Experimental Ancient Egyptian Human Mummification Tested in a Porcine Model: Excellent Preservation at a 13-Year Follow-Up
Abstract
1. Introduction
2. Material and Methods
2.1. Follow-Up Imaging
2.2. Histological Investigation
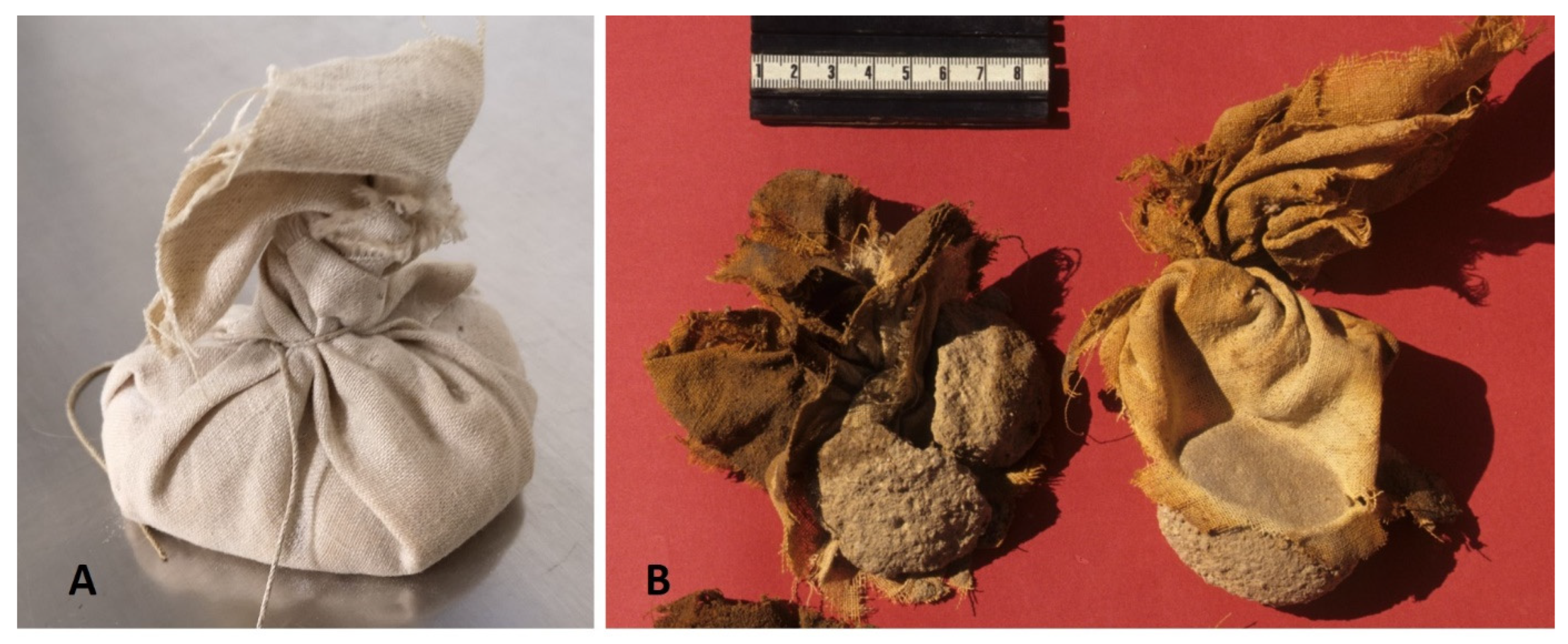
2.3. Microbiological Investigations
2.4. Evaluation of Preservation Quality
3. Results
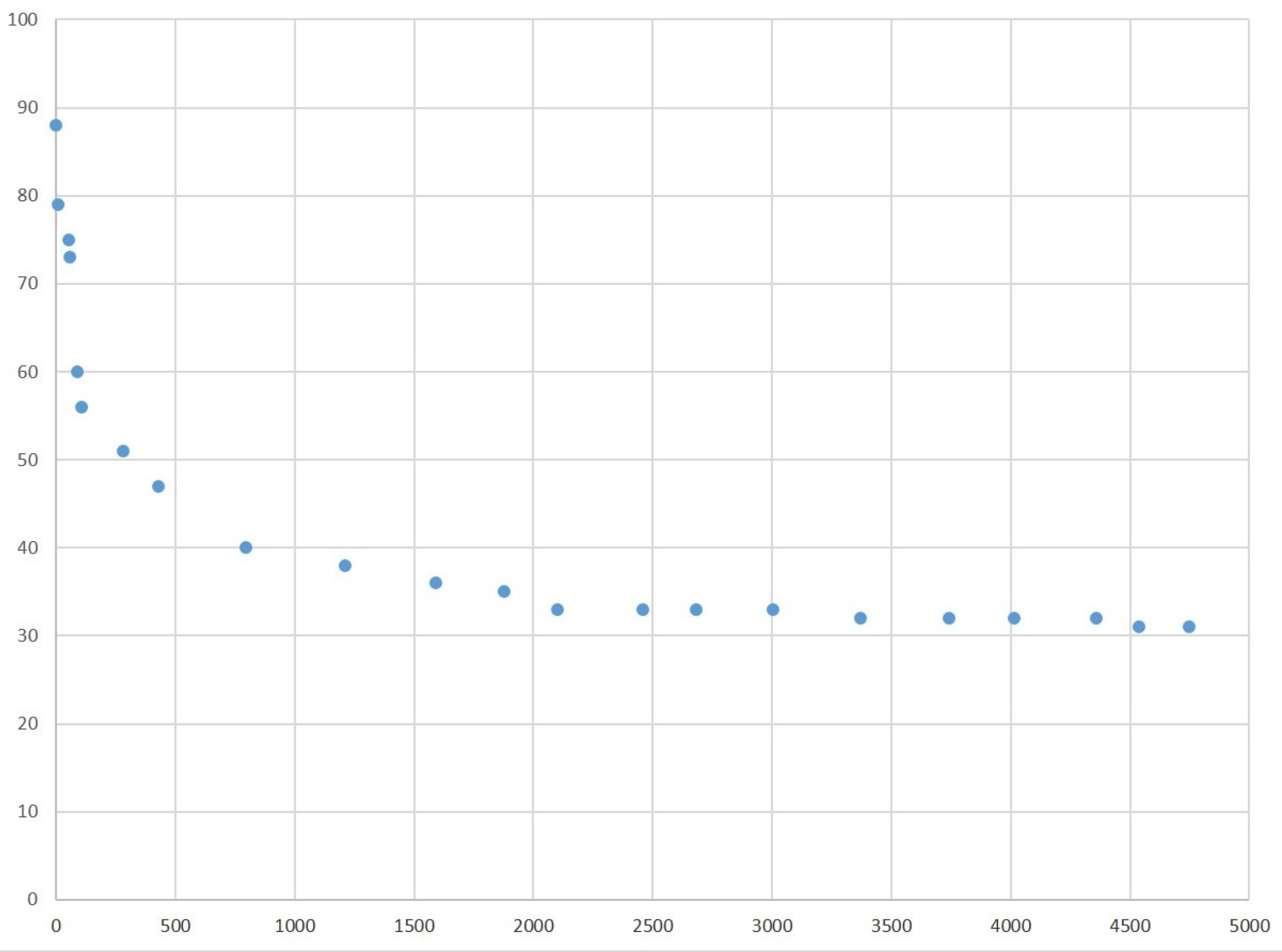
3.1. Changes in the Body Weight
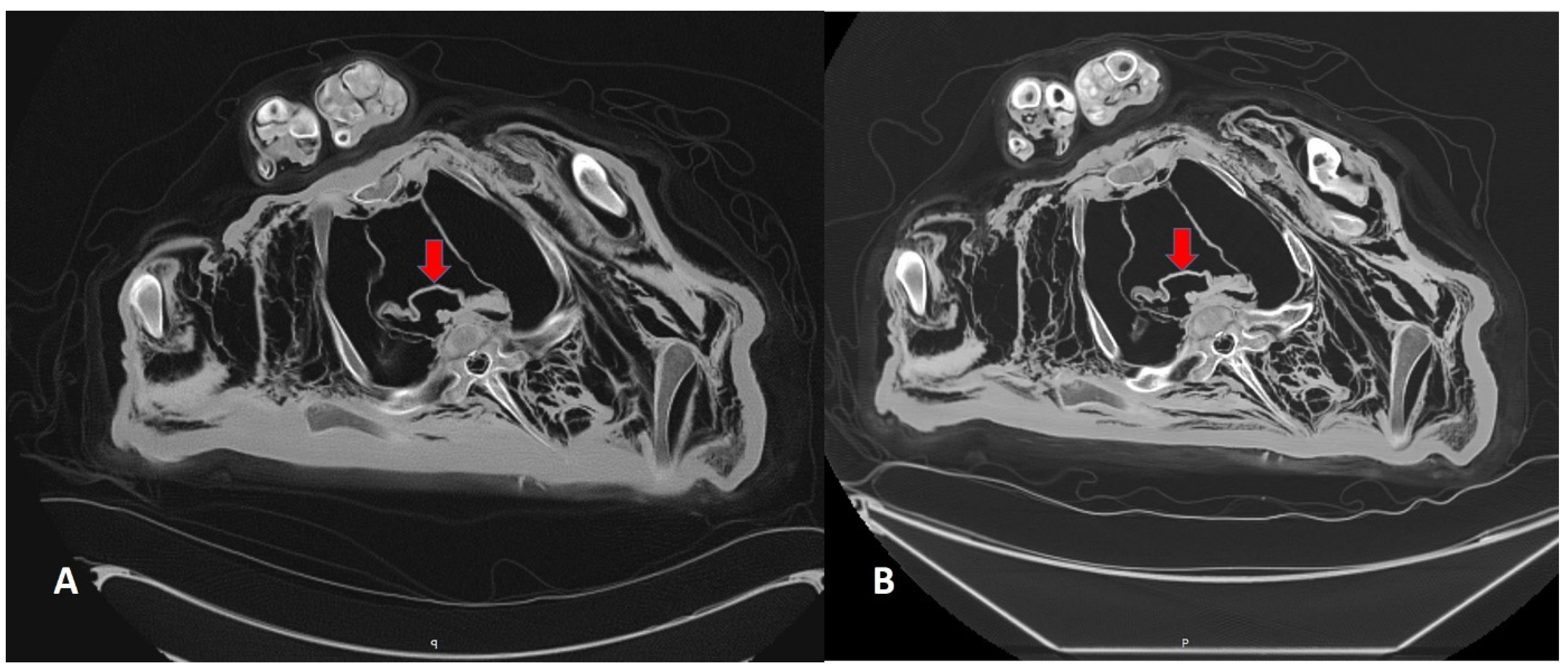
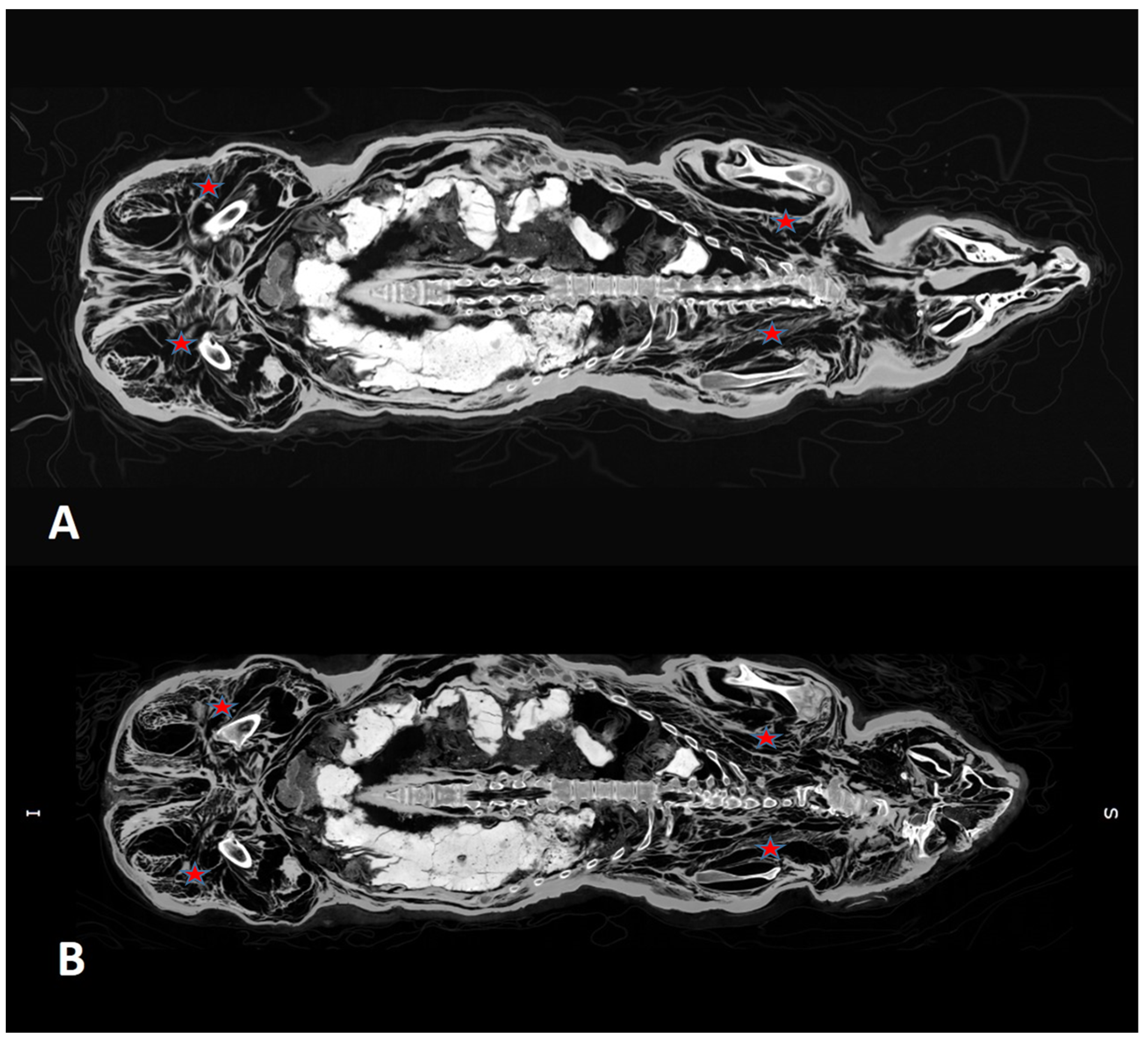
3.2. CT Scans After 7 and 13 Years
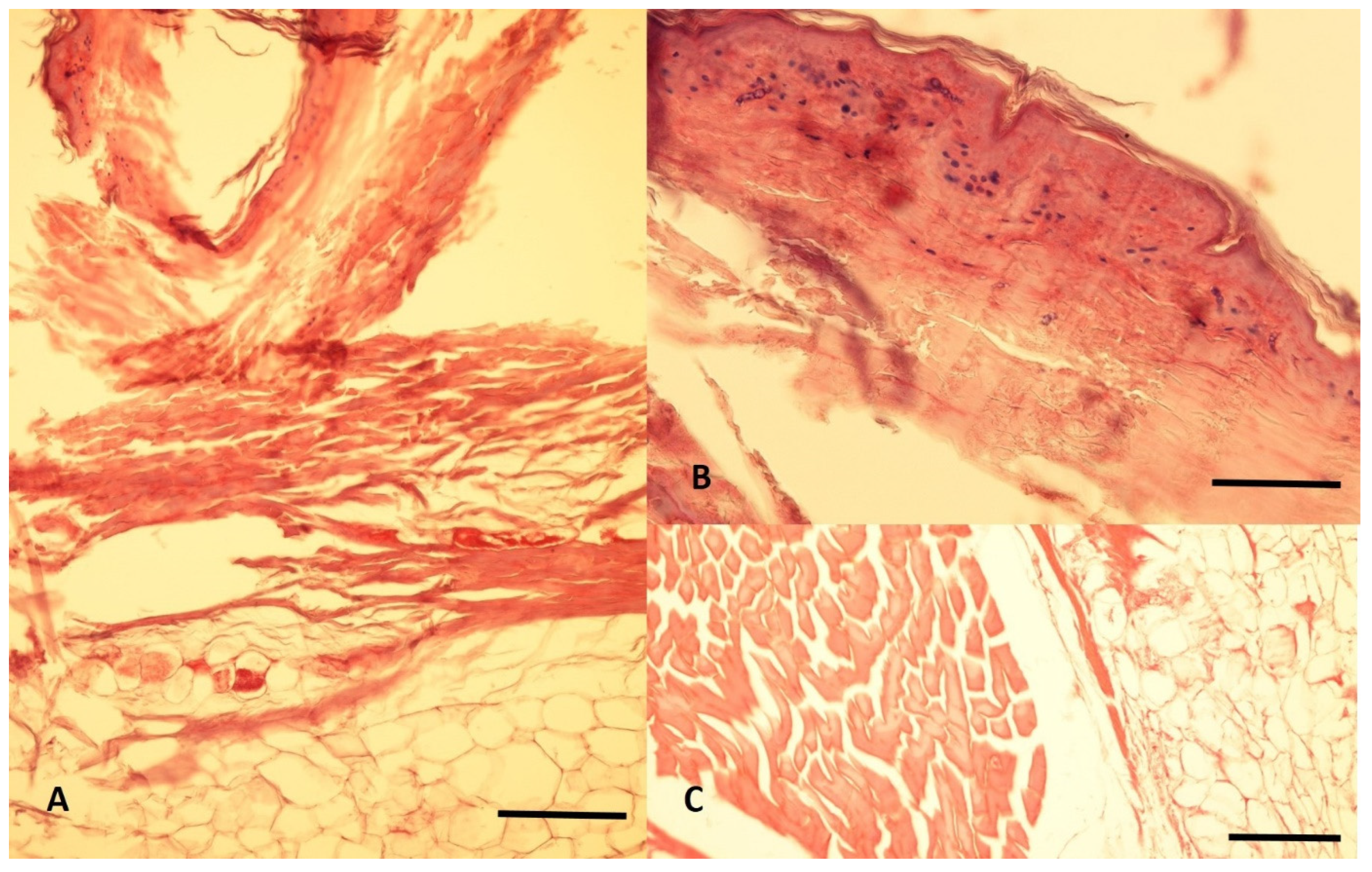
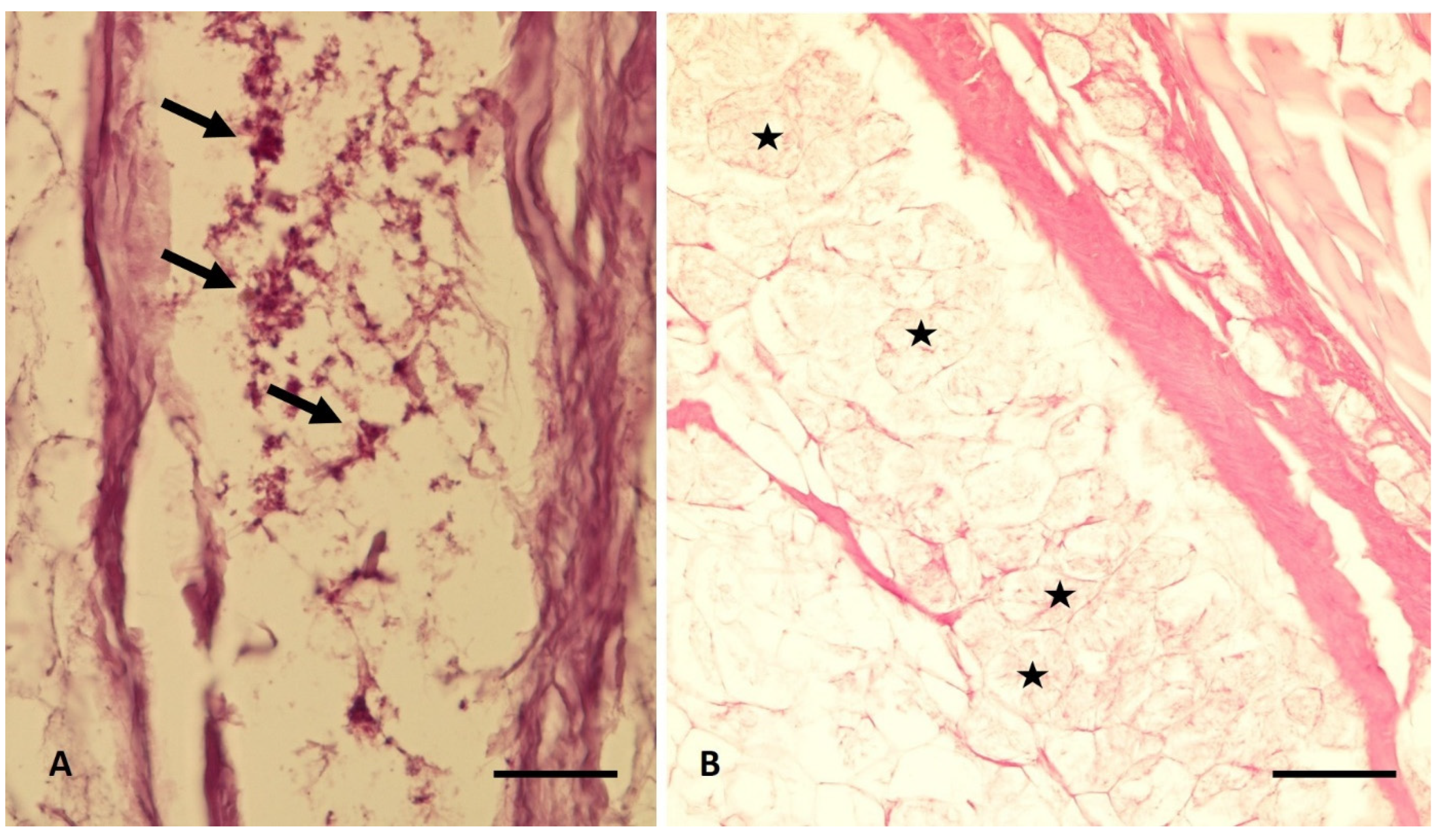
3.3. Histological Analyses
3.4. Microbiological Investigations
3.5. Overall Evaluation of the Experimental Outcome
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, R. Egyptian Mummies and Modern Science; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Ikram, S.; Dodson, A. The Mummy in Ancient Egypt: Equipping the Dead for Eternity; Thames and Hudson: London, UK, 1998. [Google Scholar]
- Smith, E.; Dawson, W. Egyptian Mummies; Dial Press: New York, NY, USA, 1924. [Google Scholar]
- Aufderheide, A.C. The Scientific Study of Mummies; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Rageot, M.; Hussein, R.B.; Beck, S.; Altmann-Wendling, V.; Ibrahim, M.I.M.; Bahgat, M.M.; Yousef, A.M.; Mittelstaedt, K.; Filippi, J.J.; Buckley, S.; et al. Biomolecular analyses enable new insights into ancient Egyptian embalming. Nature 2023, 614, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A. The use of natron in mummification. J. Egyptol. Archaeol. 1932, 18, 125–140. [Google Scholar] [CrossRef]
- Sandison, A.T. The Use of Natron in Mummification in Ancient Egypt. J. Near East. Stud. 1963, 22, 259–267. [Google Scholar] [CrossRef]
- Garner, R. Experimental mummification. In The Manchester Mummy Project; David, A.R., Ed.; Manchester University Press: Manchester, UK, 1979; pp. 19–24. [Google Scholar]
- Notman, D.N.H.; Aufderheide, A.C. Experimental mummification and computed imaging. In Actas del I Congreso Internacional de Estudios Sobre Momias; Museo Arqueológico de Tenerife: Tenerife, Spain, 1984; pp. 821–828. [Google Scholar]
- Aturaliya, S.; Wallgren, J.; Aufderheide, A.C. Studies in human taphonomy. I. an experimental animal model. In Actas del I Congreso Internacional de Estudios Sobre Momias; Museo Arqueológico de Tenerife: Tenerife, Spain, 1984; pp. 803–812. [Google Scholar]
- García-Jiménez, L. Application of oils and resins during the process of mummification: Experimental analysis and study problems. In Pharmacy and Medicine in Ancient Egypt; Dinarès Solà, R., Fernàndez Georges, M., Guasch-Jané, R.M., Eds.; Archaeopress: Oxford, UK, 2021; pp. 15–29. [Google Scholar]
- Ikram, S. Manufacturing Divinity: The Technology of Mummification. In Divine Creatures: Animal Mummies in Ancient Egypt; Ikram, S., Ed.; The American University in Cairo Press: Cairo, NY, USA, 2015; pp. 17–44. [Google Scholar]
- Brier, B.; Wade, R.S. The use of natron in human mummification: A modern experiment. Zschr. Ägyptologie 1997, 124, 89–100. [Google Scholar] [CrossRef]
- Zimmermann, M.; Brier, B.; Wade, R.S. Twentieth-century replication of an Egyptian mummy: Implications for paleopathology. Am. J. Phys. Anthropol. 1998, 107, 417–420. [Google Scholar] [CrossRef]
- Wade, A.D.; Beckett, R.G.; Conlogue, G.J.; Gonzalez, R.; Wade, R.; Brier, B. MUMAB: A conversation with the past. Anat. Rec. 2015, 298, 954–973. [Google Scholar] [CrossRef]
- Vanezis, P.; Buckley, S.; Fletcher, J.; Coe, M. Positive reaction to mummy show. The Scarborough News, 26 October 2011. [Google Scholar]
- Panzer, S.; Borumandi, F.; Wanek, J.; Papageorgopoulou, C.; Shved, N.; Colacicco, G.; Rühli, F.J. Modeling ancient Egyptian embalming: Radiological assessment of experimentally mummified human tissue by CT and MRI. Skeletal Radiol. 2013, 42, 1527–1535. [Google Scholar] [CrossRef]
- Shved, N.; Haas, C.; Papageorgopoulou, C.; Akguel, G.; Paulsen, K.; Bouwman, A.; Warinner, C.; Rühli, F.J. Post mortem DNA degradation of human tissue experimentally mummified in salt. PLoS ONE 2014, 9, e110753. [Google Scholar] [CrossRef]
- Papageorgopoulou, C.; Shved, N.; Wanek, J.; Rühli, F.J. Modeling ancient Egyptian mummification on fresh human tissue: Macroscopic and histological aspects. Anat. Rec. 2015, 298, 974–987. [Google Scholar] [CrossRef]
- Debeer, S.; Le Luduec, J.-B.; Kaiserlian, D.; Laurent, P.; Nicolas, J.-F.; Dubois, B.; Kanitakis, J. Comparative histology and immunohistochemistry of porcine versus human skin. Eur. J. Dermatol. 2013, 23, 456–466. [Google Scholar] [CrossRef]
- Sachs, D.H. The pig as a potential xenograft donor. Vet. Immunol. Immunopathol. 1994, 43, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, H.; King, T.W.; Hara, H.; Cooper, D.K.C. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Zheng, W.; Chen, M.; Zou, Q.; Tang, C.; Zhou, X. Genetically engineered pigs for xenotransplantation: Hopes and challenges. Front. Cell. Dev. Biol. 2023, 10, 1093534. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.G.; Saleh, A.; Röcker, P.; Freidank, H.; Fischer, J.; Bock, M.; Panzer, S.; Peschel, O. Experimental Mummification Using Herodotus Description—A 7-Year Longterm Experience of a Human-Sized Animal Model. In Pharmacy and Medicine in Ancient Egypt; Dinarès Solà, R., Fernàndez Georges, M., Guasch-Jané, R.M., Eds.; Archaeopress: Oxford, UK, 2021; pp. 68–77. [Google Scholar]
- Feix, J. Herodot: Historien. Buch II. Reihe Tusculum; Artemis & Winkler/Patmos Verlag: Düsseldorf, Germany, 2001. [Google Scholar]
- Nerlich, A.G.; Zink, A.; Hagedorn, H.G.; Szeimies, U.; Weyss, C. Anthropological and palaeopathological analysis of the human remains from three “Tombs of the Nobles” of the necropolis of Thebes-West, Upper Egypt. Anthropol. Anz. 2000, 58, 321–343. [Google Scholar] [CrossRef]
- Metwallya, S.M.; Abo-Elmagdb, M.; Salamaa, E. Modeling the dependency of radon concentration levels inside ancient Egyptian tombs on the ambient temperature variations. Arab. J. Nucl. Sci. Appl. 2007, 40 (Special Issue), 197–206. [Google Scholar]
- Gruber, E.; Salamaa, E.; Rühm, W. Real-time measurement of individual occupational radon exposures in tombs of the valley of the kings, Egypt. Radiat. Prot. Dosim. 2011, 144, 620–626. [Google Scholar] [CrossRef]
- Panzer, S.; Ketterl, S.; Bicker, R.; Schoske, S.; Nerlich, A.G. How to CT scan human mummies: Theoretical considerations and examples of use. Int. J. Paleopathol. 2019, 26, 122–124. [Google Scholar] [CrossRef]
- Özen, A.C.; Ludwig, U.; Öhrström, L.M.; Rühli, F.; Bock, M. Comparison of ultrashort echo time sequences for MRI of an ancient mummified human hand. Magn. Reson. Med. 2015, 75, 701–708. [Google Scholar] [CrossRef]
- Fischer, J.; Özen, A.C.; Kurzhunov, D.; Reisert, M.; Tesfai, A.; Rühli, F.; Ludwig, U.; Bock, M. Cross-modality MR image reconstruction: CT-constrained anisotropic diffusion to preserve edge information in MRI of an ancient mummified hand. In Proceedings of the 25th Annual Meeting of ISMRM, ISMRM 2017, Honolulu, HI, USA, 22–27 April 2017. [Google Scholar]
- Nerlich, A.G.; Panzer, S.; Wimmer, J.; Hamann, C.; Peschel, O.K. Adipositas and metabolic bone disorder in a 16th century Upper Austrian infant crypt mummy—An interdisciplinary palaeopathological insight into historical aristocratic life. Front. Med. 2022, 9, 979670. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Kirchhoff, S.M.; Panzer, S.; Lehn, C.; Bachmeier, B.E.; Bayer, B.; Anslinger, K.; Röcker, P.; Peschel, O.K. Chronic active non-lethal human-type tuberculosis in a high royal Bavarian officer of Napoleonic times–a mummy study. PLoS ONE 2021, 16, e0249955. [Google Scholar] [CrossRef]
- Bast, E. Mikrobiologische Methoden, 3rd ed.; Springer: Heidelberg, Germany; New York, NY, USA, 2014. [Google Scholar]
- Hiergeist, A.; Reischl, U.; Gessner, A. Multicenter quality assessment of 16S ribosomal DNA-sequencing analysis reveals high inter-venter variability. Int. J. Med. Microbiol. 2016, 306, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 00791. [Google Scholar] [CrossRef] [PubMed]





| Criterium | Excellent Outcome | Good Outcome | Poor Outcome |
|---|---|---|---|
| Macroscopic appearance | Fully intact body surface | Small defect of the body surface | Major/ large defects of the body surface |
| CT-results | Completely preserved soft tissue and remaining organs | Focal defects of soft tissue and remaining organs | Strong degradation of inner organs/ complete loss |
| Histological appearance | Almost complete preservation of skin and musculature | Partial loss of tissue integrity of skin and musculature | Extensive defects of skin and muscular integrity |
| Microbiological investigation | No microbial growth or only bacterial spores | Focal bacterial growth in the tissue | Extensive microbial growth in the tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nerlich, A.G.; Panzer, S.; Fischer, F.; Peschel, O.K. Experimental Ancient Egyptian Human Mummification Tested in a Porcine Model: Excellent Preservation at a 13-Year Follow-Up. Heritage 2025, 8, 194. https://doi.org/10.3390/heritage8060194
Nerlich AG, Panzer S, Fischer F, Peschel OK. Experimental Ancient Egyptian Human Mummification Tested in a Porcine Model: Excellent Preservation at a 13-Year Follow-Up. Heritage. 2025; 8(6):194. https://doi.org/10.3390/heritage8060194
Chicago/Turabian StyleNerlich, Andreas G., Stephanie Panzer, Florian Fischer, and Oliver K. Peschel. 2025. "Experimental Ancient Egyptian Human Mummification Tested in a Porcine Model: Excellent Preservation at a 13-Year Follow-Up" Heritage 8, no. 6: 194. https://doi.org/10.3390/heritage8060194
APA StyleNerlich, A. G., Panzer, S., Fischer, F., & Peschel, O. K. (2025). Experimental Ancient Egyptian Human Mummification Tested in a Porcine Model: Excellent Preservation at a 13-Year Follow-Up. Heritage, 8(6), 194. https://doi.org/10.3390/heritage8060194







