The Manufacture of Lake Pigments from Artificial Colours: Investigating Chemistry and Recipes in the First Book on Synthetic Dyes-Based Lakes
Abstract
1. Introduction
1.1. Contents of Jennison’s Book
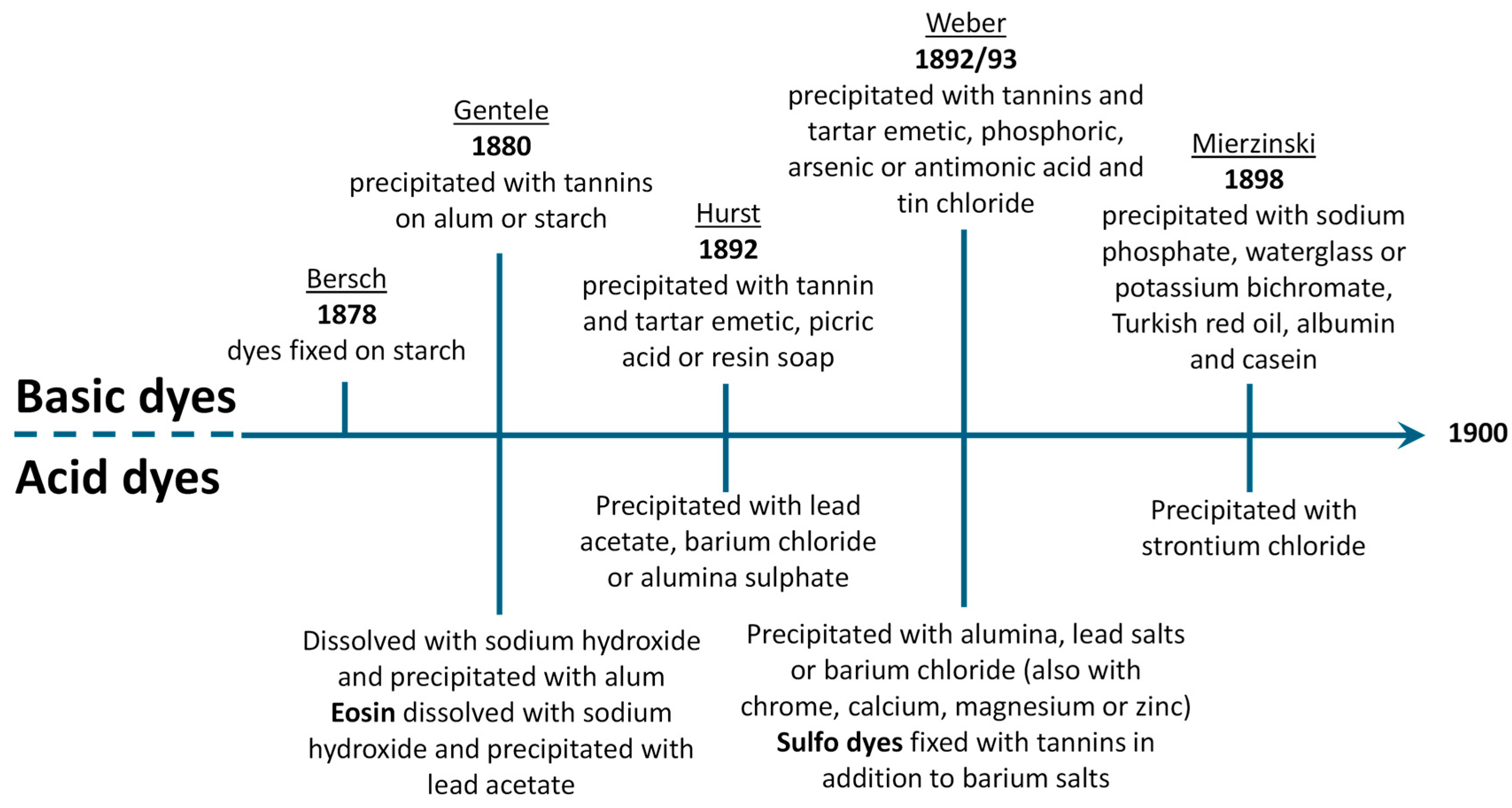
1.2. Earlier Literature on Lake Pigments
2. Materials and Methods
2.1. Provenance of the Books
2.2. Lake Pigments Investigated
2.3. Chemicals
2.4. Apparatus
2.4.1. HPLC-DAD and HPLC- ESI-Q-ToF
2.4.2. XRF
2.4.3. Raman
2.4.4. Colourimetry
3. Results
3.1. Inorganic Fillers, Precipitating Agents, or Inorganic Pigments
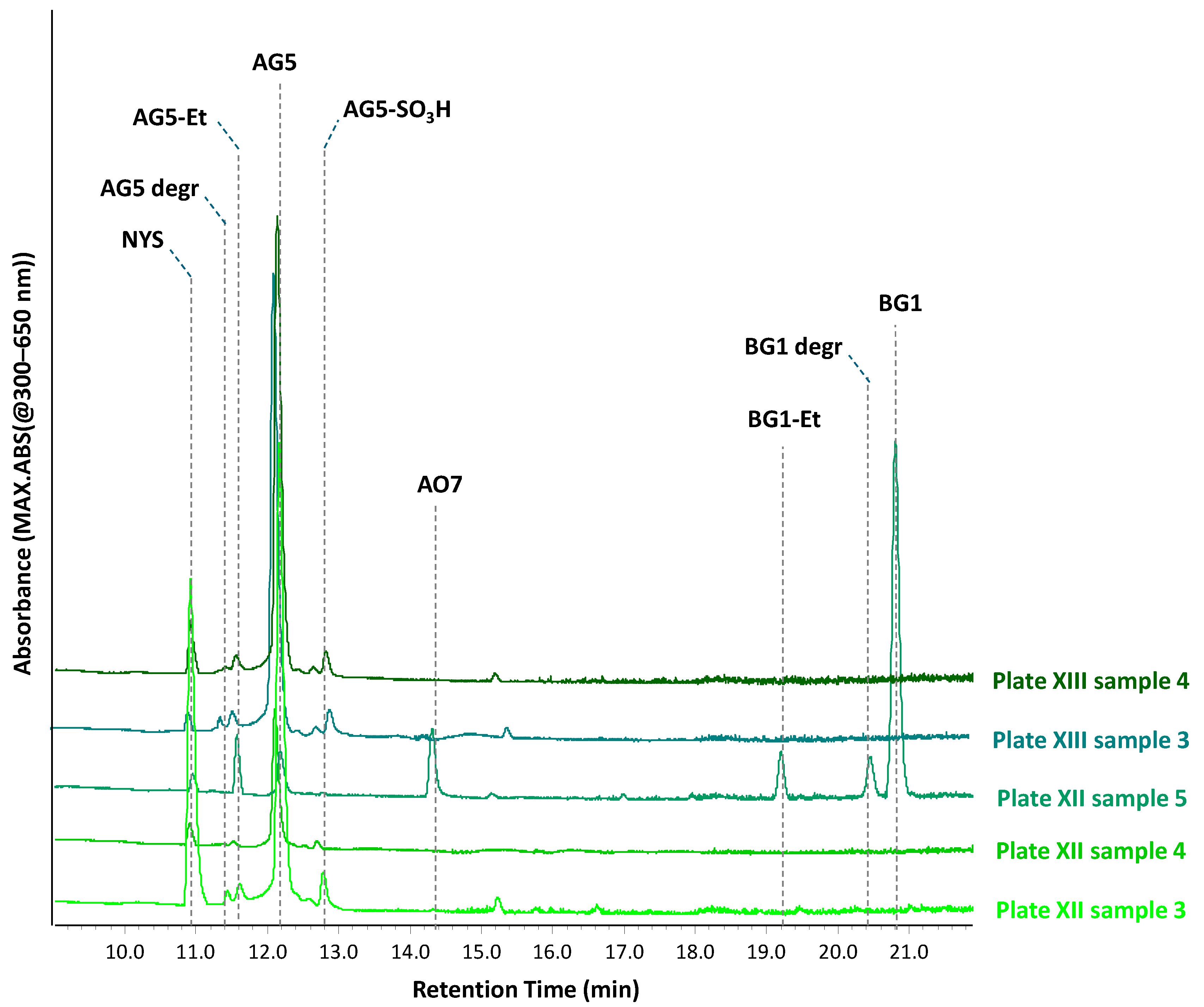
3.2. Synthetic Colourants
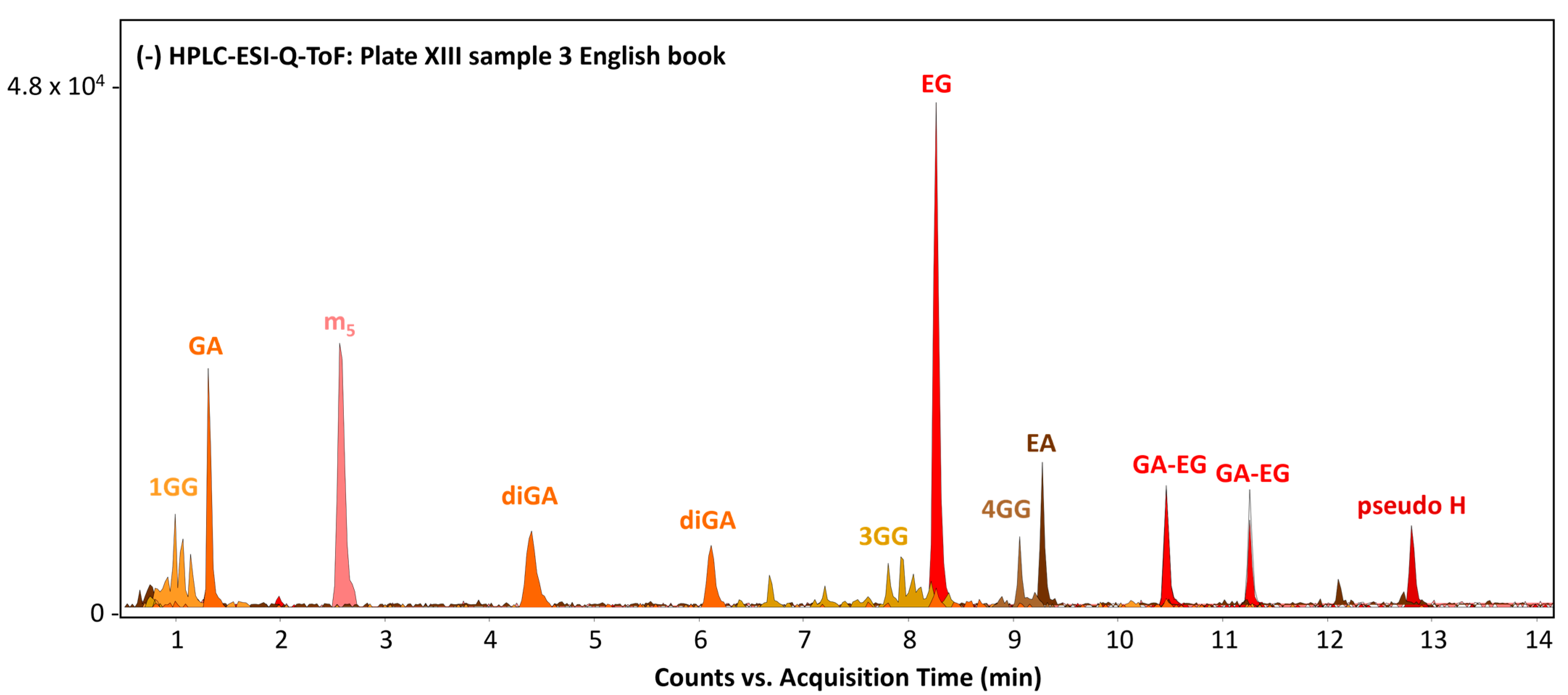
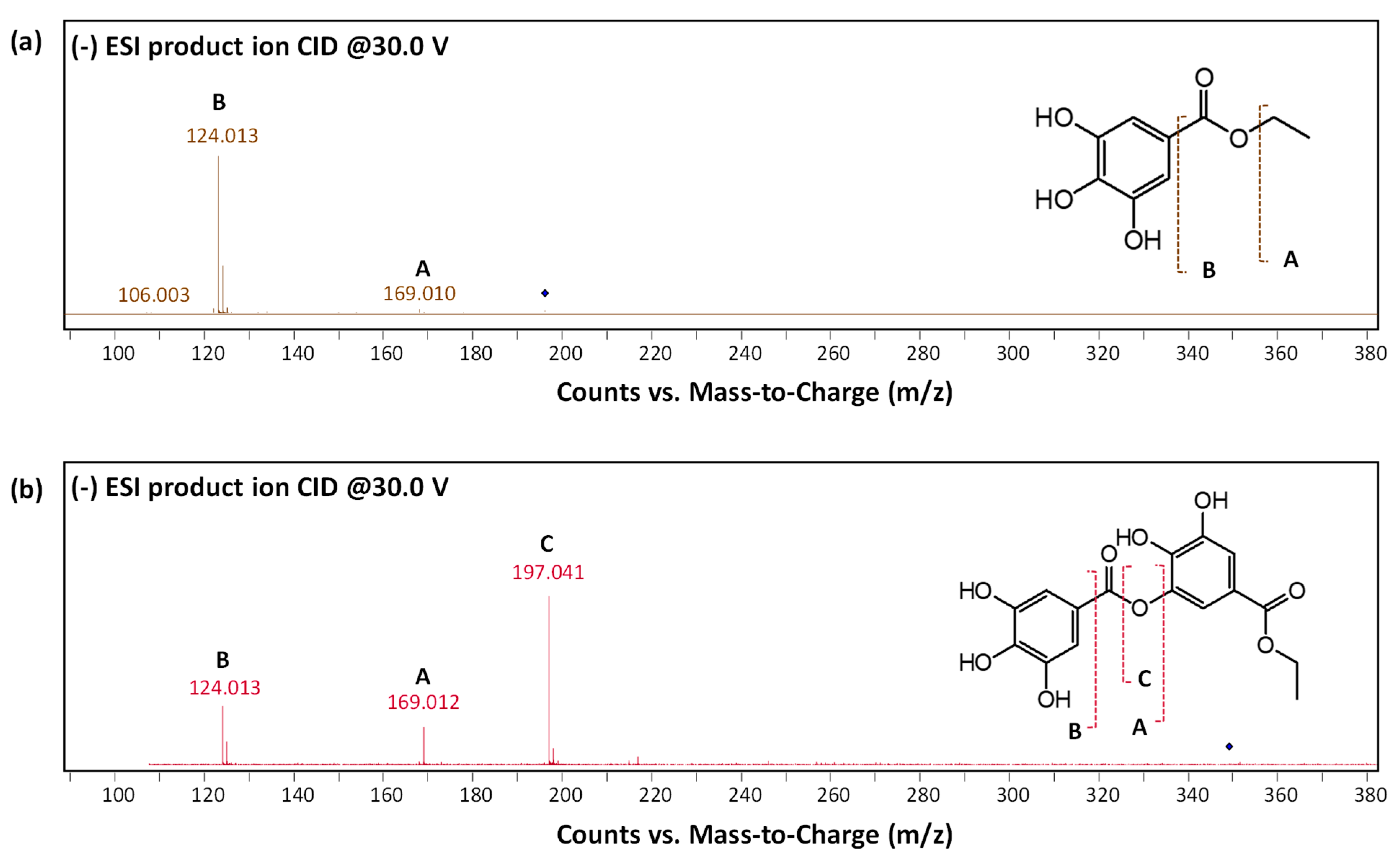
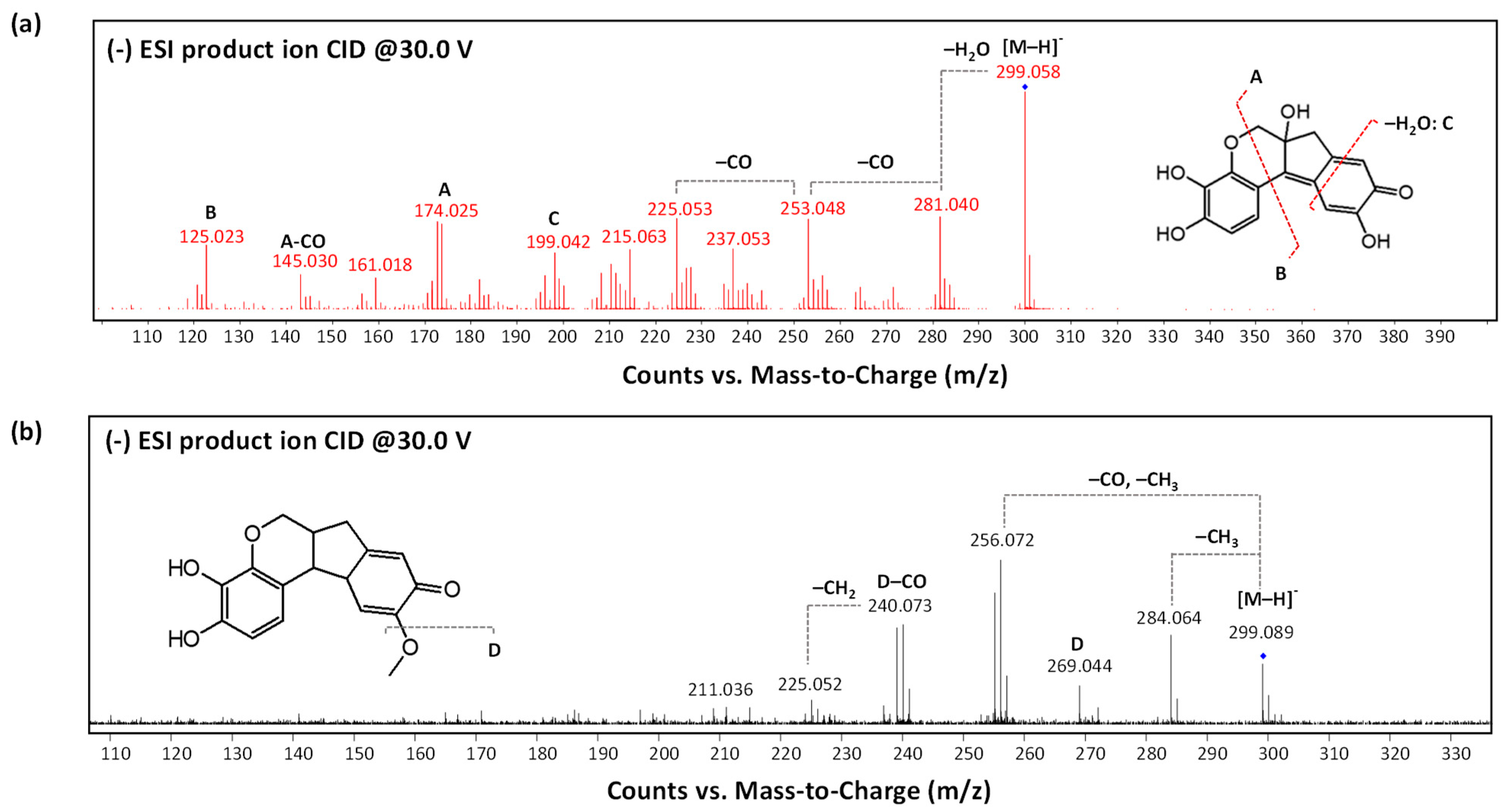
3.3. Natural Tannins
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Institute of Chemistry of Great Britain and Ireland. J. Proc. Part V 1939, 63, 435.
- Jennison, F.H. The Manufacture of Lake Pigments from Artificial Colours; Scott, Greenwood & Son: London, UK, 1900; Available online: https://ia801800.us.archive.org/26/items/manufactureoflak00jenn/manufactureoflak00jenn.pdf (accessed on 19 November 2024).
- Jennison, F.H. Die Herstellung von Farblacken aus künstlichen Farbstoffen; Rübencamp, R., Ed.; Steinkoppf & Springer: Dresden, Germany, 1901. [Google Scholar]
- Jennison, F.H. The Manufacture of Lake Pigments from Artificial Colours, 2nd ed.; Scott, Greenwood & Son: London, UK, 1920; Available online: https://archive.org/details/manufactureoflak00jenn (accessed on 19 November 2024).
- Eis, E. Die ‘Farben-Recepte‘ der Firma Heinrich Wiesel. Transkription und Auswertung der Rezeptsammlung eines Farbenfabrikanten aus dem Ausgehenden 19. Jahrhundert; Verlag Rockstuhl: Bad Langensalza, Germany, 2020; Appendix I. [Google Scholar]
- For Example the “Patentblau” Mentioned in a Recipe Dating from 1891 (See [5] WUL115/014/005-006) Was Discovered Three Years Earlier (German Patent DE000000046384A, Patented on the 18th of August 1888. Available online: https://depatisnet.dpma.de/DepatisNet/depatisnet?action=pdf&docid=DE000000046384A (accessed on 10 September 2024).
- Schonungen, Fabrikationsbuch Farbfabrik Sattler, G XVII/77. Available online: https://opac.dbu.de/ab/DBU-Abschlussbericht-AZ-35408_01-Hauptbericht.pdf (accessed on 17 June 2025).
- Neugebauer, W.; Sessa, C.; Steuer, C.; Allscher, T.; Stege, H. Naphthol Green—A Forgotten Artists’ Pigment of the Early 20th Century. History, Chemistry and Analytical Identification. J. Cult. Herit. 2018, 36, 153–165. [Google Scholar] [CrossRef]
- Cesaratto, A.; Leona, M.; Pozzi, F. Recent Advances on the Analysis of Polychrome Works of Art: SERS of Synthetic Colorants and Their Mixtures with Natural Dyes. Front. Chem. 2019, 7, 105. Available online: https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2019.00105/full (accessed on 4 December 2024). [CrossRef] [PubMed]
- Barnett, J.C. Synthetic organic dyes, 1856–1901: An introductory literature review of their use and related issues in textile conservation. Rev. Conserv. 2007, 52, 67–77. [Google Scholar] [CrossRef]
- Machill, S.; Voigtmann, M.; Nescholta, D.; Hartmann, H. Analysis of a Probe of the Historical Dye Aldehyde Green Deposited in the Historical Dyestuff Collection of the Technical University Dresden. Colorants 2025, 4, 15. [Google Scholar] [CrossRef]
- Berbers, S.V.J.; Proano Gaibor, A.N.; Ligterink, F.; Neevel, J.G.; Reissland, B.; van der Werf, I.D. Shades of violet: Study of the compositional variability of historical Methyl violet dyes. J. Cult. Herit. 2024, 66, 464–475. [Google Scholar] [CrossRef]
- Tamburini, D. On the reliability of historic books as sources of reference samples of early synthetic dyes—The case of “The Coal Tar Colours of the Farbwerke vorm. Meister, Lucius & Brüning, Höchst on the Main, Germany—A General Part” (1896). Dye. Pigment. 2024, 221, 111796. [Google Scholar] [CrossRef]
- Sundberg, B.N.; Pause, R.; van der Werf, I.D.; Astefanei, A.; Van den Berg, K.J.; van Bommel, M.R. Analytical approaches for the characterization of early synthetic organic pigments for artists’ paints. Microchem. J. 2021, 170, 106708. [Google Scholar] [CrossRef]
- Travis, A.S. The Rainbow Makers. The Origins of the Synthetic Dyestuff Industry in Western Europe; Lehigh University Press and Associated University Presses: Bethlehem, Israel; London, UK; Toronto, ON, Canada, 1993. [Google Scholar]
- Bersch, J. Die Fabrikation der Mineral- und Lackfarben. Wien, Pest, Leipzig 1878. Bersch, J. The Manufacture of Mineral and Lake Pigments; Wright, A.C., Scott, Greenwood & Son: London, UK, 1901. Available online: https://archive.org/details/manufactureofmin00bers/page/n5/mode/2up (accessed on 19 November 2024).
- Bersch, J. Die Fabrikation der Anilinfarbstoffe und aller Anderen aus dem There darstellbaren Farbstoffe (Phenyl-, Naphthalin-, Anthracen-und Resorcinfarbstoffe) und deren Anwendung in der Industrie; A. Hartleben’s Verlag: Wien, Austria; Pest, Hungary; Leipzig, Germany, 1878; Available online: https://books.google.de/books?id=9_hkG3eIBqoC&printsec=frontcover&hl=de&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 2 August 2024).
- Gentele, J.G. Lehrbuch der Farbenfabrikation; Friedrich Vieweg und Sohn: Braunschweig, Germany, 1880. [Google Scholar]
- Hurst, G.H. Painters’ Colours, Oils, and Varnishes: A Practical Manual; Charles Griffin & Company: Limited, London, UK, 1892; Available online: https://archive.org/details/painterscolourso00hursrich/page/n5/mode/2up (accessed on 27 November 2024).
- Weber, C.O. Untersuchungen über die Bildung der Farblacke. Polytech. J. 1892, 283, 159–163. Available online: https://dingler.bbaw.de/articles/ar283039.html (accessed on 10 May 2024).
- Mierzinski, S. Handbuch der Farbenfabrikation. Praxis und Theorie; Wien, A. Hartleben: Wien, Austria; Pest, Hungary; Leipzig, Germany, 1898; Volume II. [Google Scholar]
- Other authors use Turkish red oil as an additive in Alizarin lakes, but not as a precipitant. The use of “albuminous substances” is described in textile dyeing by Benedikt in 1886. In The Chemistry of the Coal-Tar Colours; Benedikt, R., Ed.; Knecht, E., Translator; George Bell and Sons: London, UK, 1886; pp. 52–53. Available online: https://archive.org/details/chemistrycoalta01knecgoog/page/n6/mode/2up (accessed on 19 August 2024).
- Olney, L.A. Textile Chemistry and Dyeing, 9th ed.; Lowell Textile Associates, US: Lowell, MA, USA, 1945; pp. 1874–1949. [Google Scholar]
- G. Siegle & Co GmbH Farbenfabriken Stuttgart. Wanderungen durch die Farben-und Lackindustrie; Stähle & Friedel: Stuttgart, Germany, 1921. [Google Scholar]
- Schultz, G.; Lehmann, L. Farbstofftabellen, 7th ed.; Akademische Verlagsgesellschaft m. b. H.: Leipzig, Germany, 1931; Volume 1. [Google Scholar]
- Ferretti, A.; Hunt, E.; Degano, I. Exploring the optimal HPLC-DAD-HRMS parameters for acid dye-based artistic materials: An analytical challenge. Microchem. J. 2024, 204, 111111. [Google Scholar] [CrossRef]
- Sabatini, F.; Giugliano, R.; Degano, I. Photo-oxidation processes of Rhodamine B: A chromatographic and mass spectrometric approach. Microchem. J. 2018, 140, 114–122. [Google Scholar] [CrossRef]
- Sabatini, F.; Degano, I.; van Bommel, M. Investigating the in-solution photodegradation pathway of Diamond Green G by chromatography and mass spectrometry. Color. Technol. 2021, 137, 456–467. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J.; Cartwright, C.; Green, A. Changes in the production materials of Burmese textiles in the nineteenth century—Dyes, mordants and fibres of Karen garments from the British Museum’s collection. Herit. Sci. 2023, 11, 1–31. [Google Scholar] [CrossRef]
- Teixeira, N.; Nabais, P.; de Freitas, V.; Lopes, J.A.; Melo, M.J. In-depth phenolic characterization of iron gall inks by deconstructing representative Iberian recipes. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Degano, I.; Mattonai, M.; Sabatini, F.; Colombini, M.P. A mass spectrometric study on tannin degradation within dyed woolen yarns. Molecules 2019, 24, 2318. [Google Scholar] [CrossRef] [PubMed]
- Hulme, A.N.; McNab, H.; Peggie, D.A.; Quye, A. Negative ion electrospray mass spectrometry of neoflavonoids. Phytochemistry 2005, 66, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, D.; Shimada, C.M.; McCarthy, B. The molecular characterization of early synthetic dyes in E. Knecht et al.’s textile sample book “A Manual of Dyeing” (1893) by high performance liquid chromatography—Diode array detector—Mass spectrometry (HPLC-DAD-MS). Dye. Pigment. 2021, 190, 109286. [Google Scholar] [CrossRef]
- Díaz Hidalgo, R.J.; Córdoba, R.; Nabais, P.; Silva, V.; Melo, M.J.; Pina, F.; Teixeira, N.; Freitas, V. New insights into iron-gall inks through the use of historically accurate reconstructions. Herit. Sci. 2018, 6, 63. [Google Scholar]
- Ferretti, A.; Sabatini, F.; Degano, I. Linking historical recipes and ageing mechanisms: The issue of 19th century iron gall inks, J. Cult. Herit. 2024, 67, 111–120. [Google Scholar] [CrossRef]
- Rübencamp, R.; Zerr, G. Handbuch der Farbenfabrikation; Union Deutsche Verlagsgesellschaft: Berlin, Germany, 1922; pp. 21–22. [Google Scholar]
- Bock, L. Herstellung von Buntfarben; Verlag von Wilhelm Knapp: Halle (Saale), Germany, 1927; p. 97. [Google Scholar]
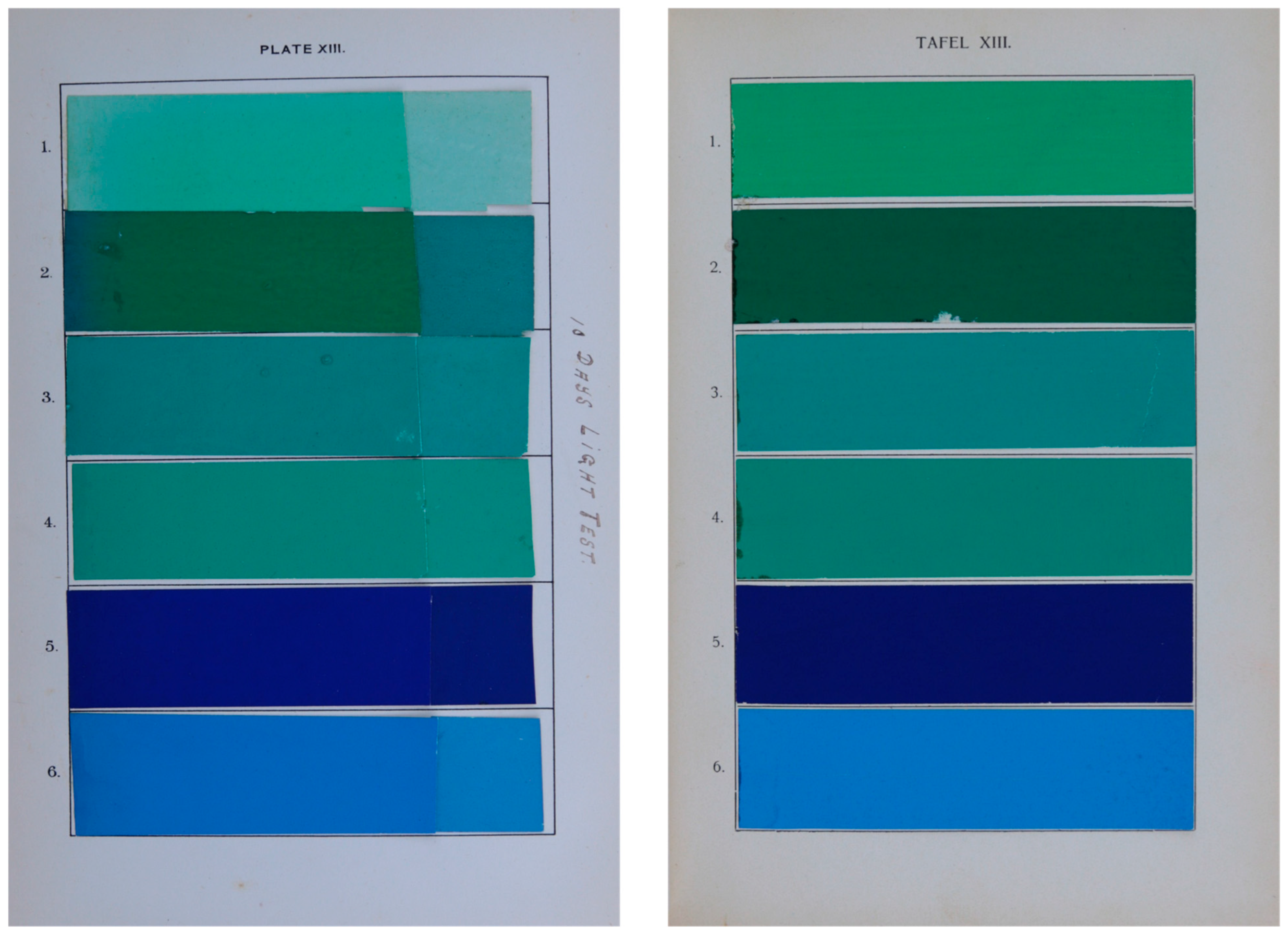
| Sample | Photo (English Book) | Photo (German Book) | Description | Results of Chemical Investigation | |
|---|---|---|---|---|---|
| English Book | German Book | ||||
| Plate XII, sample 3 |  |  | Lake pigment made of Acid green D tinted with Naphthol yellow S | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Pb, tannins | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Naphthol Yellow S (Acid Yellow 1, C.I. 13016, No. 19), Ba, S |
| Plate XII, sample 4 |  |  | Lake pigment made of Acid green D and Quinoline yellow | Diamond green (Basic Green 1, C.I. 42040, No. 760) Auramine O (Basic Yellow 2, C.I. 41000, No. 752), Pb, tannins | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Naphthol Yellow S (Acid Yellow 1, C.I. 13016, No. 19), Ba, S, barium sulphate * |
| Plate XII, sample 5 |  |  | Lake pigment of Acid green D and Metanil yellow, precipitated with barium and tannic acid | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Diamond green (Basic Green 1, C.I. 42040, No. 760) Auramine O (Basic Yellow 2, C.I. 41000, No. 752), Metanil yellow (Acid Yellow 36, C.I. 13065, No. 169), tannins | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Diamond green (Basic Green 1, C.I. 42040, No. 760), Naphthol Yellow S (Acid Yellow 1, C.I. 13016, No. 19), Orange II (Acid Orange 7, C.I. 15510, No. 189), Ba, S, Pb |
| Plate XIII, sample 3 |  |  | Barium lake of Acid Green D and Naphthol yellow S | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Ba, S, Pb, barium sulphate *, tannins | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Naphthol Yellow S (Acid Yellow 1, C.I. 13016, No. 19), Ba (traces) |
| Plate XIII, sample 4 |  |  | Barium lake of Acid green D and Naphthol yellow, but tannin and tartar were added to complete precipitation | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Ba, S, Pb, barium sulphate *, tannins | Light green SF yellowish (Acid Green 5, C.I. 42095, No. 765), Naphthol Yellow S (Acid Yellow 1, C.I. 13016, No. 19), Ba, S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eis, E.; Ferretti, A.; Sabatini, F.; Corona, V.; Legnaioli, S.; Laursen, R.; Degano, I. The Manufacture of Lake Pigments from Artificial Colours: Investigating Chemistry and Recipes in the First Book on Synthetic Dyes-Based Lakes. Heritage 2025, 8, 245. https://doi.org/10.3390/heritage8070245
Eis E, Ferretti A, Sabatini F, Corona V, Legnaioli S, Laursen R, Degano I. The Manufacture of Lake Pigments from Artificial Colours: Investigating Chemistry and Recipes in the First Book on Synthetic Dyes-Based Lakes. Heritage. 2025; 8(7):245. https://doi.org/10.3390/heritage8070245
Chicago/Turabian StyleEis, Eva, Adele Ferretti, Francesca Sabatini, Valentina Corona, Stefano Legnaioli, Richard Laursen, and Ilaria Degano. 2025. "The Manufacture of Lake Pigments from Artificial Colours: Investigating Chemistry and Recipes in the First Book on Synthetic Dyes-Based Lakes" Heritage 8, no. 7: 245. https://doi.org/10.3390/heritage8070245
APA StyleEis, E., Ferretti, A., Sabatini, F., Corona, V., Legnaioli, S., Laursen, R., & Degano, I. (2025). The Manufacture of Lake Pigments from Artificial Colours: Investigating Chemistry and Recipes in the First Book on Synthetic Dyes-Based Lakes. Heritage, 8(7), 245. https://doi.org/10.3390/heritage8070245








