Mycenaean Vitreous Artifacts: Overcoming Taxonomy Hurdles via Macro-XRF Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Macro-XRF
3. Results and Discussion
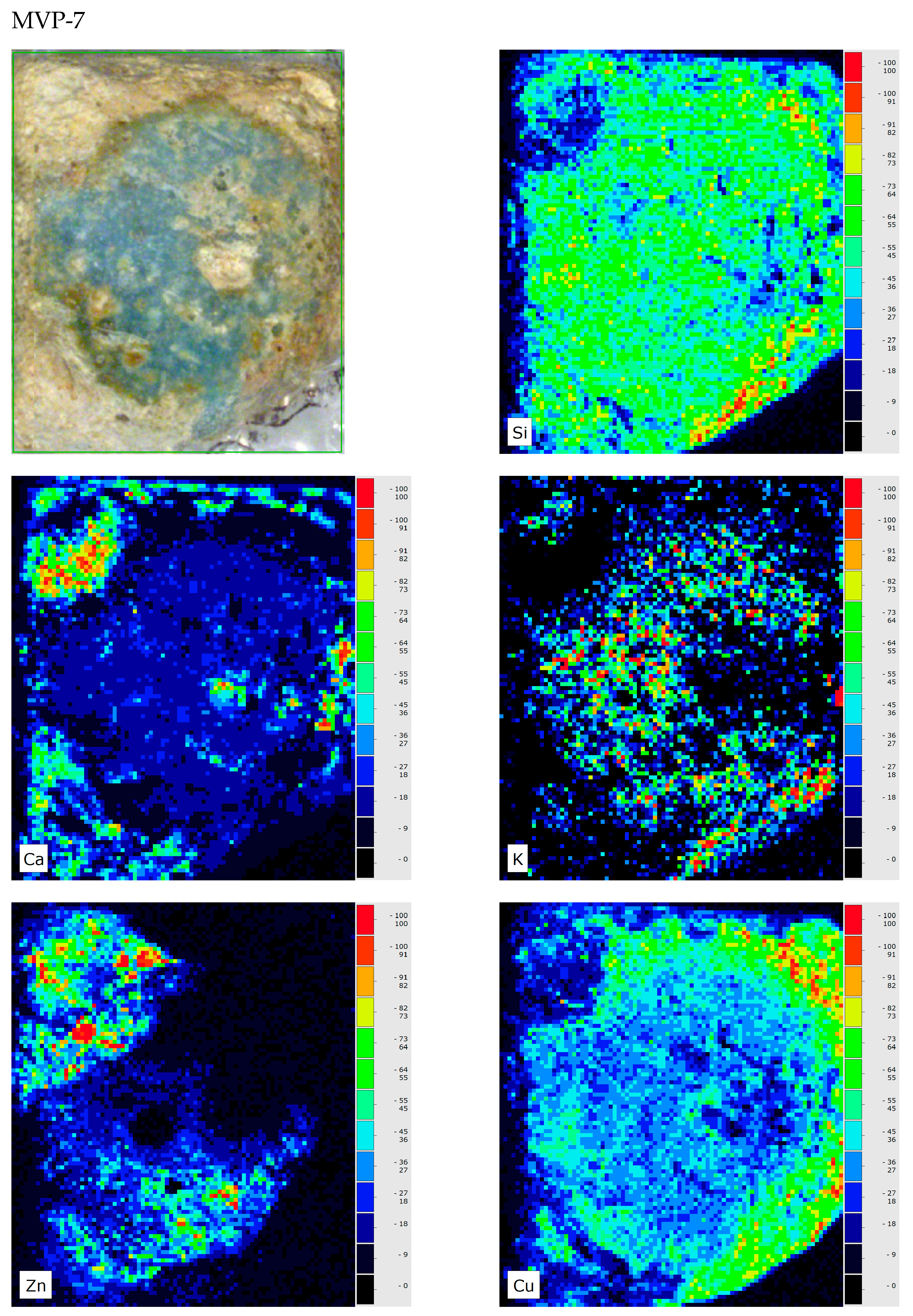
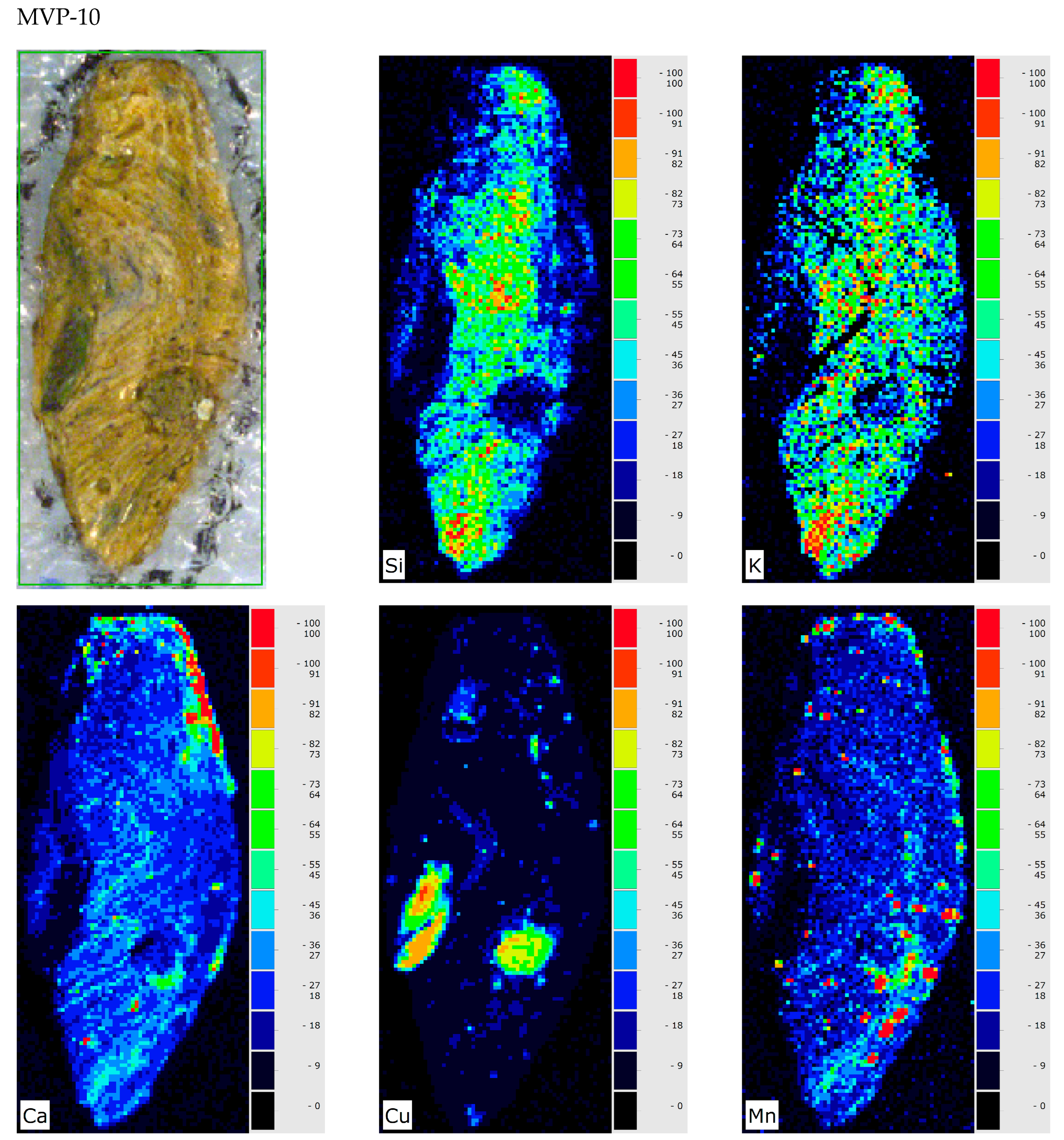
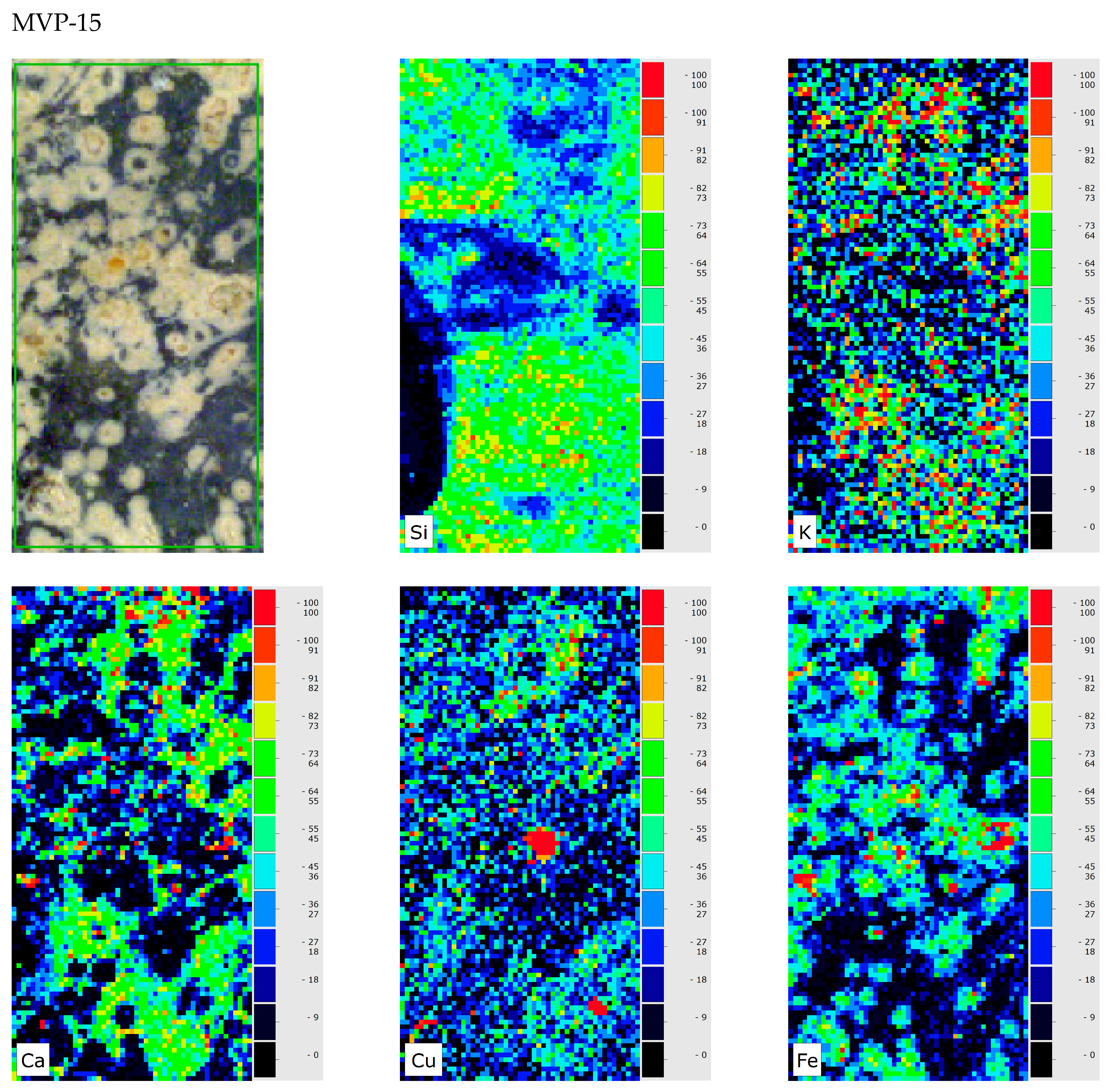
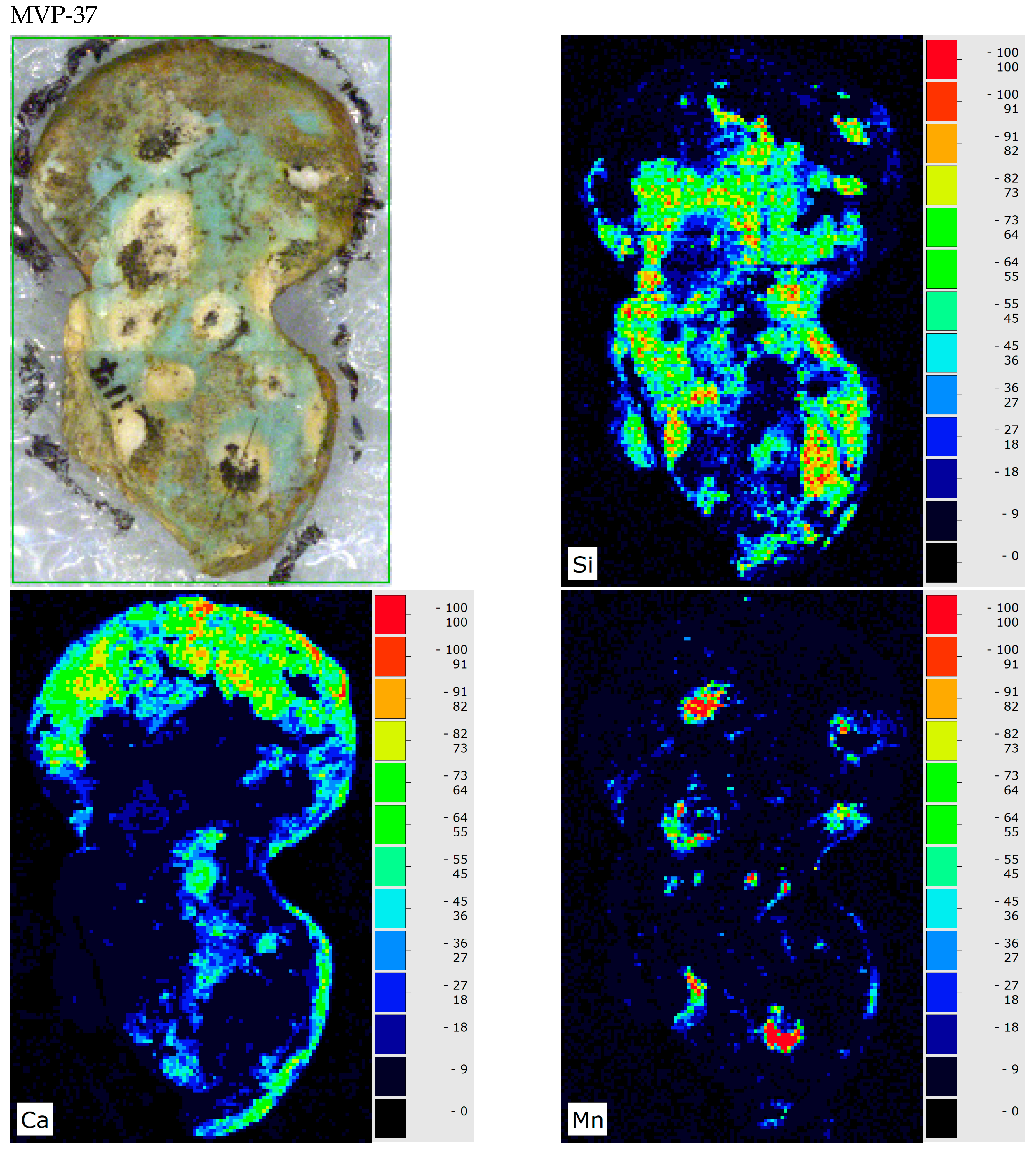
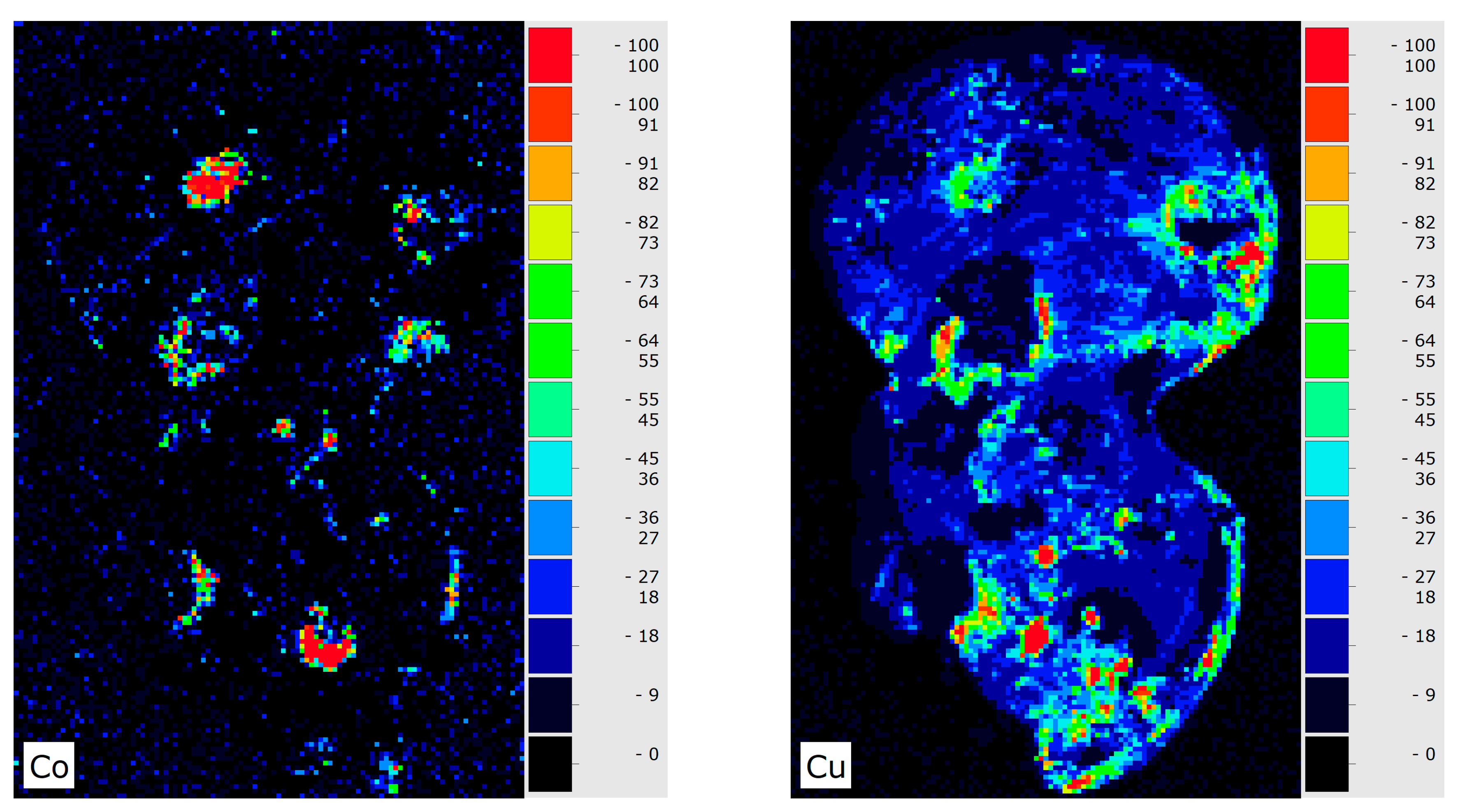

4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaparou, M.; Papageorgiou, M.; Oikonomou, A. From Mycenaeans to Romans: Reflecting on early glass technology in Greece. In Archaeology-Archaeometry: 30 Years Later, Proceedings of the 7th Symposium of the Hellenic Society for Archaeometry; Filippaki, E., Ed.; Archaeopress Publications: Birmingham, UK, 2024; pp. 170–180. [Google Scholar] [CrossRef]
- Kaparou, M.; Tsampa, K.; Zacharias, N.; Karydas, A.G. Analytical exploration of the Mycenaean glass world via micro-PIXE: A contribution to our knowledge of LBA glass technology. Archaeol. Anthropol. Sci. 2023, 15, 201. [Google Scholar] [CrossRef]
- Kaparou, M.; Oikonomou, A. Mycenaean through Hellenistic glass in Greece: Where have we got to? Archaeol. Anthropol. Sci. 2022, 14, 92. [Google Scholar] [CrossRef]
- Shortland, A.; Rogers, N.; Eremin, K. Trace element discriminants between Egyptian and Mesopotamian Late Bronze Age glasses. J. Archaeol. Sci. 2007, 34, 781–789. [Google Scholar] [CrossRef]
- Nikita, K.; Henderson, J. Glass Analyses from Mycenaean Thebes and Elateia: Compositional Evidence for a Mycenaean Glass Industry. J. Glass Stud. 2006, 48, 71–120. [Google Scholar]
- Walton, M.S.; Shortland, A.; Kirk, S.; Degryse, P. Evidence for the trade of Mesopotamian and Egyptian glass to Mycenaean Greece. J. Archaeol. Sci. 2009, 36, 1496–1503. [Google Scholar]
- Jackson, C.M.; Nicholson, P.T. The provenance of some glass ingots from the Uluburun shipwreck. J. Archaeol. Sci. 2010, 37, 295–301. [Google Scholar]
- Purowski, T.; Syta, O.; Wagner, B. Mycenaean and Egyptian faience beads discovered in southern Poland. J. Archaeol. Sci. Rep. 2019, 28, 102023. [Google Scholar] [CrossRef]
- Doremus, R.H. Interdiffusion of Hydrogen and Alkali Ions in a Glass Surface. J. Non-Cryst. Solids 1975, 19, 137. [Google Scholar] [CrossRef]
- Suza, A.D.; Pantano, C.G. Surface Layer Formation due to leaching and heat treatment of Alkali Silicate glass. Phys. Chem. Glas. 1996, 37, 79–83. [Google Scholar]
- Pantano, C.G.; Hamilton, J.P. Characterization and structure of leached surface layers on glass. Riv. Della Stn. Sper. Del Vetro 2000, 6, 81–86. [Google Scholar]
- Kaparou, M.; Oikonomou, A.; Karydas, A.G. Investigating the Degradation of Mycenaean Glass Artifacts Using Scientific Methods. Heritage 2024, 7, 1769–1783. [Google Scholar] [CrossRef]
- Tennent, N.H. (Ed.) The Conservation of Glass and Ceramics. Research, Practice and Training; James & James: London, UK, 1999. [Google Scholar]
- Doménech-Carbó, M.; Doménech-Carbó, A.; Osete-Cortina, L.; Saurí-Peris, M.C. A Study on Corrosion Processes of Archaeological Glass from the Valencian Region (Spain) and its Consolidation Treatment. Microchim. Acta 2006, 154, 123–142. [Google Scholar] [CrossRef]
- Gin, S.; Delaye, J.-M.; Angeli, F.; Schuller, S. Aqueous alteration of silicate glass: State of knowledge and perspectives. NPJ Mater. Degrad. 2021, 5, 42. [Google Scholar] [CrossRef]
- Zanini, R.; Franceschin, G.; Cattaruzza, E.; Traviglia, A. A review of glass corrosion: The unique contribution of studying ancient glass to validate glass alteration models. NPJ Mater. Degrad. 2023, 7, 38. [Google Scholar] [CrossRef]
- Newton, R.; Davison, S. Conservation of Glass; Butterworths: London, UK, 1989. [Google Scholar]
- Jackson, C.M.; Greenfield, D.; Howie, L.A. An Assessment of Compositional and Morphological Changes in Model Archaeological Glasses in an Acid Burial Matrix. Archaeometry 2012, 54, 489–507. [Google Scholar]
- van Giffen, N.A.R.; Koob, S.P. Deterioration of vitreous materials. Encycl. Archaeol. Sci. 2018, 1–4. [Google Scholar]
- Zacharias, N.; Palamara, E.; Kordali, R.; Muros, V. Archaeological Glass Corrosion Studies: Composition, Environment and Content. Sci. Cult. 2020, 6, 53–67. [Google Scholar]
- Gestels, A.; Van der Snickt, G.; Caen, J.; Nuyts, G.; Legrand, S.; Vanmeert, F.; Detry, F.; Janssens, K.; Steenackers, G. Combined MA-XRF, MA-XRPD and SEM-EDX analysis of a medieval stained-glass panel formerly from Notre Dame, Paris reveals its material history. Microchem. J. 2022, 177, 107304. [Google Scholar] [CrossRef]
- Legrand, S.; Van der Snickt, G.; Cagno, S.; Caen, J.; Janssens, K. MA-XRF imaging as a tool to characterize the 16th century heraldic stained-glass panels in Ghent Saint Bavo Cathedral. J. Cult. Herit. 2019, 40, 163–168. [Google Scholar]
- Dungworth, D. New Ways of Seeing Old Glass: Excavated Medieval Stained Glass Revealed Using Scanning µXRF. Vidimus 2017, 115. Available online: https://www.vidimus.org/issues/issue-115/feature/ (accessed on 26 March 2025).
- Van der Snickt, G.; Legrand, S.; Caen, J.; Vanmeert, F.; Alfeld, M.; Janssens, K. Chemical imaging of stained-glass windows by means of macro X-ray fluorescence (MA-XRF) scanning. Microchem. J. 2016, 124, 615–622. [Google Scholar]
- Schliemann, H. Tiryns; F. A. Brockhaus: Leipzig, Germany, 1896. [Google Scholar]
- Panagiotaki, M.; Papazoglou-Manioudaki, L.; Chatzi-Spiliopoulou, G.; Andreopoulou-Mangou, E.; Maniatis, Y.; Tite, M.S.; Shortland, A. A glass workshop at the Mycenaean citadel of Tiryns in Greece. In Annales du 16e Congrès de L’Association Internationale Pour L’Histoire Du Verre; AIHV: London, UK, 2003; pp. 14–18. [Google Scholar]
- Stamatakis, P. The Excavation Calendar; Hellenic National Archaeological Museum archive: Athens, Greece, 1877. (In Greek) [Google Scholar]
- Papadimitriou-Grammenou, A. Οι Μυκηναϊκοί τάφοι στα Σπάτα Αττικής. Ανασκαφή Παναγιώτη Σταματάκη (1877). Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2003. (In Greek). [Google Scholar]
- Nightingale, G. Tiny, Fragile, Common, Precious. Mycenaean Glass and Faience Beads and Other Objects. In Vitreous Materials in the Late Bronze Age Aegean, Sheffield Studies in Aegean; Jackson, C.M., Wager, E.C., Eds.; Oxbow Books: Oxford, UK, 2008; pp. 64–104. [Google Scholar]
- Dimakopoulou, K. Ο Θησαυρός των Αηδονιών. Σφραγίδες και κοσμήματα της Ύστερης Εποχής του Χαλκού στο Αιγαίο; Hellenic Ministry of Culture: Athens, Greece, 1996. (In Greek) [Google Scholar]
- Xenaki-Sakellariou, A. Οι θαλαμωτοί τάφοι των Μυκηνών ανασκαφής Χρ. Τσούντα (1887–1898); Publisher: Diffusion de Boccard: Paris, France, 1985. (In Greek) [Google Scholar]
- Tournié, A.; Ricciardi, P.; Colomban, P. Glass corrosion mechanisms: A multiscale analysis. Solid State Ion. 2008, 179, 2142–2154. [Google Scholar] [CrossRef]
- Abe, Y.; Harimoto, R.; Kikugawa, T.; Yazawa, K.; Nishisaka, A.; Kawai, N.; Yoshimura, S.; Nakai, I. Transition in the use of Cobalt-Blue Colorant in the New Kingdom of Egypt. J. Archaeol. Sci. 2012, 39, 1793–1808. [Google Scholar] [CrossRef]
- Hodgkinson, A.K.; Lemasson, Q.; Mäder, M.; Munnik, F.; Pichon, L.; Röhrs, S.; Reiche, I. A comparative compositional study of Egyptian glass from Amarna with regard to cobalt sources and other colourants. J. Archaeol. Sci. Rep. 2024, 54, 104412. [Google Scholar] [CrossRef]
- Smirniou, M.; Rehren, T. Shades of blue—Cobalt-copper coloured blue glass from New Kingdom Egypt and the Mycenaean world: A matter of production or colourant source? J. Archaeol. Sci. 2013, 40, 4731–4743. [Google Scholar] [CrossRef]
- Zacharias, N.; Kaparou, M.; Oikonomou, A.; Kasztovszky, Z. Mycenaean Glass from the Argolid, Peloponnese, Greece: A Technological and Provenance Study. Eur. J. Microchem. 2018, 141, 407–417. [Google Scholar]


| Vitreous Material Type | SiO2 | Na2O | K2O | MgO | Al2O3 | CaO | |
|---|---|---|---|---|---|---|---|
| Data from [4,5,6,7] | Plant ash soda-lime glass (n = 142) | 66 | 17 | 1.7 | 4 | 1.6 | 7 |
| Data from [5] (only the plant-ash glasses). | Elateia and Thebes glass (n = 73) | 67 | 17 | 1.3 | 3 | 2 | 6 |
| Data from [6] | Mesopotamia glass (n = 25) | 65 | 17 | 2.8 | 4 | 0.5 | 7 |
| Data from [7] | Egypt glass (n = 30) | 62 | 19 | 1.8 | 4.4 | 1 | 8 |
| Data from [2] | Peloponnese Mycenaean glass (n = 11) | 68 | 15 | 2.0 | 4 | 2 | 7 |
| Data from [8] | Faience Mycenaean (body, n = 4) | 84 | 2 | 1.2 | 1 | 4 | 3 |
| Data from [8] | Faience Mycenaean (glaze, n = 6) | 77 | 9 | 2.8 | 2 | 1 | 4 |
| Sample Number Location | Material | Photo | LED Microscopy | Preservation State | Corrosion Effects |
|---|---|---|---|---|---|
| MVP-7 Tiryns HΝAΜ Π 1616 | Glass |  |  | Medium corroded | Pitting with characteristic concentric layers; forming of local crust and flaking; stained by metal oxides |
| MVP-10 Spata HΝAΜ Π 2078 | Unclear (glass in the archaeological record) |  |  | Heavily corroded | Pitting with characteristic concentric layers; forming local crust; stained by metal oxides |
| MVP-15a MVP-15b Spata HΝAΜ Π 2189a HΝAΜ Π 2189b | Glass |  |  | Medium to heavily corroded | Pitting with characteristic concentric layers; forming of local crust; discolouration occurred by the semi opaque milky like surface from the concentric layers. |
| MVP-37 Mycenae HΝAΜ Π 3117 | Unclear |  |  | Good to medium preservation state | Local crusts; discolouration by metal oxides |
| MVP-83 Mycenae HΝAΜ Π 2893 | Glass |  |  | Medium to heavily corroded | Pitting with characteristic concentric layers; forming local crust |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikonomou, A.; Kaparou, M.; Asvestas, A.; Tsampa, K.; Kordali, O.; Nikolentzos, K.; Manteli, K.; Voutsa, A.; Moraitou, G.; Anagnostopoulos, D.F.; et al. Mycenaean Vitreous Artifacts: Overcoming Taxonomy Hurdles via Macro-XRF Analysis. Heritage 2025, 8, 122. https://doi.org/10.3390/heritage8040122
Oikonomou A, Kaparou M, Asvestas A, Tsampa K, Kordali O, Nikolentzos K, Manteli K, Voutsa A, Moraitou G, Anagnostopoulos DF, et al. Mycenaean Vitreous Artifacts: Overcoming Taxonomy Hurdles via Macro-XRF Analysis. Heritage. 2025; 8(4):122. https://doi.org/10.3390/heritage8040122
Chicago/Turabian StyleOikonomou, Artemios, Maria Kaparou, Anastasios Asvestas, Kalliopi Tsampa, Ourania Kordali, Konstantinos Nikolentzos, Katia Manteli, Aikaterini Voutsa, Georgianna Moraitou, Dimitrios F. Anagnostopoulos, and et al. 2025. "Mycenaean Vitreous Artifacts: Overcoming Taxonomy Hurdles via Macro-XRF Analysis" Heritage 8, no. 4: 122. https://doi.org/10.3390/heritage8040122
APA StyleOikonomou, A., Kaparou, M., Asvestas, A., Tsampa, K., Kordali, O., Nikolentzos, K., Manteli, K., Voutsa, A., Moraitou, G., Anagnostopoulos, D. F., & Karydas, A. G. (2025). Mycenaean Vitreous Artifacts: Overcoming Taxonomy Hurdles via Macro-XRF Analysis. Heritage, 8(4), 122. https://doi.org/10.3390/heritage8040122









