Conservation of a Marine Silver-Plated German Silver Cloche from the 19th-Century Shipwreck Patris
Abstract
1. Introduction
2. Background Information
2.1. The Patris Shipwreck
2.2. Nickel-Silver Alloys and Silver-Plating Techniques
- -
- In Sheffield plating, silver can be bonded to copper simply by heating the two metals in close contact with each other, without the need for an intermediate joining material or solder. Thomas Bolsover discovered this method in 1742, and most importantly, he found that the fused metals could be worked like solid silver [9]. German silver was used as a base metal for Sheffield process from the mid-1830s but only for articles that did not require complex shaping [6].
- -
- French plating was a method established in the 18th century, where the object is scored with fine grooves to provide keying and the silver foil is applied by rubbing and heating, until it is attached well into the grooves [9].
- -
- Close plating is an English process patented in 1779 by Richard Ellis, a London goldsmith, where a thin sheet of silver foil was manually fused onto a base metal object using a layer of molten tin as a solder. During the 18th and 19th centuries, this technique was used to plate silver onto a limited range of small iron or nickel-silver items [6,9].
- -
- Silvering pastes and solutions utilise the electro-differential between the plating paste or solution and the metal being plated to produce a very thin, pure, fine-grained deposit of silver with no distinguishable structure. This electrochemical process occurs when a metal from the negative end of the electromotive series is placed in an electrolyte containing ions of a more noble metal from the positive end of the series. This is a simple replacement reaction, so no external electric current is required. Silvering pastes and solutions, also known as cold silvering solutions, were undoubtedly discovered much earlier than electroplating [9].
- -
- Electroplating requires an external electric current, and difficulties were encountered in finding a suitable electrolyte during its early development. The development of electroplating as an industrial process is attributed to several English inventors, including John Wright and the Elkington cousins, George and Henry. In 1840, they patented the first commercially successful electroplating process for gold and silver [6,9]. Elkington silver plate was the thickest that could be bought at the time, around 35 μm, with most other suppliers happy to sell their EPNS (electroplated nickel silver) with coatings of 15–25 μm [1]. By the 1860s, electroplating had become standard practice in metal goods production [10].
2.3. Marine Environment and Its Effect
2.4. Corrosion of Nickel-Silver Alloys in Marine Environment
2.5. Silver Corrosion in Marine Environments
3. Methods and Materials
- A thorough visual inspection of surface technological features and corrosion stratigraphy.
- A stereomicroscope was used to perform a microscopic examination of the surface, technological features, and corrosion stratigraphy. This was initially carried out after the concretion layers had been removed and again after the object had been treated. A stereomicroscope with ×64 magnification was used for the microscopic examination.
- Sampling from areas of interest for further diagnostic analysis. Sampling was performed with a scalpel under a stereomicroscope at two selected points: one from a black area on the inside of the cloche, and a second from a whitish-grey area on the handle where the plating had worn off.
- Multi-analytical assessment:
- -
- Clean plating areas versus tarnished plating areas on both the inside and outside of the body and the handle;
- -
- Areas with plating loss and dark brown/black corrosion of the metal substrate, characterised by a layer of cupric/cuprous oxides;
- -
- Areas where redeposited copper was identified with microscopic examination.
4. Analytical Results and Discussion
4.1. Results of Examination and Spectroscopic Techniques
- Visual observation
- X-ray radiography
- Microscopic examination
- -
- Extensive black corrosion on the surface of the plating.
- -
- -
- Selective corrosion of the soldering lead alloy in the joint between the lid and handle due to object being stored in deionized water in the conservation lab (Figure 7).
- -
- Localised pitting of the silver plating and the copper alloy (Figure 8).
- -
- Blue-coloured corrosion of copper on the handle (Figure 9).


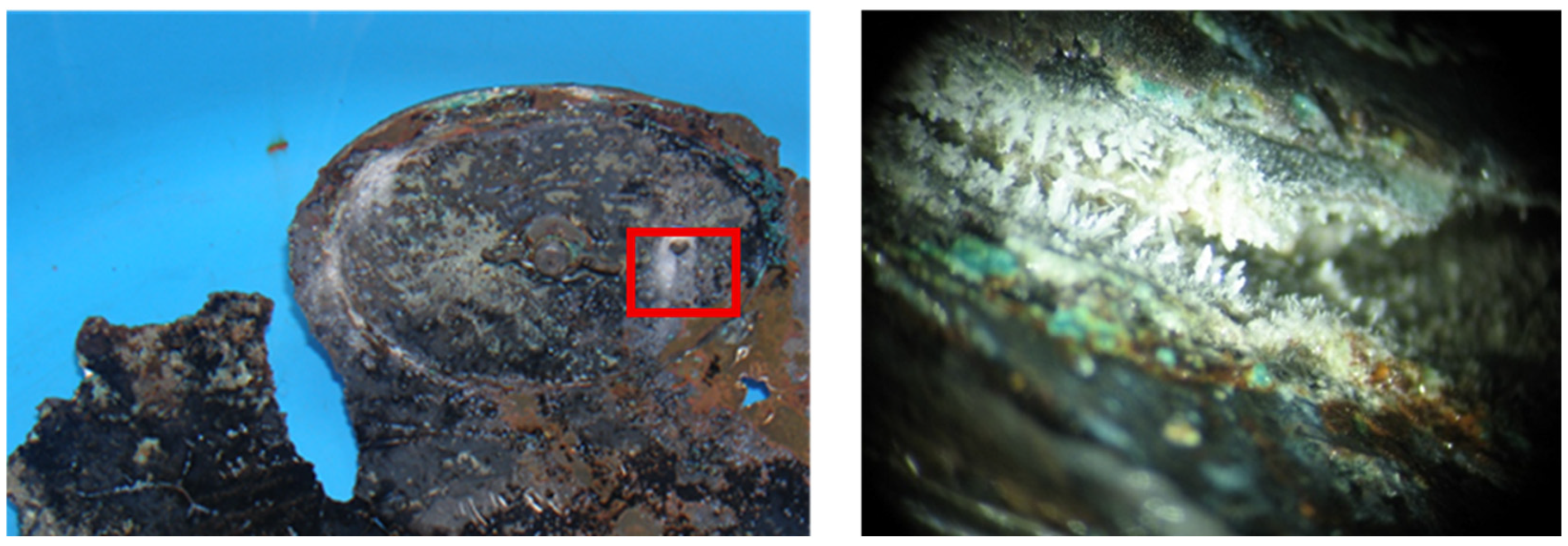


- XRF results in 2007
- SEM-EDX results in 2007
- XRF results in 2025
4.2. The Conservation Treatment
- Concretion removal
- (a)
- Application of cotton compresses containing a 10% w/w aqueous formic acid solution in deionized wather for 30 min, followed by mechanical cleaning with a scalpel;
- (b)
- Immersion of the cloche in a 5% w/w aqueous formic acid solution in deionized water for 5 min, followed by mechanical cleaning with a scalpel and a thorough rinsing with deionised water after each application.
- Stabilisation process
- Cleaning of tarnished plating
- Coating process
- Monitoring of treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLeod, I.D.; Pennec, S. Characterisation of corrosion products on artefacts recovered from the RMS Titanic (1912). In METAL 2001, Proceedings of the ICOM Committee for Conservation Metals Working Group, Santiago, Chile, 16–20 April 2001; Western Australian Museum: Perth, Australia, 2004; pp. 1–9. [Google Scholar]
- MacLeod, I.D. Corrosion and Conservation of Nickel Silver Alloys Recovered from Historic Shipwrecks. In METAL 2022, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Helsinki, Finland, 5–9 September 2022; Mardikian, P., Nasanen, L., Arponen, A., Eds.; International Council of Museums—Committee for Conservation (ICOM-CC) and The National Museums of Finland: Helsinki, Finland, 2022; pp. 71–77. [Google Scholar]
- Available online: http://www.ketepo.gr/el/2010/10/%CF%80%CE%B1%CF%84%CF%81%CE%AF%CF%82/ (accessed on 6 August 2025).
- Argyropoulos, V.; Giannoulaki, M.; Malea, E.; Pournou, A.; Rapti, S. Corrosion theory for metallic and composite artefacts in the Aegean marine environment (Chapter 4). In The Conservation of Marine Metal Shipwrecks and Artefacts from the Aegean Sea. Good Practice Guide; Argyropoulos, V., Giannoulaki, M., Charalambous, D., Eds.; Dionikos Publications: Athens, Greece, 2015; ISBN 978-618-5208-06-6. [Google Scholar]
- Argyropoulos, V.; Batis, G. Saving a marine iron paddle wheel removed from the 1868 steam engine shipwreck “Patris” during an economic crisis in Greece. In Big Stuff 2013; Zenodo: Geneva, Switzerland, 2013. [Google Scholar] [CrossRef]
- Pinn, K. Paktong: The Chinese Alloy in Europe 1680–1820; Antique Collectors’ Club Ltd.: Suffolk, UK, 1999; Chapters 1, 5, 6. [Google Scholar]
- Tuck, C.D.S.; Powell, C.A.; Nuttall, J. Corrosion of Copper and Its Alloys. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Le Niece, S. Silver Plating on copper, bronze and brass. Antiqu. J. 1990, 70, 102–114. [Google Scholar] [CrossRef]
- La Niece, S. Silvering. In Metal Plating and Patination; La Niece, S., Craddock, P., Eds.; Butterworth Heinemann: Oxford, UK, 1993; pp. 201–210. [Google Scholar]
- Gugua, E.C.; Ujah, C.O.; Asadu, C.O.; Von Kallon, D.V.; Ekwueme, B.N. Electroplating in the modern era, improvements and challenges: A review. Hybrid Adv. 2024, 7, 100286. [Google Scholar] [CrossRef]
- Nejneru, C.; Savin, C.; Perju, M.C.; Burduhos-Nergis, D.D.; Costea, M.; Bejinariu, C. Studies on galvanic corrosion of metallic materials in marine medium. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Innovative Research—ICIR EUROINVENT, Iasi, Romania, 16–17 May 2019; IOP Publishing: Galati, Romania, 2019; Volume 572. [Google Scholar] [CrossRef]
- Velaoras, D.; Kassis, D.; Perivoliotis, L.; Pagonis, P.; Hondronasios, A.; Nittis, K. Temperature and Salinity Variability in the Greek Seas Based on POSEIDON Stations Time Series: Preliminary Results. Mediterr. Mar. Sci. 2019, 14, 5–18. [Google Scholar] [CrossRef]
- Argyropoulos, V.; Boyatzis, S.; Giannoulaki, M.; Malea, E.; Petrou, M.; Rapti, S. Metals in association with organic and inorganic materials: Marine composite artefacts (Section 4, 4.4). In Bridging the Gap. Corrosion Science For Heritage Contexts, 1st ed.; Neff, D., Grassini, S., Watkinson, D., Emmerson, N., Eds.; Woodhead Publishing: Cambridge, UK, 2025; Volume 73, ISBN 9780443186905. [Google Scholar]
- Proenca, L.; Fonseca, I.T.E.; Neto, M.M.M. Electrochemical studies on the corrosion of brass in seawater under anaerobic conditions. J. Solid-State Electrochem. 2008, 12, 121–131. [Google Scholar]
- Simons, E.N. A Dictionary of Alloys; Frederick Muller: London, UK, 1969; pp. 118–119. [Google Scholar]
- Jin, T.; Zhang, W.; Li, N.; Liu, X.; Han, L.; Dai, W. Surface characterization and corrosion behaviour of 90/10 copper-nickel alloy in marine environment. Materials 2019, 12, 1869. [Google Scholar] [CrossRef]
- Santos, C.I.S.; Mendonça, M.H.; Fonseca, I.T.E. Corrosion of Brass in natural and artificial seawater. J. Appl. Electrochem. 2006, 36, 1353–1359. [Google Scholar] [CrossRef]
- Birn, J.; Skalski, I. 1-Corrosion behaviour of non-ferrous alloys in seawater in the Polish marine industry. In Corrosion Behaviour and Protection of Copper and Aluminium; Féron, D., Ed.; European Federation of Corrosion (EFC) Series; Woodhead Publishing Limited: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Gao, J.; Liu, Y.; Qin, C.; Li, J.; Li, Y.; Cao, W.; Zhai, Y.; Huang, G. Stress Corrosion Cracking of Copper–Nickel Alloys: A Review. Coatings 2023, 13, 1690. [Google Scholar] [CrossRef]
- Argyropoulos, V.; Giannoulaki, M.; Guilminot, E. Nonferrous metals (Chapter 11). In Conservation of Cultural Heritage from Marine and Freshwater Sites; Godfrey, I.M., Gregory, D.J., Eds.; Routledge Publishers: New York, NY, USA, forthcoming 2026. [Google Scholar]
- Liu, J.-F.; Esmacher, M.J.; Kotwica, D. Denickelification and Dezincification of Copper Alloys in Water Environments. Microsc. Microanal. 2015, 21 (Suppl. S3), 277–278. [Google Scholar] [CrossRef][Green Version]
- Mansfeld, F.; Little, B. Microbiologically influenced corrosion of copper-based material exposed to natural seawater. Electrochem. Acta 1992, 37, 2291–2297. [Google Scholar] [CrossRef]
- Theodoropoulou, C.; Giannoulaki, M.; Argyropoulos, V. A condition assessment of Hellenistic leaded bronze bosses from Piraeus, Greece, using scanning electron microscopy with energy dispersive x-ray analysis (SEM-EDX). Conserv. 360 Diagnosis. Before, During, After, Vol. 2. 2022. Available online: https://monografias.editorial.upv.es/index.php/con_360/article/view/400 (accessed on 5 August 2025).
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; The J. Paul Getty Museum: Los Angeles, CA, USA, 2002. [Google Scholar]
- MacLeod, I. Formation of marine concretions on copper and its alloys. Int. J. Naut. Archaeol. 1982, 11, 267–275. [Google Scholar] [CrossRef]
- North, N.A.; MacLeod, I.D. Corrosion of metals. In Conservation of Marine Archaeological Objects; Pearsoned, C., Ed.; Butterworth & Co. Ltd.: London, UK, 1987; pp. 68–98. [Google Scholar]
- Ha, H.; Payer, J. The effect of silver chloride formation on the kinetics of silver dissolution in chloride solution. Electrochim. Acta 2011, 56, 2781–2791. [Google Scholar] [CrossRef]
- Costa, V. The deterioration of silver alloys and some aspects of their conservation. Rev. Conserv. 2001, 2, 19–35. [Google Scholar] [CrossRef]
- Sinclair, J.D. The tarnishing of silver by organic sulfur vapors. J. Electrochem. Soc. 1982, 129, 33–40. [Google Scholar] [CrossRef]
- McNeil, M.B.; Little, B.J. Corrosion mechanisms for copper and silver objects in near-surface environments. J. Am. Inst. Conserv. 1992, 31, 355–366. [Google Scholar] [CrossRef]
- Abdel-Kareem, O.; Al-Zahrani, A.; Al-Sadoun, A. Authentication and conservation of marine archaeological coins excavated from underwater of the Red Sea, Saudi Arabia. Mediterr. Archaeol. Archaeom. 2016, 16, 107–118. [Google Scholar]
- MacLeod, I.D.; Schindelholz, E. Surface Analysis of Corroded Silver Coins from the Wreck of the San Pedro De Alcantara (1786). In Proceedings of the Metal 2004 National Museum of Australia Canberra ACT, Canberra, ACT, Australia, 4–8 October 2004. [Google Scholar]
- Dungworth, D. Roman copper alloys: Analysis of artefacts from Northern Britain. J. Archaeol. Sci. 1997, 24, 901–910. [Google Scholar] [CrossRef]
- Loeper-Attia, M.-A.; Maniguet, T. Twenty years of conservation of plated brass instruments at the Musse de la musique in Paris. In Proceedings of the I COM Committee for Conservation 18th Triennial Meeting, Copenhagen, Denmark, 4–8 September 2017; ISBN 978-92-9012-426-9. [Google Scholar]
- Palomar, T.; Cano, E.; Ramirez, B. A comparative study of cleaning methods for tarnished silver. J. Cult. Herit. 2016, 17, 20–26. [Google Scholar] [CrossRef]
- Palomar, T.; Cano, E.; Ramirez, B. Evaluation of Cleaning Treatments for Tarnished Silver: The Conservator’s Perspective. Int. J. Conserv. Sci. 2018, 9, 81–90. [Google Scholar]
- Wharton, G.; Lansing Maish, S.; Ginell, W.S. A Comparative Study of Silver Cleaning Abrasives. J. Am. Inst. Conserv. 2013, 29, 13–31. [Google Scholar] [CrossRef]
- Novakovic, J.M.; Vassiliou, P.; Georgiza, E. Electrochemical Cleaning of Artificially Tarnished. Int. J. Electrochem. Sci. 2013, 8, 7223–7232. [Google Scholar] [CrossRef]
- Degrigny, C.; Baudin, C.; Jeanneret, R.; Bussy, G. A new electrolytic pencil for the local cleaning of silver tarnish. Stud. Conserv. 2025, 61, 150612094103006. [Google Scholar] [CrossRef]
- Degrigny, C.; Jerome, M.; Lacoudre, N. Surface Cleaning of Silvered Brass Wind Instruments Belonging to the Sax Collection. Corros. Australas. 1993, 18, 16–18. [Google Scholar]
- Aldaz, A.; Espana, T.; Montiel, V.; Lopez-Segura, M. A Simple Tool for the Electrolytic Restoration of Archaeological Objects with Localized Corrosion. Stud. Conserv. 1986, 31, 175–176. [Google Scholar] [CrossRef]










| Analysis Area | Elemental Composition | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Ni | Ag | Fe | Pb | Ca | Cl | |
| Area a—body, where plating is not preserved | × | × | × | traces | ||||
| Area b—body, area where plating is preserved | × | × | traces | × | × | |||
| Area c—handle, whitish layer where plating is not preserved | × | × | × | |||||
| Area d—handle, brown corrosion layer where plating is not preserved | × | × | traces | × | ||||
| Area e—soldering area of handle to the body | × | × | traces | × | ||||
| Sample | Electron Image | Elemental Analysis (Weight %) * |
|---|---|---|
| Black layer (internal surface of lid) |  | Cu (47.7, ±0.9), O (20.7, ±0.7), Ag (17.5, ±0.7), Zn (7.1, ±0.6), Ca (3.6, ±0.2), S (1.5, ±0.2) Si (1.0, ±0.3) (Al) (0.9, ±0.3) |
| Whitish layer (handle, under the plating layer) |  | Zn (65.0, ±0.6), O (20.0, ±0.2), Ni (14.3, ±0.4), (Ca) (0.6, ±0.1) |
| Analysis Area | Elemental Composition wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ag | Cu | Zn | Ni | Fe | Pb | S | Si | Bi | |
| Spot a—body outside, clean silver plating area | 87.25 (±0.46) | 7.01 (±0.08) | 4.77 (±0.06) | 0.40 (±0.01) | - | - | - | - | 0.55 (±0.08) |
| Spot b—body inside, clean silver plating area | 93.96 (±0.48) | 3.52 (±0.06) | 1.99 (±0.04) | 0.15 (±0.01) | - | 0.32 (±0.07) | - | - | - |
| Spot c—body outside, base of the handle, clean silver plating area | 89.69 (±0.54) | 3.55 (±0.07) | 1.15 (±0.03) | 0.25 (±0.02) | 3.95 (±0.07) | 0.40 (±0.08) | 0.98 (±0.02) | - | - |
| Spot d—body outside, black/brown area on silver plating | 61.07 (±0.29) | 8.32 (±0.07) | 2.81 (±0.03) | 0.31 (±0.01) | 22.93 (±0.17) | 1.69 (±0.07) | 2.83 (±0.02) | - | - |
| Spot e—body outside, black/brown area on silver plating | 48.80 (±0.26) | 17.96 (±0.11) | 3.98 (±0.04) | 0.77 (±0.02) | 24.89 (±0.16) | 1.40 (±0.06) | 1.51 (±0.02) | 0.64 (±0.03) | - |
| Spot f—handle, black/brown area on silver platin | 60.84 (±0.30) | 18.89 (±0.11) | 2.06 (±0.03) | 1.80 (±0.14) | 14.17 (±0.14) | 0.40 (±0.09) | 1.80 (±0.01) | - | - |
| Analysis Area | Elemental Composition wt.% | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Ni | Fe | Ag | Pb | Si | Al | |
| Spot a—body outside | 64.67 (±0.20) | 22.07 (±0.11) | 7.24 (±0.07) | 1.31 (±0.03) | 0.43 (±0.04) | 0.49 (±0.03) | 2.45 (±0.09) | 0.67 (±0.20) |
| Spot b—handle | 65.91 (±0.16) | 18.03 (±0.09) | 14.52 (±0.08) | 0.23 (±0.01) | 0.43 (±0.04) | 0.04 (±0.01) | 0.36 (±0.04) | 0.29 (±0.14) |
| Spot c—body inside | 73.04 (±0.22) | 15.64 (±0.09) | 5.40 (±0.0) | 0.12 (±0.01) | 3.73 (±0.08) | 0.44 (±0.03) | 0.65 (±0.06) | 0.52 (±0.22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoulaki, M.; Argyropoulos, V. Conservation of a Marine Silver-Plated German Silver Cloche from the 19th-Century Shipwreck Patris. Heritage 2025, 8, 451. https://doi.org/10.3390/heritage8110451
Giannoulaki M, Argyropoulos V. Conservation of a Marine Silver-Plated German Silver Cloche from the 19th-Century Shipwreck Patris. Heritage. 2025; 8(11):451. https://doi.org/10.3390/heritage8110451
Chicago/Turabian StyleGiannoulaki, Maria, and Vasilike Argyropoulos. 2025. "Conservation of a Marine Silver-Plated German Silver Cloche from the 19th-Century Shipwreck Patris" Heritage 8, no. 11: 451. https://doi.org/10.3390/heritage8110451
APA StyleGiannoulaki, M., & Argyropoulos, V. (2025). Conservation of a Marine Silver-Plated German Silver Cloche from the 19th-Century Shipwreck Patris. Heritage, 8(11), 451. https://doi.org/10.3390/heritage8110451







