From Waste to Multi-Hybrid Layering of High Carbon Steel to Improve Corrosion Resistance: An In-Depth Analysis Using EPMA and AFM Techniques
Abstract
1. Introduction
2. Materials and Methods
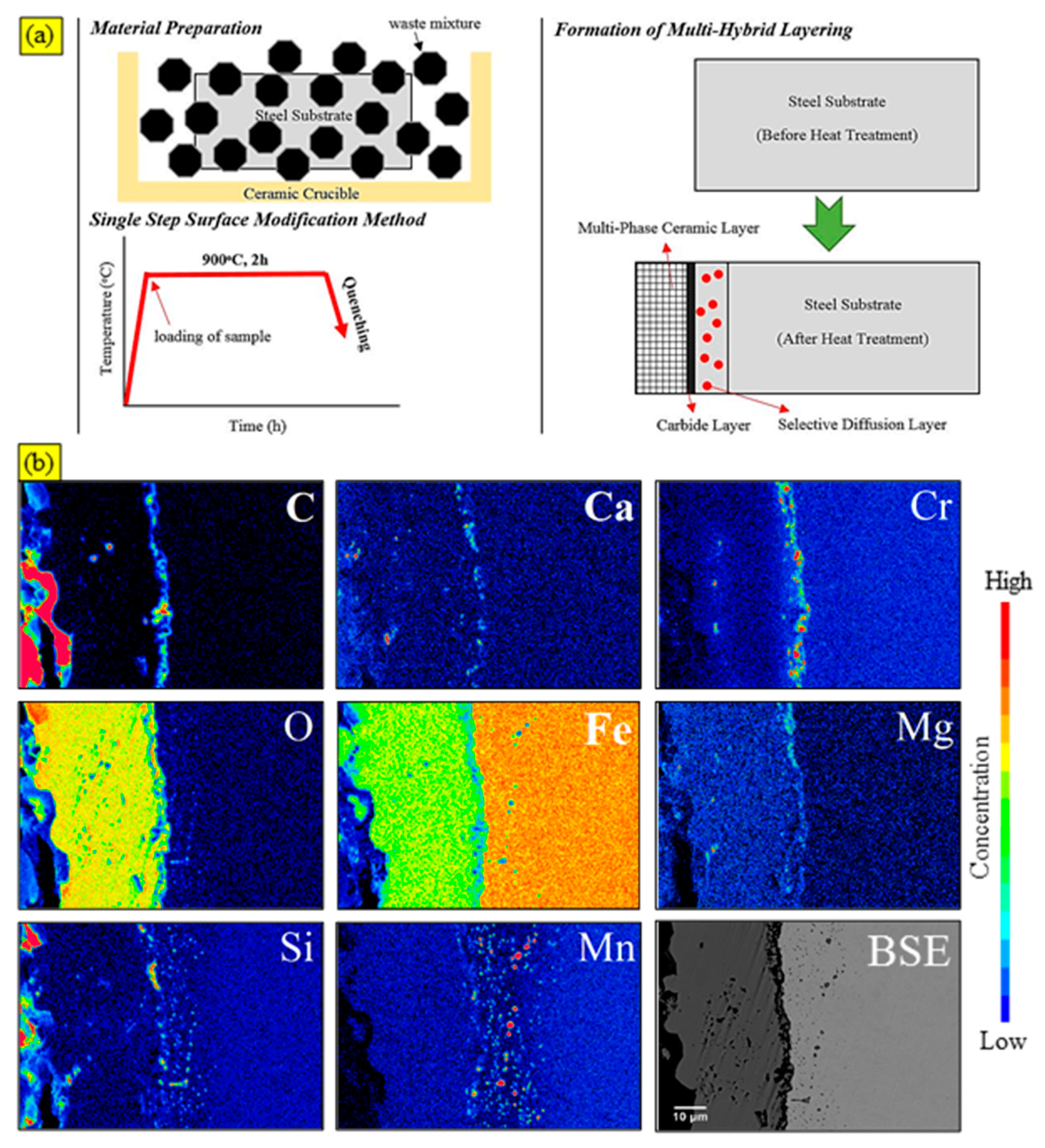
2.1. Material Preparation
2.2. Analytical Methods
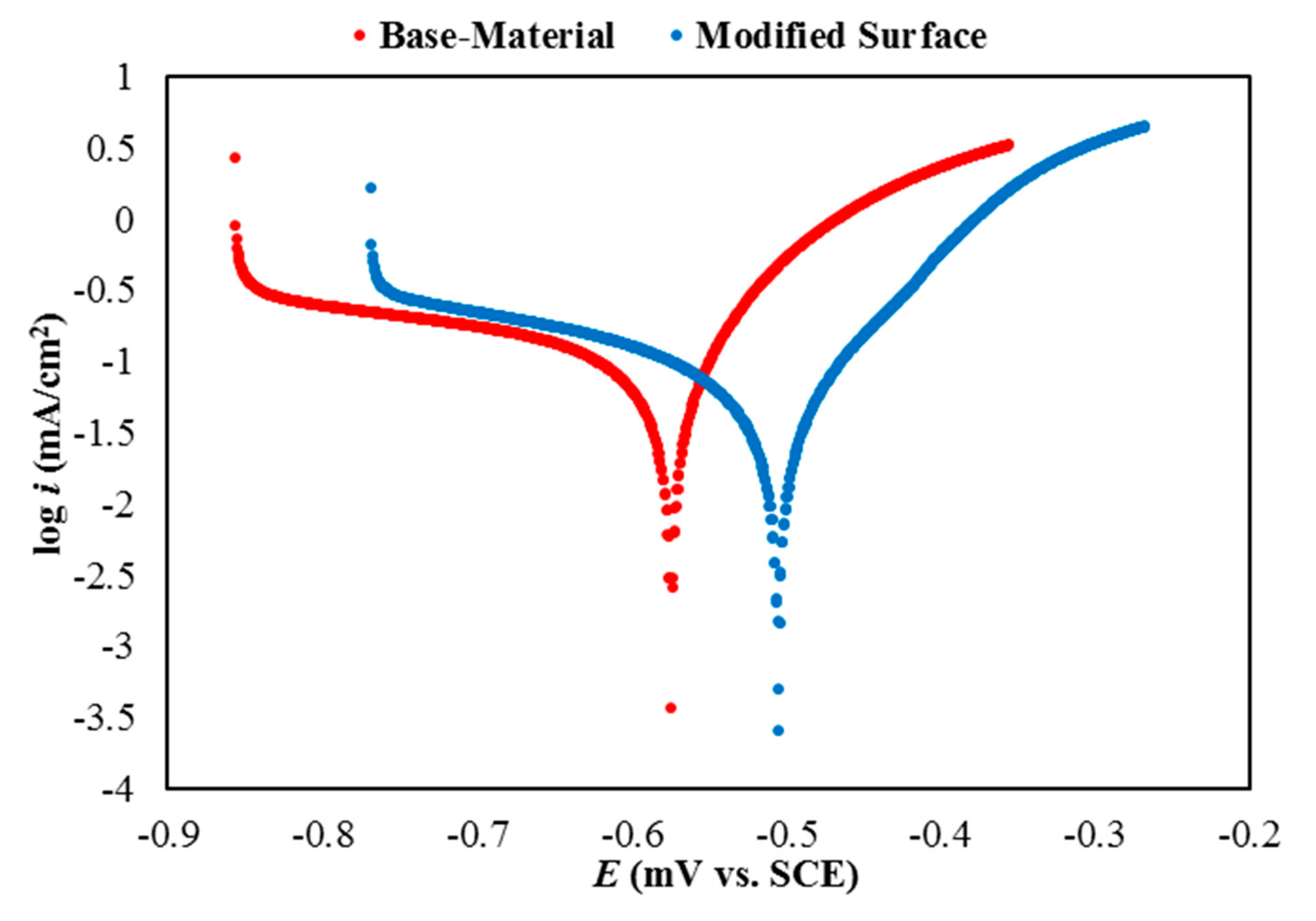
3. Results and Discussions
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tewary, N.; Kundu, A.; Nandi, R.; Saha, J.; Ghosh, S. Microstructural characterisation and corrosion performance of old railway girder bridge steel and modern weathering structural steel. Corros. Sci. 2016, 113, 57–63. [Google Scholar] [CrossRef]
- Yu, L.; François, R.; Dang, V.H.; L’Hostis, V.; Gagné, R. Distribution of corrosion and pitting factor of steel in corroded RC beams. Constr. Build. Mater. 2015, 95, 384–392. [Google Scholar] [CrossRef]
- Yaro, A.S.; Abdul-Khalik, K.R.; Khadom, A.A. Effect of CO2 corrosion behavior of mild steel in oilfield produced water. J. Loss Prev. Process. Ind. 2015, 38, 24–38. [Google Scholar] [CrossRef]
- Tavakoli, H.; Khoie, S.M. An electrochemical study of the corrosion resistance of boride coating obtained by thermo-reactive diffusion. Mater. Chem. Phys. 2010, 124, 1134–1138. [Google Scholar] [CrossRef]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloy. Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Xiang, Z.; Datta, P. Effects of pack composition on the formation of aluminide coatings on alloy steels at 650 °C. J. Mater. Sci. 2005, 40, 1959–1966. [Google Scholar] [CrossRef]
- Kumar, S.; Jyothirmayi, A.; Wasekar, N.; Joshi, S.; Joshi, S. Influence of annealing on mechanical and electrochemical properties of cold sprayed niobium coatings. Surf. Coat. Technol. 2016, 296, 124–135. [Google Scholar] [CrossRef]
- Kim, S.H.; Subramanian, G.O.; Kim, C.; Jang, C.; Park, K.M. Surface modification of austenitic stainless steel for corrosion resistance in high temperature supercritical-carbon dioxide environment. Surf. Coat. Technol. 2018, 349, 415–425. [Google Scholar] [CrossRef]
- Carvalho, S.; Vernilli, F.; Almeida, B.; Demarco, M.; Silva, S. The recycling effect of BOF slag in the Portland cement properties. Resour. Conserv. Recycl. 2017, 127, 216–220. [Google Scholar] [CrossRef]
- Al-Zubaid, A.B.; Shabeeb, K.M.; Ali, A.I. Study the Effect of Recycled Glass on the Mechanical Properties of Green Concrete. Energy Procedia 2017, 119, 680–692. [Google Scholar] [CrossRef]
- Handoko, W.; Pahlevani, F.; Emmanuelawati, I.; Sahajwalla, V. Transforming automotive waste into TiN and TiC ceramics. Mater. Lett. 2016, 176, 17–20. [Google Scholar] [CrossRef]
- Mayyas, M.; Pahlevani, F.; Handoko, W.; Sahajwalla, V. Preliminary investigation on the thermal conversion of automotive shredder residue into value-added products: Graphitic carbon and nano-ceramics. Waste Manag. 2016, 50, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Rogge, M.; Stark, R.W. Atomic force microscopy measurements probing the mechanical properties of single collagen fibrils under the influence of UV light in situ. J. Mech. Behav. Biomed. Mater. 2018, 88, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Gaveau, A.; Coetsier, C.; Roques, C.; Bacchin, P.; Dague, E.; Causserand, C. Bacteria transfer by deformation through microfiltration membrane. J. Membr. Sci. 2017, 523, 446–455. [Google Scholar] [CrossRef]
- Derjaguin, K.L.; Muller, V.M.; Toporov, Y.P. Effect of contact deformation on the adhesion of particles. J. Colloid Interface Sci. 1971, 53, 314–326. [Google Scholar] [CrossRef]
- Heu, C.; Berquand, A.; Elie-Caille, C.; Nicod, L.; Nicod, L.P. Glyphosate-induced stiffening of HaCaT keratinocytes, a Peak Force Tapping study on living cells. J. Struct. Boil. 2012, 178, 1–7. [Google Scholar] [CrossRef]
- Eich, S.; Schmitz, G. Embedded-atom study of grain boundary segregation and grain boundary free energy in nanosized iron–chromium tricrystals. Acta Mater. 2018, 147, 350–364. [Google Scholar] [CrossRef]
- Windus, C.; Sujishi, S.; Giering, W.P. Relative iron-carbon and iron-silicon bond strengths in derivatives of (.eta.-cyclopentadienyl) dicarbonyliron. J. Am. Chem. Soc. 1974, 96, 1951–1952. [Google Scholar] [CrossRef]
- Tusche, C.; Meyerheim, H.L.; Jedrecy, N.; Renaud, G.; Ernst, A.; Henk, J.; Bruno, P.; Kirschner, J. Oxygen-Induced Symmetrization and Structural Coherency inFe/MgO/Fe(001)Magnetic Tunnel Junctions. Phys. Rev. Lett. 2005, 95, 176101. [Google Scholar] [CrossRef]
- Wang, S.; Han, G.; Yu, G.; Jiang, Y.; Wang, C.; Kohn, A.; Ward, R. Evidence for FeO formation at the Fe/MgO interface in epitaxial TMR structure by X-ray photoelectron spectroscopy. J. Magn. Magn. Mater. 2007, 310, 1935–1936. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, S.; Wang, X.; Han, H.; Bai, Z.; Yang, L. Hierarchical Co9S8 hollow microspheres as multifunctional electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2017, 246, 380–390. [Google Scholar] [CrossRef]
- Chen, E.; Leung, C.K. A coupled diffusion-mechanical model with boundary element method to predict concrete cover cracking due to steel corrosion. Corros. Sci. 2017, 126, 180–196. [Google Scholar] [CrossRef]
- Miyake, K.; Hirata, Y.; Shimonosono, T.; Sameshima, S. The Effect of Particle Shape on Sintering Behavior and Compressive Strength of Porous Alumina. Materials 2018, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Ciria, M.; Proietti, M.G.; Corredor, E.C.; Coffey, D.; Begué, A.; De La Fuente, C.; Arnaudas, J.I.; Ibarra, A. Crystal structure and local ordering in epitaxial Fe100−Ga/MgO(001) films. J. Alloy. Compd. 2018, 767, 905–914. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Yang, J.; Sang, S.; Wang, Q. Fabrication of MgO-NiO-Fe2O3 materials and their corrosion in Na3AlF6-AlF3-K3AlF6 bath. J. Alloy. Compd. 2017, 723, 64–69. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Kong, D.; Wang, L.; Li, X. Beneficial effect of reversed austenite on the intergranular corrosion resistance of martensitic stainless steel. Corros. Sci. 2019, 151, 108–121. [Google Scholar] [CrossRef]
- Park, I.-J.; Kim, S.-T.; Lee, I.-S.; Park, Y.-S.; Moon, M.B. A Study on Corrosion Behavior of DP-Type and TRIP-Type Cold Rolled Steel Sheet. Mater. Trans. 2009, 50, 1440–1447. [Google Scholar] [CrossRef]
- Hossain, R.; Pahlevani, F.; Quadir, M.Z.; Sahajwalla, V. Stability of retained austenite in high carbon steel under compressive stress: an investigation from macro to nano scale. Sci. Rep. 2016, 6, 34958. [Google Scholar] [CrossRef]
- Gouné, M.; Danoix, F.; Allain, S.; Bouaziz, O. Unambiguous carbon partitioning from martensite to austenite in Fe–C–Ni alloys during quenching and partitioning. Scr. Mater. 2013, 68, 1004–1007. [Google Scholar] [CrossRef]
- Stern, M. and Geary, A.L. Electrochemical polarization I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 55–63. [Google Scholar] [CrossRef]
- Bockris, J.; Reddy, A.K.N. Modern Electrochemistry; Kluwer Academic Publishers: New York, NY, USA, 2000. [Google Scholar]
- Tao, Q.; Wang, J.; Fu, L.; Chen, Z.; Shen, C.; Zhang, D.; Sun, Z. Ultrahigh hardness of carbon steel surface realized by novel solid carburizing with rapid diffusion of carbon nanostructures. J. Mater. Sci. Technol. 2017, 33, 1210–1218. [Google Scholar] [CrossRef]
- Ueji, R.; Inoue, T. Acceleration of diffusional transformation in a high-carbon steel layer composed of a sandwich-like clad steel sheet. Mater. Sci. Eng. A 2019, 764, 138217. [Google Scholar] [CrossRef]
- Chen, M.; Bai, Z.; Tan, S. Microstructure and friction behavior of polymeric composite coatings on alumina/aluminum composite. Surf. Coat. Technol. 2002, 151, 478–482. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Zucchi, F. Corrosion and tribo corrosion behaviour of thermally sprayed ceramic coatings on steel. Surf. Coat. Technol. 2011, 205, 3683–3691. [Google Scholar] [CrossRef]
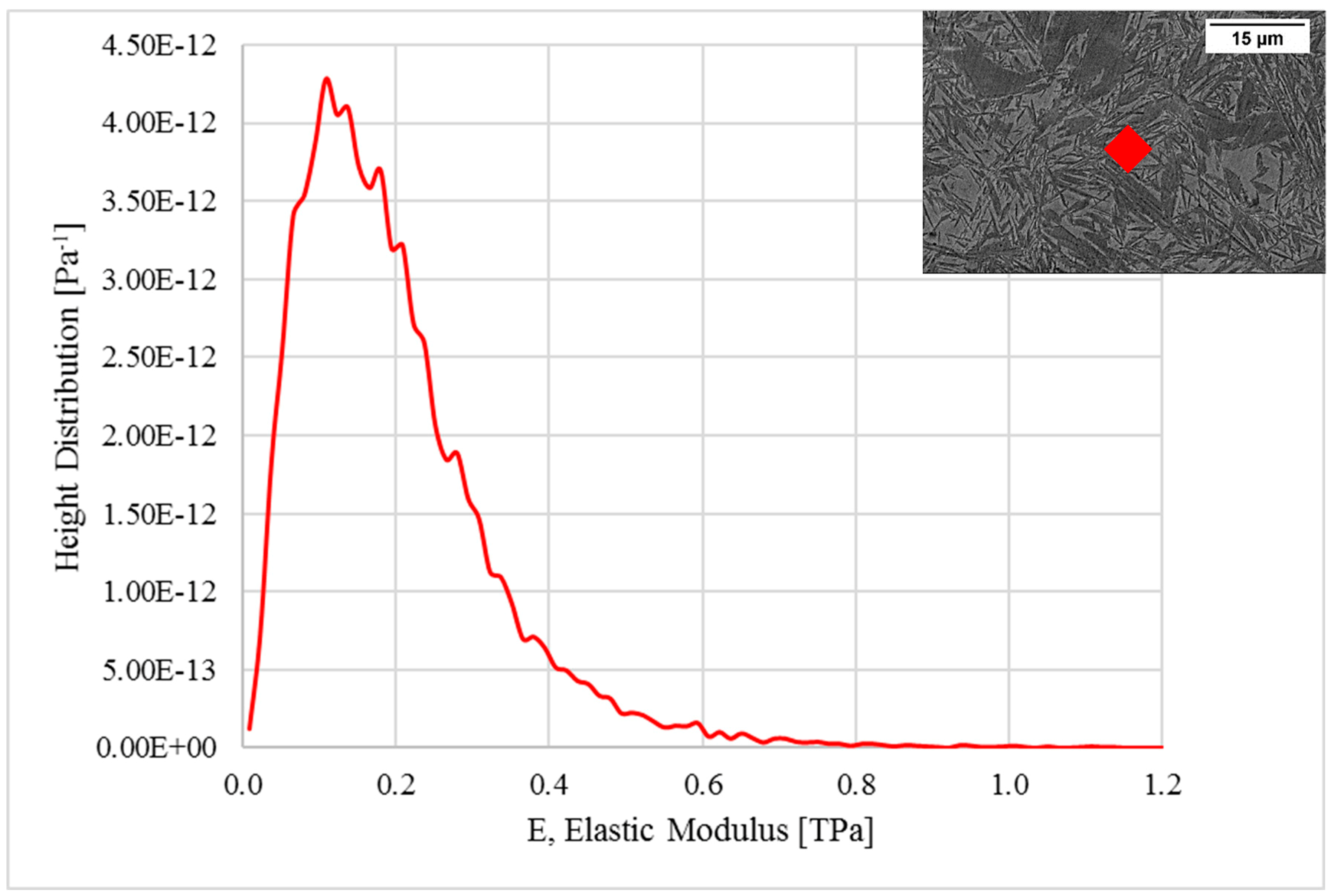

| Sample-ID | Young’s Modulus (GPa) | Standard Deviation (GPa) |
|---|---|---|
| Steel Substrate (Base-Material, Before Surface Modification) | 204.0 | 20.92 |
| Multi-Phase Ceramic Layer (After Surface Modification) | 359.3 | 17.12 |
| Selective Diffusion Layer (After Surface Modification) | 278.0 | 20.34 |
| Steel Substrate (After Surface Modification) | 200.4 | 18.72 |
| Sample-ID | Electrochemical Corrosion Measurements | PEF (%) | ||
|---|---|---|---|---|
| Ecorr (mV vs. SCE) | icorr (mA/cm2) | Rp (kΩ·cm2) | ||
| Base-Material | −577 ± 8 | 0.282 ± 0.012 | 13.87 | - |
| Modified Surface | −507 ± 11 | 0.0186 ± 0.0015 | 0.346 | 39.08 |
| Sample-ID | Hardness (HV) | in GPa |
|---|---|---|
| Steel Substrate (Base-Material, Before Surface Modification) | 780 ± 10 | 7.65 |
| Multi-Phase Ceramic Layer (After Surface Modification) | 1385 ± 19 | 13.58 |
| Selective Diffusion Layer (After Surface Modification) | 992 ± 14 | 9.73 |
| Steel Substrate (After Surface Modification) | 781 ± 12 | 7.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handoko, W.; Pahlevani, F.; Yao, Y.; Privat, K.; Sahajwalla, V. From Waste to Multi-Hybrid Layering of High Carbon Steel to Improve Corrosion Resistance: An In-Depth Analysis Using EPMA and AFM Techniques. Surfaces 2019, 2, 485-496. https://doi.org/10.3390/surfaces2030036
Handoko W, Pahlevani F, Yao Y, Privat K, Sahajwalla V. From Waste to Multi-Hybrid Layering of High Carbon Steel to Improve Corrosion Resistance: An In-Depth Analysis Using EPMA and AFM Techniques. Surfaces. 2019; 2(3):485-496. https://doi.org/10.3390/surfaces2030036
Chicago/Turabian StyleHandoko, Wilson, Farshid Pahlevani, Yin Yao, Karen Privat, and Veena Sahajwalla. 2019. "From Waste to Multi-Hybrid Layering of High Carbon Steel to Improve Corrosion Resistance: An In-Depth Analysis Using EPMA and AFM Techniques" Surfaces 2, no. 3: 485-496. https://doi.org/10.3390/surfaces2030036
APA StyleHandoko, W., Pahlevani, F., Yao, Y., Privat, K., & Sahajwalla, V. (2019). From Waste to Multi-Hybrid Layering of High Carbon Steel to Improve Corrosion Resistance: An In-Depth Analysis Using EPMA and AFM Techniques. Surfaces, 2(3), 485-496. https://doi.org/10.3390/surfaces2030036







