Sci. 2008, 43, 1389–1399. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays to Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Murata, Y.; Fukuta, S.; Ishikawa, S.; Yokoyama, S. Photoelectrochemical properties of TiO2 rutile microalloyed with 4d and 5d transition elements. Sol. Energy Mater. Sol. Cells 2000, 62, 157–165. [Google Scholar] [CrossRef]
- Seetharaman, A.; Sivasubramanian, D.; Gandhiraj, V.; Rao Soma, V. Tunable Nanosecond and Femtosecond Nonlinear Optical Properties of C−N−S-Doped TiO2 Nanoparticles. J. Phys. Chem. C 2017, 121, 24192–24205. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Matsushima, S.; Takehara, K.; Yamane, H.; Yamada, K.; Nakamura, H.; Arai, M.; Kobayashi, K. First-principles energy band calculation for undoped and S-doped TiO2 with anatase structure. J. Phys. Chem. Solids 2007, 68, 206–210. [Google Scholar] [CrossRef]
- Lin, Y.M.; Jiang, Z.Y.; Zhu, C.Y.; Hu, X.Y.; Zhang, X.D.; Fan, J. Visible-light photocatalytic activity of Ni-doped TiO2 from ab initio calculations. Mater. Chem. Phys. 2012, 133, 746–750. [Google Scholar] [CrossRef]
- Sun, T.; Fan, J.; Liu, E.; Liu, L.; Wang, Y.; Dai, H.; Yang, Y.; Hou, W.; Hu, X.; Jiang, Z. Fe and Ni co-doped TiO2 nanoparticles prepared by alcohol-thermal method: Application in hydrogen evolution by water splitting under visible light irradiation. Powder Technol. 2012, 228, 210–218. [Google Scholar] [CrossRef]
- Serpone, N.; Pelizzetti, E. Photocatalysis: Fundamentals and Applications; Wiley: New York, NY, USA, 1989; ISBN 0471626031. [Google Scholar]

| Sample | NiSO4 (mM)/(g L−1) | Anatase/Rutile (wt %) | EBG (eV) | CBedge (VSCE, pH=7) |
|---|---|---|---|---|
| S0 | 0/0 | 55.1/44.9 | 3.08 | −0.28 |
| S2 | 0.2/0.054 | 66.4/33.6 | 3.09 | −0.31 |
| S4 | 0.4/0.1123 | 60.7/39.3 | 3.10 | −0.23 |
| S6 | 0.6/0.1695 | 18.0/82.0 | 3.06 | −0.23 |
| SA | 0/0 | 100/0 | 3.18 | - |
| SR | 0/0 | 0/100 | 2.94 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arab, H.; Chiarello, G.L.; Selli, E.; Bomboi, G.; Calloni, A.; Bussetti, G.; Albani, G.; Bestetti, M.; Franz, S. Ni-Doped Titanium Dioxide Films Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes. Surfaces 2020, 3, 168-181. https://doi.org/10.3390/surfaces3020013
Arab H, Chiarello GL, Selli E, Bomboi G, Calloni A, Bussetti G, Albani G, Bestetti M, Franz S. Ni-Doped Titanium Dioxide Films Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes. Surfaces. 2020; 3(2):168-181. https://doi.org/10.3390/surfaces3020013
Chicago/Turabian StyleArab, Hamed, Gian Luca Chiarello, Elena Selli, Giacomo Bomboi, Alberto Calloni, Gianlorenzo Bussetti, Guglielmo Albani, Massimiliano Bestetti, and Silvia Franz. 2020. "Ni-Doped Titanium Dioxide Films Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes" Surfaces 3, no. 2: 168-181. https://doi.org/10.3390/surfaces3020013
APA StyleArab, H., Chiarello, G. L., Selli, E., Bomboi, G., Calloni, A., Bussetti, G., Albani, G., Bestetti, M., & Franz, S. (2020). Ni-Doped Titanium Dioxide Films Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes. Surfaces, 3(2), 168-181. https://doi.org/10.3390/surfaces3020013