1. Introduction
“Stress markers” are signs used in bioarcheology to investigate lifestyle, among which enamel hypoplasia is included. Enamel hypoplasia (EH), defined as a quantitative defect of dental enamel by the Developmental Defects of Enamel index, is a dental sign denoting a physiological disruption in enamel formation during amelogenesis due to infections, nutritional deficiencies, and broader environmental shifts [
1,
2,
3,
4,
5]. The thickness of the defect depends on various factors, including the number of active ameloblasts, the amount of enamel secreted, the duration of ameloblast activity [
4], the distance between the hydroxyapatite prism and the DEJ (dentin-enamel junction) [
6], as well as the severity [
7] and duration of the environmental stress that caused it [
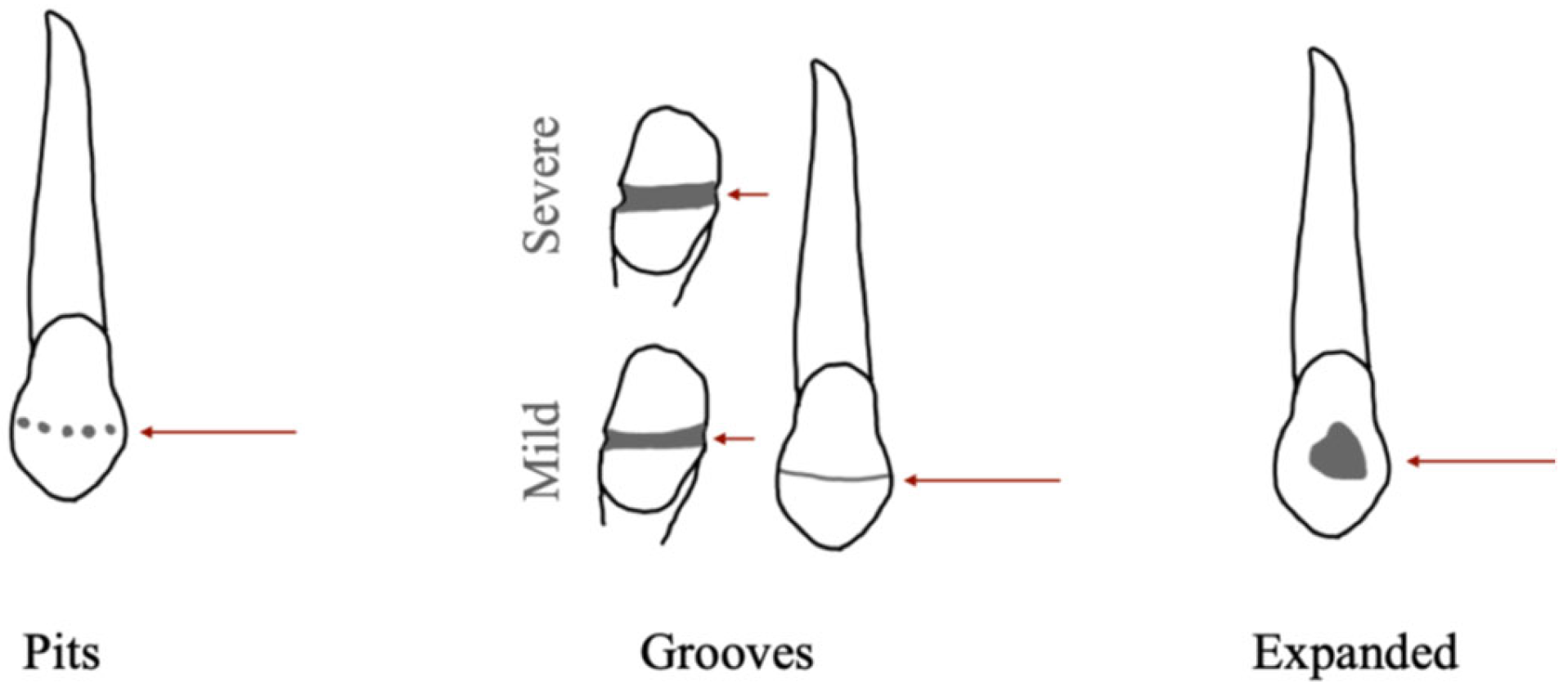
8]. This defect manifests as a reduction in enamel thickness, presenting as grooves (referred to as linear enamel hypoplasia—LEH), pits, or expanded along the tooth surface [
4,
8,
9,
10] (
Figure 1). Perikymata morphology associated with hypoplastic defects confirms that EH develops during the process of enamel deposition [
9]. Specifically, the perikymata of LEH are aligned parallel [
11,
12], whereas pitted EH is related to the apoptosis of groups of 10 to 100 ameloblasts, while neighboring enamel cells continue to secrete matrix, albeit in smaller quantities [
9]. Conversely, expanded EH defects impact the Retzius stria more consistently, resulting in a more evident sign [
9].
1.1. Etiology
The etiology of EH is recognized as multifactorial and related to calcium metabolism and the ARNT pathway (aryl hydrocarbon receptor nuclear translocator) [
13,
14,
15]. Environmental factors seem to exert a more significant influence on its development than genetic and epigenetic alterations [
4,
16]. Prenatal and postnatal environmental shifts and pathological conditions can equally co-occur in EH formation, e.g., alterations of oxygen and glucose levels in blood (due to neonatal hypoxia and gestational diabetes), infectious diseases (rubella, HIV, chickenpox, measles, mumps, cytomegalovirus, respiratory infections, medium ear infections, urinary tract infections) [
17,
18,
19,
20,
21], malnutrition [
22], and birth characteristics [
15,
23,
24,
25]. Prenatal and perinatal conditions (e.g., low body weight, respiratory and cardiovascular problems, gastrointestinal and renal disorders, hematological problems, immune deficiencies, intracranial hemorrhages), as well as birth characteristics (twin birth, podalic position, preterm birth), usually provoke defects on the deciduous dentition [
19,
26,
27,
28,
29,
30,
31,
32,
33]. Malnutrition can occur during intra-uterine life [
22,
34], and, especially after birth, can be related to gastrointestinal infections, parasitic infestations, renal (e.g., nephrotic syndrome) or liver failure [
12,
20,
32,
35,
36], socioeconomical conditions [
37,
38,
39,
40,
41], metabolic condition (e.g., celiac diseases) [
19], and breastfeeding [
42,
43,
44,
45,
46,
47]. Additionally, the use of amoxicillin and tetracycline [
19,
48], dioxin [
49,
50], and iron intoxications [
51,
52] have been reported in correlation with EH. Common infant infections, postnatal traumatic injuries (e.g., from laryngoscopy, orotracheal intubation, maxillary or mandibular trauma), and the mechanical pressure from deciduous teeth on permanent ones may also disrupt enamel deposition for a short period [
14,
19,
31,
53,
54]. EH, in its most severe forms, can also be related to genetic alterations, entering the category of Amelogenesis Imperfecta (AI) [
54,
55,
56].
1.2. Research Hypotheses
This study applies a bioarchaeological approach to the analysis of EH within a contemporary clinical sample. The primary objective is to examine the distribution of EH as a marker of systemic stress in individuals whose medical records and socioeconomic context are known. Through this comparison, it becomes possible to evaluate the reliability and limitations of EH as a proxy for early-life stress, especially in contexts where historical or biographical information is lacking.
Based on prior research [
57], two main hypotheses are proposed:
Hypothesis 1. EH frequency will be higher among individuals from socioeconomically disadvantaged backgrounds, regardless of their country of origin [57,58]. This would support the hypothesis that EH is more closely linked to the biocultural environment during growth and differences in structural vulnerabilities rather than to biogeographical origin (i.e., genetics and epigenetic factors). To test this, we investigate correlations between EH presence and childhood socioeconomic status. This hypothesis is based on the multifactorial etiology of the lesions; therefore, we do not expect to find a specific factor to be associated with EH, but a sum of them, which could be summarized in the access to healthcare and food. Hypothesis 2. No significant sex-based differences in EH distribution are expected, as males and females in the sample are presumed to have experienced comparable living conditions and have relatively equal access to resources. However, the study explores whether female biological buffering [59]—a greater resilience of females to environmental stress—plays a role in the manifestation of EH. This will be assessed by comparing EH frequency and severity between sexes in light of each individual’s known contextual background. 2. Materials and Methods
Two distinct samples were considered: the first comprised 91 living individuals who were examined by one of the authors (CM) for the presence of EH. This assemblage only included adult volunteers willing to take part in the analysis. The second sample consisted of 41 cadavers (10 females and 31 males) subjected to autopsy between May and November 2023 at the Institute of Legal Medicine of Milan (
Table 1); EH was observed by two authors (CM, GL). The following information was obtained for all individuals: biological sex, age, nationality, and socioeconomic status (with three possible answers: low, middle, high). For three individuals, the exact age could not be obtained, and therefore, they were excluded from the age-based evaluation of the EH. The criteria for the selection of the sample were the following: presence of a complete dentition (with the exclusion of the third molar) and known medical history, even if partial.
Data collection was performed in accordance with ethical standards of the institutional and national research committee and with the Helsinki declaration (1964). Also, data acquired on cadavers were obtained during a forensic judicial investigation, in accordance with the Italian Police Mortuary Regulation. The data collected are part of the standard procedures of juridical autopsies, and informed consent was not required. All methods were performed in accordance with Italian law, institutional guidelines, and regulations. The approval from the ethical committee was obtained (UMIL 92/14, UMIL 63/22). To ensure anonymization, the individuals were recorded with alphanumeric values, and no other identifying elements were requested or kept by the authors.
Prior to examination, each tooth was cleaned with a toothbrush, and, in the case of the cadaver sample, no teeth were extracted. For this study, only permanent teeth were analyzed, and all dentition was observed macroscopically. Recorded data included the presence/absence of EH, observability on the dentition, morphology (linear, pitted, and expanded), and location.
The questionnaire was structured as a series of closed dichotomous questions regarding the participants’ medical history, with spaces provided for further specifications [
60]. Questions regarding health status and lifestyle pertained to the period between birth and the 16th year of age (during the period of amelogenesis) to facilitate etiological evaluation of EH.
Information requested from all individuals included biological sex, date of birth or age, nationality (grouped in the present study in two broad categories as European (EU) or non-European (non-EU); place of birth was considered as a proxy for biogeographical origin and its genetic ancestry), economic level (with three possible answers based on annual income: high, if higher than 51.000 euros/year; middle, when included between 25.500–51.000 euros/years; or low, if lower than 25.500 euros/year), medical history (number and reasons for hospitalizations, frequency of dental appointments, medication and vaccines), potential conditions during intrauterine life, characteristics at birth (birth order, weight at birth, potential neonatal hypoxia, cesarean delivery, abruptio placentae, preterm birth) and breastfeeding (duration, supplements), metabolic disorders and nutritional deficiencies (diabetes, scurvy, rickets, malnutrition, calcium deficiency, vitamin C deficiency, food poisoning), other diseases (caries, fluorosis, respiratory disease, blood and vascular disease), trauma on mandibular or maxillary bones, infections (respiratory infections, rubella, chickenpox). Additionally, information regarding the mother’s age at childbirth, nationality, habits, and pregnancy-related pathologies (e.g., smoking, alcohol abuse, gestational diabetes, gestational undernutrition, other problems during pregnancy) was collected. All the information collected referred to the period of dental enamel formation.
Statistical Analyses
Fisher’s test and chi-square test were performed to investigate the relationship between EH, biological sex, socioeconomical status, and etiological factors. All frequencies were obtained through crude data. Statistical analyses were performed using JASP® software (version 0.19.1).
3. Results
The demographic composition of the study sample is detailed in
Table 1. Unless otherwise specified, the results presented below refer to the entire sample, which includes both deceased and living individuals.
EH was observed in 15 individuals (11.3% of the total sample), including 5 females (3.8% of the total sample; 7.9% of females) and 10 males (7.6% of the total sample; 18.5% of males) (Chi-square, p = 0.94). Among the non-European individuals (n = 14), 50% (n = 7) exhibited EH. Of these, 14.2% were female (n = 1) and 85.8% were male (n = 6). In contrast, the frequency of EH in the European group was 7.3% (n = 8), with an equal distribution between sexes (females: 50%, n = 4; males: 50%, n = 4). Excluding the adults for whom a specific age category could not be determined (“adults ND”), the highest frequency of EH was observed in the 31–45 age group (n = 3, 27%). No EH defects were found in individuals aged 13–20 or over 60. Qualitative assessment revealed that the majority of defects were LEH, present in 14 individuals (93.3%). Pitting defects were observed in 5 individuals (33.3%), chromatic alterations (i.e., hypocalcification and fluorosis) in 8 individuals (53.3%), and expanded hypoplasia in 2 individuals (13.3%). EH distribution across the dentition was not uniform. A greater frequency was observed in the upper dentition (58%, n = 52/88 affected teeth), particularly among maxillary central incisors (35.2%, n = 18/52). In the lower dentition, EH was equally distributed between mandibular central incisors and canines (each 25%, n = 9/36).
Among the various biogeographical and medical variables examined through questionnaires, only socioeconomic status showed a statistically significant correlation with the presence of EH (
Table 2).
4. Discussion
4.1. Socioeconomical Status and Etiological Factors
The data derived from the questionnaires were limited, particularly for deceased individuals, for whom only demographic data were generally available. A complete medical history could only be reconstructed for living participants. As a result, the number of individuals with EH in certain subcategories was too low for robust statistical analysis.
4.1.1. Socioeconomic Status
Among all variables derived from the questionnaires, only annual family income showed a statistically significant correlation with EH. Specifically, individuals with a low family income during growth were significantly more likely to exhibit EH (Fisher’s test,
p = 0.05). This suggests that socioeconomic status acts as a proxy for a complex array of stressors—including limited access to healthcare, malnutrition, and hygiene—that influence EH development. However, this result does not imply that low income alone causes EH. Rather, it reflects the broader effects of poverty on early-life health. This has two important implications: first, EH may serve as an indicator of an individual’s socioeconomic condition in the contemporary sample; second, the multifactorial etiology of EH is reinforced, as no consistent correlations were found with other individual variables. Nearly half (45.4%) of individuals in the middle-income bracket displayed at least one EH defect, but this finding did not reach statistical significance. This is probably related to the relatively stable living conditions typical of this income group in the European context [
34,
39,
41,
45,
46,
61,
62,
63,
64,
65,
66]. In the case of individuals subjected to autopsy, low annual income may indicate early exposure to socioeconomic disadvantage, including limited access to healthcare and adequate nutrition. Nevertheless, the correlation between annual income and EH is not unequivocal [
10,
67,
68], and comparisons with other studies should be approached with caution, as income threshold and reporting standards vary widely. Therefore, the correlation with EH and socioeconomic status is statistically supported, and this result confirms the first hypothesis.
The investigation of the context of origin of the individuals can be difficult, especially for an assemblage characterized by individual mobility, as is the one under analysis. For the living individuals, information about this variable was easily collected. However, for the cadavers, the reconstruction of the context of origin and growth was extrapolated from the nationality (i.e., place of birth, declared nationality on ID card). This represents a clear bias due to the lack of direct data about the individuals’ experience. To mitigate this limit, the grouping criteria were limited to two categories (EU, non-EU) and were based on the composition of the sample itself, and current data on GDP were considered to provide a socioeconomic contextualization. Nonetheless, and in spite of these efforts, the interpretation of the results based on the nationality of the individuals must be cautious, especially given the low frequency of non-EU individuals. The findings (significantly higher frequency of EH among non-EU individuals, Fisher’s test,
p = 0.01) align with previous research by Goodman [
37]. However, the association between EH and biogeographical origin cannot be ascertained due to the small sample size and lack of complete information.
4.1.2. Maternal Health
Regarding maternal demographic variables, only age at childbirth between 31 and 35 years approached significance (
p-value = 0.05), consistent with other studies [
31,
62,
64,
69,
70,
71,
72]. This is likely due to the demographic characteristics of the sample and coincides with the typical reproductive age range. Based on questionnaire responses, no instances of deprivation were reported. In the contemporary era, it is plausible that these women received prenatal care, mitigating potential nutritional or health-related risks, as they would have been granted access to health services guaranteed by the State.
None of the variables regarding the mother’s health status during pregnancy appeared to influence the occurrence of EH in newborns (
Table 2). None of the mothers of the individuals with EH disclosed the consumption of alcohol or smoking cigarettes during pregnancy and breastfeeding, although exposure to passive smoke from external sources cannot be strictly ruled out [
51]. Cigarette smoke exposure poses risks to the health of the child, increasing the likelihood of respiratory infections and directly impacting the formation of EH by impeding ameloblast activity [
51]. No other instances of stress during gestation were reported.
4.1.3. Access to Healthcare
All individuals with EH had received standard vaccinations in Italy and had been administered supplements or medications during their childhood. Most (4 out of 5; 75%) also attended regular dental check-ups, and the majority (3 out of 5; 60%) did not require hospitalization during the first 16 years of life, suggesting the absence of severe medical conditions. The absence of statistically significant correlations between EH and variables such as hospitalizations, supplement consumption, and vaccination may suggest that medical support reduced the likelihood of EH development. In fact, income and access to healthcare are considered indicative of an optimal lifestyle and, thus, related to a lower occurrence of EH defects [
11,
39,
63]. However, dental intervention could alter the visibility of EH, potentially leading to underreporting. It appears to act as a protective factor by ensuring adequate medical supervision during growth and reducing risks such as severe infections or malnutrition—factors known to affect enamel formation [
10,
41,
73]. Conversely, limited access to healthcare may increase EH risk and should be considered alongside other socioeconomic indicators [
45].
4.1.4. Order at Birth and Weight
All individuals with EH were firstborns; therefore, the evaluation of the impact of subsequent pregnancies on EH development, due to cumulative maternal stress, cannot be addressed. However, any potential maternal vulnerability might have been mitigated by access to prenatal care.
Individuals with EH had a normal weight at birth (2500–3500 g), indicating that this variable did not influence the development of EH in the present study, despite evidence from previous studies [
70,
73,
74], though the finding was not unprecedented [
34]. Low weight at birth is associated with complications, including cardiovascular, metabolic, and respiratory issues that can necessitate invasive procedures (e.g., laryngoscopy), which can cause mechanical pressures on the mandibular and maxillary bones, causing interruptions in the deposition of the enamel [
31,
33]. However, such complications were not reported in this sample.
4.1.5. Health at Birth
Neonatal hypoxia, premature birth, cesarean section, childbirth complications, and problems or abruptio placentae were not documented in the medical history of individuals with EH. Although premature birth or cesarean section is frequently associated with the development of EH [
31,
46,
70,
75,
76,
77], in this sample, their influence on amelogenesis did not appear to play a role in this study. Hypoxia has been included among the etiological factors of EH because any alterations of oxygen level (bronchitis, asthma, hypoxia, and hypoventilation) could lead to respiratory acidosis (i.e., the increase in acidity in the blood, caused by an excess of carbon dioxide), inhibiting proteolytic enzymes. Consequently, proteins may not be eliminated from the enamel matrix, leaving too little space for mineral deposition and compromising the secretion of hydroxyapatite [
23,
78]. Hypoxia can interrupt ameloblast metabolism by altering their vital functions [
32,
40]. However, hypoxic episodes are typically brief and therefore may not be directly linked to EH but could increase ameloblast sensitivity to other stressors [
79].
4.1.6. Breastfeeding and Weaning
All individuals in the sample, regardless of EH status, were weaned before 12 months of age. Higher frequencies of EH might be expected with early weaning, as the World Health Organization (1990) recommends breastfeeding for at least 12 months to ensure the proper development of the newborn [
43,
45]. This sample—drawn from a relatively wealthy socioeconomic context—may not reflect populations at greater nutritional risk. Previous studies have shown similar findings despite WHO recommendations [
42,
44,
46,
47].
One individual with EH (25%) reported malnutrition during breastfeeding due to nutritional sterility of breast milk, which could represent, along with short weaning, a remarkable risk factor.
4.1.7. Malnutrition
No individual with EH was reported to have suffered from general malnutrition or specific deficiencies in calcium, vitamin C, or vitamin D. This is consistent with the sample’s overall high socioeconomic status and good access to healthcare [
34,
80]. EH has been linked to severe nutritional deficits in several studies [
38,
39,
41,
46,
69,
70,
81,
82], and its absence in this study may be attributed to differences in sample biogeographical origin and composition. Experimental studies suggest that significant caloric (at least equal to 287–521 kcal/day, compared to a normal diet of 1200 kcal/day) and protein restriction (9.6–20 g/day) is required to disrupt enamel formation [
37,
82]. Although not found in this sample, vitamin D deficiency (related to diet, duration of sun exposure, and ability of the gastrointestinal tract to absorb nutrients) can trigger protein malfunction resulting in enamel hypomineralisation [
19,
40,
83,
84]. Celiac disease, which causes nutrient and mineral malabsorption, could also contribute to EH [
19]. In this study, no cases were reported. EH related to celiac disease may assume a specific morphology: hydroxyapatite crystals near EH are arranged irregularly and are less cohesive, thus leaving space for the accumulation of organic matrix, dark pigmentation may be observed from chromogenic microorganisms (i.e., the presence of oral flora) [
85], the defect may be characterized by low severity, and be found on multiple dental elements [
85,
86,
87]. None of the EH defects observed corresponded to this description.
4.1.8. General Health
The evaluation of the medical history of the individual with EH did not reveal any correlation with diabetes, respiratory failure [
32,
70] (in contrast to previous studies [
10,
88]), hypothyroidism, or cardiovascular disease. Hypothyroidism leads to hypocalcemia, which could slow down tooth mineralization and increase the risk of EH formation [
29,
89]. Similarly, excess glucose in the bloodstream associated with diabetes could inhibit the activity of ameloblasts [
15,
24,
25,
29,
90].
While no direct link was found between EH and caries, associations between the two have been documented in the literature. Indeed, periapical lesions resulting from caries could potentially arrest the development of the tooth germ, leading to enamel defects [
91,
92]. Additionally, teeth with EH may be more susceptible to carious lesions due to their porous and soluble enamel in acid in correspondence to the defect [
48,
93]. However, frequent dental care visits, as indicated in the questionnaires, might have contributed to a reduction in the frequency of caries.
Trauma was also considered: 13.3% of respondents reported fractures to the maxillary bones or mandible during growth, but 92% of these individuals did not show EH. In the single case where trauma and EH co-occurred, the injury did not affect the tooth.
Childhood infectious diseases were common in the sample (94.1%), and this can explain the lack of a statistical correlation with EH. The association between EH and infections could stem from direct effects of pathogens on ameloblasts or inhibition of amelogenesis from high body temperature [
51]. Particular attention was given to diseases such as chickenpox, rubella, otitis, urinary tract infections, and respiratory infections. Although there was no significant statistical correlation in the sample, the frequency observed was higher than that proposed by Ford [
51]. For instance, rubella was found in 25% of individuals with EH and in 28% of individuals without EH. The condition is associated with early apoptosis of ameloblasts and interference with the secretion process [
94,
95]. Congenital rubella syndrome (CRS) was noted to have teratogenic effects (i.e., hearing loss, cataracts, heart problems, neurological deficits, diabetes, encephalopathy, thyroid disorders, and EH) due to the production of proteins able to inhibit the mitosis process and cause malfunction of the circulatory system [
96,
97], potentially leading to EH from lack of differentiation or necrosis of the ameloblasts. Urinary tract infections, respiratory infections, and otitis did not occur in the sample; therefore, their correlation with EH could not be investigated.
4.2. Differences Between Females and Males
No statistically significant difference between sexes was found (chi-square test,
p = 0.69). This finding may reflect sampling variability or contextual factors specific to the sample analyzed. Specifically, the low overall frequency of EH within the Italian (and broader European) group may have limited the ability to detect sex-based patterns. Nonetheless, this distribution reflects the studied context, where structural access to food and healthcare is relatively uniform, and overt sex-based disparities in early-life health conditions are not widely reported [
39]. The low frequency of EH was expected, given the relatively high socioeconomic status of the EU-born group and consistent access to healthcare and food resources for both sexes. Given that the theory of female biological buffering posits that, under similar conditions, males should embody environmental stress (i.e., display stress markers) more consistently than females, the obtained results suggest a re-evaluation of this factor. Indeed, in the present context, EH frequencies are similar between the two sexes. This suggests that the presence of EH in this context should not be strictly linked to biological factors related to a greater susceptibility of one sex over the other.
These findings support the utility of EH as a tool for exploring sex-related differences in bioarcheological contexts, particularly in societies where lifestyle conditions between sexes are not equal. In such cases, EH may reflect disparities in access to resources and health stressors during development.
Although the sample size is not large enough for broad epidemiological conclusions, it nonetheless provides useful insights into patterns of EH in a contemporary sample. Additionally, the characteristics of the sample (biological sex, biographical origin) offer a valuable point of comparison for both bioarchaeological and clinical studies.
4.3. Study Limitations
The study comprises several limitations that should be acknowledged: macroscopic observation entails a loss of information, as smaller defects may go unnoticed [
9]. Defect visibility could have been compromised by abrasions and dental wear. Additionally, the absence of juveniles in the examination means that defects in deciduous teeth are not detected. The available data did not permit the identification of a representative statistical trend regarding the analysis of etiology. In fact, it was possible to obtain a complete medical history for only four of 15 individuals with EH.
Despite these constraints, this study presents an innovative combination of EH dating and questionnaire data, allowing for a more comprehensive interpretation of the results. The absence of a straightforward correlation between stress and EH underscores its multifactorial etiology. Rather than being caused by a single variable, EH appears to result from the interplay between structural vulnerability and environmental factors. To substantiate this hypothesis, enlarging the sample size to include more individuals and considering the inclusion of juveniles to analyze deciduous dentition would be necessary. Furthermore, the questionnaire could be improved by adding details about the mother’s health throughout her life and by asking for specific information about individuals’ diets during childhood.
5. Conclusions
This study aimed to evaluate whether biological sex and different etiological factors influence the formation of EH by analyzing a contemporary clinical sample with known demographic and contextual information.
The distribution of EH within the sample supported both initial hypotheses. Specifically, from the comparison with the data from the questionnaire emerges an association between EH and low socioeconomic status. These findings underscore the potential of EH to reflect biocultural conditions and structural vulnerabilities, demonstrating that even in a European context, stress markers can effectively differentiate social variables such as access to resources and structural disadvantage.
The nonspecific etiology of EH—often viewed as a limitation—may, in fact, be one of its strengths. Its ability to capture general physiological stress makes it a powerful indicator of adverse living conditions. In this study, EH was statistically correlated with low income, supporting a multifactorial model in which nutritional deficits and pathological stressors interact during growth. With the synergistic role of nutritional and pathological aspects established, the next step towards better understanding EH may involve exploring the interactions between these factors. Consequently, progressing from the isolation of individual etiological factors, a more comprehensive and accurate approach could entail the development of a statistical model capable of elucidating which factors are involved in the formation of the defect and how they interact. The incorporation of additional factors into the questionnaire may unveil previously unexplored elements.
Although the limited sample size and incomplete medical records constrained some aspects of the analysis, the study nonetheless confirms the value of EH as a marker of physiological stress with applications in both bioarchaeology and forensic anthropology. This research also contributes a rare European clinical dataset to the field, offering a valuable comparative reference for interpreting bioarchaeological samples across different historical periods and geographic contexts.
By applying a bioarchaeological perspective to a clinical population, this study bridges methodological approaches and demonstrates the relevance of EH as a tool for exploring the embodied effects of social inequality in both past and present populations.
Author Contributions
Conceptualization, C.M. and L.B.-G.; methodology, L.B.-G. and D.D.A.; software, C.M.; validation, L.B.-G., G.L.A., D.D.A. and C.C.; formal analysis, C.M. and G.L.A.; investigation, C.M., G.L.A.; resources, C.M.; data curation, C.M.; writing—original draft preparation, C.M.; writing—review and editing, C.M., L.B.-G., D.D.A., F.B., D.M.G., and C.C.; visualization, C.M.; supervision, L.B.-G., F.B., and C.C.; project administration, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the support of the FAITH (Fighting Against Injustice Through Humanities) project of the University of Milan.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
References
- Armelagos, G.J.; Goodman, A.H.; Harper, K.N.; Blakey, M.L. Enamel Hypoplasia and Early Mortality: Bioarcheological Support for the Barker Hypothesis. Evol. Anthropol. 2009, 18, 261–271. [Google Scholar] [CrossRef]
- Clarkson, J.; O’Mullane, D. A Modified DDE Index for Use in Epidemiological Studies of Enamel Defects. J. Dent. Res. 1989, 68, 445–450. [Google Scholar] [CrossRef]
- Hillson, S. Dental Enamel. In Dental Anthropology; Hillson, S., Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 148–181. ISBN 978-0-521-56439-7. [Google Scholar]
- Simalcsik, R.D.; Simalcsik, A.; Groza, V.M. Dental Enamel Hypoplasia. Investigations on the Bones Echumed from the Medieval Necropole of Lozova (Republic of Moldova), XIVth–XVth Centuries. Mem. Sci. Sect. Rom. Acad. 2014, 37, 85–96. [Google Scholar]
- Zsigmondy, O. On Congenital Defects of the Enamel. Dent. Cosm. 1893, 35, 709–717. [Google Scholar]
- Goodman, A.H.; Armelagos, G.J.; Rose, J.C. The Chronological Distribution of Enamel Hypoplasias from Prehistoric Dickson Mounds Populations. Am. J. Phys. Anthropol. 1984, 65, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Shklar, G.; McCarthy, P.L. The Oral Manifestations of Systemic Disease; Butterworth: Boston, MA, USA, 1976. [Google Scholar]
- Guita, J.L. Oral Pathology; Willianx and Wilkins: Philadelphia, PA, USA, 1984. [Google Scholar]
- Hillson, S.; Bond, S. Relationship of Enamel Hypoplasia to the Pattern of Tooth Crown Growth: A Discussion. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 1997, 104, 89–103. [Google Scholar] [CrossRef]
- Mackay, T.D.; Thomson, W. Enamel Defects and Dental Caries among Southland Children. NZ Dent. J. 2005, 101, 35–43. [Google Scholar]
- Hillson, S. Dental Pathology. In Biological Anthropology of the Human Skeleton; Katzenberg, M.A., Saunders, S.R., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 293–333. [Google Scholar]
- King, T.; Humphrey, L.T.; Hillson, S. Linear Enamel Hypoplasias as Indicators of Systemic Physiological Stress: Evidence from Two Known Age-at-Death and Sex Populations from Postmedieval London. Am. J. Phys. Anthropol. 2005, 128, 547–559. [Google Scholar] [CrossRef]
- Collignon, A.-M.; Vergnes, J.-N.; Germa, A.; Azogui, S.; Breinig, S.; Hollande, C.; Bonnet, A.-L.; Nabet, C. Factors and Mechanisms Involved in Acquired Developmental Defects of Enamel: A Scoping Review. Front. Pediatr. 2022, 10, 836708. [Google Scholar] [CrossRef]
- Seow, W.K.; Masel, J.P.; Weir, C.; Tudehope, D.I. Mineral Deficiency in the Pathogenesis of Enamel Hypoplasia in Prematurely Born, Very Low Birthweight Children. Int. J. Paediatr. Dent. 1989, 11, 297–302. [Google Scholar]
- Tolomeu, J.S.O.; Soares, M.E.C.; Mourão, P.S.; Ramos-Jorge, M.L. Is Gestational Diabetes Mellitus Associated with Developmental Defects of Enamel in Children? A Systematic Review with Meta-Analysis. Arch. Oral Biol. 2022, 141, 105488. [Google Scholar] [CrossRef] [PubMed]
- Taji, S.S.; Seow, W.K.; Townsend, G.C.; Holcombe, T. Enamel Hypoplasia in the Primary Dentition of Monozygotic and Dizygotic Twins Compared with Singleton Controls. Int. J. Paediatr. Dent. 2011, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Atar, M.; Körperich, E.J. Systemic Disorders and Their Influence on the Development of Dental Hard Tissues: A Literature Review. J. Dent. 2010, 38, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Jaskoll, T.; Abichaker, G.; Htet, K. Cytomegalovirus Induces Stage-Dependent Enamel Defects and Misexpression of Amelogenin, Enamelin and Dentin Sialophosphoprotein in Developing Mouse Molars. Cells Tissues Organs 2010, 192, 221–239. [Google Scholar] [CrossRef]
- Salanitri, S.; Seow, W.K. Developmental Enamel Defects in the Primary Dentition: Aetiology and Clinical Management. Aust. Dent. J. 2013, 58, 133–140. [Google Scholar] [CrossRef]
- Seow, W.K. Enamel Hypoplasia in the Primary Dentition: A Review. J. Dent. Child. 1991, 58, 441–452. [Google Scholar]
- Seow, W.K.; Shepherd, R.W.; Ong, T.H. Oral Changes Associated with End-Stage Liver Disease and Liver Transplantation: Implications for Dental Management. ASDC J. Dent. Child. 1991, 58, 474–480. [Google Scholar]
- Goodman, A.H.; Rose, J.C. Assessment of Systemic Physiological Perturbations from Dental Enamel Hypoplasias and Associated Histological Structures. Am. J. Phys. Anthropol. 1990, 33, 59–110. [Google Scholar] [CrossRef]
- Franco, K.M.D.; Line, S.R.P.; de Moura-Ribeiro, M.V.L. Prenatal and Neonatal Variables Associated with Enamel Hypoplasia in Deciduous Teeth in Low Birth Weight Preterm Infants. J. Appl. Oral Sci. 2007, 15, 518–523. [Google Scholar] [CrossRef]
- Ghapanchi, J.; Kamali, F.; Siavash, Z.; Ebrahimi, H.; Pourshahidi, S.; Ranjbar, Z. The Relationship between Gestational Diabetes, Enamel Hypoplasia and DMFT in Children: A Clinical Study in Southern Iran. Br. J. Med. Med. Res. 2015, 10, 1–6. [Google Scholar] [CrossRef]
- Grahnén, H.; Möller, E.-B.; Bergström, A.-L. Maternal Diabetes and Changes in the Hard Tissues of Primary Teeth. Caries Res. 1968, 2, 333–337. [Google Scholar] [CrossRef]
- Pimlott, J.F.; Howley, T.P.; Nikiforuk, G.; Fitzhardinge, P.M. Enamel Defects in Prematurely Born, Low Birth-Weight Infants. Pediatr. Dent. 1985, 7, 218–223. [Google Scholar]
- Seow, W.K. Effects of Preterm Birth on Oral Growth and Development. Aust. Dent. J. 1997, 42, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.K.; Young, W.G.; Tsang, A.K.L.; Daley, T. A Study of Primary Dental Enamel from Preterm and Full-Term Children Using Light and Scanning Electron Microscopy. Pediatr. Dent. 2005, 27, 374–379. [Google Scholar] [PubMed]
- Singh, S.; Rathore, M.; Kapur, A. Decrypting the Role of Systemic Illnesses in Developmental Defects of Enamel. J. Postgrad. Med. Educ. Res. 2022, 56, 21–28. [Google Scholar] [CrossRef]
- Taji, S.; Hughes, T.; Rogers, J.; Townsend, G. Localised Enamel Hypoplasia of Human Deciduous Canines: Genotype or Environment? Aust. Dent. J. 2000, 45, 83–90. [Google Scholar] [CrossRef]
- Takaoka, L.A.M.V.; Goulart, A.L.; Kopelman, B.I.; Weiler, R.M.E. Enamel Defects in the Complete Primary Dentition of Children Born at Term and Preterm. Pediatr. Dent. 2011, 33, 171–176. [Google Scholar]
- Via, W.F.; Churchill, J.A. Relationship of Enamel Hypoplasia to Abnormal Events of Gestation and Birth. J. Am. Dent. Assoc. 1959, 59, 702–707. [Google Scholar] [CrossRef]
- Norén, J.G. Enamel Structure in Deciduous Teeth from Low-Birth-Weight Infants. Acta Odontol. Scand. 1983, 41, 355–362. [Google Scholar] [CrossRef]
- Enwonwu, C.O. Influence of Socio-Economic Conditions on Dental Development in Nigerian Children. Arch. Oral Biol. 1973, 18, 95-IN15. [Google Scholar] [CrossRef]
- Koch, M.J.; Bührer, R.; Pioch, T.; Schärer, K. Enamel Hypoplasia of Primary Teeth in Chronic Renal Failure. Pediatr. Nephrol. 1999, 13, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.J.; Owings, C.L.; Brown, W.E.; Shapiro, B.A. Hypoplastic Enamel Associated with the Nephrotic Syndrome. Pediatrics 1963, 32, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.H.; Rose, J.C. Dental Enamel Hypoplasias as Indicators of Nutritional Status. Adv. Dent. Antropol. 1991, 5, 279–293. [Google Scholar]
- MacGregor, A.B. Diet and Dental Disease in Ghana: Hunterian Lecture Delivered at the Royal College of Surgeons of England on 24th May 1963. Ann. R. Coll. Surg. Engl. 1964, 34, 179. [Google Scholar]
- May, R.L.; Goodman, A.H.; Meindl, R.S. Response of Bone and Enamel Formation to Nutritional Supplementation and Morbidity among Malnourished Guatemalan Children. Am. J. Phys. Anthropol. 1993, 92, 37–51. [Google Scholar] [CrossRef]
- Neto, M.B.C.; da Silva-Souza, K.P.; Maranhão, V.F.; Botelho, K.V.G.; Heimer, M.V.; Dos Santos-Junior, V.E. Enamel Defects in Deciduous Dentition and Their Association with the Occurrence of Adverse Effects from Pregnancy to Early Childhood. Oral Health Prev. Dent. 2020, 18, 741–746. [Google Scholar]
- Sweeney, E.A.; Saffir, A.J.; Leon, R. Linear Hypoplasia of Deciduous Incisor Teeth in Malnourished Children. Am. J. Clin. Nutr. 1971, 24, 29–31. [Google Scholar] [CrossRef]
- Haschke, F.; van’t Hof, M.A. Euro-Growth References for Breast-Fed Boys and Girls: Influence of Breast-Feeding and Solids on Growth until 36 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2000, 31, S60–S71. [Google Scholar] [CrossRef]
- Kramer, M.S.; Guo, T.; Platt, R.W.; Shapiro, S.; Collet, J.-P.; Chalmers, B.; Hodnett, E.; Sevkovskaya, Z.; Dzikovich, I.; Vanilovich, I. Breastfeeding and Infant Growth: Biology or Bias? Pediatrics 2002, 110, 343–347. [Google Scholar] [CrossRef]
- Masumo, R.; Bårdsen, A.; Åstrøm, A.N. Developmental Defects of Enamel in Primary Teeth and Association with Early Life Course Events: A Study of 6–36 Month Old Children in Manyara, Tanzania. BMC Oral Health 2013, 13, 21. [Google Scholar] [CrossRef]
- Pinho, J.R.O.; Thomaz, E.B.A.F.; Ribeiro, C.C.C.; Alves, C.M.C.; da Silva, A.A.M. Factors Associated with the Development of Dental Defects Acquired in the Extrauterine Environment. Braz. Oral Res. 2019, 33, e094. [Google Scholar] [CrossRef]
- Vargas-Ferreira, F.; Peres, M.A.; Dumith, S.C.; Thomson, W.M.; Demarco, F.F. Association of Pre-Peri-and Postnatal Factors with Developmental Defects of Enamel in Schoolchildren. J. Clin. Pediatr. Dent. 2018, 42, 125. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.G.; Paul, A.A. Growth Charts and the Assessment of Infant Feeding Practices in the Western World and in Developing Countries. Early Hum. Dev. 1984, 9, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Levy, S.M.; Warren, J.J.; Broffitt, B. Association between Enamel Hypoplasia and Dental Caries in Primary Second Molars: A Cohort Study. Caries Res. 2009, 43, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Alaluusua, S.; Lukinmaa, P.; Koskimies, M.; Pirinen, S.; Hölttä, P.; Kallio, M.; Holttinen, T.; Salmenperä, L. Developmental Dental Defects Associated with Long Breast Feeding. Eur. J. Oral Sci. 1996, 104, 493–497. [Google Scholar] [CrossRef]
- Jälevik, B.; Klingberg, G.; Barregård, L.; Norén, J.G. The Prevalence of Demarcated Opacities in Permanent First Molars in a Group of Swedish Children. Acta Odontol. Scand. 2001, 59, 255–260. [Google Scholar] [CrossRef]
- Ford, D.; Seow, W.K.; Kazoullis, S.; Holcombe, T.; Newman, B. A Controlled Study of Risk Factors for Enamel Hypoplasia in the Permanent Dentition. Pediatr. Dent. 2009, 31, 382–388. [Google Scholar]
- Lawson, B.F.; Stout, F.W.; Ahern, D.E.; Sneed, W.D. The Incidence of Enamel Hypoplasia Associated with Chronic Pediatric Lead Poisoning. South. Carol. Dent. J. 1971, 29, 5–10. [Google Scholar]
- Norén, J.G.; Ranggård, L.; Klingberg, G.; Persson, C.; Nilsson, K. Intubation and Mineralization Disturbances in the Enamel of Primary Teeth. Acta Odontol. Scand. 1993, 51, 271–275. [Google Scholar] [CrossRef]
- Wong, H.M. Aetiological Factors for Developmental Defects of Enamel. Austin J. Anat. 2014, 1, 1003. [Google Scholar]
- Collins, M.A.; Mauriello, S.M.; Tyndall, D.A.; Wright, J.T. Dental Anomalies Associated with Amelogenesis Imperfecta: A Radiographic Assessment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1999, 88, 358–364. [Google Scholar] [CrossRef]
- Winter, G.B.; Brook, A.H. Enamel Hypoplasia and Anomalies of the Enamel. Dent. Clin. North. Am. 1975, 19, 3–24. [Google Scholar] [CrossRef]
- Biehler-Gomez, L.; Palamenghi, A.; Baudu, M.; Caccia, G.; Lanza Attisano, G.; Gibelli, D.; Mazzarelli, D.; Cattaneo, C. Skeletal Markers of Physiological Stress as Indicators of Structural Violence: A Comparative Study between the Deceased Migrants of the Mediterranean Sea and the CAL Milano Cemetery Skeletal Collection. Biology 2023, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, J.S.; Soler, A. Skeletal Indicators of Stress: A Component of the Biocultural Profile of Undocumented Migrants in Southern Arizona. J. Forensic Sci. 2016, 61, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.J.; Nesse, R.M. Economic Transition, Male Competition, and Sex Differences in Mortality Rates. Evol. Psychol. 2007, 5, 147470490700500213. [Google Scholar] [CrossRef]
- Rattray, J.; Jones, M.C. Essential Elements of Questionnaire Design and Development. J. Clin. Nurs. 2007, 16, 234–243. [Google Scholar] [CrossRef]
- Gurri, F.D. Agricultural Transformation and Ontogeny in Rural Populations from the Yucatan Peninsula at the Turn of the Century: Studying Linear Enamel Hypoplasias and Body Composition in Adolescents. In Culture, Environment and Health in the Yucatan Peninsula: A Human Ecology Perspective; Springer: Cham, Switzerland, 2019; pp. 137–157. [Google Scholar]
- Correa-Faria, P.; Martins-Junior, P.A.; Vieira-Andrade, R.G.; Marques, L.S.; Ramos-Jorge, M.L. Perinatal Factors Associated with Developmental Defects of Enamel in Primary Teeth: A Case-Control Study. Braz. Oral Res. 2013, 27, 363–368. [Google Scholar] [CrossRef]
- Daneshkazemi, A.R.; Davari, A. Assessment of DMFT and Enamel Hypoplasia among Junior High School Children in Iran. J. Contemp. Dent. Pr. 2005, 6, 85–92. [Google Scholar] [CrossRef][Green Version]
- Tourino, L.F.P.; Zarzar, P.M.; Corrêa-Faria, P.; Paiva, S.M.; do Vale, M.P.P. Prevalence and Factors Associated with Enamel Defects among Preschool Children from a Southeastern City in Brazil. Cienc. Saude Coletiva 2018, 23, 1667–1674. [Google Scholar] [CrossRef]
- Durmus, B.; Abbasoglu, Z.; Peker, S.; Kargul, B. Possible Medical Aetiological Factors and Characteristics of Molar Incisor Hypomineralisation in a Group of Turkish Children/Moguci Medicinski Etioloski Cimbenici i Znacajke Molarno Incizivne Hipomineralizacije u Skupini Turske Djece. Acta Stomatol. Croat. 2013, 47, 297–306. [Google Scholar] [CrossRef]
- Lopes-Fatturi, A.; Menezes, J.V.N.B.; Fraiz, F.C.; Assunção, L.R.D.S.; de Souza, J.F. Systemic Exposures Associated with Hypomineralized Primary Second Molars. Pediatr. Dent. 2019, 41, 364–370. [Google Scholar]
- Lukacs, J.R.; Walimbe, S.R.; Floyd, B. Epidemiology of Enamel Hypoplasia in Deciduous Teeth: Explaining Variation in Prevalence in Western India. Am. J. Hum. Biol. Off. J. Hum. Biol. Assoc. 2001, 13, 788–807. [Google Scholar] [CrossRef]
- Mariam, S.; Goyal, A.; Dhareula, A.; Gauba, K.; Bhatia, S.K.; Kapur, A. A Case–Controlled Investigation of Risk Factors Associated with Molar Incisor Hypomineralization (MIH) in 8–12 Year-Old Children Living in Chandigarh, India. Eur. Arch. Paediatr. Dent. 2022, 23, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Slayton, R.L.; Warren, J.J.; Kanellis, M.J.; Levy, S.M.; Islam, M. Prevalence of Enamel Hypoplasia and Isolated Opacities in the Primary Dentition. Pediatr. Dent. 2001, 23, 32–43. [Google Scholar] [PubMed]
- Lunardelli, S.E.; Peres, M.A. Breast-Feeding and Other Mother-Child Factors Associated with Developmental Enamel Defects in the Primary Teeth of Brazilian Children. J. Dent. Child. 2006, 73, 70–78. [Google Scholar]
- Dourado, D.G.; Lima, C.C.B.; Silva, R.N.C.; Tajra, F.S.; Moura, M.S.; Lopes, T.S.P.; de Deus Moura, L.D.F.A.; de Deus Moura de Lima, M. Molar-incisor Hypomineralization in Quilombola Children and Adolescents: A Study of Prevalence and Associated Factors. J. Public Health Dent. 2021, 81, 178–187. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.; Menon, I.; Singh, J.; Rai, V.; Aswal, G.S. Molar Incisor Hypomineralization: Prevalence and Risk Factors among 7-9 Years Old School Children in Muradnagar, Ghaziabad. Open Dent. J. 2018, 12, 714. [Google Scholar] [CrossRef]
- Li, Y.; Navia, J.M.; Bian, J. Prevalence and Distribution of Developmental Enamel Defects in Primary Dentition of Chinese Children 3–5 Years Old. Community Dent. Oral Epidemiol. 1995, 23, 72–79. [Google Scholar] [CrossRef]
- Seow, W.K.; Humphrys, C.; Tudehope, D.I. Increased Prevalence of Developmental Dental Defects in Low Birth-Weight, Prematurely Born Children: A Controlled Study. Pediatr. Dent. 1987, 9, 221–225. [Google Scholar]
- Aine, L.; Backström, M.C.; Mäki, R.; Kuusela, A.; Koivisto, A.; Ikonen, R.; Mäki, M. Enamel Defects in Primary and Permanent Teeth of Children Born Prematurely. J. Oral Pathol. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef]
- Seow, W.K.; Brown, J.P.; Tudehope, D.I.; O’callaghan, M. Developmental Defects in the Primary Dentition of Low Birth-Weight Infants: Adverse Effects of Laryngoscopy and Prolonged Endotracheal Intubation. Pediatr. Dent. 1984, 6, 28–31. [Google Scholar] [PubMed]
- Stein, G. Enamel Damage of Systemic Origin in Premature Birth and Diseases of Early Infancy. Am. J. Orthod. Oral Surg. 1947, 33, B831–B841. [Google Scholar] [CrossRef]
- Rizzardi, K.F.; da Silva Toledo, E.; Ferraz, L.F.C.; Darrieux, M.; Girardello, R.; de Lima Marson, F.A.; Parisotto, T.M. Association between Asthma and Enamel Defects in Primary and Young Permanent Teeth–a Systematic Review. Pediatr. Pulmonol. 2022, 57, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sabel, N.; Klingberg, G.; Dietz, W.; Nietzsche, S.; Norén, J.G. Polarized Light and Scanning Electron Microscopic Investigation of Enamel Hypoplasia in Primary Teeth. Int. J. Paediatr. Dent. 2010, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Istat. Resta Stabile La Povertà Assoluta, La Spesa Media Cresce Ma Meno Dell’inflazione; Istat: Rome, Italy, 2024. [Google Scholar]
- Infante, P.F.; Gillespie, G.M. An Epidemiologic Study of Linear Enamel Hypoplasia of Deciduous Anterior Teeth in Guatemalan Children. Arch. Oral Biol. 1974, 19, 1055–1061. [Google Scholar] [CrossRef]
- Zhou, L.; Corruccini, R.S. Enamel Hypoplasias Related to Famine Stress in Living Chinese. Am. J. Hum. Biol. 1998, 10, 723–733. [Google Scholar] [CrossRef]
- Goodman, J.R.; Gelbier, M.J.; Bennett, J.H.; Winter, G.B. Dental Problems Associated with Hypophosphataemic Vitamin D Resistant Rickets. Int. J. Paediatr. Dent. 1998, 8, 19–28. [Google Scholar] [CrossRef]
- Rabbani, A.; Rahmani, P.; Ziaee, V.; Ghodoosi, S. Dental Problems in Hypophosphatemic Rickets, a Cross Sectional Study. Iran. J. Pediatr. 2012, 22, 531. [Google Scholar]
- Bossù, M.; Bartoli, A.; Orsini, G.; Luppino, E.; Polimeni, A. Enamel Hypoplasia in Coeliac Children: A Potential Clinical Marker of Early Diagnosis. Eur. J. Paediatr. Dent. 2007, 8, 31. [Google Scholar]
- Aine, L. Coeliac-Type Permanent-Tooth Enamel Defects. Ann. Med. 1996, 28, 9–12. [Google Scholar] [CrossRef]
- Priovolou, C.; Vanderas, A.; Papagiannoulis, L.A. Comparative Study on the Prevalence of Enamel Defects and Dental Caries in Children and Adolescents with and without Coeliac Disease. Eur. J. Paediatr. Dent. 2004, 5, 102–106. [Google Scholar] [PubMed]
- Nik-Hussein, N.N.; Majid, Z.A.; Mutalib, K.A.; Abdullah, F.; Abang, A.; Wan, M.N. Prevalence of Developmental Defects of Enamel among 16-Year-Old Children in Malaysia. Ann. Dent. Univ. Malaya 1999, 6, 11–16. [Google Scholar]
- Papageorgopoulou, C.; Suter, S.K.; Rühli, F.J.; Siegmund, F. Harris Lines Revisited: Prevalence, Comorbidities, and Possible Etiologies. Am. J. Hum. Biol. 2011, 23, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Pascon, T.; Barbosa, A.M.P.; Cordeiro, R.C.L.; Bussaneli, D.G.; Prudencio, C.B.; Nunes, S.K.; Pinheiro, F.A.; Bossolan, G.; Oliveira, L.G.; Calderon, I.M.P. Prenatal Exposure to Gestational Diabetes Mellitus Increases Developmental Defects in the Enamel of Offspring. PLoS ONE 2019, 14, e0211771. [Google Scholar] [CrossRef]
- McCormick, J.; Filostrat, D.J. Injury to the Teeth of Succession by Abscess of the Temporary Teeth. J. Dent. Child. 1967, 34, 501–504. [Google Scholar]
- Souza, M.A.; Soares Junior, L.A.V.; dos Santos, M.A.; Vaisbich, M.H. Dental Abnormalities and Oral Health in Patients with Hypophosphatemic Rickets. Clinics 2010, 65, 1023–1026. [Google Scholar] [CrossRef]
- Hoffmann, R.H.S.; Sousa, M.D.L.R.D.; Cypriano, S. Prevalence of Enamel Defects and the Relationship to Dental Caries in Deciduous and Permanent Dentition in Indaiatuba, São Paulo, Brazil. Cad. Saúde Pública 2007, 23, 435–444. [Google Scholar] [CrossRef]
- Bhatia, S.; Goyal, A.; Dubey, M.; Kapur, A.; Ritwik, P. Congenital Rubella Syndrome: Dental Manifestations and Management in a 5 Year Old Child. J. Clin. Pediatr. Dent. 2013, 37, 71–75. [Google Scholar] [CrossRef]
- Guggenheimer, J.; Nowak, A.J.; Michaels, R.H. Dental Manifestations of the Rubella Syndrome. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 30–37. [Google Scholar] [CrossRef]
- Musselman, R.J. Dental Defects and Rubella Embryopathy: A Clinical Study of Fifty Children. Ph.D. Dissertation, Indiana University-Purdue University Indianapolis, Indiana, IN, USA, 1968. [Google Scholar]
- Seow, W.K. Oral Complications of Premature Birth. Aust. Dent. J. 1986, 31, 23–29. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).