1. Introduction
One of the main forms of degradation affecting cellulosic textile substrates is depolymerisation caused by oxidation and hydrolysis phenomena. The breakdown of the cellulose chain occurs at the oxygen bridge between two β-D-glucopyranose units when the pH deviates significantly from neutrality. Hydrolysis readily takes place at ambient temperature, especially under humid conservation conditions and in the presence of acidity (e.g., air pollutants or oxidation products from pictorial materials). These processes act on the long cellulose chains, causing fragmentation and a progressive alteration of the fibres’ physical and mechanical properties, which is directly proportional to the extent of degradation. On a macroscopic level, this results in a loss of flexibility and an increase in the fragility of the textile support. For objects made from cellulose-based fabrics, this represents a serious issue in terms of the artwork’s structural integrity and preservation. In the case of canvas paintings, where the textile serves as the support for the pictorial layers, such degradation can result in serious damage to or even loss of the painted surface. In the past, highly depolymerised textile substrates were subjected to invasive and irreversible consolidation treatments. These involved the impregnation of cellulose fibres with natural or synthetic adhesive substances, or the drastic intervention of lining [
1]. The current trend towards minimal intervention favours a more conservative approach, whereby direct intervention on the product is limited to what is strictly necessary.
This includes the preventive action of treatment through deacidifying products on textile materials. The utilisation of these products enables the buffering and deceleration of depolymerisation through the neutralisation of carboxylic functions. This is achieved through the formation of insoluble salts (traditionally salts of Ca and Mg) in water. Additionally, a surface alkaline reserve with a buffer function is created. The objective is to maintain the surface pH within the range of 6.5 to 7.5, a range in which the hydrolysis of glycosidic bonds and the oxidation of cellulose are significantly reduced [
2,
3].
While the use of deacidification treatments in the conservation of paper artefacts has a tradition that can be traced back to the end of the nineteenth century, its use in the conservation of paintings is more recent. It was not until 1974 that Gustav Berger introduced the possibility of using this treatment for the conservation of paintings on canvas. Over the decades, the use of non-aqueous treatments, derived from the restoration of paper objects, has become increasingly common, given that the utilisation of water can result in the swelling of fibres, the contribution to alkaline hydrolysis reactions and the interaction with and swelling of the preparatory and pictorial layers [
4].
In light of these considerations, recent studies [
2,
5,
6,
7,
8,
9] have sought to explore the potential of some alternative formulations on textiles. These include techniques derived from the field of paper restoration, such as the Bookkeeper
TM method (Preservation Technologies, L.P., Cranberry, USA) [
10], and others with new formulations, such as nano-dispersions of alkaline earth metal hydroxides in non-polar and apolar solvents [
11,
12], which were developed within the framework of the European project NanoRestArt (CSGI Florence). A recent evaluation of these products has been conducted to ascertain their suitability for use in the restoration of paintings on canvas [
2,
13,
14], but there is a paucity of systematic and comparative studies in the existing literature that are useful for restorers in practice in terms of understanding the actual behaviour of these materials and the interaction they may have with the constituent materials. The objective of this study is, therefore, to evaluate and compare the behaviour of some of these products concerning their use on textile fibres, intending to simulate an application context as close as possible to that which occurs in the practice of restoration.
2. Materials and Methods
To evaluate the efficacy of three distinct commercially available deacidifying products, canvas mock-ups were constructed. The types of canvas were selected based on their historical utilisation as textile supports for paintings. The most representative materials for this purpose have been identified as flax, cotton and jute yarns [
15]; hemp was excluded because its physical–mechanical properties are like those of flax. The mock-ups, each measuring approximately 16 cm
2, were derived from unaltered raw canvases with minimal canvas reinforcement (1:1), referring to the weave structure of the selected fabrics, namely one weft thread for each warp thread (1:1 ratio) and were not subjected to any treatment. A total of 129 mock-ups were prepared, comprising 43 for each type of yarn. The specimens were subjected to a chemical degradation process based on the protocol proposed by Nechyporchuk [
16].
The procedure entailed the immersion of fabric specimens in a solution composed of hydrogen peroxide (35 wt%) and sulphuric acid (95–98%), mixed in varying ratios to induce acidification. The protocol identified in the literature was evaluated to determine the optimal proportion required to achieve a target surface pH of 4.
To identify the most suitable formulation for the specific experimental conditions, preliminary trials were conducted using both of the solutions described in the original reference: 1 mL of H2SO4 in 200 mL of H2O2 and 10 mL of H2SO4 in 200 mL of H2O2. These formulations were tested on four fabric samples (two linen and two cotton) to assess the resulting degree of acidification. The samples were immersed in the respective solutions for 72 continuous hours, followed by rinsing in deionised water for five minutes, repeated twice, to remove residual reagents.
Based on the results of these trials, the experimental protocol proceeded with the immersion of the mock-ups—divided by fibre type—in a solution consisting of 330 mL of H2O2 and 1.65 mL of H2SO4 for a total duration of 72 h. Following treatment, the samples were rinsed in demineralised water (5 min, repeated twice) and dried in a ventilated oven at a constant temperature of 40 °C for 48 h.
The products tested included the following:
- -
Bookkeeper Spray Products
TM → nano-particles of magnesium oxide (MgO) dispersed in and suspended in an inert liquid material (a blend of C
5-C
18 perfluoroalkanes) at a concentration of 4,3 gl
−1. The dispersion contains less than 0∙1% surfactant, in the form of a perfluorinated Mg-soap [
8].
- -
Nanorestore Paper
TM → Calcium hydroxide nano-dispersions available in both ethanol and 2-propanol formulations at 3 g/L and 5 g/L concentrations. In this study, products available in concentrations of 3 g/L in both ethanol and 2-propanol were selected for testing [
13].
The decision to utilise these products was predicated on the fact that they are both new-generation products, characterised by a nanostructured composition. The decision to select BookkeeperTM was based on the premise that, despite the absence of a concrete case study on the treatment of paintings on canvas, the product displays characteristics that suggest it could be a viable alternative. A comparison was therefore sought between its application for the treatment of textile fibres and that of NanorestoreTM products, which are currently one of the most widely used in Italian restoration.
The products were applied in accordance with the instructions provided in the respective data sheets. Although the claims reported by Nanorestore
TM dispersion manufacturers were known, the decision was made to simulate a process that would reflect the realistic conditions in which paintings on canvas could be treated in the context of a restoration laboratory. To ensure comparability between the mock-ups, a single application was conducted on both sides of each specimen. The products were stored in new, unopened bottles and shaken for two minutes before application [
11]. The Bookkeeper
TM solution was applied using the spray nozzle provided in the package, at a distance of 15 cm from the mock-ups. The two products, NanoRestorePaper
TM in ethanol and 2-propanol, were applied using both a brush and a spray, following the instructions provided by the manufacturer, which indicate both methods as possible [
17,
18]. The definition of sample codes based on products and application mode is indicated in
Table 1. The application protocol, the yarns and the product tested represent practical applications in the conservation of easel paintings.
The artificial ageing process was carried out by alternating two environmental conditions over time. For half of the duration between each measurement point, the samples were placed in a humidification chamber at room temperature (approximately 20–22 °C) with a relative humidity of 90%. For the remaining half, the samples were transferred to a ventilated oven maintained at a constant temperature of 60 °C under dry conditions.
The total ageing time was calculated based on this alternating exposure schedule and corresponded to the cumulative duration of both phases.
This protocol was designed to simulate realistic environmental fluctuations, such as those that may occur in uncontrolled storage or exhibition conditions, by exposing the samples to alternating cycles of high temperature with low relative humidity and high humidity at lower temperatures. This allowed for the monitoring and objective evaluation of the behaviour of the deacidifying agents under stress conditions that are representative of actual conservation contexts.
Given the objective of this study, which was to identify trends in the behaviour of the products under observation, the decision was made to conduct the data acquisition campaigns at progressive and exponential times of 1, 4, 11, 26, 57 and 128 days, resulting in a total ageing period of 3072 h. This sampling method is used in the field of chemistry to highlight both rapid variations (in the initial part of the curve) and slower changes (in the final part).
The data collection protocol included the measurement of surface pH, the evaluation of treatment-induced chromatic variation and the morphological observation of the surface. Data were acquired at each step of the process, commencing with the canvases before degradation and concluding with the final extraction from the artificial ageing cycle.
2.1. pH Measurements
The pH of the test pieces was determined using a Hanna Instrument HI14142 HALO flat-tip pH meter with a glass electrode and a 3% (
w/
v) agarose cylinder with a height of 0.45 cm [
18]. Ten measurements were obtained for each mock-up, from which the mean value was calculated. The instrument utilises a precision range of 4 to 9, with values falling below or above this threshold indicated as <4 and >9, respectively, during treatment.
2.2. Colourimetric Measurements
Colourimetric measurements were conducted utilising a Konica Minolta CM-700d portable contact spectropolarimeter calibrated with the Konica Minolta CM-A177 white standard. The measurements were conducted in the SCE mode within the L*a*b* colour space, utilising a 10° observer, standard illuminant D65 and an analysis diameter of 11 mm. Ten measurements were taken for each area. The colour differences between the various areas were calculated using both the classical formula and the latest CIEDE2000 formula, represented by the values ΔE and ΔE00 [
19,
20,
21].
2.3. Scanning Electron Microscopy with Energy-Dispersive X-Ray Spectroscopy (SEM/EDS)
The canvas samples were observed and analysed with a Zeiss EVO60 electron microscope, equipped with a lanthanum hexaboride source (LaB6) and a silicon drift detector (SDD), coupled with a 40 mm2 Ultim Max Oxford EDS microprobe for semi-quantitative elemental analysis. The samples were analysed without any preliminary treatment in a variable pressure environment, with an acceleration voltage of 20 kV and a pressure of 20 Pa.
3. Results and Discussion
3.1. pH Variation
The pH value of the surface of all samples was measured at each stage of the experiment. The initial pH values for the unprocessed fabrics were 7.24 for cotton, 7.19 for flax and 5.9 for jute, respectively. The chemical acidification process resulted in a reduction in these values to 4.67 for cotton, 4.55 for flax and <4 for jute. While cotton and flax fibres exhibited comparable pH and chemical degradation behaviour, jute displayed a more acidic profile from the outset. This is attributable to the lower proportion of cellulose present in jute fibres (58–63%) in comparison to flax (64.10–71.90%) and cotton (82.70–90%), coupled with a higher concentration of crusting substances (lignin, waxes, hemicellulose, etc.) [
18]. This characteristic renders jute less resilient to degradation than other cellulosic fibres.
To ensure comparability between the data sets, the processes applied to the fibres were maintained consistent across all samples, although it is correct to emphasise that the quantities applied on the same surfaces cannot be considered completely identical, as different application methods were used.
This choice was intentional and motivated by the desire to simulate an in operando condition, that is, an application carried out by a conservator according to the operational guidelines provided by the manufacturers of the tested products.
The most intriguing findings from this study pertain to the alteration in pH levels after the utilisation of the products above (
Table 2). The cotton samples to which Nanorestore products were applied exhibited a sudden increase in pH, reaching values exceeding 9, except the NE3B sample, which reached a value of 8.4 following treatment. The cotton swabs to which Bookkeeper was applied exhibited a comparatively modest initial pH increase, reaching a value of 6.75. The behaviour of the products to the flax samples was analogous: among the Nanorestore products, only NE3B reached a value below 9, specifically 7.38. Similarly, the application of Bookkeeper to flax resulted in a reduced pH rise, reaching 5.75. The jute displayed a distinctive behavioural pattern. BS, following its application, demonstrated a pH value below 4, whereas the spray-applied Nanorestore products reached values of 4.75 for 2-propanol and 4.70 for ethanol, respectively. The Nanorestore products applied by brush also resulted in a notable rise in pH. NP3B reached a value of 6.75, while NE3B reached 8.09. It is crucial to emphasise that it is vital to consider the relative delta between values, rather than solely focusing on the numerical value. This is particularly pertinent in the case of jute, where the pH values were markedly low.
In consideration of the data mentioned above, it is feasible to formulate an opinion regarding the requisite level of detail when utilising these products on the reverse of paintings on canvas. As previously highlighted by P. Cremonesi [
14], the potential for inducing or facilitating alkaline hydrolysis processes of cellulose chains is heightened in a pH range exceeding 9. Furthermore, when considering a painting on canvas, it is essential to consider the complex system of stratified and heterogeneous materials that can interact with an alkaline environment more severely. The necessity of assessing the compatibility between the materials and the substrate before treatment is already highlighted in the technical data sheet of the products, as recalled by the CSGI group [
22]. Considering the recommendations put forth by the manufacturers of Nanorestore
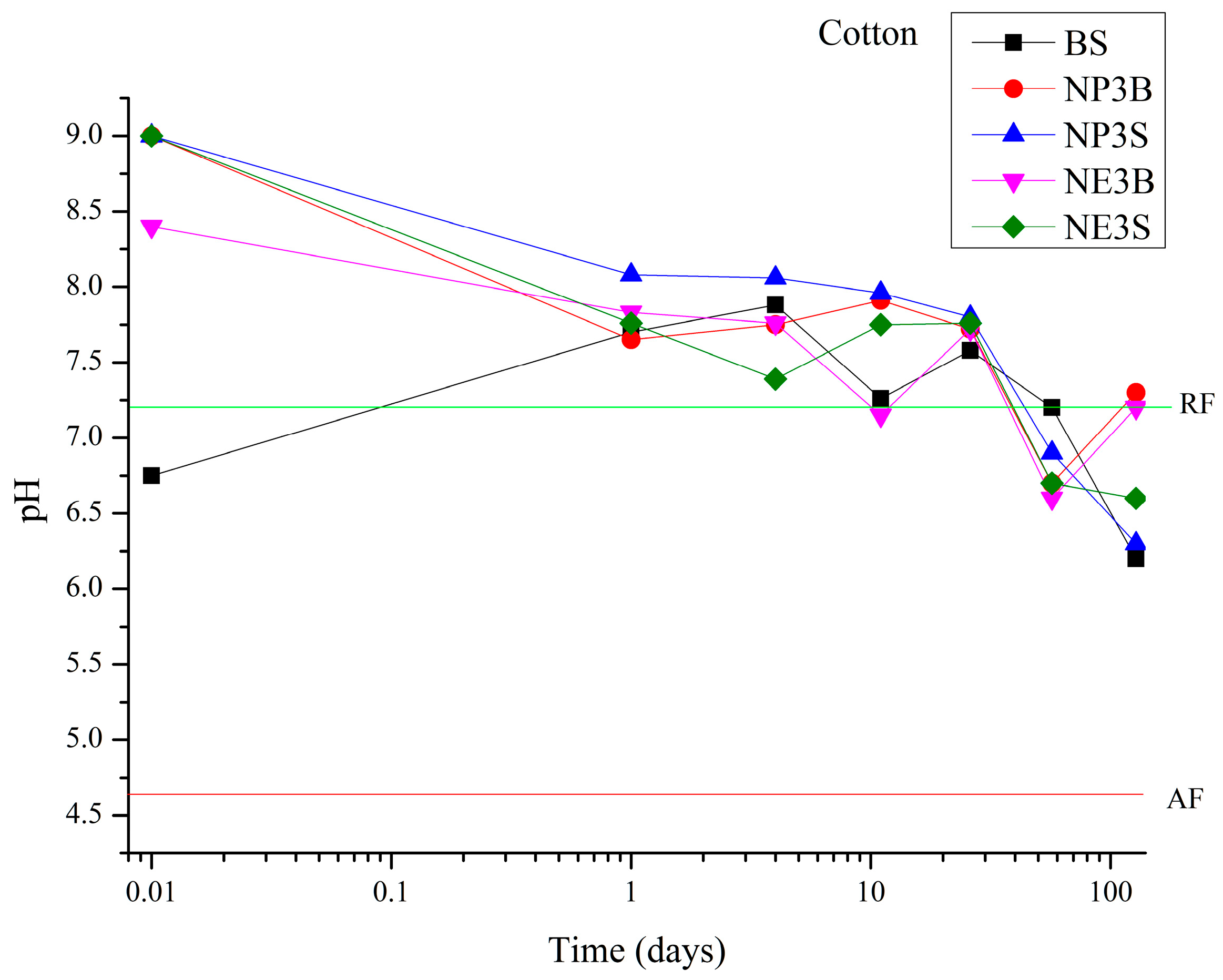
TM products, it is evident that the induction of a highly alkaline environment is not advised to minimise interaction with constructive materials. In contrast, the Bookkeeper formulation is gentler towards the substrate. One advantage of its composition is the use of magnesium oxide, which allows for a maximum pH value of 10.4, in comparison to calcium oxide, which allows for even higher values. Furthermore, the Bookkeeper formulation does not include solvents, which enables the inert liquid to evaporate without interacting with the original materials. The pH values of the samples were monitored at regular intervals throughout the artificial ageing process to gain insight into the behaviour of the products over time. Cotton fibre exhibited a decline in performance across all products, accompanied by a reduction in pH values from the fourth extraction onwards (26 days of ageing,
Figure 1). During the final extraction period (128 days), it was observed that the two Nanorestore products applied by brush were maintained at a pH value close to 7, while those applied by spray were maintained at a pH value near 6.5. BS exhibited a more pronounced decline in performance over the 128 days. Untreated cotton fibre subjected to the ageing cycle exhibited a pH value of 5.3, which was corroborated by comparison with the treated samples, thereby confirming the persistence of all products applied beyond 128 days.
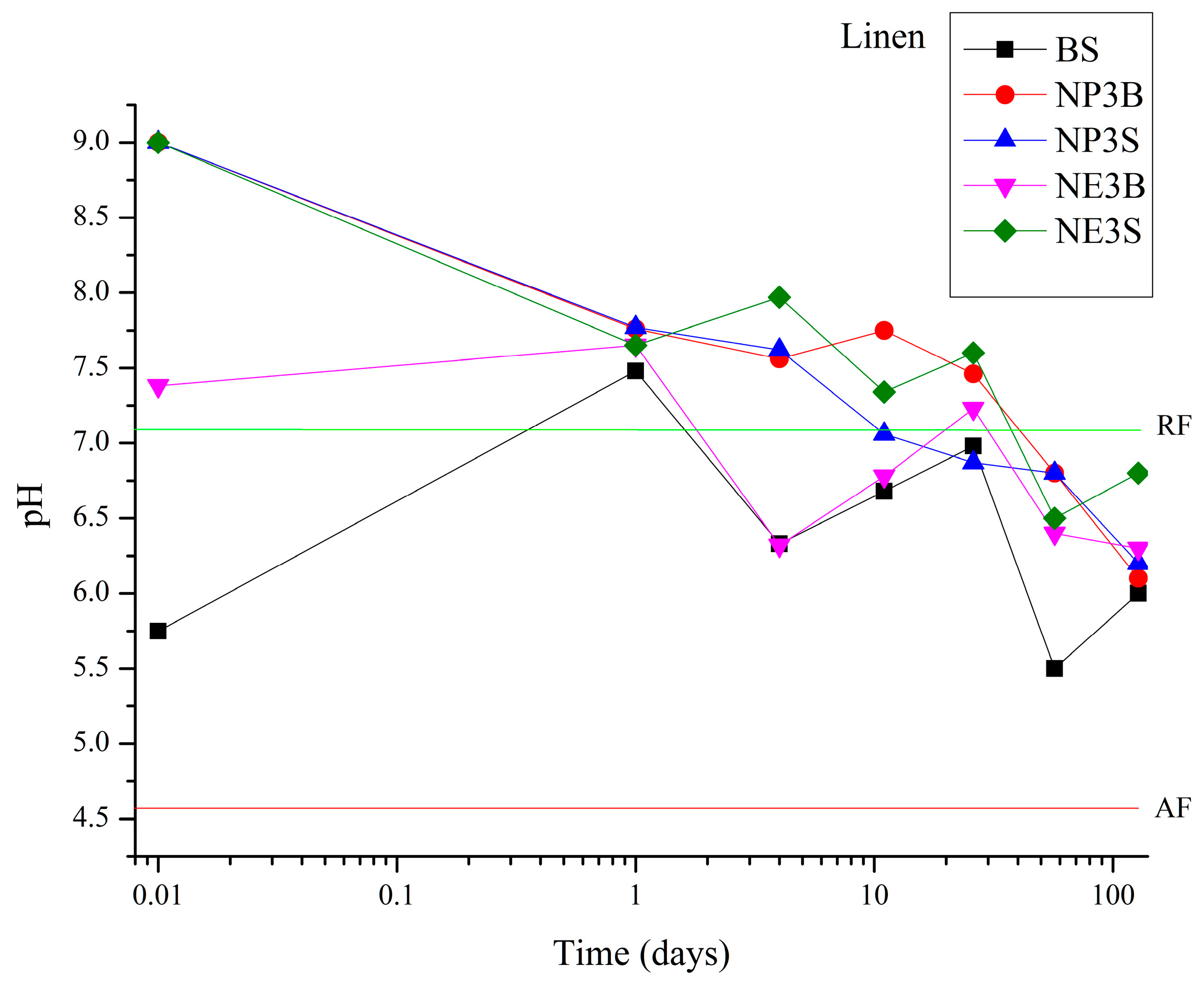
A similar trend was observed in the case of flax fibre (
Figure 2): after 26 days, a decline in pH was noted. In this instance, after 128 days, the BS, NP3B, NP3S and NE3B samples exhibited comparable pH values of approximately 6, except NE3S, which reported a higher value of 6.8.
Regarding the anomalous, and positive, result observed for the NE3B treatment compared to the other three Nanorestore Paper® treatments, the authors attributed it to the higher volatility of ethanol, which altered the rheological properties of the suspension on the brush, thereby reducing the amount of product transferred to the textile support.
As previously mentioned, these aspects are inherent to the application method for which these products were designed and inevitably reflect a certain degree of subjectivity, although controlled, inherent in manual application by the restorer.
The pH value of untreated aged fibre was observed to be 4.7 after 128 days of artificial ageing.
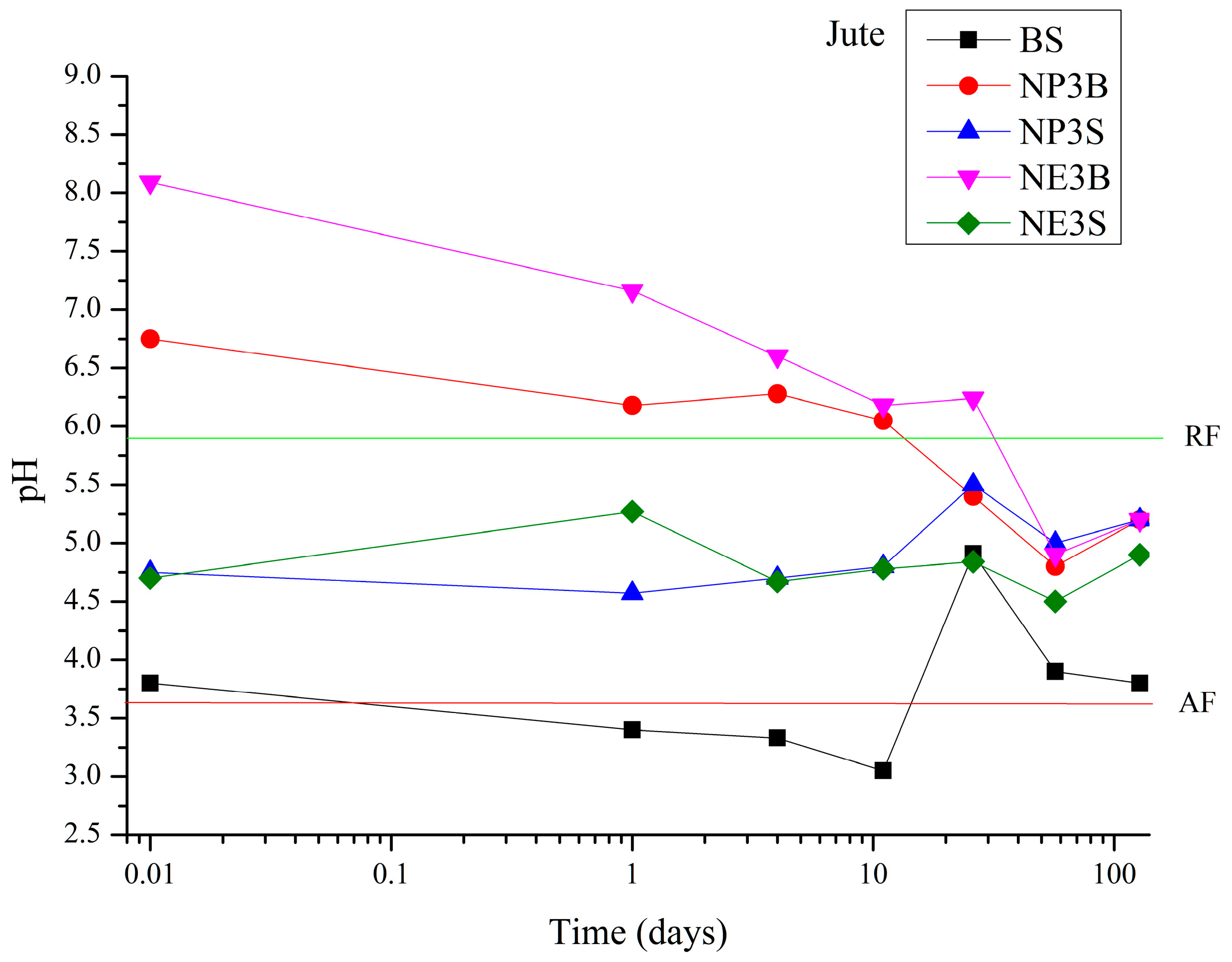
Jute fibre exhibited a distinct behavioural pattern, diverging from the observed trends in the other two fibres (
Figure 3). In this instance, Bookkeeper proved to be an ineffective agent, failing to elevate the pH values above 6. At the time of the third extraction, the pH value of the treated sample (3.8) was comparable to that of the aged but untreated fibre (3.6). The efficacy of Nanorestore products was observed to decline from the 26th day onwards; on the 128th day of ageing, the pH values remained at a level close to 5.
3.2. Colour Variation
After treatment, an initial macroscopic observation immediately revealed a change in the colour of the fabrics caused by the application of the products. The data obtained from the colourimetric measurements made after the treatment of the test specimens, when compared with the acidified but untreated fabrics, confirm notable colour variations caused by four of the five products tested (
Figure 4,
Figure 5 and
Figure 6).
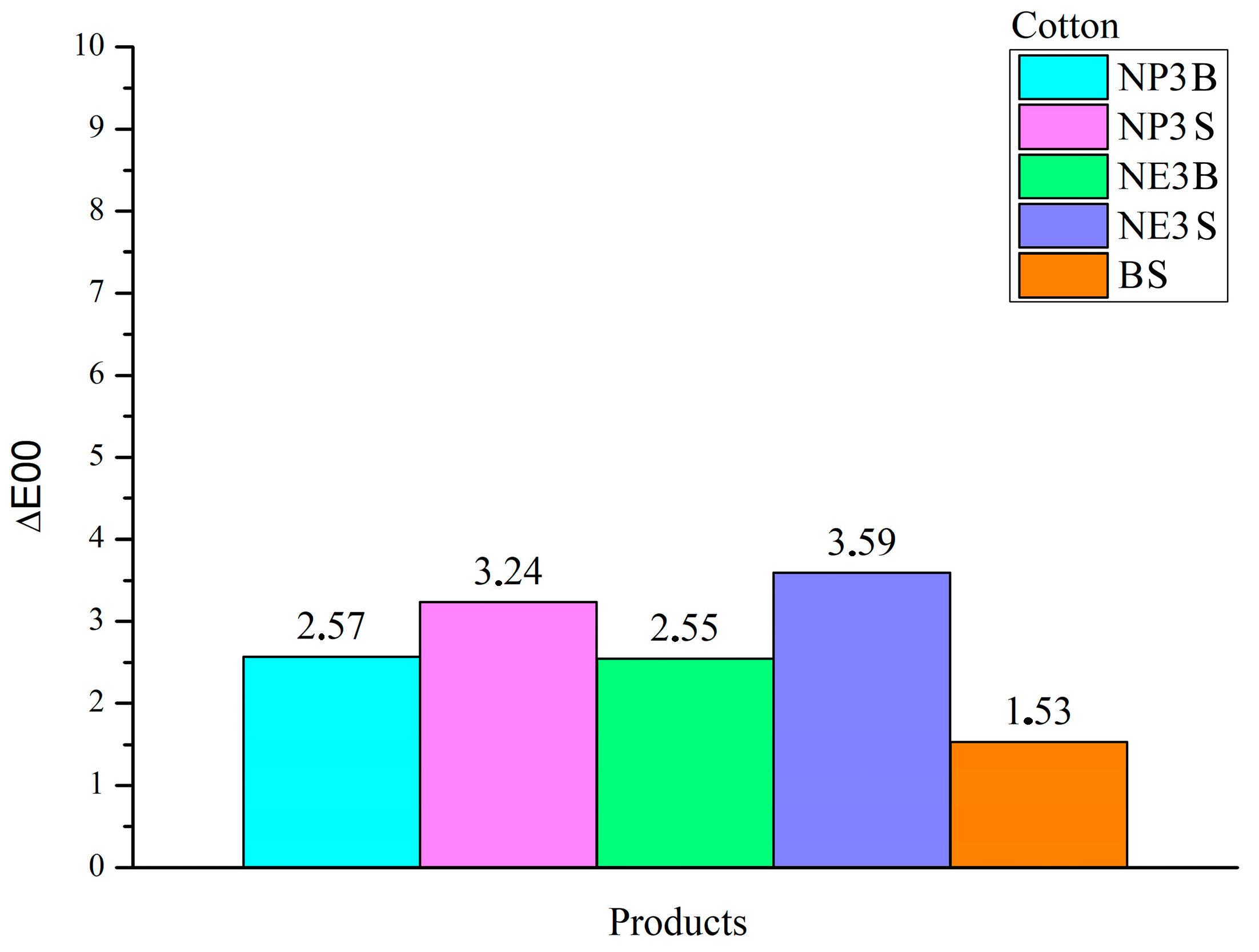
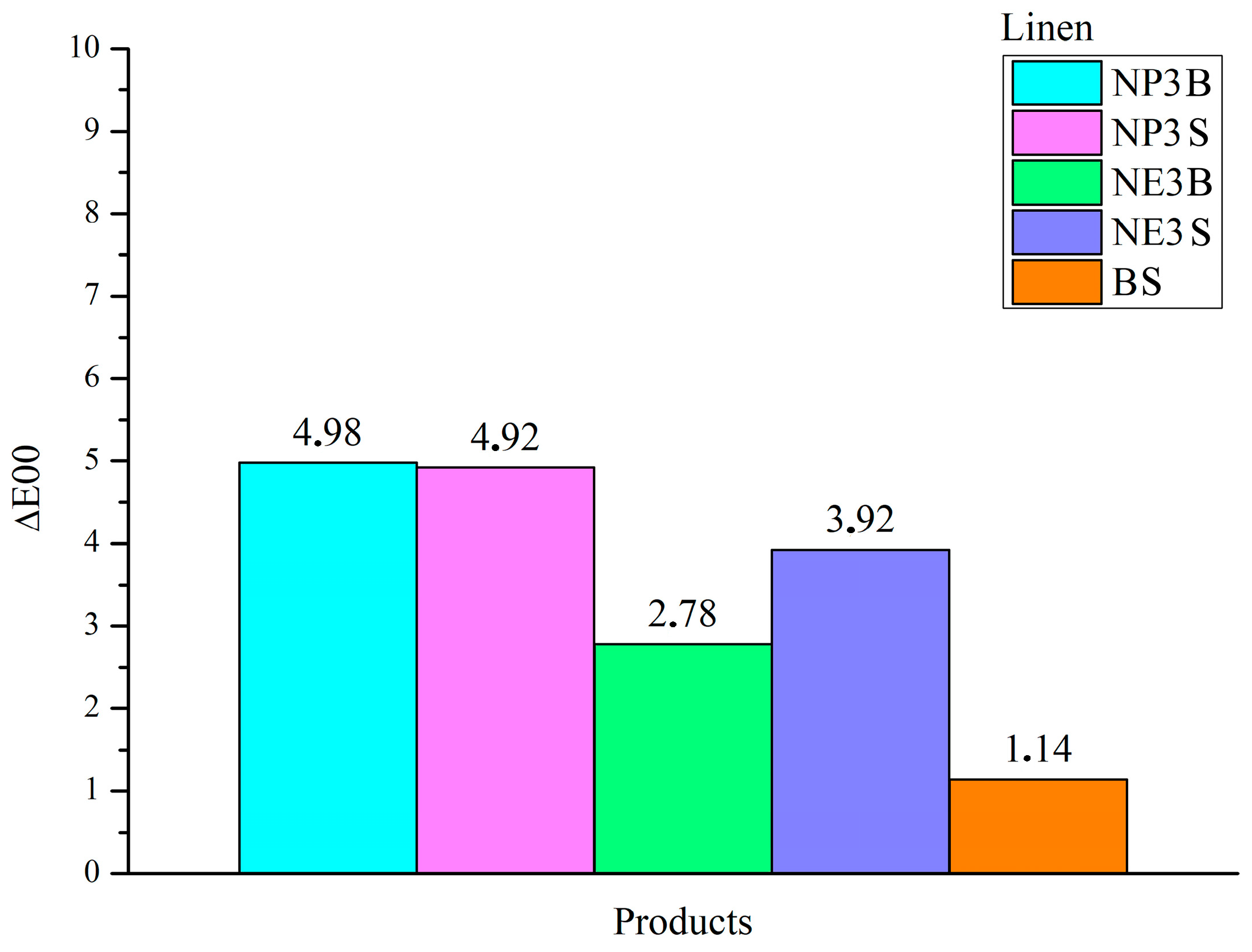
A value of ∆E00 > 1 was deemed a significant threshold, as it represents the point at which a colour change is perceived by the human eye. As for cotton fibres, all dispersions of the NanorestoreTM line in each type of solvent and application exhibited ∆E00 values > 2. BookkeeperTM-treated samples showed a variation, albeit only slightly above 1. The most pronounced variations were observed in the spray application treatments: NP3S exhibited ΔE00 3.24, while NE3S demonstrated a value of 3.59.
The linen samples exhibited a notable alteration in their chromatic characteristics following the application of the treatments, with the greatest impact observed in the case of the NanorestoreTM dispersions, which exhibited ΔE00 values that exceeded the significant threshold of 1. The 2-propanol dispersions exhibited the poorest performance. NP3B exhibited the highest value for ΔE00, reaching 4.98. The largest contribution to this result was made by the L*coordinate, which indicates a significant loss of brightness (ΔL* −4.64). Additionally, NP3S exhibited a notable ∆E00 value of 4.92, predominantly due to a discernible loss of brightness (∆L* −4.45). The ethanol dispersions exhibited a comparatively minor alteration in ΔE00 in comparison to the 2-propanol products yet remained > 1. Of note was the spray application (NE3S), with ΔE00 3.92, predominantly attributable to a loss of brightness and saturation (ΔL −3.99, Δa −2.24; Δb −1.11). In contrast, the application of BookkeeperTM resulted in a minimal change (∆E00 1.14).
The jute fibre exhibited a distinctive behaviour following the application of the NE3B product, displaying a pronounced colour alteration after the treatment (∆E00 7.25), predominantly due to a notable loss of brightness (∆L −7.93) and a reduction in warm tones (∆b* −7.59; ∆a* −2.68). The remaining NanorestoreTM dispersions exhibited a moderate alteration, whereas BookkeeperTM demonstrated a minimal change.
The data collected during the ageing cycle were compared with the measurements taken after the application of the products at zero time to monitor the colour variation as a function of time. In the case of cotton and linen fibres, the samples treated with BS exhibited considerable fluctuations in ΔE00, with an upward trend observable from the third extraction (11 days). The NanorestoreTM products demonstrated favourable ageing characteristics, exhibiting heightened variability when applied by brush in comparison to spraying. The treated jute samples exhibited notable alterations in colour over time for the NP3B, NP3S, NE3S and BS products. Once more, the general trend is a significant increase from the third extraction onwards, except for the NP3B-treated samples. Notably, the propanol dispersions exhibit particularly high ∆E00 values in comparison to those in ethanol. The samples treated with NE3B demonstrated the most favourable ageing performance, with a significantly lower ∆E00 at the final time point of 128 days in comparison to the other treatments.
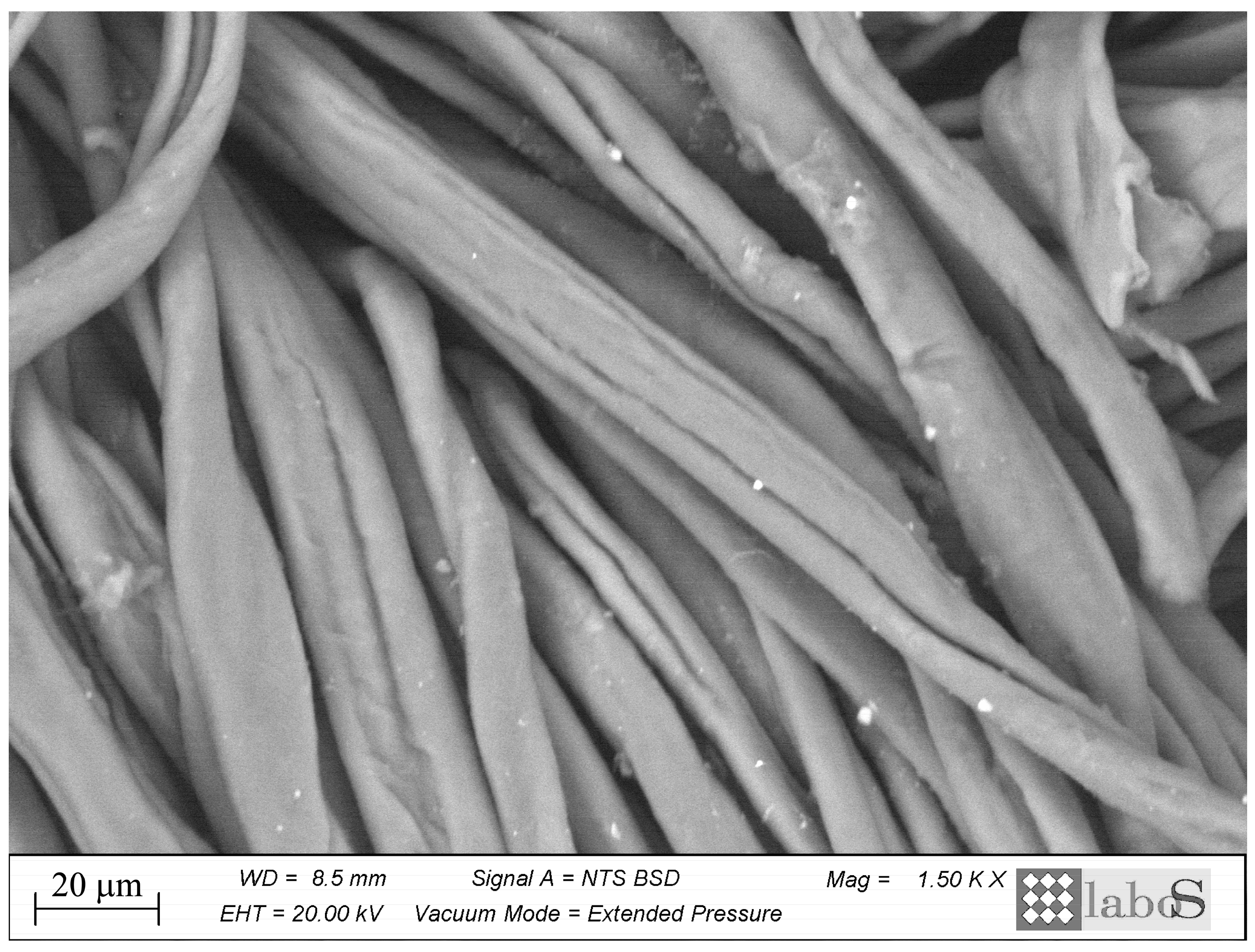
3.3. Morphological Observations (SEM-EDS)
Tissue samples were examined by scanning electron microscope and energy-dispersive X-ray spectroscopy (SEM-EDS) following the application of the products to ascertain the presence, distribution and appearance of the particles applied. Regarding BookkeeperTM, the particles were not readily discernible by morphological analysis in any of the three fibre types. Despite the presence of identifiable regions of Mg associated with F due to the product, no clusters of particles are visible. The NanorestoreTM products typically display the presence of clusters on the fibres, which can be discerned through morphological analysis and subsequently confirmed through elemental analysis, indicating the presence of Ca. The absence of agglomerates in the samples treated with Bookkeeper can be considered a direct consequence of the presence of a fluorinated surfactant in the formulation, as confirmed by the presence of fluorine (F) in the elemental analysis. The absence of surfactants in the formulation of Nanorestore products can be attributed to the clustering observed on the surface.
It may be surmised that a greater quantity of particles would be discernible on the cotton fibres, likely due to the denser weave of the fabric, which allows for a greater accumulation to be retained on the surface, in comparison to the jute, which is distinguished by a sparse weave. The linen fabric exhibits an intermediate weave in comparison to cotton and jute, which demonstrate a lesser degree of agglomeration. The samples that underwent a total ageing cycle of 57 days, corresponding to extraction no. 5, were examined by scanning electron microscopy to assess the differences in the time of application of the products. In the case of the samples treated with Bookkeeper
TM, there is no evidence of deposition, which is consistent with the observations made after application (
Figure 7). The samples treated with NanorestorePaper
TM demonstrate a modestly detectable deposit, which is still discernible (
Figure 8). This is the case for both cottons treated with a 2-propanol solution (applied by spray or brush) and for the linen treated with propanol by brush. The deposition of material on jute fibres and 2-propanol-treated linen that were treated by spraying is minimal or non-existent.
4. Conclusions
The objective of this study is to provide restorers with data that will assist informed decision-making regarding the selection of intervention materials. It is recognised that the data collected are representative only of the specific context under consideration. While maintaining full respect for the work of others, it is essential for restorers to be aware of the materials employed and to test them under realistic application conditions.
The results of this trial demonstrated that NanoRestorePaper products require the adoption of specific precautions during application which, in a practical context, may prove difficult to follow within the limits of a standard conservation laboratory. This could result in an excessive alkalinisation of the surface, potentially promoting alkaline hydrolysis mechanisms that may further compromise the textile support. The considerable variation in pH levels observed following application also suggests that these products should be applied with caution on canvas paintings, given the likely presence of paint layers. Indeed, many materials used within the pictorial stratigraphy may be sensitive to highly alkaline environments (e.g., azurite [
23]). Moreover, attention should be paid to the observed chromatic changes which, in addition to indicating interactions with the constituent materials, could raise concerns even at a purely aesthetic level. It is generally inadvisable to apply a product that may alter the original appearance of the material in question.
A comparison with BookkeeperTM indicated that it has a gentler impact on the surface, resulting in colour changes not perceptible to the naked eye. However, its efficacy appears to be of shorter duration, requiring reapplication at more frequent intervals. The presence of surfactants in the formulation aids distribution across the surface, while also contributing to the preservation of fluorinated compounds.
The aim of this study is not to provide definitive recommendations to restorers regarding material selection, but rather to compile systematic data to support informed and context-sensitive decision-making.