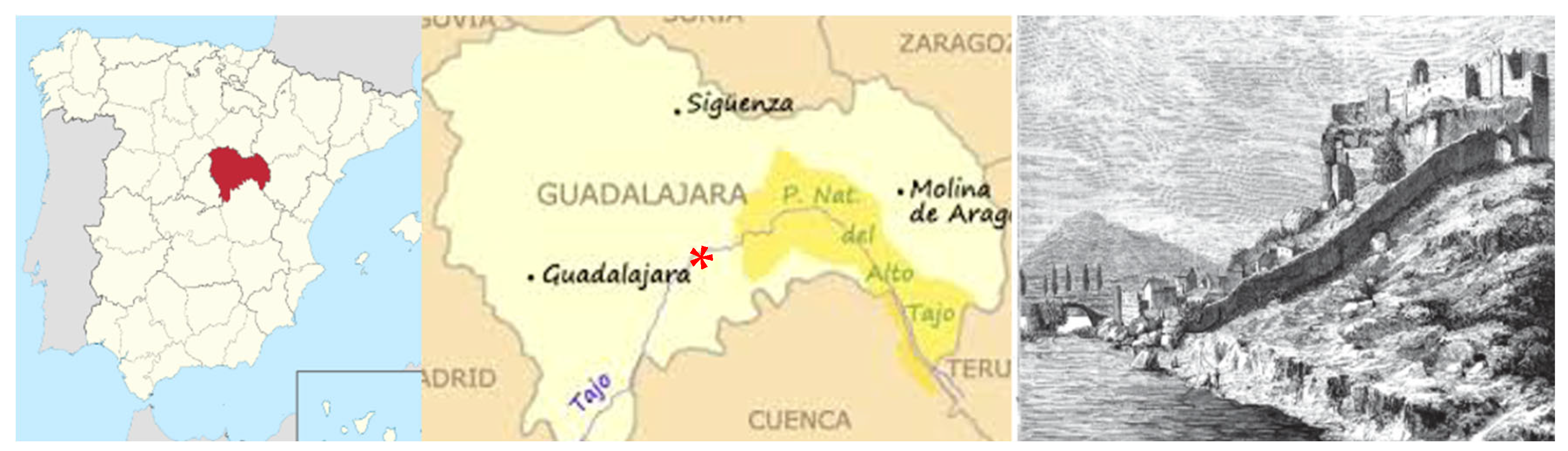
An Ultradolichocephaly in a Knight of the Order of Calatrava from the Castle of Zorita de los Canes (Guadalajara, Spain) Dated Between the 13th and 15th Centuries
Abstract
1. Introduction
2. Materials and Methods
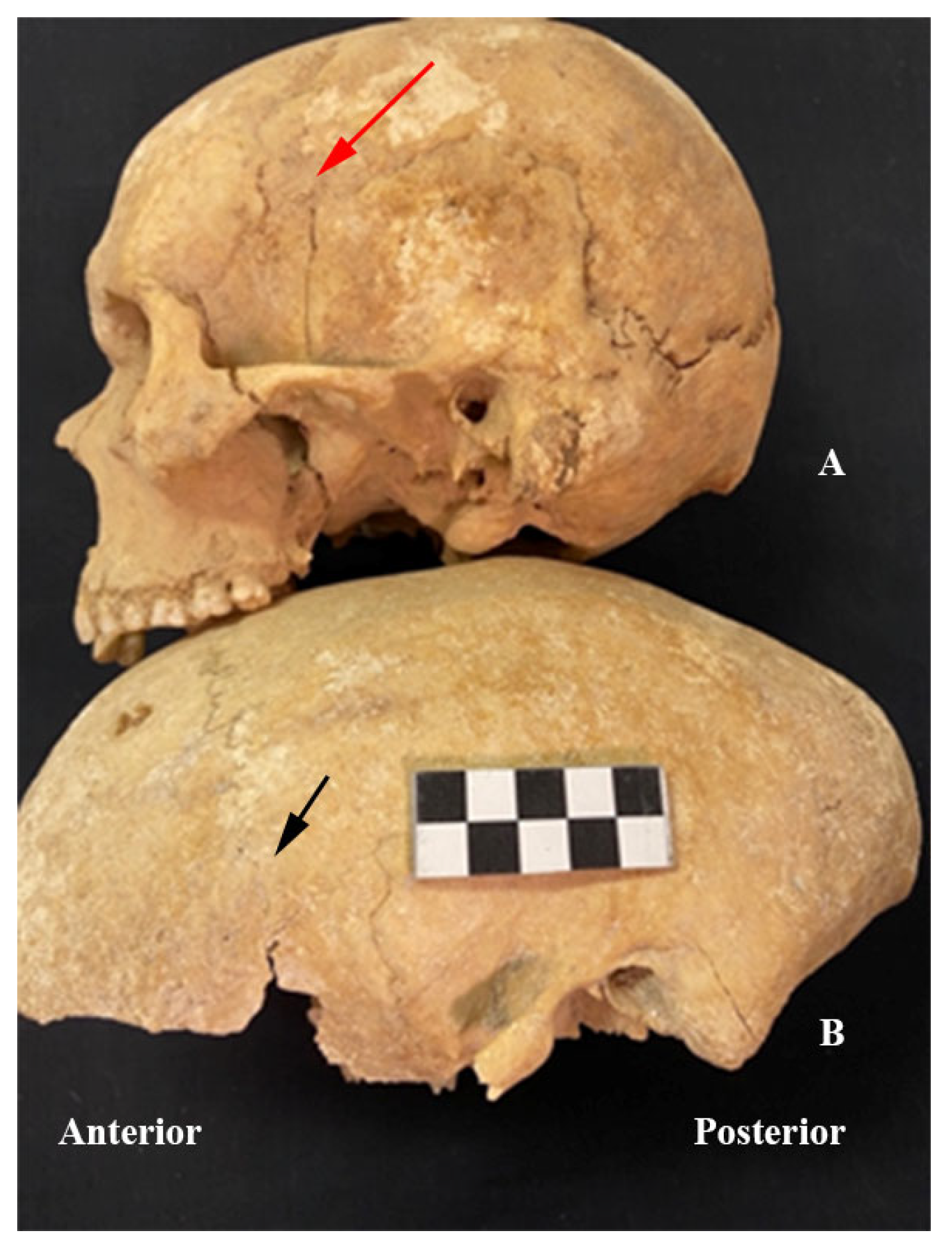
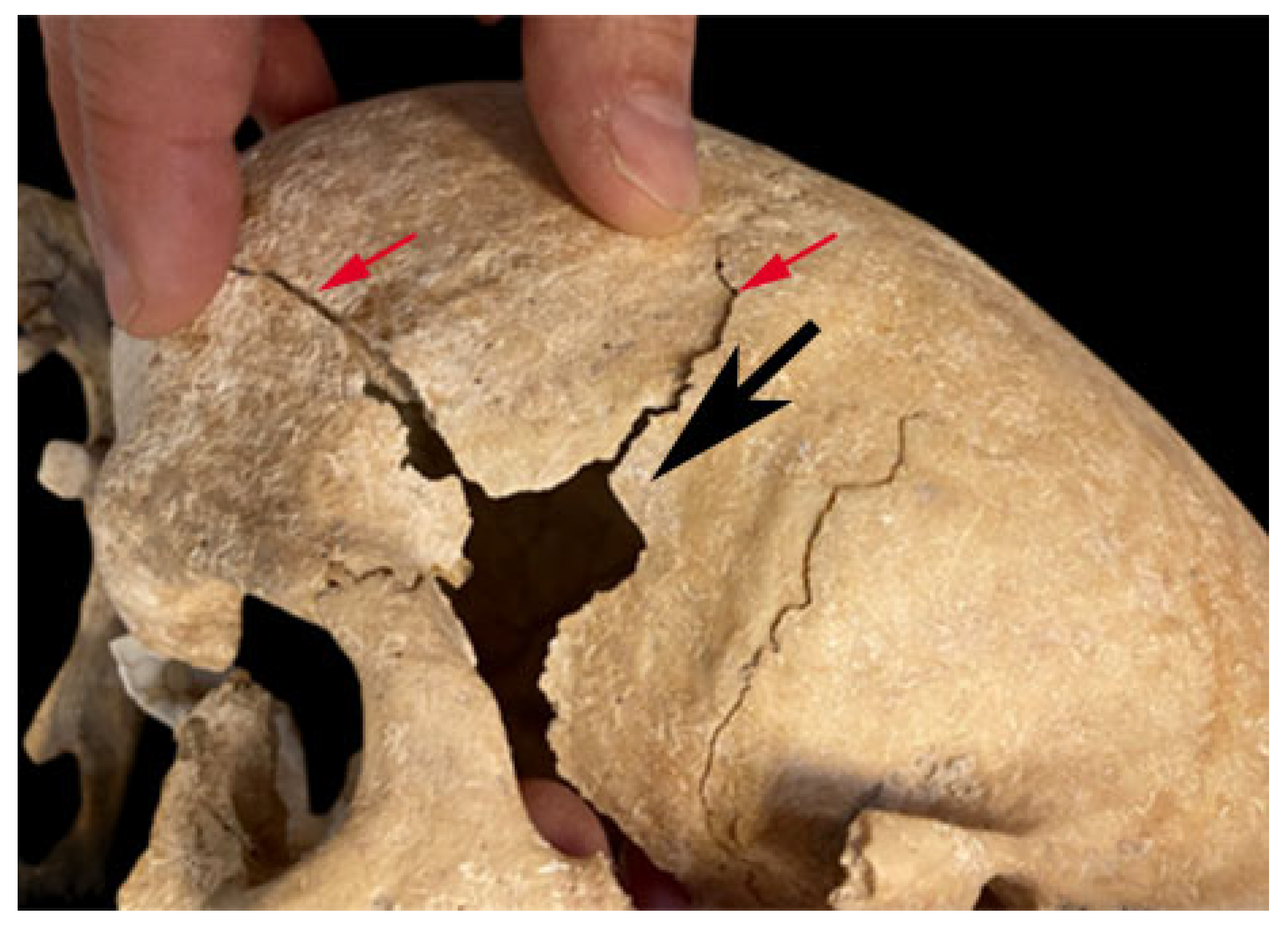
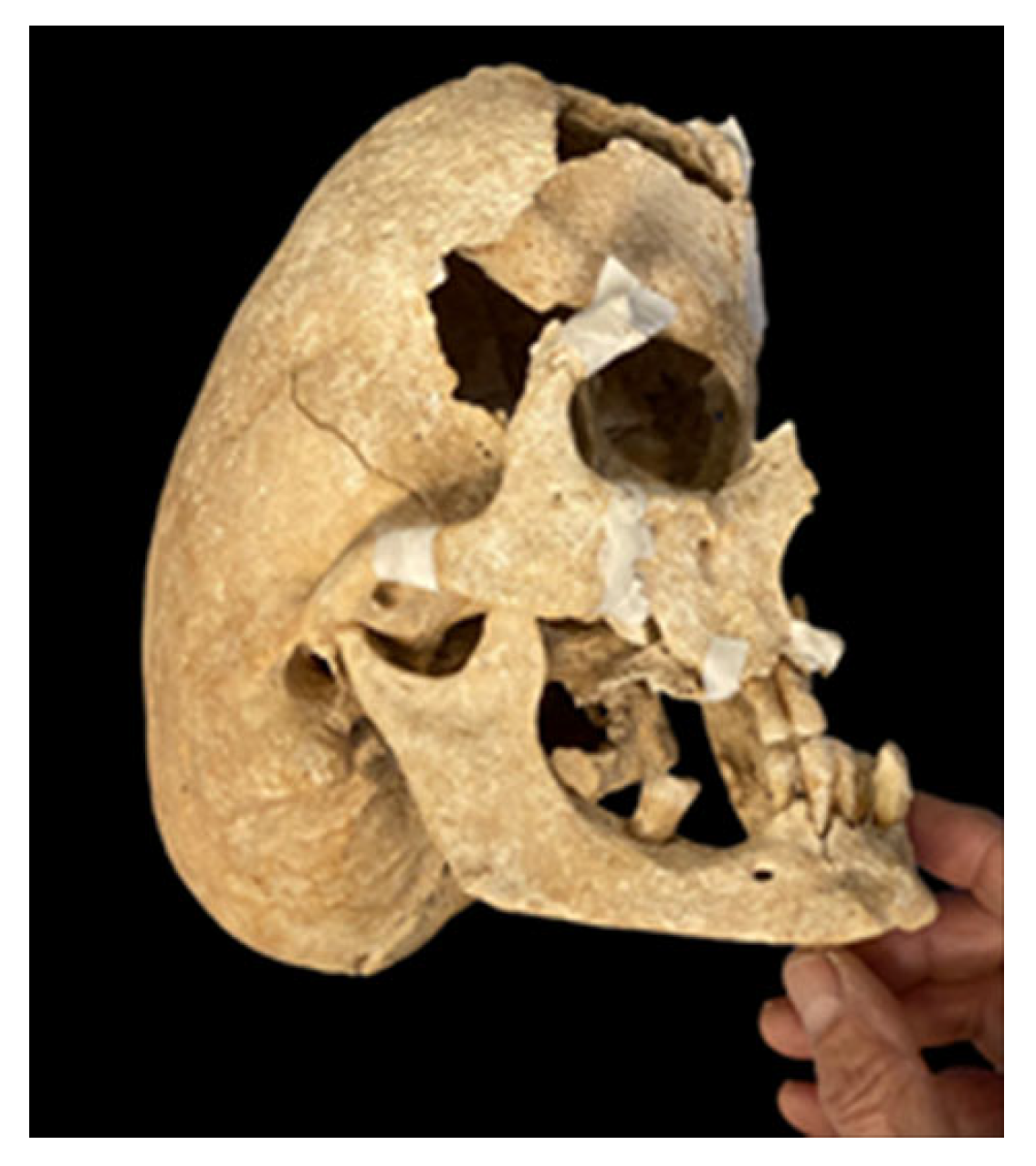
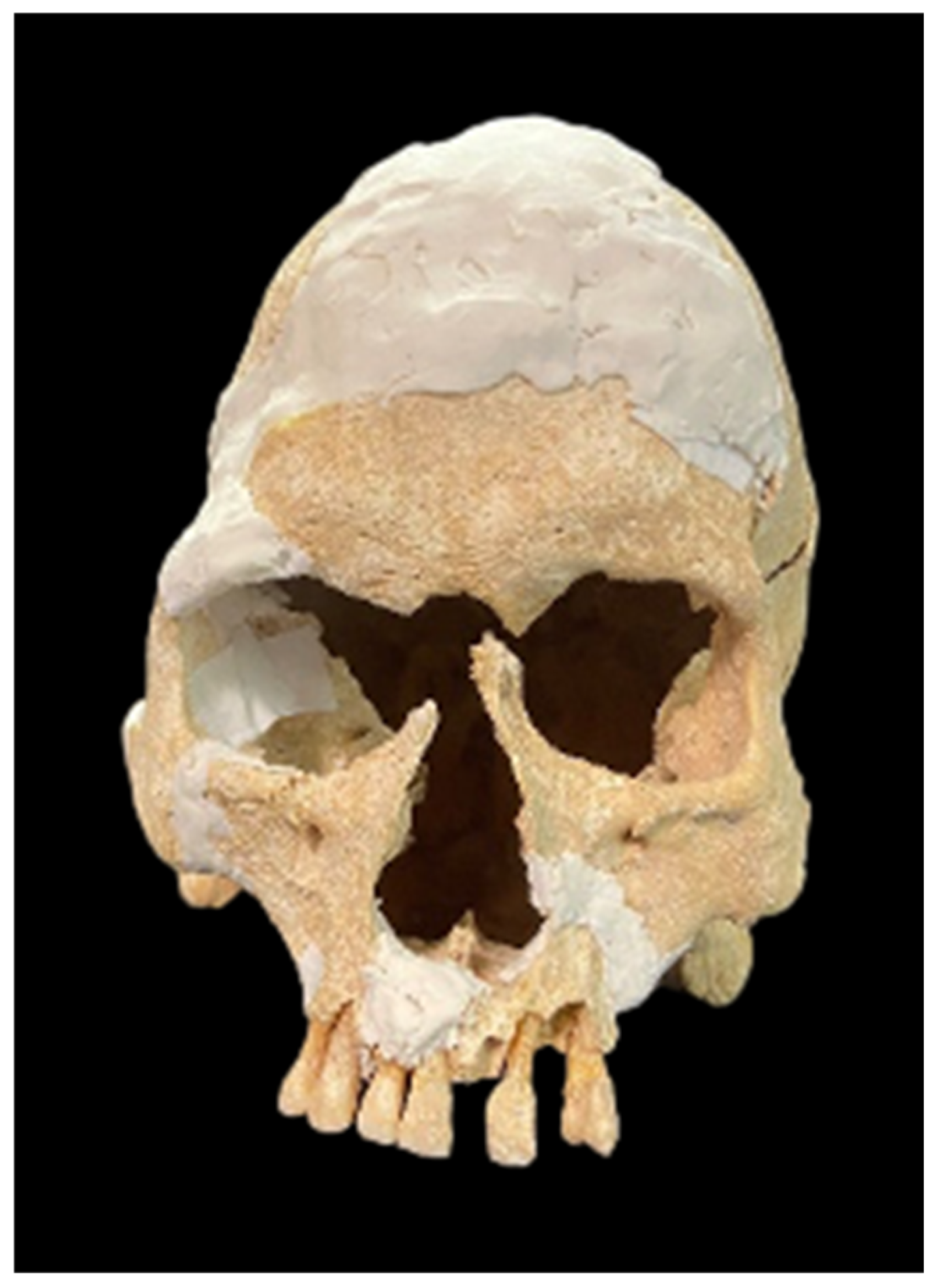
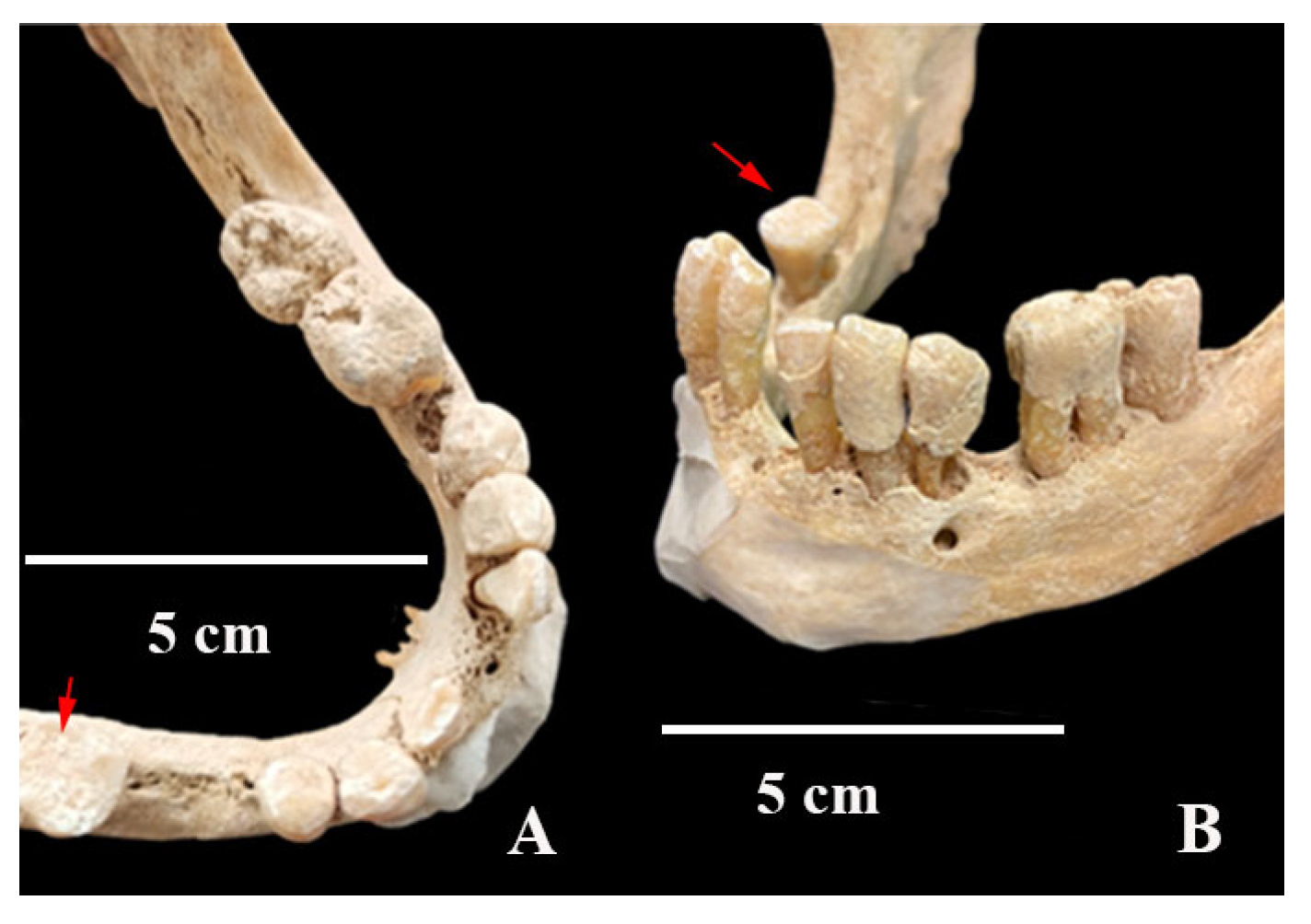
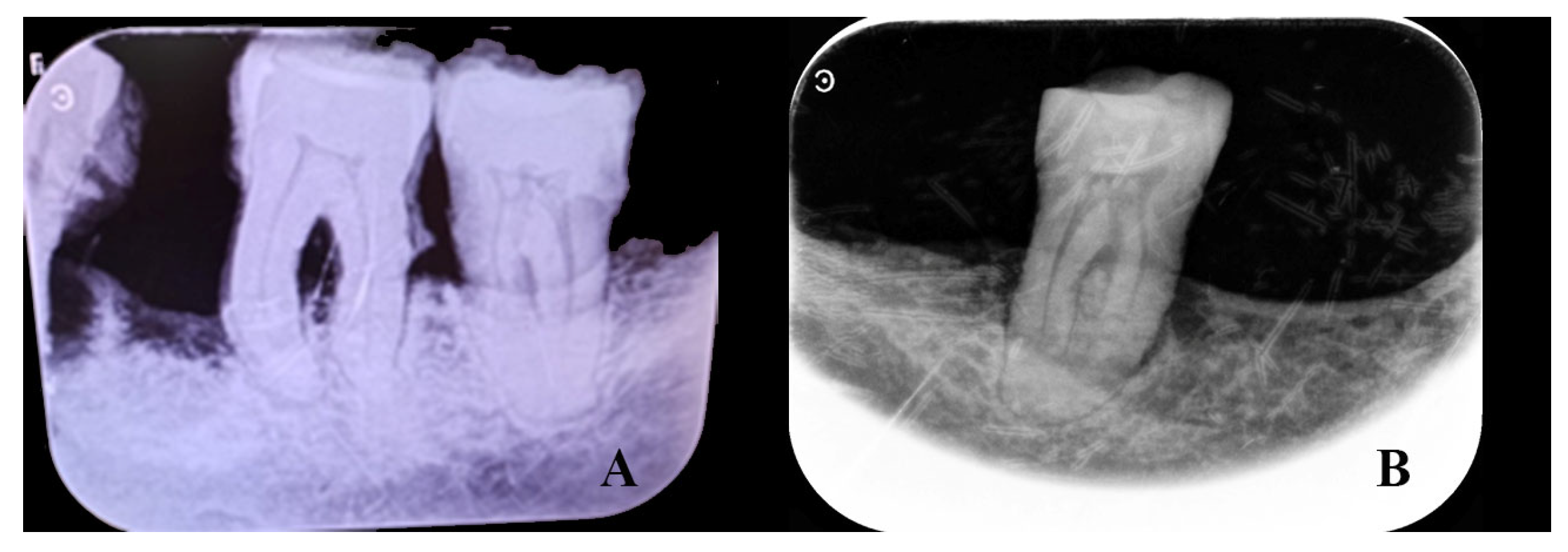
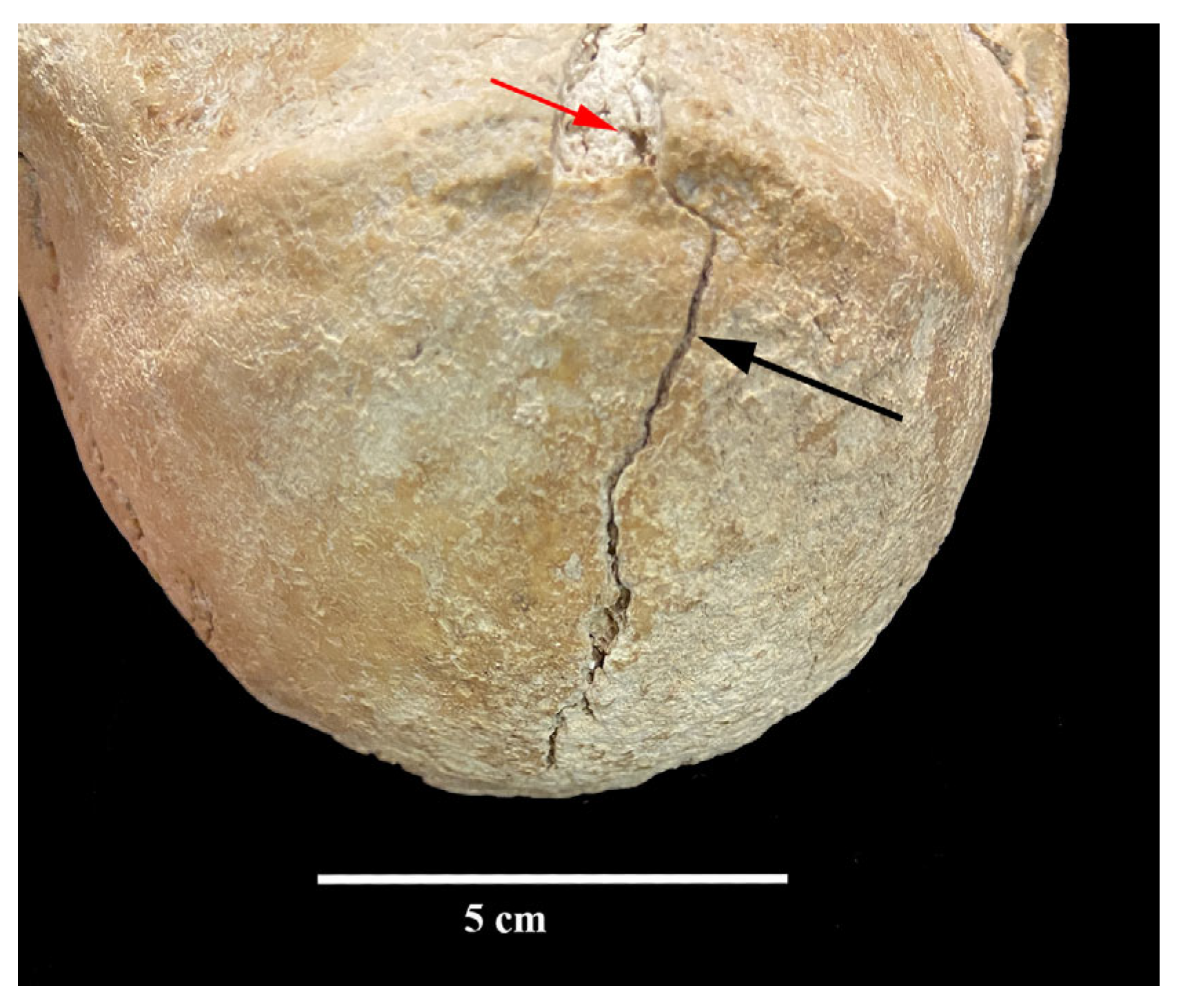
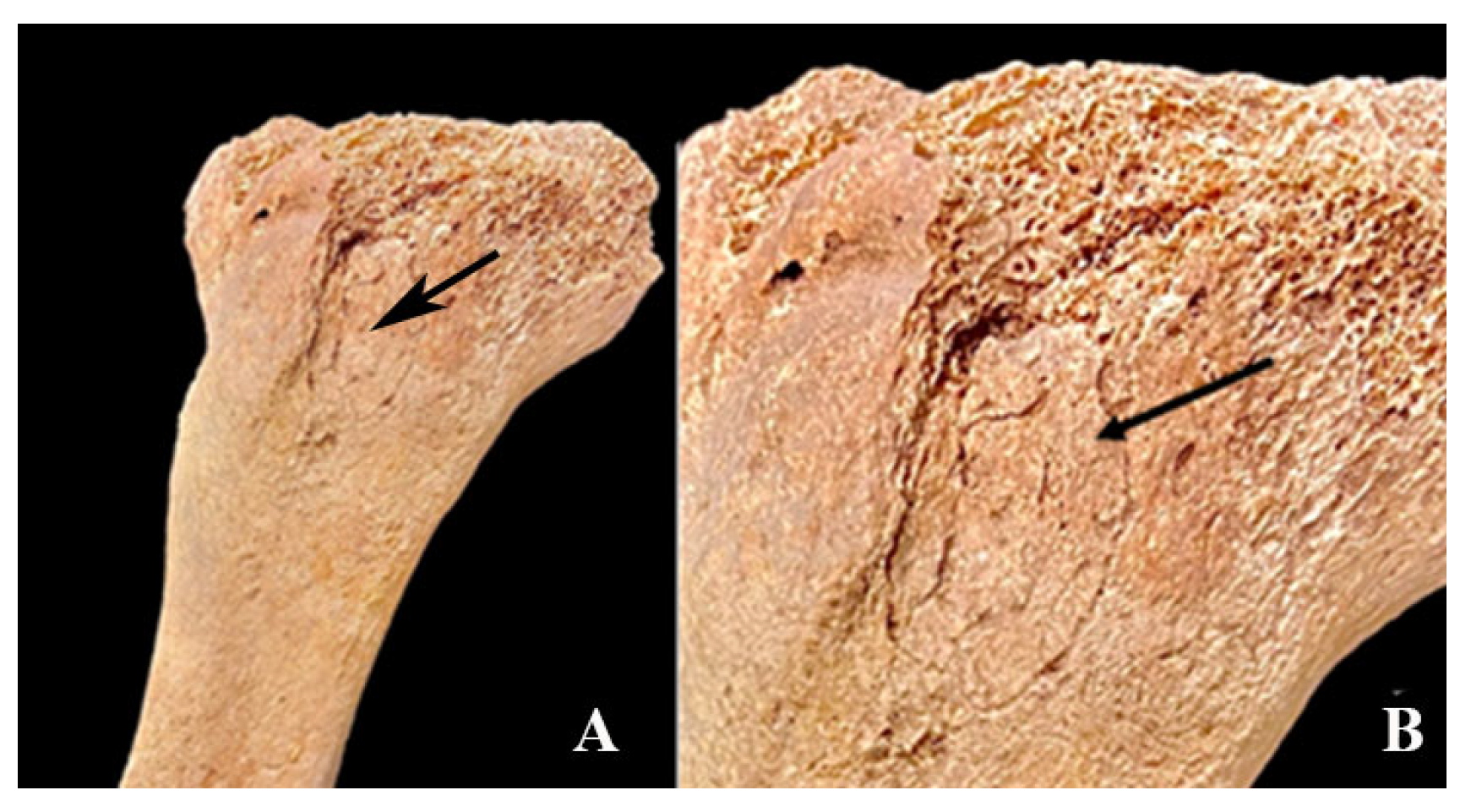
3. Results
4. Discussion
4.1. Differential Diagnosis
4.1.1. Secondary or Primary Craniosynostosis?
4.1.2. What Type of Primary Craniosynostosis: Syndromic or Non-Syndromic?
- Crouzon Syndrome (Table 1)
- b.
- Treacher Collins Syndrome (Table 1)
- c.
- Apert Syndrome (Table 1)
- d.
- Pfeiffer Syndrome (Table 1)
- e.
- Saethre–Chotzen Syndrome (Table 1)
- f.
- Craniofrontonasal Syndrome (Table 1)
- g.
- Noonan Syndrome (Table 1)
- h.
- Muenke Syndrome (Table 1)
- i.
- Neurofibromatosis Syndrome (Table 1)
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Humerus | R | L | Femur | R | L | Radius | R | L |
|---|---|---|---|---|---|---|---|---|
| Minimum perim | 64 | - | Midshaft Perim | 89 | 91 | Minimum perim | - | 43 |
| Midshaft perím | 76 | - | Subtrochanteric perim | 93 | 118 | Midshaft perim. | - | 48 |
| Midshaft minimum diam | 22 | - | Head transvers diam | 30 | 36 | Midshaft minimum. perim. | - | 11 |
| Midshaft. maximum diam | 25 | - | Subtrochanteric antero-pos diam | 28 | 36 | Midshaft minimum. diam. | - | 16 |
| Tibia | R | L | Clavicula | - | 34 | Radial tuberosity. perim | - | 58 |
| Minimum perim | 84 | - | Midshaft perim. | - | 19 | Head perim | - | 72 |
| Midshfta perim. | 88 | - | Acromium maximum width | - | 34 | |||
| Nut-foram A-P Ø | - | 33 | ||||||
| Nut-foram T Ø | - | 29 | ||||||
| Midshaft A-P Ø. | 24 | - | ||||||
| Midshaft T Ø. | 30 | - | ||||||
| Postcranial indices | ||||||||
| Diaphyseal index at R humeral midshaft 88 Cnemic index of the Tibia 88 | ||||||||
| Diaphyseal index at L radial midshaft 69 Diaphyseal index at R tibial midshaft 125 | ||||||||
| Neurocranium | Cranial Indices | ||
|---|---|---|---|
| Maximum length | 230 | Cranial index | 53.04 |
| Maximum width | 122 | Cranial height indices | |
| Biasterionic breadth | 100 | Auricular height-width index (R) | 106.36 |
| Porion-bregmatic height (R) | 117 | Auricular height-width index (L) | 107.27 |
| Porion-bregmatic height (L) | 118 | Longitudinal auricular index (R) | 50.87 |
| Minimum frontal breadth | 102 | Longitudinal auricular index (L) | 51.30 |
| Maximum frontal breadth | 101 | Mean height index (R) | 68.82 |
| Total facial breadth | 100 | Mean height index (L) | 69.41 |
| Parietal sagittal chord | 168 | Facial indices | |
| Occipital sagittal chord | 44 | Frontal transversal index | 100.99 |
| Occipital squama sagittal chord | 68 | Frontoparietal transversal index | 83.61 |
| Parietal sagittal arc | 83 | Orbital index (L) | 103.23 |
| Occipital sagittal arc | 43 | Mandibular indices | |
| Occipital squama sagittal arc | 160 | Yugomandibular index | 73.02 |
| Facial skeleton | Mandibular ramus index (R) | 46.48 | |
| Facial breadth | 126 | Mandibular ramus index (L) | 47.83 |
| Bigonial breadth | 99 | Mandibular index (Thompson) | 98.30 |
| Orbital height (L) | 32 | Mandibular robus index (symphysis) | 80.95 |
| Orbital breadth (L) | 31 | Mandibular robus index (mentalia foramina) (R) | 55.00 |
| Mandibular ramus breadth (R) | 33 | Mandibular Robus index (mentalia foramina) (L) | 47.62 |
| Mandibular ramus breadth (L) | 33 | Mandibular symphysis breadth | 17 |
| Mandibular ramus height (R) | 71 | Mandibular symphsys length | 21 |
| Mandibular ramus height (L) | 69 | Breadth at the mentalia foramina (R) | 11 |
| Mandibular length | 116 | Breadth at the mentalia foramina (L) | 10 |
| Bicondylar breadth | 118 | Height at the mentalia foramina (R) | 20 |
| Height at the mentalia foramina (L) | 21 |
References
- Urbina, D.; Urquijo, C. El Castillo de Zorita (Guadalajara). Historia y Arqueología; El Tercer Sello: Madrid, Spain, 2022. [Google Scholar]
- Rodriguez-Picavea, E. Los Monjes Guerreros en Los Reinos Hispánicos; La Esfera de Los Libros: Madrid, Spain, 2008. [Google Scholar]
- Ayala Martinez, C. Las Órdenes Militares Hispánicas en La Edad Media (Siglos XII–XV); Latorre Literaria: Madrid, Spain, 2003. [Google Scholar]
- Perez-Ramallo, P.; Rissech, C.; Lloveras, L.; Lucas, M.; Urbina, D.; Urquijo, C.; Roberts, P. Unravelling social status in the first medieval military order of the Iberian Peninsula using isotope analysis. Sci. Rep. 2024, 14, 11074. [Google Scholar] [CrossRef] [PubMed]
- Rissech, C.; Creo, O.; Revuelta, B. Estudio preliminar de los restos humanos encontrados en el yacimiento Castillo de Zorita (Guadalajara, España). In El Castillo de Zorita (Guadalajara). Historia y Arqueología; Martínez, D.U., de Toledo, C.U.Á., Eds.; El Tercer Sello: Madrid, Spain, 2022; pp. 265–333. [Google Scholar]
- Alesan, A. Estudi d’una Població Subadulta de l’Edat del Ferro: Demografia, Antropometria i Creixement. Master’s Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 1990. [Google Scholar]
- Ferembach, D.; Schwidetzky, I.; Stloukal, M. Recommendations for Age and Sex Diagnoses of Skeletons. J. Hum. Evol. 1980, 9, 517–549. [Google Scholar] [CrossRef]
- Ubelaker, D.H. Enterramientos Humanos: Excavación, Análisis, Interpretación; Sociedad de Ciencias Aranzadi: San Sebastián, Spain, 2007. [Google Scholar]
- Krenzer, U. Compendio de Métodos Antropológico-Forenses; CAFCA, Centro de Análisis Forense y Ciencias Aplicadas: Guate, Guatemala, 2006. [Google Scholar]
- Aleman, I.; Botella, M.C.; Ruiz, L. Determinación del sexo en el esqueleto postcraneal. Estudio de una población mediterránea actual. Arch. Esp. Morfol. 1997, 2, 7–17. [Google Scholar]
- Krogman, W.M.; Icşan, M.Y. The Human Skeleton in Forensic Medicine; Charles C Thomas Publisher: Springfield, IL, USA, 1986. [Google Scholar]
- Lovejoy, C.O.; Meindl, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronical Metamorphosis of the Auricular Surface of the Ilium: A New Method for the Determination of Adult Skeletal Age at Death. Am. J. Phys. Anthr. 1985, 68, 15–28. [Google Scholar] [CrossRef]
- Rissech, C.; Winburn, A.P.; San-Millán, M.; Sastre, J.; Rocha, J. The acetabulum as an adult age marker and the new IDADE2 (the IDADE2 web page). Am. J. Phys. Anthr. 2019, 169, 757–764. [Google Scholar] [CrossRef]
- Isçan, Y.M.; Loth, S.R.; Wright, R.K. Metamorphosis at the sternal rib end. A new method to estimate age at death in white males. Am. J. Phys. Anthr. 1984, 65, 147–156. [Google Scholar] [CrossRef]
- Márquez-Grant, N.; Rissech, C.; López-Costas, O.; Caro-Dobón, L. Spain/España. In The Routledge Handbook of Archaeological Human Remains and Legislation: An International Guide to Laws and Practice in the Excavation, Study and Treatment of Archaeological Human Remains; Márquez-Grant, N., Fibiger, L., Eds.; Routledge: London, UK, 2010; pp. 423–438. [Google Scholar]
- Martin, R.; Saller, K. Lehrbuch der Anthropologie; Georg Fischer: Stuttgart, Germany, 1957; pp. 429–597. [Google Scholar]
- Olivier, G. Pratique Antropologique; Vigot Frères: Paris, France, 1960; pp. 202–245. [Google Scholar]
- Pearson, K. Mathematical contributions to the Theory of evolution. V. On the reconstruction of the stature of prehistoric races. Phil. Trans. R. Soc. 1899, 192, 169–244. [Google Scholar]
- Fernández-Suárez, M.E. Marcadores Óseos de Actividad Ocupacional en las Poblaciones Históricas de Palat de Rey (León) y de Gormaz (Soria). Ph.D. Thesis, Universidad de León, León, Spain, 2008. [Google Scholar]
- Capasso, L. Atlas of Occupational Markers on Human Remains; Edigrafital, S.P.A.: Teramo, Italy, 1998. [Google Scholar]
- Kennedy, K.A.R. Skeletal markers of occupational stress. In Reconstruction of Life from the Skeleton; Isçan, M.Y., Kennedy, K.A.R., Eds.; Alan, R. Liss: New York, NY, USA, 1989; pp. 129–160. [Google Scholar]
- Hillson, S. Dental Anthropology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Cobo, S. Estudio Dental de los Individuos del Hipogeo Neolítico Final-Calcolítico del Carrer París de Cerdanyola del Vallès (Barcelona). Master’s Thesis, Universitat de Barcelona, Barcelona, Spain, 2022. [Google Scholar]
- Ortner, D.J. Identification of Pathological Conditions in Human Skeletal Remains, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 1–645. [Google Scholar]
- Del Río Múñoz, P.A. Estudio Antropológico-Forense, Antropométrico y Morfológico, de la Colección de la Escuela de Medicina Legal de Madrid. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2000. [Google Scholar]
- Testut, L.; Latarjet, A. Tratado de Anatomía Humana, Vol. 1, Osteología, Artrología, Miología; Salvat: Barcelona, Spain, 1975. [Google Scholar]
- Villa, P.; Mahieu, E. Breakage patterns of human long bones. J. Hum. Evol. 1991, 21, 27–48. [Google Scholar] [CrossRef]
- Mann, R.W.; Hunt, D. Non-metric traits and anatomical variants that can mimic trauma in the human skeleton. Forensic Sci. Int. 2019, 301, 202–224. [Google Scholar] [CrossRef]
- Yilmaz, E.; Mihci, E.; Nur, B.; Alper, Ö.; Taçoy, Ş. Recent Advances in Craniosynostosis. Pediatr. Neurol. 2019, 99, 7–15. [Google Scholar]
- Coussens, A.K.; Wilkinson, C.R.; Hughes, I.P.; Morris, C.P.; van Daal, A.; Anderson, P.J.; Powell, B.C. Unravelling the molecular control of calvarial suture fusion in children with craniosynostosis. BMC Genom. 2007, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.G.; Martín, M.C.; Rodríguez, F.R.; Abascal, I.P. Evidencias paleopatológicas de raquitismo en España. In Sistematización Metodológica en Paleopatología; Actas V Congreso Nacional Asociación Española de Paleopatología: Alcalá la Real, Spain, 1999; pp. 139–145. [Google Scholar]
- Brickley, M.; Ives, R. The Bioarchaeology of Metabolic Bone Disease; Elsevier: London, UK, 2008; pp. 114–150. [Google Scholar]
- Kranzler, J.H.; Rosenbloom, A.L.; Proctor, B.; Diamond, F.B.; Watson, M. Is short stature a handicap? A comparison of the psychosocial functioning of referred and nonreferred children with normal short stature and children with normal stature. J. Pediatr. 2000, 136, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Darbà, J.; Marsà, A. Epidemiology and management of parathyroid gland disorders in Spain over 15 years: A retrospective multicentre analysis. PLoS ONE 2020, 15, e0230130. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Pérez, I.S.; Sequeda-Monterroza, J.F.; Zarate-Vergara, A.C. Craneosinostosis: Revisión de literatura. Univ. Salud 2016, 18, 182–189. [Google Scholar] [CrossRef]
- Reina, F.; Carrera, A.; Rissech, C.; Coromina, N.; Burch, J.; Sagrera, J.; Vivo, J. Descripció morfològica de vuit cranis procedents del sector de l’antic cementiri parroquial del jaciment arqueològic de Sant Julià de Ramis (Girona). In What Bones Tell Us. El que ens Expliquen els Ossos; Lloveras, L., Rissech, C., Nadal, J., Fullola, J.M., Eds.; Monografies del SERP 12; Universitat de Barcelona: Barcelona, Spain, 2016; pp. 105–111. [Google Scholar]
- Kimonis, V.; Gold, J.A.; Hoffman, T.L.; Panchal, J.; Boyadjiev, S.A. Genetics of Craniosynostosis. Semin. Pediatr. Neurol. 2007, 14, 150–161. [Google Scholar] [CrossRef]
- Wilkie, A.O.; Byren, J.C.; Hurst, J.A.; Jayamohan, J.; Johnson, D.; Knight, S.J.; Wall, S.A. Prevalence and complications of single-gene and chromosomal disorders in craniosynostosis. Pediatrics 2010, 126, e391–e400. [Google Scholar] [CrossRef]
- Ko, J.M. Genetic syndromes associated with craniosynostosis. J. Korean Neurosur. Soc. 2016, 59, 187–191. [Google Scholar] [CrossRef]
- Governale, L.S. Craniosynostosis. Pediatr. Neurol. 2025, 53, 394–401. [Google Scholar] [CrossRef]
- Díaz, P.A.; Hernández, J.A. Síndrome de Crouzon: Revisión de tema y reporte de caso. Rev. Estomatol. 2016, 24, 26–32. [Google Scholar] [CrossRef]
- de León, C.P.F. Craneoestenosis. II. Análisis de las craneoestenosis sindromáticas y diferentes tipos de tratamiento. Med. Hosp. Infant. Mex. 2011, 68, 409–418. [Google Scholar]
- Ibarra, L.; Pérez, J.I.; Baltar, F.; Lucas, L.; Costa, G.; Borbonet, D.; González, G. Guía clínica: Alteraciones de la forma del cráneo. Arch. Pediatría Urug. 2022, 93, e804. [Google Scholar] [CrossRef]
- Orfila, D.; Tiberti, L. Atresia congénita del oído y su manejo. Med. Clínica Condes 2016, 27, 880–891. [Google Scholar] [CrossRef]
- Mollinedo, M.; Quisbert, I.J. Síndrome de Treacher Collins (STC). Rev. Actual. Clín. UMSA 2014, 46, 2347–2441. [Google Scholar]
- Papp, E. Síndrome de Apert (Acrocefalosindactilia): Presentación de dos casos clínicos. Acta Odontológica Venez. 1999, 37, 163–167. [Google Scholar]
- Kaplan, L.C. Clinical assessment and multispecialty management of Apert syndrome. Clin. Plast. Surg. 1991, 18, 217–225. [Google Scholar] [CrossRef]
- Kaloust, S.; Ishii, K.; Vargervik, K. Dental development in Apert syndrome. Cleft Palate-Craniofacial J. 1997, 34, 117–121. [Google Scholar] [CrossRef]
- Reardon, C. Craniosynostosis. Diagnosis, evaluation and management. J. Med. Genet. 2000, 37, 727. [Google Scholar] [CrossRef]
- De Los Ríos Quintanero, B.D.; Gracia Rojas, E.; Ortiz-Movilla, R.; Cabrejas-Núñez, M.J.; Marín-Gabriel, M. Saethre-Chotzen syndrome: A case report. Arch. Argent. Pediatr. 2021, 119, E129–E132. [Google Scholar] [CrossRef]
- Santana-Hernández, E.E.; Llauradó-Robles, R.A. Síndrome de Saethre Chotzen. Rev. Cienc. Médicas 2017, 21, 271–276. [Google Scholar]
- Marzin, P.; Cormier-Daire, V. Enfermedades óseas constitucionales. EMC Pediatría 2021, 56, 1–13. [Google Scholar] [CrossRef]
- Ortube, M.C.; Vélez, F. Estrabismo en craneosinostosis. Acta Estrabológica 2022, 2, 64–78. [Google Scholar]
- Carcavilla, A.; Suárez, L.; Rodríguez, A.; Gonzalez, I.; Ramón, M.; Labarta, J.; Quinteiro, S.; Riaño Galán, I.; Ezquieta Zubicaray, B.; López, J.P. Noonan syndrome: Genetic and clinical update and treatment options. An. Pediatr. 2020, 93, 61.e1–61.e14. [Google Scholar] [CrossRef] [PubMed]
- Carcavilla, A.; Santomé, J.; Galbis, L.; Ezquieta, B. Mesa redonda ¿de la clínica al gen, o del gen a la clínica? Síndrome de Noonan. Rev. Esp. Endocrinol. Pediatr. 2013, 4 (Suppl. S1), 71–86. [Google Scholar] [CrossRef]
- Domínguez-Carrillo, L.G. Sinostosis Radiocubital Proximal Congénita en Femenino. Rev. Med. Clin. 2020, 9, 57–58. [Google Scholar] [CrossRef]
- Doherty, E.S.; Lacbawan, F.; Hadley, D.W.; Brewer, C.; Zalewski, C.; Kim, H.J.; Muenke, M. Muenke syndrome (FGFR3-related craniosynostosis): Expansion of the phenotype and review of the literature. Am. J. Med. Gene. Part A 2007, 143, 3204–3215. [Google Scholar] [CrossRef]
- Sharafi, P.; Ayter, S. Possible modifier genes in the variation of neurofibromatosis type 1 clinical phenotypes. J. Neurogenet. 2018, 32, 65–77. [Google Scholar] [CrossRef]
- Ueda, K.; Yaoita, M.; Niihori, T.; Aoki, Y.; Okamoto, N. Craniosynostosis in patients with RASopathies: Accumulating clinical evidence for expanding the 30 phenotype. Am. J. Med. Gene. Part A 2017, 173, 2346–2352. [Google Scholar] [CrossRef]
- Gabilondo, F.E. Paleopatología de los restos humanos de San Andrés de Astigarribia (Motrico, Guipuzcoa). Zainak 1987, 4, 279–288. [Google Scholar]
- Amar, T.; Krishna, V.; Sona, K. Apert syndrome: A rarepresentation. J. Indian Acad. Clin. Med. 2007, 8, 245–246. [Google Scholar]
- Rathore, E.; Rathore, A.H. Apert Syndrome: Report of a rare congenital malformation. Pak. J. Med. Sci. 2017, 33, 773–775. [Google Scholar] [CrossRef]
- Ruggiere, M.; Gentile, A.E.; Ferrara, V.; Papi, M.; Praticò, A.D.; Mudry, A.; Taruscio, D.; Micali, G.; Polizzi, A. Neurocutaneous syndromes in art and antiquities. Am. J. Med. Genet. 2021, 187, 224–234. [Google Scholar] [CrossRef]
- Cevallos, A.; Tomàs, X.; Lloveras, L.; Rissech, C. Reconstructing a Lay Individual’s Elbow Fracture at Santa Caterina Friary, Barcelona (15th–16th Century): The Contribution of Paleopathology to the Valorization of Bioarcheological Heritage. Heritage 2024, 7, 4182–4192. [Google Scholar] [CrossRef]
- Argote, N.; Botella, M.; Etxebarría, F. Necrópolis medievales del País Vasco: Estado actual de la investigación antropológica. Sautuola Rev. Inst. Prehist. Arqueol. Sautuola 2013, 18, 275–289. [Google Scholar]
- Campillo, D. Craneoencephalic pathology in paleopathology. Int. Congr. Ser. 2006, 1296, 41–54. [Google Scholar] [CrossRef]
- Lloret, F.R.; Díaz, J.A.S.; de Togores-Muñoz, C.R.; Rodrigo, J.C.; Rodrigo, B.C.; Lloret, J.B.M. Paleopatología traumática en dos cráneos encontrados en el nivel III de la Cova d’En Pardo (Planes, Alicante). MARQ Arqueol. Mus. 2006, 1, 9–24. [Google Scholar]



| Syndrome | Gen | Gen Dominance | Affected Sutures | Craniofacial Malformations | Associated Malformations |
|---|---|---|---|---|---|
| Crouzon | FGFR2/FGFR3 (10q25–10q26) | Autosomal Dominant. | Sagittal (the most prevalent), Coronal, and/or lambdoidea. Variability | High phenotypic variability Hypertelorism, maxillary hypoplasia, choanal atrophy, receding maxilla, a sunken palate, and atresia of the auditory meatus. | Normal cognitive level, possible cervical vertebral fusion. |
| Treacher Collins | TCOF1 POLR1C POLR1D | Depending on the gene can be autosomal dominant or recessive. | Underdevelopment of the zygomatic bones. The facial sunken bone is characteristic | Midface hypoplasia, micrognathia as well as sporadically cleft palate and choanal atresia or stenosis. | Digestive and respiratory systems, visual and hearing problems, language and dental disorders, as well as malformations of the hands bones and absence of the first finger. |
| Apert | FGFR2 (10q25–10q26) | Autosomal Dominant. | Mostly coronal. However, it can affect any cranial suture. | Acrocephaly, brachytrurrycephaly, facial hypoplasia, ogival upper jaw, dental crowding. | Syndactyly in hands, cognitive and hearing deficits. Aplasia of the shoulders, elbows, and hips. |
| Pfeiffer | FGFR1/FGFR2 (8p11.22-p12 y 10q26-q26) | Autosomal Dominant. | Coronal and/or sagittal. Possible cloverleaf skull | Maxillary hypoplasia, choanal atresia | Partial syndactyly of the hands and feet. Normal cognitive level. Patients with cloverleaf skull have a high mortality rate. |
| Saethre–Chotzen | TWIST1/FGFR2 (7p 21–22) | Autosomal Dominant. | Coronal, lambdoid and/or metopic. | Acrocephaly and brachycephaly, maxillary hypoplasia. | Brachydactyly in hands and feet. They do not present cognitive deficit. |
| Craniofrontonasal | EFNB1 in X chromosome | Autosomal dominant | Cranial synostosis | Hypertelorism | Drooping shoulders, clavicular dysplasia, cleft palate, and duplication of the first finger |
| Noonan | PTPN11 SOS1, RAF1, KRAS | Autosomal dominant | Craniofacial abnormalities | Ptosis, low-set ears, wide neck, and broad forehead. | Short stature, growth retardation, cardiovascular problems. Syndactyly. |
| Muenke | FGFR3 (4p) | Autosomal dominant | Coronal (unilateral or bilateral) | Exophthalmia, midfacial hypoplasia | Clinodactyly, tarsal and carpal fusion. Hearing loss |
| Neurofibromatosis | Gen NF1 | Autosomal dominant | Sagittal | Increased size and prominence of the frontal and occipital bones | Cardiovascular problems Frontal hypertrichosis, café-au-lait spots on the dermis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissech, C.; Creo, O.; Revuelta, B.; Cobo, S.; Urbina, D.; Urquijo, C.; Banks, P.; Lloveras, L. An Ultradolichocephaly in a Knight of the Order of Calatrava from the Castle of Zorita de los Canes (Guadalajara, Spain) Dated Between the 13th and 15th Centuries. Heritage 2025, 8, 414. https://doi.org/10.3390/heritage8100414
Rissech C, Creo O, Revuelta B, Cobo S, Urbina D, Urquijo C, Banks P, Lloveras L. An Ultradolichocephaly in a Knight of the Order of Calatrava from the Castle of Zorita de los Canes (Guadalajara, Spain) Dated Between the 13th and 15th Centuries. Heritage. 2025; 8(10):414. https://doi.org/10.3390/heritage8100414
Chicago/Turabian StyleRissech, Carme, Oscar Creo, Blanca Revuelta, Susana Cobo, Dionisio Urbina, Catalina Urquijo, Philip Banks, and Lluís Lloveras. 2025. "An Ultradolichocephaly in a Knight of the Order of Calatrava from the Castle of Zorita de los Canes (Guadalajara, Spain) Dated Between the 13th and 15th Centuries" Heritage 8, no. 10: 414. https://doi.org/10.3390/heritage8100414
APA StyleRissech, C., Creo, O., Revuelta, B., Cobo, S., Urbina, D., Urquijo, C., Banks, P., & Lloveras, L. (2025). An Ultradolichocephaly in a Knight of the Order of Calatrava from the Castle of Zorita de los Canes (Guadalajara, Spain) Dated Between the 13th and 15th Centuries. Heritage, 8(10), 414. https://doi.org/10.3390/heritage8100414





