An Invisible Threat to Natural Heritage: Examples of Large Protected Areas with Hg-Enriched Freshwater Environments
Abstract
1. Introduction
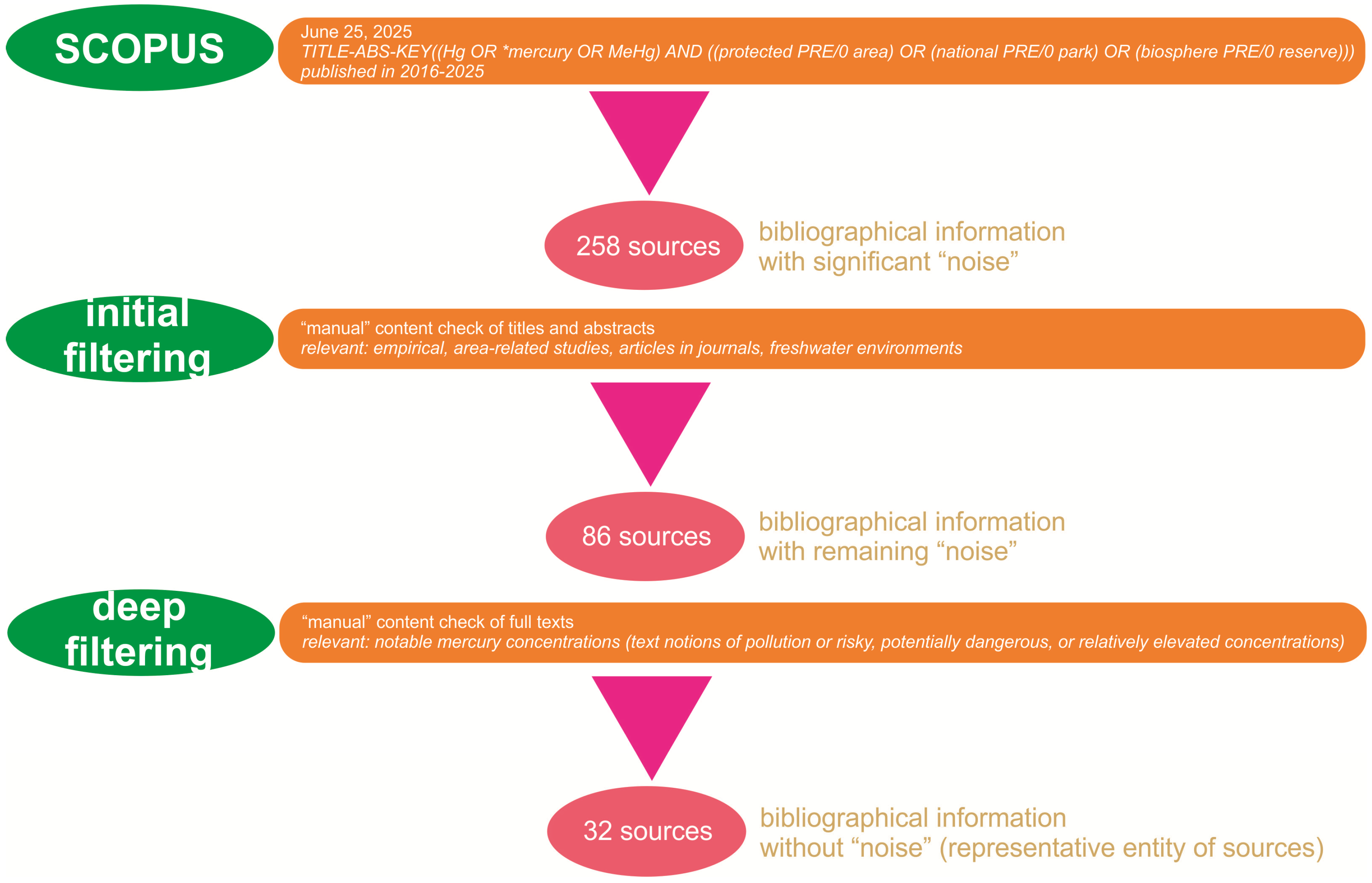
2. Literature Selection Principles
3. Results: Found Examples and Tentative Synthesis of the Extracted Information
3.1. Geographical Pattern
3.2. Water
3.3. Sediments
3.4. Biota
3.5. Factors of Pollution
4. Discussion: Applications to Natural Heritage
4.1. Threat to Natural Heritage: Principal Questions and Research Biases
4.2. Geographical Biases?
4.3. Heritage-Related Inferences
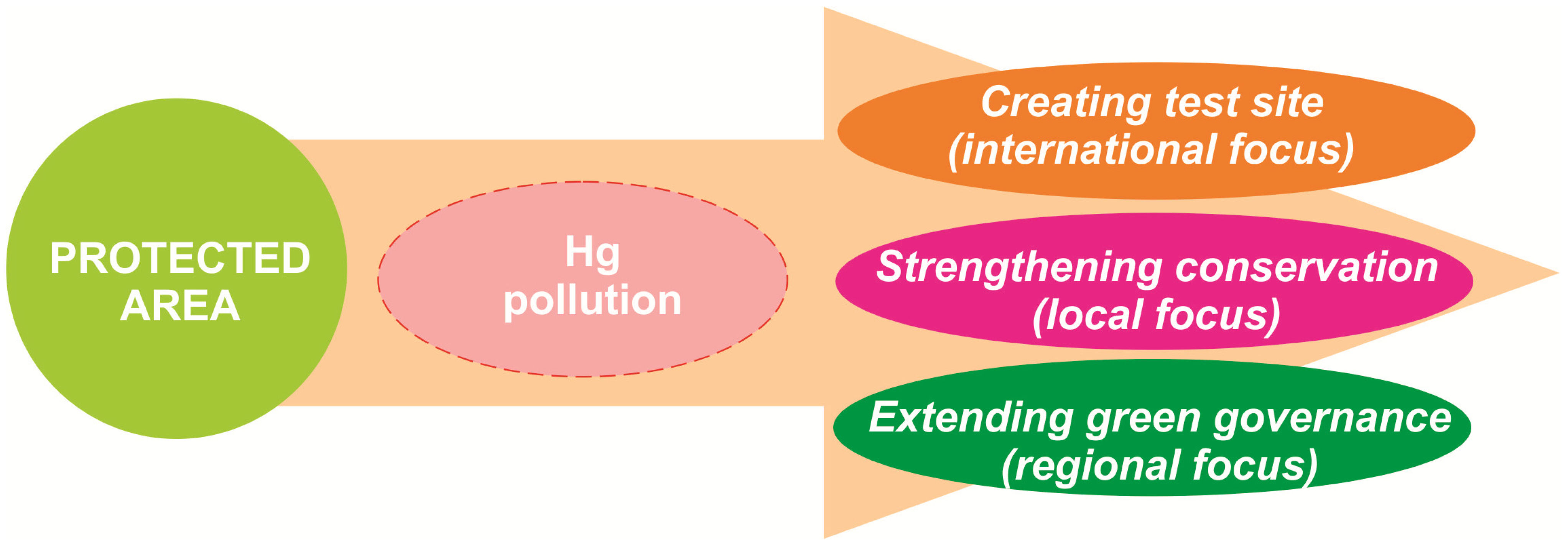
4.4. Managerial Perspectives and Recommendations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groves, C.R.; Klein, M.L.; Breden, T.F. Natural Heritage Programs: Public-private partnerships for biodiversity conservation. Wildl. Soc. Bull. 1995, 23, 784–790. [Google Scholar]
- Reyes-Fornet, A.; García, J.F.S.; Igarza, L.M.Z.; Hernández, E.B.F. Conceptual model of natural heritage in environmental management for ecosystem conservation. Ecosistemas 2020, 29, 2003. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, K.; Liu, Z.; He, L. Research progress on world natural heritage conservation: Its buffer zones and the implications. Herit. Sci. 2022, 10, 102. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, K.; Huang, D. Natural world heritage conservation and tourism: A review. Herit. Sci. 2023, 11, 55. [Google Scholar] [CrossRef]
- Zhao, Y.; Umair, M.; Gulzar, F.; Guliyeva, S.; Xabibullayev, D. The role of private natural heritage conservation areas in promoting sustainable development Goals Insight. Ecol. Indic. 2025, 176, 113658. [Google Scholar] [CrossRef]
- Bridgewater, P. A commentary on ecohydrology as a science-policy interface in implementing the UN Sustainable Development Goals. Ecohydrol. Hydrobiol. 2021, 21, 387–392. [Google Scholar] [CrossRef]
- Lucchi, E.; Turati, F.; Colombo, B.; Schito, E. Climate-responsive design practices: A transdisciplinary methodology for achieving sustainable development goals in cultural and natural heritage. J. Clean. Prod. 2024, 457, 142431. [Google Scholar] [CrossRef]
- Roque, A.M.; Madi, R.R.; Coelho, A.S.; de Melo, C.M. Conservation units and sustainable development goals: The private natural heritage reserves of Brazil. Environ. Dev. Sustain. 2024, 26, 2183–2202. [Google Scholar] [CrossRef]
- Lowell, K. A socio-environmental monitoring system for a UNESCO biosphere reserve. Environ. Monit. Assess. 2017, 189, 601. [Google Scholar] [CrossRef]
- Kubak, M.; Gavurova, B.; Legutka, K. Economic value estimation of the natural heritage of the Tatra National Park. Int. J. Environ. Res. Public Health 2020, 17, 3032. [Google Scholar] [CrossRef]
- Nurkassimova, M.; Omarova, N.; Zinicovscaia, I.; Yushin, N.; Chaligava, O. Mosses as bioindicators of air pollution with potentially toxic elements in the Burabay State National Natural Park, Kazakhstan. Environ. Monit. Assess. 2024, 196, 442. [Google Scholar] [CrossRef]
- Basu, M. Impact of Mercury and Its Toxicity on Health and Environment: A General Perspective. Environ. Sci. Eng. 2023, F1792, 95–139. [Google Scholar]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L. Mercury in the environment: Sources, toxicities, and prevention of exposure. Pediatr. Ann. 2004, 33, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kayani, K.F.; Mohammed, S.J. Mercury in aquatic environments: Toxicity and advances in remediation using covalent organic frameworks. Mater. Adv. 2025, 6, 3371–3385. [Google Scholar] [CrossRef]
- Patra, M.; Sharma, A. Mercury toxicity in plants. Bot. Rev. 2000, 66, 379–422. [Google Scholar] [CrossRef]
- Gnamuš, A.; Byrne, A.R.; Horvat, M. Mercury in the soil-plant-deer-predator food chain of a temperate forest in Slovenia. Environ. Sci. Technol. 2000, 34, 3337–3345. [Google Scholar] [CrossRef]
- Kumar, V.; Umesh, M.; Shanmugam, M.K.; Chakraborty, P.; Duhan, L.; Gummadi, S.N.; Pasrija, R.; Jayaraj, I.; Dasarahally Huligowda, L.K. A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects. Sustainability 2023, 15, 13292. [Google Scholar] [CrossRef]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef]
- Barocas, A.; Vega, C.; Alarcon Pardo, A.; Araujo Flores, J.M.; Fernandez, L.; Groenendijk, J.; Pisconte, J.; Macdonald, D.W.; Swaisgood, R.R. Local Intensity of Artisanal Gold Mining Drives Mercury Accumulation in Neotropical Oxbow Lake Fishes. Sci. Total Environ. 2023, 886, 164024. [Google Scholar] [CrossRef]
- Cheng, Y.; Watari, T.; Seccatore, J.; Nakajima, K.; Nansai, K.; Takaoka, M. A review of gold production, mercury consumption, and emission in artisanal and small-scale gold mining (ASGM). Resour. Policy 2023, 81, 103370. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef]
- Kohio, E.N.; Karoui, H.; Sossou, S.K.; Yacouba, H. Review of pollution trends and impacts in artisanal and small-scale gold mining in Sub-Saharan Africa: Advancing towards sustainable practices through equitable redistribution of gold spin-offs. J. Clean. Prod. 2024, 476, 143754. [Google Scholar] [CrossRef]
- Pang, Q.; Gu, J.; Wang, H.; Zhang, Y. Global health impact of atmospheric mercury emissions from artisanal and small-scale gold mining. iScience 2022, 25, 104881. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.E.; Kotalik, C.J.; Willacker, J.J.; Tate, M.T.; Flanagan Pritz, C.M.; Nelson, S.J.; Krabbenhoft, D.P.; Walters, D.M.; Eagles-Smith, C.A. Geographic Drivers of Mercury Entry into Aquatic Food Webs Revealed by Mercury Stable Isotopes in Dragonfly Larvae. Environ. Sci. Technol. 2024, 58, 13444–13455. [Google Scholar] [CrossRef] [PubMed]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Yang, H.; Macario-González, L.; Cohuo, S.; Whitmore, T.J.; Salgado, J.; Peréz, L.; Schwalb, A.; Rose, N.L.; Holmes, J.; Riedinger-Whitmore, M.A.; et al. Mercury Pollution History in Tropical and Subtropical American Lakes: Multiple Impacts and the Possible Relationship with Climate Change. Environ. Sci. Technol. 2023, 57, 3680–3690. [Google Scholar] [CrossRef]
- Abell, R.; Allan, J.D.; Lehner, B. Unlocking the potential of protected areas for freshwaters. Biol. Conserv. 2007, 134, 48–63. [Google Scholar] [CrossRef]
- Han, Y.; Xu, W.; Wang, K.; Wang, D.; Mei, Z. Effects of Freshwater Protected Areas on Survival of a Critically Endangered Cetacean. Conserv. Lett. 2025, 18, e13081. [Google Scholar] [CrossRef]
- Tsavdaridou, A.I.; Doxa, A.; Mazaris, A.D. Towards achieving a twenty-fold increase in the coverage of freshwater species distributions within protected areas in Europe. Biol. Conserv. 2023, 285, 110233. [Google Scholar] [CrossRef]
- Dias-Silva, K.; Vieira, T.B.; Moreira, F.F.F.; Juen, L.; Hamada, N. Protected areas are not effective for the conservation of freshwater insects in Brazil. Sci. Rep. 2021, 11, 21247. [Google Scholar] [CrossRef]
- Hermoso, V.; Abell, R.; Linke, S.; Boon, P. The role of protected areas for freshwater biodiversity conservation: Challenges and opportunities in a rapidly changing world. Aquatic Conserv. Mar. Freshw. Ecosyst. 2016, 26, 3–11. [Google Scholar] [CrossRef]
- Nogueira, J.G.; Teixeira, A.; Varandas, S.; Lopes-Lima, M.; Sousa, R. Assessment of a terrestrial protected area for the conservation of freshwater biodiversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 520–530. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boultron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Quaff, A.R. Bibliometric Analysis on Global Research Trends in Air Pollution Prediction Research Using Machine Learning from 1991–2023 Using Scopus Database. Aerosol Sci. Eng. 2024, 8, 288–306. [Google Scholar] [CrossRef]
- Bhatt, G.D.; Emdad, A. Blockchain’s role in environmental sustainability: Bibliometric insights from Scopus. Green Technol. Sustain. 2025, 3, 100236. [Google Scholar] [CrossRef]
- Pellinen, V.; Cherkashina, T.; Gustaytis, M. Assessment of Metal Pollution and Subsequent Ecological Risk in the Coastal Zone of the Olkhon Island, Lake Baikal, Russia. Sci. Total Environ. 2021, 786, 147441. [Google Scholar] [CrossRef]
- Lemaire, J.; Bustamante, P.; Shirley, M.H. Preliminary Assessment of Blood Mercury Contamination in Four African Crocodile Species. Environ. Int. 2024, 190, 108877. [Google Scholar] [CrossRef]
- Schneider, T.; Musa Bandowe, B.A.; Bigalke, M.; Mestrot, A.; Hampel, H.; Mosquera, P.V.; Fränkl, L.; Wienhues, G.; Vogel, H.; Tylmann, W.; et al. 250-Year Records of Mercury and Trace Element Deposition in Two Lakes from Cajas National Park, SW Ecuadorian Andes. Environ. Sci. Pollut. Res. 2021, 28, 16227–16243. [Google Scholar] [CrossRef]
- Dalu, T.; Mwedzi, T.; Wasserman, R.J.; Madzivanzira, T.C.; Nhiwatiwa, T.; Cuthbert, R.N. Land Use Effects on Water Quality, Habitat, and Macroinvertebrate and Diatom Communities in African Highland Streams. Sci. Total Environ. 2022, 846, 157346. [Google Scholar] [CrossRef]
- Dalu, T.; Dube, T.; Dondofema, F.; Cuthbert, R.N. Illegal Mining Impacts on Freshwater Potamonautid Crab in a Subtropical Austral Highland Biosphere Reserve. Sci. Total Environ. 2023, 896, 165251. [Google Scholar] [CrossRef]
- Janssen, S.E.; Tate, M.T.; Poulin, B.A.; Krabbenhoft, D.P.; DeWild, J.F.; Ogorek, J.M.; Varonka, M.S.; Orem, W.H.; Kline, J.L. Decadal Trends of Mercury Cycling and Bioaccumulation within Everglades National Park. Sci. Total Environ. 2022, 838, 156031. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Valencia, O.M.; Murillo-García, O.E.; Rodriguez-Salazar, G.A.; Bolívar-García, W. Bioaccumulation of Mercury in Direct-Developing Frogs: The Aftermath of Illegal Gold Mining in a National Park. Herpetol. J. 2023, 33, 6–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.-P.; Ellison, A.M.; Liu, W.-G.; Chen, D. Establish an Environmentally Sustainable Giant Panda National Park in the Qinling Mountains. Sci. Total Environ. 2019, 668, 979–987. [Google Scholar] [CrossRef]
- Carling, G.T.; Rupper, S.B.; Fernandez, D.P.; Tingey, D.G.; Harrison, C.B. Effect of Atmospheric Deposition and Weathering on Trace Element Concentrations in Glacial Meltwater at Grand Teton National Park, Wyoming, U.S.A. Arct. Antarct. Alp. Res. 2017, 49, 427–440. [Google Scholar] [CrossRef]
- Magnuson, J.T.; Sandheinrich, M.B. Relation among Mercury, Selenium, and Biomarkers of Oxidative Stress in Northern Pike (Esox lucius). Toxics 2023, 11, 244. [Google Scholar] [CrossRef]
- Clarke, R.G.; Klapstein, S.J.; Hillier, N.K.; O’Driscoll, N.J. Methylmercury in Caddisflies and Mayflies: Influences of Water and Sediment Chemistry. Chemosphere 2022, 286, 131785. [Google Scholar] [CrossRef]
- Graves, S.D.; Kidd, K.A.; Batchelar, K.L.; Cowie, A.M.; O’Driscoll, N.J.; Martyniuk, C.J. Response of Oxidative Stress Transcripts in the Brain of Wild Yellow Perch (Perca flavescens) Exposed to an Environmental Gradient of Methylmercury. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2017, 192, 50–58. [Google Scholar] [CrossRef]
- Graves, S.D.; Kidd, K.A.; Houlahan, J.E.; Munkittrick, K.R. General and Histological Indicators of Health in Wild Fishes from a Biological Mercury Hotspot in Northeastern North America. Environ. Toxicol. Chem. 2017, 36, 976–987. [Google Scholar] [CrossRef]
- Roberts, S.; Kirk, J.L.; Wiklund, J.A.; Muir, D.C.G.; Yang, F.; Gleason, A.; Lawson, G. Mercury and Metal(loid) Deposition to Remote Nova Scotia Lakes from Both Local and Distant Sources. Sci. Total Environ. 2019, 675, 192–202. [Google Scholar] [CrossRef]
- Thera, J.C.; Kidd, K.A.; Bertolo, R.F.; O’Driscoll, N.J. Tissue Content of Thiol-Containing Amino Acids Predicts Methylmercury in Aquatic Invertebrates. Sci. Total Environ. 2019, 688, 567–573. [Google Scholar] [CrossRef]
- Thera, J.C.; Kidd, K.A.; Stewart, A.R.; Bertolo, R.F.; O’Driscoll, N.J. Using Tissue Cysteine to Predict the Trophic Transfer of Methylmercury and Selenium in Lake Food Webs. Environ. Pollut. 2022, 311, 119936. [Google Scholar] [CrossRef] [PubMed]
- Mijošek, T.; Šariri, S.; Kljaković-Gašpić, Z.; Fiket, Ž.; Filipović Marijić, V. Interrelation between Environmental Conditions, Acanthocephalan Infection and Metal(loid) Accumulation in Fish Intestine: An In-Depth Study. Environ. Pollut. 2024, 356, 124358. [Google Scholar] [CrossRef] [PubMed]
- du Preez, M.; Govender, D.; Kylin, H.; Bouwman, H. Metallic Elements in Nile Crocodile Eggs from the Kruger National Park, South Africa. Ecotoxicol. Environ. Saf. 2018, 148, 930–941. [Google Scholar] [CrossRef]
- Daga, R.; Ribeiro Guevara, S.; Pavlin, M.; Rizzo, A.; Lojen, S.; Vreča, P.; Horvat, M.; Arribére, M. Historical Records of Mercury in Southern Latitudes over 1600 Years: Lake Futalaufquen, Northern Patagonia. Sci. Total Environ. 2016, 553, 541–550. [Google Scholar] [CrossRef]
- Juárez, A.; Arribére, M.A.; Arcagni, M.; Williams, N.; Rizzo, A.; Ribeiro Guevara, S. Heavy Metal and Trace Elements in Riparian Vegetation and Macrophytes Associated with Lacustrine Systems in Northern Patagonia Andean Range. Environ. Sci. Pollut. Res. 2016, 23, 17995–18009. [Google Scholar] [CrossRef]
- Aranda-Coello, J.M.; Mendoza-Velázquez, O.M.; Gutiérrez-Olvera, C. Heavy Metals in Caudal Scales of Crocodylus moreletii in the Southern Portion of the Lacandona Jungle, Chiapas, Mexico. Rev. Int. Contam. Ambient. 2023, 39, 85–94. [Google Scholar] [CrossRef]
- Soto Cárdenas, C.; Diéguez, M.D.C.; Queimaliños, C.; Rizzo, A.; Fajon, V.; Kotnik, J.; Horvat, M.; Ribeiro Guevara, S. Mercury in a Stream-Lake Network of Andean Patagonia (Southern Volcanic Zone): Partitioning and Interaction with Dissolved Organic Matter. Chemosphere 2018, 197, 262–270. [Google Scholar] [CrossRef]
- Soto Cárdenas, C.S.; Fernandez, Z.; Arcagni, M.; Rizzo, A.; Diéguez, M.C. Mercury Patterns in Lakes within a Natural Hotspot in the Southern Volcanic Zone of the Andes (Nahuel Huapi National Park, Patagonia, South America). Environ. Chem. 2025, 22, EN24088. [Google Scholar] [CrossRef]
- Camacho-delaCruz, A.A.; Espinosa-Reyes, G.; Rebolloso-Hernández, C.A.; Carrizales-Yáñez, L.; Ilizaliturri-Hernández, C.A.; Reyes-Arreguín, L.E.; Díaz-Barriga, F. Holistic Health Risk Assessment in an Artisanal Mercury Mining Region in Mexico. Environ. Monit. Assess. 2021, 193, 541. [Google Scholar] [CrossRef]
- Acharyya, S.; Majumder, S.; Nandi, S.; Ghosh, A.; Saha, S.; Bhattacharya, M. Uncovering Mercury Accumulation and the Potential for Bacterial Bioremediation in Response to Contamination in the Singalila National Park. Sci. Rep. 2025, 15, 3664. [Google Scholar] [CrossRef]
- Bartz, K.K.; Hannam, M.P.; Wilson, T.L.; Lepak, R.F.; Ogorek, J.M.; Young, D.B.; Eagles-Smith, C.A.; Krabbenhoft, D.P. Understanding Drivers of Mercury in Lake Trout (Salvelinus namaycush), a Top-Predator Fish in Southwest Alaska’s Parklands. Environ. Pollut. 2023, 330, 121678. [Google Scholar] [CrossRef] [PubMed]
- Cesário, R.; Monteiro, C.E.; Nogueira, M.; O’Driscoll, N.J.; Caetano, M.; Hintelmann, H.; Mota, A.M.; Canário, J. Mercury and Methylmercury Dynamics in Sediments on a Protected Area of Tagus Estuary (Portugal). Water Air Soil Pollut. 2016, 227, 475. [Google Scholar] [CrossRef]
- Germ, M.; Golob, A.; Zelnik, I.; Klink, A.; Polechońska, L. Contents of Metals in Sediments and Macrophytes Differed between the Locations in an Alpine Lake Revealing Human Impacts—A Case Study of Lake Bohinj (Slovenia). Water 2023, 15, 1254. [Google Scholar] [CrossRef]
- Mezhevova, A.; Berestneva, Y.; Belyaev, A. The Bodies of the Volga-Akhtuba Floodplain (Russia). J. Geogr. Inst. Jovan Cvijic SASA 2024, 74, 147–164. [Google Scholar] [CrossRef]
- Hebert, C.E. The River Runs through It: The Athabasca River Delivers Mercury to Aquatic Birds Breeding Far Downstream. PLoS ONE 2019, 14, e0206192. [Google Scholar] [CrossRef]
- Rolón, E.; Rosso, J.J.; Mabragaña, E.; Tripodi, P.; Bavio, M.; Bidone, C.; Volpedo, A.V.; Avigliano, E. Distribution and Accumulation of Major and Trace Elements in Water, Sediment, and Fishes from Protected Areas of the Atlantic Rainforest. Environ. Sci. Pollut. Res. 2022, 29, 58843–58868. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.B.; Qiu, G.L.; Shang, L.H.; Li, Z.G. Mercury pollution in Asia: A review of the contaminated sites. J. Hazard. Mater. 2009, 168, 591–601. [Google Scholar] [CrossRef]
- Granados-Sánchez, R.R.; Sedeño-Díaz, J.E.; López-López, E. Microplastic pollution and associated trace metals in freshwater ecosystems within protected natural areas: The case of a biosphere reserve in Mexico. Front. Environ. Sci. 2024, 12, 1441340. [Google Scholar] [CrossRef]
- Acharyya, S.; Majumder, S.; Bhattacharya, M. Presence of pesticide-tolerant microorganisms in high-altitude pristine lakes within Singalila Ridge of the Himalayas. Environ. Monit. Assess. 2024, 196, 925. [Google Scholar] [CrossRef]
- Vimal, R.; Navarro, L.M.; Jones, Y.; Wolf, F.; Le Moguedec, G.; Rejou-Mechain, M. The global distribution of protected areas management strategies and their complementarity for biodiversity conservation. Biol. Conserv. 2021, 256, 109014. [Google Scholar] [CrossRef]
- Fernández-Trujillo, S.; López-Perea, J.J.; Jiménez-Moreno, M.; Martín-Doimeadios, R.C.R.; Mateo, R. Metals and Metalloids in Freshwater Fish from the Floodplain of Tablas de Daimiel National Park, Spain. Ecotoxicol. Environ. Saf. 2021, 208, 111602. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, T.; Hamada, Y.K.; Baransano, C.; Kodamatani, H.; Matsuyama, A.; Imura, R.; Hidayati, N.; Rahajoe, J.S. Mercury Concentrations in Paddy Field Soil and Freshwater Snails around a Small-Scale Gold Mining Area, West Java, Indonesia. Toxicol. Environ. Health Sci. 2020, 12, 23–29. [Google Scholar] [CrossRef]
- Budnik, L.G.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef]
- Arrighi, S.; Franceschini, F.; Petrini, R.; Fornasaro, S.; Ghezzi, L. The Legacy of Hg Contamination in a Past Mining Area (Tuscany, Italy): Hg Speciation and Health Risk Assessment. Toxics 2024, 12, 436. [Google Scholar] [CrossRef]
- Baptista-Salazar, C.; Biester, H. The role of hydrological conditions for riverine Hg species transport in the Idrija mining area. Environ. Pollut. 2019, 247, 716–724. [Google Scholar] [CrossRef]
- Barquero, J.I.; Hidalgo, J.J.; Esbrí, J.M.; Higueras, P.; García-Ordiales, E. A preliminary assessment of mercury, methylmercury and other potentially toxic elements in largemouth bass (Micropterus salmoides) from the Almadén mining district. Environ. Geochem. Health 2025, 47, 27. [Google Scholar] [CrossRef]
- Gosar, M.; Teršič, T. Environmental geochemistry studies in the area of Idrija mercury mine, Slovenia. Environ. Geochem. Health 2012, 34, 27–41. [Google Scholar] [CrossRef]
- Jiménez-Moreno, M.; Barre, J.P.G.; Perrot, V.; Bérail, S.; Rodríguez Martín-Doimeadios, R.C.; Amouroux, D. Sources and fate of mercury pollution in Almadén mining district (Spain): Evidences from mercury isotopic compositions in sediments and lichens. Chemosphere 2016, 147, 430–438. [Google Scholar] [CrossRef]
- Nannoni, A.; Morelli, G.; Lattanzi, P.; Fagotti, C.; Friani, R.; Fornasaro, S.; Ciani, F.; Manca, R.; Monnanni, A.; Rimondi, V.; et al. Toxic trace elements transport in stream sediments from the world-class Monte Amiata Hg mining district: Potential impact to the Mediterranean Sea. Environ. Pollut. 2025, 372, 126088. [Google Scholar] [CrossRef]
- Ordóñez, A.; Álvarez, R.; Loredo, J. Soil pollution related to the mercury mining legacy at Asturias (Northern Spain). Int. J. Min. Reclam. Environ. 2014, 28, 389–396. [Google Scholar] [CrossRef]
- Rimondi, V.; Costagliola, P.; Lattanzi, P.; Morelli, G.; Cara, G.; Cencetti, C.; Fagotti, C.; Fredduzzi, A.; Marchetti, G.; Sconocchia, A.; et al. A 200 km-long mercury contamination of the Paglia and Tiber floodplain: Monitoring results and implications for environmental management. Environ. Pollut. 2019, 255, 113191. [Google Scholar] [CrossRef]
- Alvárez, C.R.; Moreno, M.J.; Alonso, L.L.; Gómara, B.; Bernardo, F.J.G.; Martín-Doimeadios, R.C.R.; González, M.J. Mercury, methylmercury, and selenium in blood of bird species from Doñana National Park (Southwestern Spain) after a mining accident. Environ. Sci. Pollut. Res. 2013, 20, 5361–5372. [Google Scholar] [CrossRef]
- Boente, C.; Albuquerque, M.T.D.; Gerassis, S.; Rodríguez-Valdés, E.; Gallego, J.R. A coupled multivariate statistics, geostatistical and machine-learning approach to address soil pollution in a prototypical Hg-mining site in a natural reserve. Chemosphere 2019, 218, 767–777. [Google Scholar] [CrossRef]
- Buono, F.; Pediaditi, K.; Carsjens, G.J. Local Community Participation in Italian National Parks Management: Theory versus Practice. J. Environ. Policy Plan. 2012, 14, 189–208. [Google Scholar] [CrossRef]
- Edge, S.; McAllister, M.L. Place-based local governance and sustainable communities: Lessons from Canadian biosphere reserves. J. Environ. Plan. Manag. 2009, 52, 279–295. [Google Scholar] [CrossRef]
- Hailemicheal, H.G.; Senbeta, F.; Negash, T.T.; Seyoum, A. The influence of socio-economic variables on protected area governance and outcomes: Insights from local communities and experts in Kafta-Sheraro National Park, Tigray, Northern Ethiopia. Sustain. Environ. 2025, 11, 2498782. [Google Scholar] [CrossRef]
- Hernes, M.I.; Metzger, M.J. Understanding local community’s values, worldviews and perceptions in the Galloway and Southern Ayrshire Biosphere Reserve, Scotland. J. Environ. Manag. 2017, 186, 12–23. [Google Scholar] [CrossRef]
- Shahi, K.; Khanal, G.; Jha, R.R.; Bhusal, P.; Silwal, T. What drives local communities’ attitudes toward the protected area? Insights from Bardia National Park, Nepal. Conserv. Sci. Pract. 2023, 5, e12883. [Google Scholar] [CrossRef]
- Chape, S.; Harrison, J.; Spalding, M.; Lysenko, I. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 443–455. [Google Scholar] [CrossRef]
- Chen, R.; Peng, Y.; Ren, Q.; Wu, J. Optimizing global protected areas to address future land use threats to biodiversity. Land Use Policy 2024, 154, 107560. [Google Scholar] [CrossRef]
- Casarim, R.; Caldeira, Y.M.; Pompeu, P.S. Representativeness of national parks in protecting freshwater biodiversity: A case of Brazilian savanna. Ecol. Freshw. Fish 2020, 29, 705–721. [Google Scholar] [CrossRef]
- Acreman, M.; Hughes, K.A.; Arthington, A.H.; Tickner, D.; Dueñas, M.-A. Protected areas and freshwater biodiversity: A novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 2020, 13, e12684. [Google Scholar] [CrossRef]
- Alic, E.; Trottier, L.L.; Twardek, W.M.; Bennett, L.L.; Chisholm, S.; Tremblay, P.; Tuononen, E.; Bennett, J.R.; Bowe, S.D.; Lennox, R.J.; et al. Recreational fisheries activities and management in national parks: A global perspective. J. Nat. Conserv. 2021, 59, 125948. [Google Scholar] [CrossRef]
- Echeverri, A.; Batista, N.M.; Wolny, S.; Herrera-R, G.A.; Andrade-Rivas, F.; Bailey, A.; Cardenas-Navarrete, A.; Dávila Arenas, A.; Díaz-Salazar, A.F.; Hernandez, K.V.; et al. Toward Sustainable Biocultural Ecotourism: An Integrated Spatial Analysis of Cultural and Biodiversity Richness in Colombia. People Nat. 2025, 7, 194–214. [Google Scholar] [CrossRef]
- Lei, P.; Zhong, H.; Duan, D.; Pan, K. A review on mercury biogeochemistry in mangrove sediments: Hotspots of methylmercury production? Sci. Total Environ. 2019, 680, 140–150. [Google Scholar] [CrossRef]
- Ackerman, J.T.; Eagles-Smith, C.A. Agricultural wetlands as potential hotspots for mercury bioaccumulation: Experimental evidence using caged fish. Environ. Sci. Technol. 2010, 44, 1451–1457. [Google Scholar] [CrossRef]
- Buck, D.G.; Evers, D.C.; Adams, E.; DiGangi, J.; Beeler, B.; Samánek, J.; Petrlik, J.; Turnquist, M.A.; Speranskaya, O.; Regan, K.; et al. A global-scale assessment of fish mercury concentrations and the identification of biological hotspots. Sci. Total Environ. 2019, 687, 956–966. [Google Scholar] [CrossRef]
- Chen, C.Y.; Driscoll, C.T.; Kamman, N.C. Mercury hotspots in freshwater ecosystems drivers, processes, and patterns. In Mercury in the Environment: Pattern and Process; Bamk, M., Ed.; University of California Press: Oakland, CA, USA, 2012; pp. 143–166. [Google Scholar]
- Evers, D.C.; Han, Y.-J.; Driscoll, C.T.; Kamman, N.C.; Goodale, M.W.; Lambert, K.F.; Holsen, T.M.; Chen, C.Y.; Clair, T.A.; Butler, T. Biological mercury hotspots in the northeastern United States and southeastern Canada. BioScience 2007, 57, 29–43. [Google Scholar] [CrossRef]
- Winder, V.L.; Anteau, M.J.; Fisher, M.R.; Wilcox, M.K.; Igl, L.D.; Ackerman, J.T. Wetland water-management may influence mercury bioaccumulation in songbirds and ducks at a mercury hotspot. Ecotoxicology 2020, 29, 1229–1239. [Google Scholar] [CrossRef]
- Enders, M.S.; Knickerbocker, C.; Titley, S.R.; Southam, G. The role of bacteria in the supergene environment of the Morenci porphyry copper deposit, Greenlee County, Arizona. Econ. Geol. 2006, 101, 59–70. [Google Scholar] [CrossRef]
- Gao, M.; Ning, Y.; Liu, C.; Song, X.; Xu, J.; Cui, L.; Liu, J. The “Fe-S wheel”: A new perspective on methylmercury production dynamics in subalpine peatlands. J. Hazard. Mater. 2025, 493, 138401. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.C.; González-Delgado, S.; Aliende-Hernández, M.; Alfonso, B.; Rufino-Navarro, A.; Hernández, C.A. Natural acidified marine systems: Lessons and predictions. Adv. Mar. Biol. 2024, 97, 59–78. [Google Scholar]
- Mikhailenko, A.V.; Ruban, D.A. Geoheritage in Deltaic Environments: Classification Notes, Case Example, and Geopark Implication. Environments 2019, 6, 18. [Google Scholar] [CrossRef]
- Taylor, K.G.; Perry, C.T.; Greenaway, A.M.; Machent, P.G. Bacterial iron oxide reduction in a terrigenous sediment-impacted tropical shallow marine carbonate system, north Jamaica. Mar. Chem. 2007, 107, 449–463. [Google Scholar] [CrossRef]
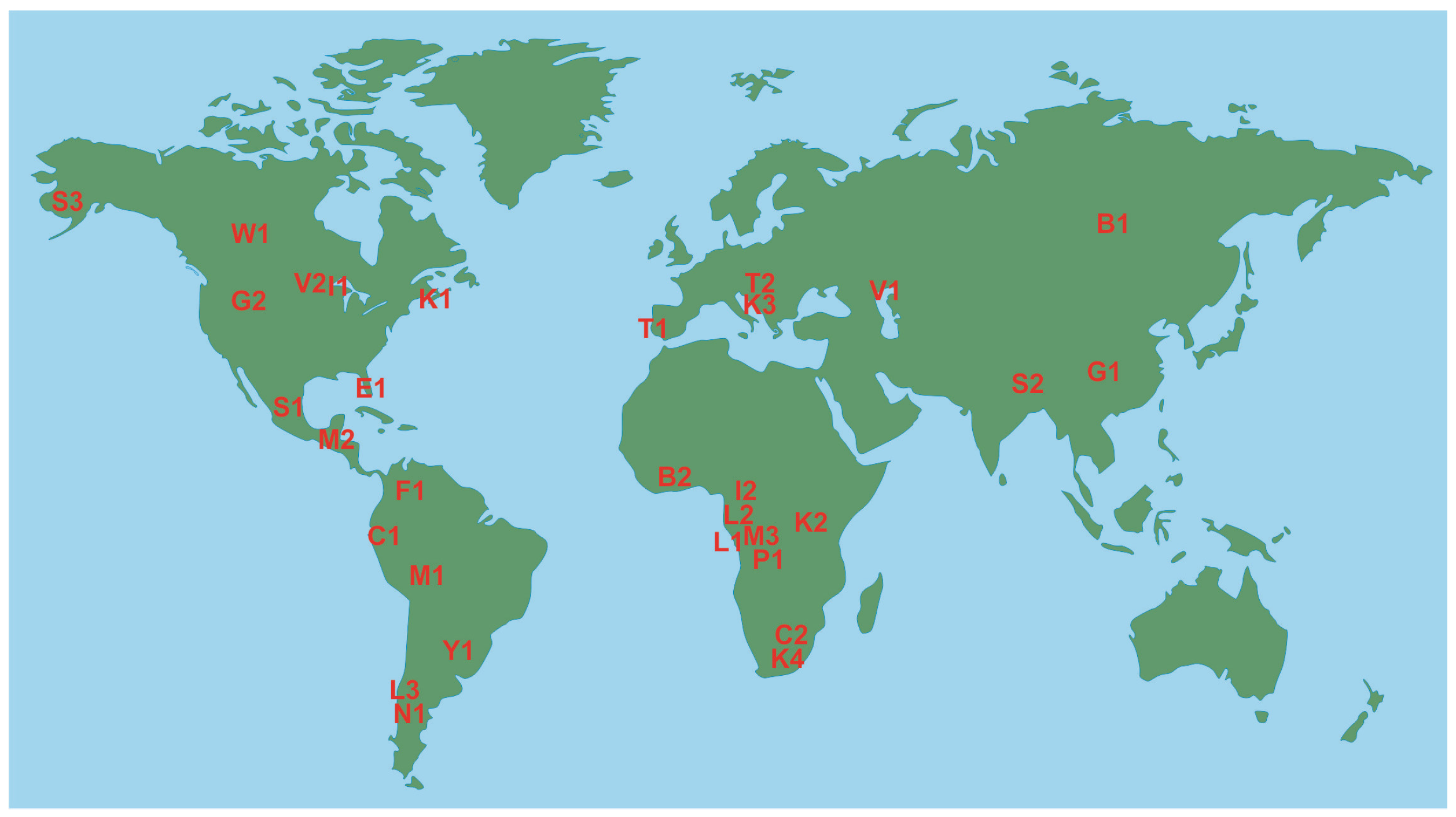
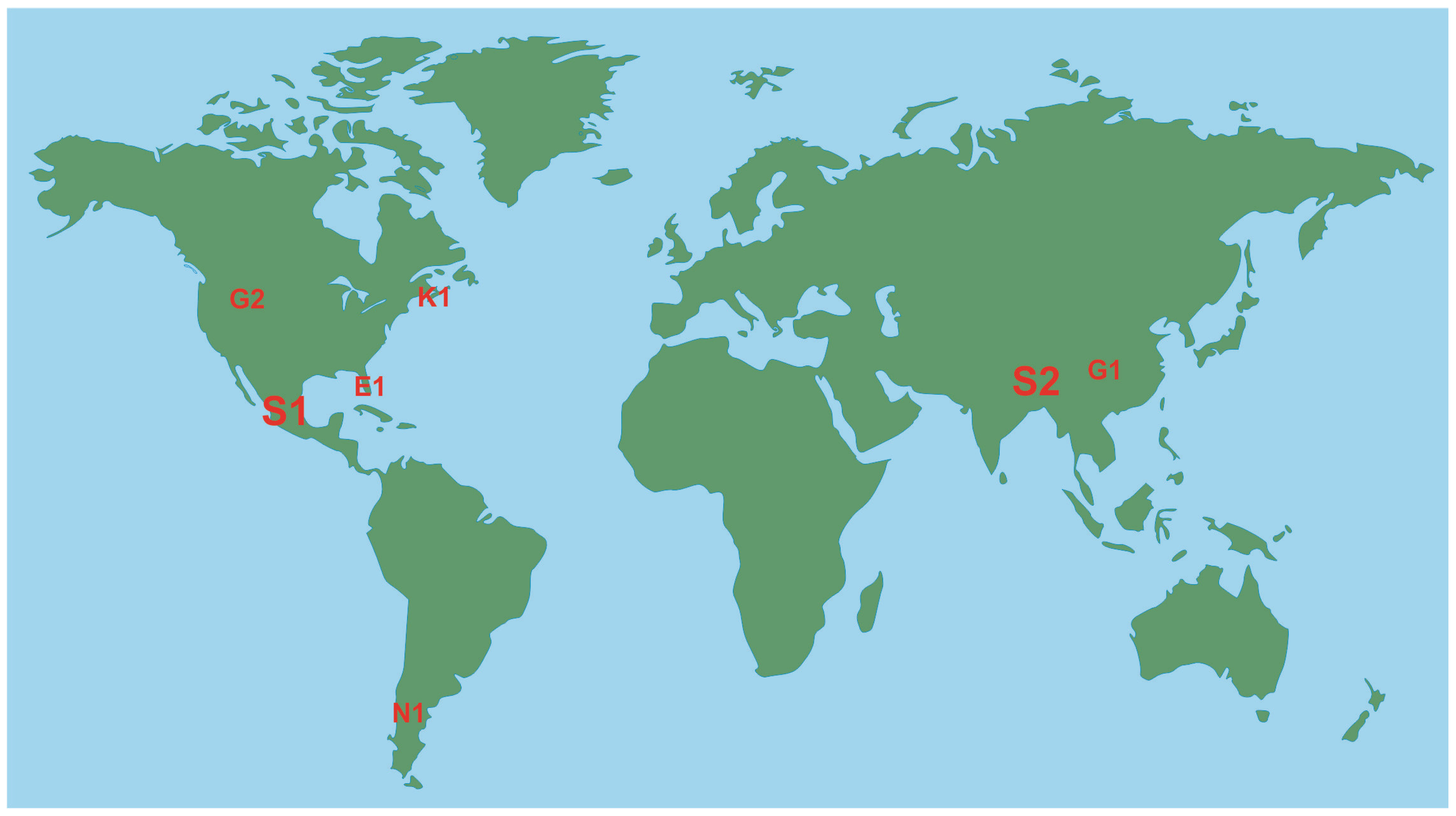
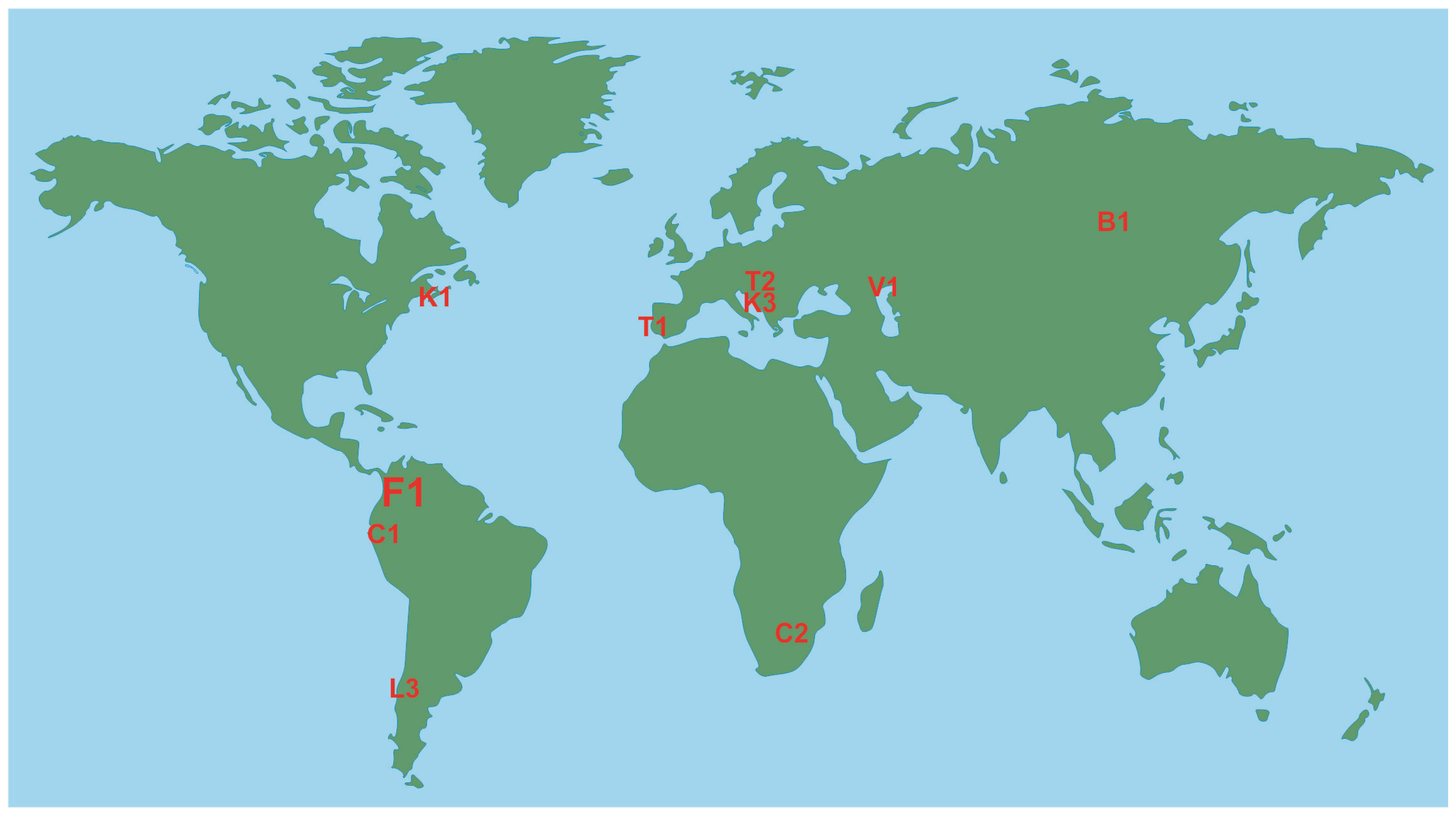


| ID | Protected Area | Country | Area, km2 | Geographical Domain | Considered Literature on Mercury in Freshwater Environments |
|---|---|---|---|---|---|
| B1 | Baikal (Pribaikalskiy) National Park | Russia | 4173 | Russian Siberia | [37] |
| B2 | Bui National Park | Ghana | 1821 | West African savannah | [38] |
| C1 | Cajas National Park | Ecuador | 285 | Andes | [39] |
| C2 | Chimanimani national parks * | Zimbabwe | - | Southern Africa | [40,41] |
| E1 | Everglades National Park | USA | 6105 | Florida | [42] |
| F1 | Farallones de Cali National Park | Colombia | 1500 | Andes | [43] |
| G1 | Giant Panda National Park ** | China | - | Qinling Mountains | [44] |
| G2 | Grand Teton National Park | USA | 1255 | Rocky Mountains | [45] |
| I1 | Isle Royale National Park | USA | 2314 | Great Lakes | [46] |
| I2 | Ivindo National Park | Gabon | 3000 | Central Africa | [38] |
| K1 | Kejimkujik National Park and Historic Site | Canada | 404 | Nova Scotia | [47,48,49,50,51,52] |
| K2 | Kidepo Valley National Park | Uganda | 1442 | Central Africa | [38] |
| K3 | Krka National Park | Croatia | 109 | Western Balkans | [53] |
| K4 | Kruger National Park | South Africa | 19,485 | Southern Africa | [54] |
| L1 | Loango National Park | Gabon | 1550 | Central Africa | [38] |
| L2 | Lope National Park | Gabon | 4912 | Central Africa | [38] |
| L3 | Los Alerces National Park | Argentina | 2596 | Patagonia | [55,56] |
| M1 | Manu National Park | Peru | 17,163 | Eastern Andes–Western Amazonia | [20] |
| M2 | Montes Azules Biosphere Reserve | Mexico | 3312 | Chiapas | [57] |
| M3 | Moukalaba-Doudou National Park | Gabon | 4500 | Central Africa | [38] |
| N1 | Nahuel Huapi National Park | Argentina | 7050 | Patagonia | [56,58,59] |
| P1 | Plateau Bateke National Park | Gabon | 2034 | Central Africa | [38] |
| S1 | Sierra Gorda Biosphere Reserve | Mexico | 3836 | Central Mexico | [60] |
| S2 | Singalila National Park | India | 204 | Darjeeling (West Bengal) | [61] |
| S3 | Southwest Alaska national parks * | USA | - | Alaska | [62] |
| T1 | Tagus Estuary Natural Reserve | Portugal | 142 | Iberian Peninsula | [63] |
| T2 | Triglav National Park | Slovenia | 880 | Alps | [64] |
| V1 | Volga-Akhtuba Natural Park | Russia | 1539 | Caspian Region | [65] |
| V2 | Voyageurs National Park | USA | 883 | West of Great Lakes (Minnesota) | [46] |
| W1 | Wood Buffalo National Park | Canada | 44,741 | Boreal Plains | [66] |
| Y1 | Yabotí Biosphere Reserve | Argentina | 2216 | Misiones’s forests | [67] |
| ID | Concentrations *, ** | Explanations *** | Literature |
|---|---|---|---|
| E1 | Hg = 0.59–1.08 ng/L MeHg = 0.05–0.28 ng/L | Different water objects; range reflects differences in mean values for 11 years between geographic regions | [42] |
| G1 | Hg = 30–250 ng/L | Streams; range reflects differences in mean values between areas | [44] |
| G2 | Hg ~ 0.2–0.6 ng/L | Meltwater and streams; range reflects differences in values between different types of water objects, different localities, and different times of measurement | [45] |
| K1 | MeHg = 0.05–0.46 ng/g | Lakes and stream; range reflects differences in values between samples and sites | [47] |
| Hg = 1–2.28 ng/L MeHg = 0.03–0.09 ng/L | Lakes; range reflects differences in average values between lakes | [50] | |
| N1 | Hg = 40.7–363 ng/L MeHg = 0.01–0.30 ng/L | Streams; range reflects differences in values between samples | [58] |
| Hg = 16.8–268 ng/L MeHg = 0.01–0.16 ng/L | Lakes; range reflects differences in values between samples | ||
| Hg = 1.73–61.5 ng/L | Lakes; range reflects differences in values between samples | [59] | |
| S1 | Hg = 7000 ng/L | Tap water; maximum value | [60] |
| S2 | Hg = 10,000–16,000 ng/L | Lakes, man-made water body; range reflects differences in values between sampled water objects | [61] |
| ID | Concentrations *, ** | Explanations *** | Literature |
|---|---|---|---|
| B1 | Hg = 10–25 ng/g | Island beach sand; range reflects differences in values between samples | [37] |
| C1 | Hg ~ 150–200 ng/g | Upper layers of lake sediments; range reflects differences in values between lakes | [39] |
| C2 | Hg = 40 ng/g | Stream sediments; mean value | [41] |
| F1 | Hg = 5.3–2200 ng/g | Stream sediments; range reflects differences in values between samples | [43] |
| K1 | MeHg = 0.02–28.94 ng/g | Lake and stream sediments; range reflects differences in values between samples and sites | [47] |
| K3 | Hg = 36 ng/g | Lake sediments; mean value | [53] |
| L3 | Hg = 81 ng/g | Upper layers of lake sediments; maximum value | [55] |
| T1 | Hg < 1000 ng/g MeHg < 4.4 ng/g | Estuary sediments; common values | [63] |
| T2 | Hg = 30 ng/g | Lake sediments; mean value | [64] |
| V1 | Hg = 180–750 ng/g | Shallow channel sediments; range reflects differences in values between channels | [65] |
| ID | Concentrations *, ** | Explanations *** | Literature |
|---|---|---|---|
| B2 | Hg = 258–495 ng/g | Crocodiles, blood; range reflects differences in values between samples | [38] |
| E1 | MeHg = 59.8–163.5 ng/g | Fishes; range reflects differences in mean values for 2018 between geographic regions and species | [38] |
| F1 | Hg = 0.4–42.6 ng/g | Frogs, muscle tissues; range reflects differences in values between species and sites | [43] |
| I1 | Hg = 1034 ng/g MeHg = 311 ng/g | Fishes, liver; mean value | [46] |
| I2 | Hg = 2025–10,162 ng/g | Crocodiles, blood; range reflects differences in values between samples | [38] |
| K1 | MeHg = 14.28–276.96 ng/g | Insects; range reflects differences in values between samples, sites, and groups of insects | [47] |
| MeHg = 380–2000 ng/g | Fishes, brain; range reflects differences in values between samples | [48] | |
| Hg = 180–2130 ng/g | Fishes, muscles; range reflects differences in values between samples | ||
| Hg = 310–980 ng/g | Fishes, liver; range reflects differences in mean values between sites | ||
| Hg = 270–340 ng/g | Fishes, muscles; range reflects differences in mean values between species | [49] | |
| MeHg = 40–320 ng/g | Invertebrates; range reflects differences in mean values between groups of invertebrates | [51] | |
| MeHg = 40–2180 ng/g | Epilithic biofilm, macroinvertebrates, zooplankton; range reflects differences in mean values between organisms | [52] | |
| K2 | Hg = 253–1002 ng/g | Crocodiles, blood; range reflects differences in values between samples | [38] |
| K3 | Hg ~ 50 ng/g | Fishes, intestine; approximate maximum value | [53] |
| Hg ~ 120 ng/g | Parasites (acanthocephalans); approximate maximum value | ||
| K4 | Hg = 30–1800 ng/g | Crocodiles, eggs; range reflects differences in values between samples | [54] |
| L1 | Hg = 390–11228 ng/g | Crocodiles, blood; range reflects differences in values between samples of different species | [38] |
| L2 | Hg = 450–2328 ng/g | Crocodiles, blood; range reflects differences in values between samples | [38] |
| L3 + N1 | Hg = 2000–7600 ng/g | Aquatic plants; range reflects differences in values between samples | [56] |
| M1 | Hg = 320–3370 ng/g | Fishes, muscle tissues; range reflects differences in mean values between sites | [20] |
| M2 | Hg = 827.7 ng/g | Crocodiles; mean value | [57] |
| M3 | Hg = 739–4993 ng/g | Crocodiles, blood; range reflects differences in values between samples | [38] |
| P1 | Hg = 928–8608 ng/g | Crocodiles, blood; range reflects differences in values between samples | [38] |
| S3 | Hg = 101–3046 ng/g | Fishes, muscles; range reflects differences in values between samples | [62] |
| V2 | Hg = 2922 ng/g MeHg = 177 ng/g | Fishes, liver; mean value | [46] |
| W1 | Hg = 160–1440 ng/g | Birds, eggs; range reflects differences in values for 2017 between samples | [66] |
| Y1 | Hg = 1340 ng/g | Fishes, muscles; maximum value | [67] |
| ID | Proposed Sources * | Principal Factors of Pollution ** | Literature | ||
|---|---|---|---|---|---|
| Natural | Anthropogenic | Complex | |||
| B1 | Anthropogenic | + | [37] | ||
| B2 | Natural and anthropogenic | + | + | [38] | |
| C1 | Atmospheric deposition, mining, road transport | + | [39] | ||
| C2 | Illegal gold mining | + | [41] | ||
| E1 | Atmospheric deposition | + | [42] | ||
| F1 | Illegal gold mining | + | [43] | ||
| G1 | Combustion of coal, waste, fuel | + | [44] | ||
| G2 | Atmospheric deposition | + | [45] | ||
| I1 | Atmospheric deposition | + | [46] | ||
| I2 | Natural and anthropogenic, also oil extraction, agriculture | + | + | [38] | |
| K1 | Atmospheric deposition, local gold mining | + | + | [50,52] | |
| K2 | Natural and anthropogenic | + | + | [38] | |
| K3 | Anthropogenic | + | [53] | ||
| K4 | Long-distance river transportation | + | [54] | ||
| L1 | Natural and anthropogenic, also oil extraction, agriculture | + | + | [38] | |
| L2 | Natural and anthropogenic | + | + | [38] | |
| L3 | Anthropogenic fires, volcanism | + | + | [55] | |
| M1 | Artisanal and small-scale gold mining | + | [20] | ||
| M2 | Boat transport | + | [57] | ||
| M3 | Natural and anthropogenic | + | + | [38] | |
| N1 | Volcanism | + | [59] | ||
| P1 | Natural and anthropogenic | + | + | [38] | |
| S1 | Mercury mining | + | [60] | ||
| S2 | Atmospheric deposition, cold trapping | + | [61] | ||
| S3 | Volcanism, other natural | + | [62] | ||
| T1 | Local industrial activity | + | [63] | ||
| T2 | Geological, agriculture and sewage leaching | + | + | [64] | |
| V1 | Anthropogenic | + | [65] | ||
| V2 | Atmospheric deposition | + | [46] | ||
| W1 | Various anthropogenic, long-distance river transportation | + | + | [66] | |
| Y1 | Anthropogenic | + | [67] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailenko, A.V.; Ruban, D.A. An Invisible Threat to Natural Heritage: Examples of Large Protected Areas with Hg-Enriched Freshwater Environments. Heritage 2025, 8, 384. https://doi.org/10.3390/heritage8090384
Mikhailenko AV, Ruban DA. An Invisible Threat to Natural Heritage: Examples of Large Protected Areas with Hg-Enriched Freshwater Environments. Heritage. 2025; 8(9):384. https://doi.org/10.3390/heritage8090384
Chicago/Turabian StyleMikhailenko, Anna V., and Dmitry A. Ruban. 2025. "An Invisible Threat to Natural Heritage: Examples of Large Protected Areas with Hg-Enriched Freshwater Environments" Heritage 8, no. 9: 384. https://doi.org/10.3390/heritage8090384
APA StyleMikhailenko, A. V., & Ruban, D. A. (2025). An Invisible Threat to Natural Heritage: Examples of Large Protected Areas with Hg-Enriched Freshwater Environments. Heritage, 8(9), 384. https://doi.org/10.3390/heritage8090384







