Osseous Variants of the Cervical Spine with Potential Pathological Significance: Possible Evidence of Vertebrobasilar Insufficiency in a Skeletal Sample from the Post-Classical Cemetery of Corfinio (12th–15th Centuries CE, L’Aquila, Italy)
Abstract
1. Introduction
Cervical Spine Anatomy Overview
2. Materials and Methods
2.1. Original and Study Skeletal Samples
2.2. Archaeological Background of the Funerary Area
2.3. Analytical and Methodological Procedures
2.3.1. Morphological Data Collection
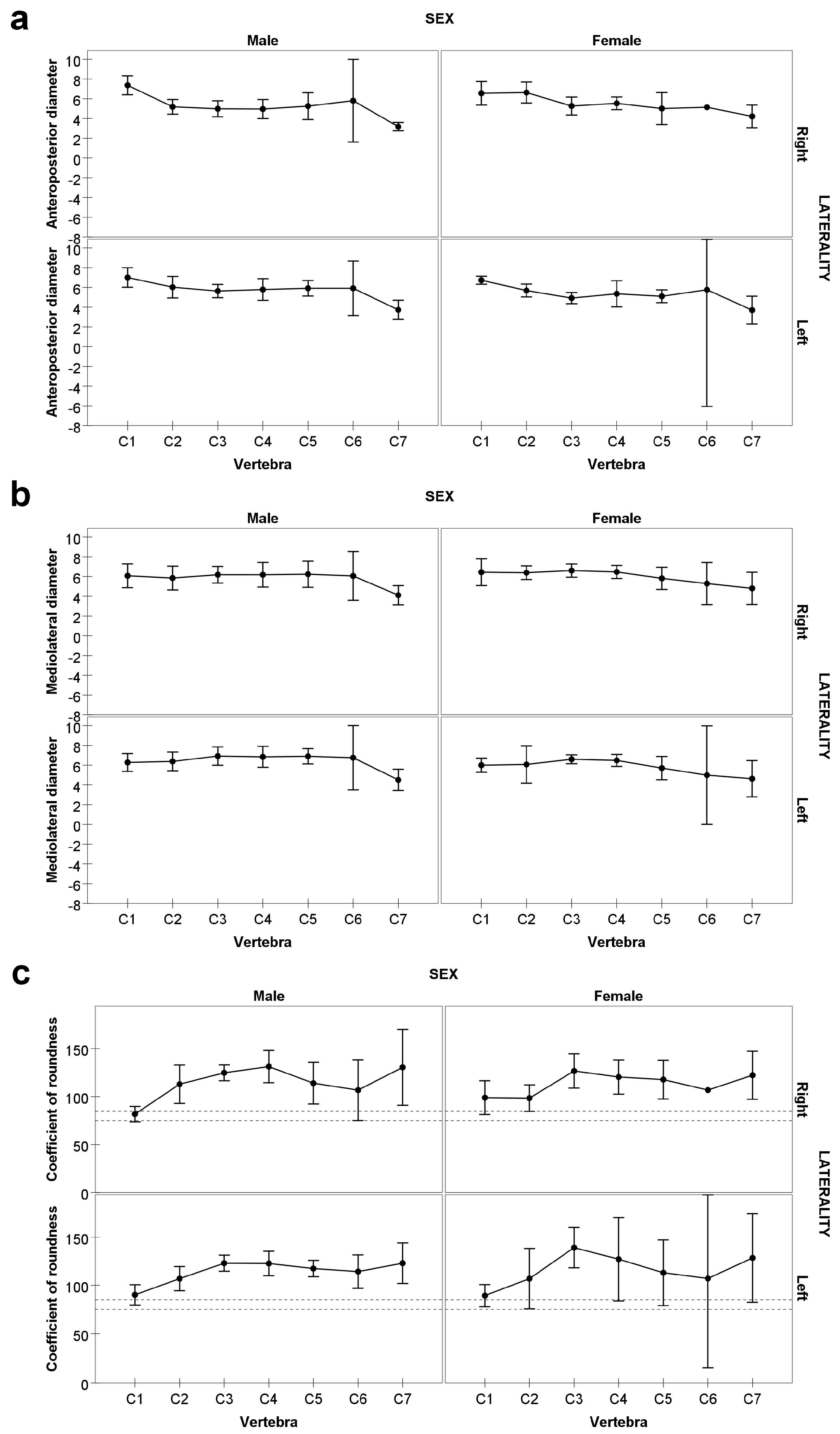
2.3.2. Metric Data Collection
2.3.3. Statistical Analysis
3. Results
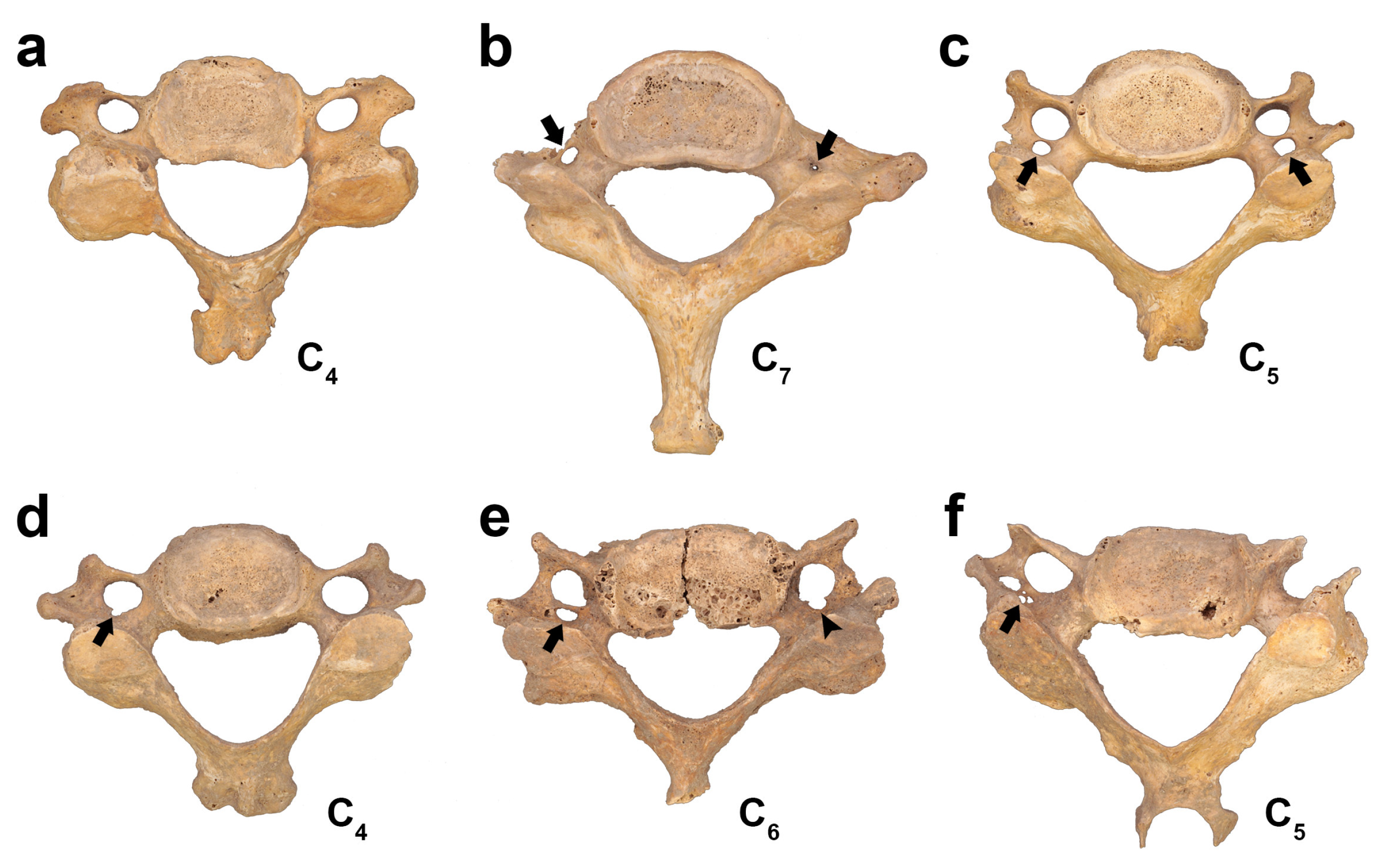
3.1. Osseus Variations of FT
3.1.1. Variations in Number
3.1.2. Variations in Size
Normal FT
3.1.3. Variations in Shape
Normal FT
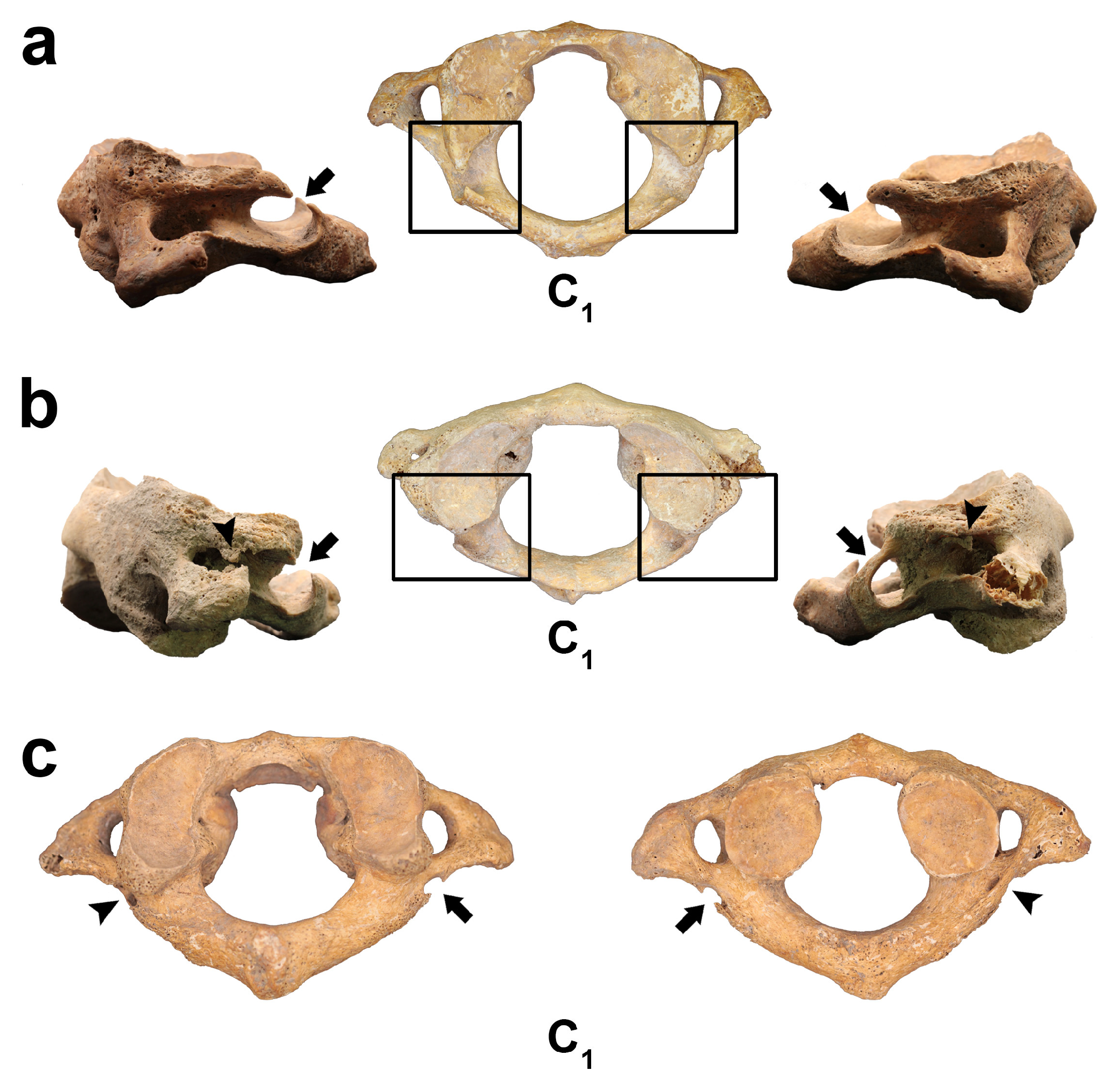
3.2. Osseous Variations of Vertebra C1
3.2.1. Ponticulus Posticus (PP)
3.2.2. Ponticulus Lateralis (PL)
3.2.3. Retrotransverse Foramen (RTF)
4. Discussion
4.1. Osseous Variations of FT
4.1.1. Variations in Number
4.1.2. Variations in Size
Normal FT
Complete Double FT
4.1.3. Variations in Shape
Normal FT
4.2. Osseous Variations of Vertebra C1
4.2.1. Ponticulus Posticus (PP) and Ponticulus Lateralis (PL)
4.2.2. Retrotransverse Foramen
5. Concluding Remarks and Suggestions for Further Research
- The prevalence of osseous variations of the FT (hypoplastic + double FT) in C1 is high (35.7%) compared with previous studies.
- The coefficient of roundness for C1, when pooling both sexes and sides, is classified as brachymorph (i.e., mediolateral diameter exceeding anteroposterior), contrasting with previous studies in which C1 FT is typically classified as mesomorph.
- The prevalence of bony bridges in C1, particularly PP (52.9%) and RTF (64.7%), is markedly higher than in most published reports.
- The distribution of FT variations in number, size, and shape, as well as the prevalence of bony bridges in C1, appears relatively homogeneous between sexes, with no statistically significant sex-based differences.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| VA | Vertebral artery |
| SCA | Subclavian artery |
| BA | Basilar artery |
| VBI | Vertebrobasilar insufficiency |
| FT | Foramen transversarium |
| PP | Ponticulus posticus |
| PL | Ponticulus lateralis |
| RTF | Retrotransverse foramen |
| R | Coefficient of roundness |
References
- Alraddadi, A. Literature Review of Anatomical Variations: Clinical Significance, Identification Approach, and Teaching Strategies. Cureus 2021, 13, e14451. [Google Scholar] [CrossRef] [PubMed]
- Suhani, S.; Mamatha, H.; Rao, I.; Shree, A.; Kumar, N. Supernumerary Heads of Biceps Brachii Muscle in South Indian Cadavers. Anat. J. Afr. 2013, 2, 108–113. [Google Scholar]
- Kachlík, D.; Varga, I.; Báča, V.; Musil, V. Variant Anatomy and Its Terminology. Medicina 2020, 56, 713. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, J.R.; Vázquez, R.; Puerta, J. Meaning and Clinical Interest of the Anatomical Variations in the 21st Century. Eur. J. Anat. 2003, 7, 1–3. [Google Scholar]
- Żytkowski, A.; Tubbs, R.S.; Iwanaga, J.; Clarke, E.; Polguj, M.; Wysiadecki, G. Anatomical Normality and Variability: Historical Perspective and Methodological Considerations. Transl. Res. Anat. 2021, 23, 100105. [Google Scholar] [CrossRef]
- Raikos, A.; Smith, J.D. Anatomical Variations: How Do Surgical and Radiology Training Programs Teach and Assess Them in Their Training Curricula? Clin. Anat. 2015, 28, 717–724. [Google Scholar] [CrossRef]
- Viciano, J.; Urbani, V.; D’Anastasio, R. Congenital Anatomical Variant of the Clavicle. Anat. Rec. 2017, 300, 1401–1408. [Google Scholar] [CrossRef]
- Viciano, J.; Tanga, C.; Somma, M.C. Cervical Rib in a Young Individual from the Late Medieval Cemetery of Corfinio (12th–13th Century CE, Italy): A Case Report and Review of the Literature. Anthropol. Anz. 2021, 78, 237–252. [Google Scholar] [CrossRef]
- Barnes, E. Atlas of Developmental Field Anomalies of the Human Skeleton: A Paleopathology Perspective; Wiley-Blackwell: Hoboken, NJ, USA, 2012; ISBN 9781118013885. [Google Scholar]
- Nikolova, S.Y.; Toneva, D.H.; Yordanov, Y.A.; Lazarov, N.E. Multiple Wormian Bones and Their Relation with Definite Pathological Conditions in a Case of an Adult Cranium. Anthropol. Anz. 2014, 71, 169–190. [Google Scholar] [CrossRef]
- Buzic, I.; Giuffra, V. The Paleopathological Evidence on the Origins of Human Tuberculosis: A Review. J. Prev. Med. Hyg. 2020, 61, e3–e8. [Google Scholar]
- Dutton, M. Vertebral Artery. In Dutton’s Orthopaedic: Examination, Evaluation, and Intervention; Dutton, M., Ed.; McGraw Hill: New York, NY, USA, 2020; pp. 1246–1255. [Google Scholar]
- Benzel, E.C. The Cervical Spine, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, BA, USA, 2012. [Google Scholar]
- George, B. Extracranial Vertebral Artery Anatomy and Surgery. In Advances and technical standards in Neurosurgery; Picard, J.D., Dolenc, V.V., Reulen, H.J., de Tribolet, N., Vapalahti, M., Eds.; Springer-Verlag: Vienna, Austria, 2002; Volume 27, pp. 179–216. [Google Scholar]
- Mohr, J.P.; Caplan, L.R. Vertebrobasilar Disease. In Stroke; Mohr, J.P., Wolf, P.A., Grotta, J.C., Moskowitz, M.A., Mayberg, M.R., von Kummer, R., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 446–484. [Google Scholar]
- Mitchell, J. The Incidence of the Lateral Bridge of the Atlas Vertebra. J. Anat. 1998, 193, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Pirau, L.; Lui, F. Vertebrobasilar Insufficiency; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Viciano, J.; Remigio, M.; D’Anastasio, R.; Capasso, L. Anatomical Variations of the Foramen Transversarium of Cervical Vertebrae from the Ancient Population of Herculaneum (79 CE; Naples, Italy). Homo 2021, 72, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Kültür, T.; Bayar Muluk, N.; Iyem, C.; Inal, M.; Burulday, V.; Alpua, M.; Çelebi, U.O. Anatomic Considerations: The Relationship between the Vertebral Artery and Transverse Foramina at Cervical Vertebrae 1 to 6 in Patients with Vertigo. ENT Updates 2018, 8, 185–194. [Google Scholar] [CrossRef]
- Taitz, C.; Nathan, H.; Arensburg, B. Anatomical Observations of the Foramina Transversaria. J. Neurol. Neurosurg. Psychiatry 1978, 41, 170–176. [Google Scholar] [CrossRef]
- Zibis, A.H.; Mitrousias, V.; Baxevanidou, K.; Hantes, M.; Karachalios, T.; Arvanitis, D. Anatomical Variations of the Foramen Transversarium in Cervical Vertebrae: Findings, Review of the Literature, and Clinical Significance during Cervical Spine Surgery. Eur. Spine J. 2016, 25, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Karapetian, M.K. Discrete Morphological Variants of Human Cervical Vertebrae: Exploring Pattern of Distribution and Biological Significance. Homo 2017, 68, 176–198. [Google Scholar] [CrossRef]
- Song, M.S.; Lee, H.J.; Kim, J.T.; Kim, J.H.; Hong, J.T. Ponticulus Posticus: Morphometric Analysis and Its Anatomical Implications for Occipito-Cervical Fusion. Clin. Neurol. Neurosurg. 2017, 157, 76–81. [Google Scholar] [CrossRef]
- Paraskevas, G.; Papaziogas, B.; Tsonidis, C.; Kapetanos, G. Gross Morphology of the Bridges over the Vertebral Artery Groove on the Atlas. Surg. Radiol. Anat. 2005, 27, 129–136. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Madhukar, P.K.; Rahman, S.; Kashyap, N. A Morphometric Study of Foramen Transversarium of Dried Cervical Vertebrae. Int. J. Res. Med. Sci. 2015, 3, 912–916. [Google Scholar] [CrossRef]
- Taitz, C.; Nathan, H. Some Observations on the Posterior and Lateral Bridge of the Atlas. Acta Anat. (Basel) 1986, 127, 212–217. [Google Scholar] [CrossRef]
- Ferembach, D.; Schwindezky, I.; Stoukal, M. Recommendations for Age and Sex Diagnoses of Skeletons. J. Hum. Evol. 1980, 9, 517–549. [Google Scholar] [CrossRef]
- Brůžek, J.; Santos, F.; Dutailly, B.; Murail, P.; Cunha, E. Validation and Reliability of the Sex Estimation of the Human Os Coxae Using Freely Available DSP2 Software for Bioarchaeology and Forensic Anthropology. Am. J. Phys. Anthropol. 2017, 164, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Getz, S.M. The Use of Transition Analysis in Skeletal Age Estimation. WIREs Forensic Sci. 2020, 2, e1378. [Google Scholar] [CrossRef]
- Vallois, H.V. Vital Statistics in Prehistoric Populations as Determined from Archaeological Data. In The Application of Quantitative Methods in Archaeology; Heizer, R.F., Cook, S.F., Eds.; Quadrangle Books: Chicago, IL, USA, 1960; pp. 186–222. [Google Scholar]
- De Nino, A. Corfinio: Notizie Degli Scavi Di Antichità; Reale Accademia dei Lincei: Roma, Italy, 1879. [Google Scholar]
- Van Wonterghem, F. Superaequum, Corfinium, Sulmo; L.S. Olschki: Firenze, Italy, 1984. [Google Scholar]
- Giuntella, A.M. Ricerche Sul Complesso Episcopale Valvense: Un Singolare Sarcofago Vescovile? In Quaeritur Inventus Colitur: Miscellanea in Onore Di Padre U.M. Fasola; Pontificio Istituto di Archeologia Cristiana: Vatican City, Vatican, 1989; pp. 363–379. [Google Scholar]
- Cavallari, C. Le Aree Funerarie Tardoantiche e Medievali Di Corfinio (AQ). In Proceedings of the Atti del VII Congresso Nazionale di Archeologia Medievale; Arthur, P., Leo Imperiale, M., Eds.; All’Insegna del Giglio: Firenze, Italy, 2015; pp. 55–60. [Google Scholar]
- Somma, M.C. Da Corfinio a Pentima: Trasformazioni Urbane Di Un Centro a Continuità Di Vita. In Ancient Modern Towns. I Centri Urbani a Continuità di vita: Archeologia e Valorizzazione; Somma, M.C., Ed.; Edizioni Quasar: Roma, Italy, 2021; pp. 69–84. [Google Scholar]
- Casolino, C. Ri-Pensare Le Trasformazioni in Età Postclassica: Valutazione e Analisi Del Dato Archeologico. PhD Dissertation, “G. d’Annunzio” University of Chieti-Pescara, Chieti, Italy, 2024. [Google Scholar]
- Barbiera, I. Sepolture e Necropoli Medievali Nei Quarant’anni Di Vita Di Archeologia Medievale. Archeol. Mediev. Cult. Mater. Insediamenti Territ. 2014, Special Issue. 111–121. [Google Scholar]
- Tanga, C. Ll Cimitero Bassomedievale Presso Il Complesso Monumentale Valvense Di S. Pelino a Corfinio (AQ): Riflessioni Sui Primi Dati. In Temporis signa: Archeologia della tarda antichità e del medioevo; Stasolla, F.R., Annoscia, G., De Lellis, L., Guerrini, P., Marchetti, M.I., Somma, M.C., Eds.; Fondazione CISAM: Spoleto, Italy, 2020; Volume XIII, pp. 153–164. [Google Scholar]
- Miller, C.A.; Hwang, S.J.; Cotter, M.M.; Vorperian, H.K. Cervical Vertebral Body Growth and Emergence of Sexual Dimorphism: A Developmental Study Using Computed Tomography. J. Anat. 2019, 234, 764–777. [Google Scholar] [CrossRef]
- Travan, L.; Saccheri, P.; Gregoraci, G.; Mardegan, C.; Crivellato, E. Normal Anatomy and Anatomic Variants of Vascular Foramens in the Cervical Vertebrae: A Paleo-Osteological Study and Review of the Literature. Anat. Sci. Int. 2015, 90, 308–323. [Google Scholar] [CrossRef]
- Cagnie, B.; Barbaix, E.; Vinck, E.; D’Herde, K.; Cambier, D. Extrinsic Risk Factors for Compromised Blood Flow in the Vertebral Artery: Anatomical Observations of the Transverse Foramina from C3 to C7. Surg. Radiol. Anat. 2005, 27, 312–316. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows 2017; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Kaya, S.; Yilmaz, N.D.; Pusat, S.; Kural, C.; Kirik, A.; Izci, Y. Double Foramen Transversarium Variation in Ancient Byzantine Cervical Vertebrae: Preliminary Report of an Anthropological Study. Turk. Neurosurg. 2011, 21, 534–538. [Google Scholar] [CrossRef]
- Nagar, Y.; Taitz, C.; Reich, R. What Can We Make of These Fragments? Excavation at “Mamilla” Cave, Byzantine Period, Jerusalem. Int. J. Osteoarchaeol. 1999, 9, 29–38. [Google Scholar] [CrossRef]
- Yadav, Y.; Goswami, P.; Bharihoke, V. An Osteological Study of Foramen Transversarium: Variations and Clinical Aspects. J. Evol. Med. Dent. Sci. 2014, 3, 14562–14566. [Google Scholar] [CrossRef]
- Molinet Guerra, M.; Robles Fuentes, P.; Roa, I. Anatomical Variations of the Foramen Transversarium in Cervical Vertebrae. Int. J. Morphol. 2017, 35, 719–722. [Google Scholar] [CrossRef]
- Kimura, K.; Konishi, M.; Hu, S.Y. Shape and Size of the Transverse Foramina in Japanese. Okajimas Foria Anat. Jpn. 1985, 62, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ambali, M.P.; Jadhav, S.D. Anatomical Variations in Foramen Transversarium of Typical Cervical Vertebrae and Its Clinical Significance. Int. J. Anat. Res. 2017, 5, 3426–3429. [Google Scholar] [CrossRef]
- Chandravadiya, L.; Patel, S.; Goda, J.; Chavda, V.; Ruparelia, S.; Patel, S. Double Foramen Transversarium in Cervical Vertebra: Morphology and Clinical Importance. Int. J. Res. Med. Sci. 2013, 2, 103–105. [Google Scholar]
- Chaudhari, M.L.; Maheria, P.B.; Bachuwar, S.P. Double Foramen Transversarium in Cervical Vertebra: Morphology and Clinical Importance. Indian J. Basic Appl. Med. Res. 2013, 2, 1084–1088. [Google Scholar]
- Esakkiammal, N.; Chauhan, R. Clinical Significance of Presence of Accessory Foramen Transversarium in Typical Cervical Vertebrae. Int. J. Res. Med. Sci. 2016, 4, 5231–5236. [Google Scholar] [CrossRef][Green Version]
- Gujar, S.M.; Oza, S.G.; Shekhawat, J.P. A Study of Accessory Foramen Transversarium in Dry Cervical Vertebrae and Its Clinical Implications. Natl. J. Integr. Res. Med. 2015, 6, 27–30. [Google Scholar] [CrossRef]
- Karthikeyan Variations in Foramen Transversarium. Univ. J. Pre. Para Clin. Sci. 2019, 5, 1.
- Katikireddi, R.S.; Setty, S.N.R.S. A Study of Double Foramen Transversarium in Dried Cervical Vertebra. Int. J. Health Sci. Res. 2014, 4, 59. [Google Scholar]
- Kumari, M.; Omar, S.; Deb, S.; Alam, K. An Osteological Study on Accessory Transverse Foramina in Cervical Vertebrae and Their Clinical Significance. Int. J. Recent Sci. Res. 2015, 6, 4514–4516. [Google Scholar]
- Malik, V.S.; Soni, G.; Garsa, V.; Rathee, S.; Gupta, S. An Osteological Study of Double Foramina Transversaria of Cervical Vertebrae. Int. J. Anat. Res. 2017, 5, 3527–3529. [Google Scholar] [CrossRef]
- Mehta, G.; Shamkuwar, S.; Mokhasi, V. Foramina Transversaria “Bipartita”: A Study of Cervical Vertebrae. Int. J. Curr. Res. Rev. 2014, 6, 31–34. [Google Scholar]
- Mishra, G.P.; Bhatnagar, S.; Singh, B.; Mishra, P.P.; Mishra, A. Anatomical Variations in Foramen Transversarium of Typical Cervical Vertebrae and Clinical Significance. Int. J. Biomed. Res. 2014, 5, 405–407. [Google Scholar]
- Murlimanju, B.V.; Prabhu, L.V.; Shilpa, K.; Rai, R.; Dhananjaya, K.V.N.; Jiji, P.J. Accessory Transverse Foramina in the Cervical Spine: Incidence, Embryological Basis, Morphology and Surgical Importance. Turk. Neurosurg. 2011, 21, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Verma, S. A Study on Variations of Foramen Transversarium of Cervical Vertebrae. Natl. J. Clin. Anat. 2014, 3, 4–7. [Google Scholar] [CrossRef]
- Nayak, G.; Mohanty, B.B.; Baisakh, P.; Das, S.R.; Panda, S.K.; Chinara, P.K. Study of Accessory Foramina Transversaria in Cervical Vertebrae and Their Surgical and Morphological Importance. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1370–1373. [Google Scholar]
- Patil, N.P.; Dhapate, S.S.; Porwal, S.; Bhagwat, V.B. The Study of Incidence of Accessory Foramen Transversaria in the Cervical Vertebrae. IOSR J. Dent. Med. Sci. 2014, 13, 85–87. [Google Scholar] [CrossRef]
- Patra, A.; Kaur, H.; Chhabra, U.; Kaushal, S.; Kumar, U. Double Foramen Transversarium in Dried Cervical Vertebra: An Osteological Study with Its Clinical Implications. Indian J. Oral Sci. 2015, 6, 7–9. [Google Scholar] [CrossRef]
- Ramachandran, K.; Ravikumar, P.C.; Manavalan, M.S. A Study on the Foramen Transversarium in Cervical Vertebrae. Int. J. Health Sci. Res. 2014, 4, 178–183. [Google Scholar]
- Rathnakar, P.; Remya, K. Swathi Study of Accessory Foramen Transversaria in Cervical Vertebrae. Nitte Univ. J. Health Sci. 2013, 3, 97–99. [Google Scholar]
- Saxena, A.K.; Aneja, P.S.; Sharma, N.K.; Madan, H.S. Variations in the Number of Foramen Transversarium: An Osteological Study. J. Evol. Med. Dent. Sci. 2016, 5, 531–533. [Google Scholar] [CrossRef]
- Shah, S.T.; Arora, K.; Shah, K.P. Study of Accessory Foramen Transversarium in Cervical Vertebrae. GCSMC J. Med. Sci. 2014, 3, 21–24. [Google Scholar]
- Sharma, A.; Singh, K.; Gupta, V.; Srivastava, S. Double Foramen Transversarium in Cervical Vertebra an Osteological Study. J. Anat. Soc. India 2010, 59, 229–231. [Google Scholar] [CrossRef]
- Singh, A.P.; Anand, C.; Singh, S. A Study of Anatomical Variations in Transverse Foramen of Cervical Vertebrae for Morphological and Clinical Importance. Int. J. Contemp. Med. Res. 2019, 6, F9–F11. [Google Scholar] [CrossRef]
- Sumalatha, T.; Manasa, B. Variations in Foramen Transversarium of Cervical Vertebrae - An Observational Study. Int. J. Anat. Radiol. Surg. 2018, 7, 13–17. [Google Scholar] [CrossRef]
- Vikani, S.; Patel, S.; Suthar, K.; Maheria, P. Morphological Study of Accessory Foramen Transversarium in Dry Cervical Vertebra and Its Clinical Importance. Int. J. Anat. Res. 2016, 4, 2847–2849. [Google Scholar] [CrossRef]
- Sheik Abdul, R.; Lazarus, L.; Rennie, C.; Satyapal, K.S. The Foramen Transversarium of Typical and Atypical Cervical Vertebrae: Morphology and Morphometry. Int. J. Morphol. 2018, 36, 1439–1446. [Google Scholar] [CrossRef]
- Sanchis-Gimeno, J.A.; Quiles-Guiñau, L.; Llido-Torrent, S.; Aparicio, L.; Nalla, S.; Miquel-Feutch, M. Possible Clinical Implications of Geographic Differences in Prevalence of Double Transverse Foramen. World Neurosurg. 2019, 126, e570–e572. [Google Scholar] [CrossRef]
- Aparicio Bellver, L.; Calatayud Fombuena, M.; Pérez Moltó, F. Variaciones Morfológicas y Numéricas En La Columna Vertebral. Rev. Soc. Andal. Traumatol. Ortop. 1998, 18, 215–221. [Google Scholar]
- Sanchis-Gimeno, J.A.; Martínez-Soriano, F.; Aparicio-Bellver, L. Degenerative Anatomic Deformities in the Foramen Transversarium of Cadaveric Cervical Vertebrae. Osteoporos. Int. 2005, 16, 1171–1172. [Google Scholar] [CrossRef]
- Aydınlıoğlu, A.; Kavaklı, A.; Yeşilyurt, H.; Erdem, S.; Eroğlu, C. Foramen Transversarium Bipartita. Van Tıp Derg. 2001, 8, 110–112. [Google Scholar]
- Lacy, S.A.; Trinkaus, E. The Foramina Transversaria of the Sunghir 2 and 3 Cervical Vertebrae. Archaeol. Ethnol. Anthropol. Eurasia 2013, 41, 126–131. [Google Scholar] [CrossRef]
- Jaén, M.T. Variedades Anatómicas En Vértebras de La Colección Tlatelolco. Inst. Nac. Antropol. Hist. 1974, 52, 71–82. [Google Scholar]
- Kwiatkowska, B.; Szczurowski, J.; Nowakowski, D. Variation in Foramina Transversaria of Human Cervical Vertebrae in the Medieval Population from Sypniewo (Poland). Anthropol. Rev. 2014, 77, 175–188. [Google Scholar] [CrossRef]
- Wysocki, J.; Bubrowski, M.; Reymond, J.; Kwiatkowski, J. Anatomical Variants of the Cervical Vertebrae and the First Thoracic Vertebra in Man. Folia Morphol. 2003, 62, 357–363. [Google Scholar]
- Quiles-Guiñau, L.; Gómez-Cabrero, A.; Miquel-Feucht, M.; Sanchis-Gimeno, J.A. Double Transverse Foramen in Cervical Vertebrae in a Spanish Rural Population of the Late 17th and 18th Centuries. Ital. J. Anat. Embryol. 2017, 122, 27–38. [Google Scholar] [CrossRef]
- Abd El Gawad, F.A.; Shaaban, M.H.; Shuaib, D.M.; Shallan, H.M. Anatomical Variations of the Vertebral Artery and Its Relation to the Atlas Vertebra-Radiological and Dry Bone Study. Eur. J. Anat. 2019, 23, 49–58. [Google Scholar]
- Katsanos, A.H.; Kosmidou, M.; Kyritsis, A.P.; Giannopoulos, S. Is Vertebral Artery Hypoplasia a Predisposing Factor for Posterior Circulation Cerebral Ischemic Events? A Comprehensive Review. Eur. Neurol. 2013, 70, 78–83. [Google Scholar] [CrossRef]
- Ikegami, A.; Ohtani, Y.; Ohtani, O. Bilateral Variations of the Vertebral Arteries: The Left Originating from the Aortic Arch and the Left and Right Entering the C5 Transverse Foramina. Anat. Sci. Int. 2007, 82, 175–179. [Google Scholar] [CrossRef]
- Shoja, M.M.; Tubbs, R.S.; Khaki, A.A.; Shokouhi, G.; Farahani, R.M.; Moein, A. A Rare Variation of the Vertebral Artery. Folia Morphol. 2006, 65, 167–170. [Google Scholar]
- Roh, J.; Jessup, C.; Yoo, J.; Bohlman, H. The Prevalence of Accessory Foramen Transversaria in the Human Cervical Spine. In Proceedings of the NASS 19th Annual Meeting, New Orleans, LA, USA, 15–19 July 2004; Elsevier BV: Amsterdam, The Netherlands, 2004; Volume 4, p. 92. [Google Scholar]
- Quiles-Guiñau, L.; Gomez-Cabrero, A.; Miquel-Feucht, M.; Blanco-Pérez, E.; Mata-Escolano, F.; Sanchis-Gimeno, J.A. Analysis of the Cervical Double Transverse Foramen in Present Spanish Population. Eur. J. Anat. 2016, 20, 337–346. [Google Scholar]
- Chaiyamoon, A.; Yannasithinon, S.; Sae-Jung, S.; Samrid, R.; Thongbuakaew, T.; Iamsaard, S. Anatomical Variation and Morphometric Study on Foramen Transversarium of the Upper Cervical Vertebrae in the Thai Population. Asian Spine J. 2021, 15, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Moreira Moreira, J.J.; Herrero, C.F.P.S. Anatomical Variations and Morphometric Features of the Foramen Transversarium in the Cervical Vertebrae of a Latin American Population: A Brazilian Study. World Neurosurg. 2020, 137, e18–e26. [Google Scholar] [CrossRef] [PubMed]
- Ouies, S.M. A Morphologic and Morphometric Study of the Foramen Transversarium of the Cervical Vertebrae: An Osteological Study in Upper Egypt. J. Am. Sci. 2018, 14, 25–29. [Google Scholar] [CrossRef]
- Ranjan, R.; Hussain, M.Z.; Kumari, S.; Singh, M.K.; Prasad, R. The Morphology and Incidence of the Accessory Foramen Transversarium in Human Dried Cervical Vertebrae as Well as Their Clinical Significance in the Eastern Indian Population. Asian J. Med. Sci. 2022, 13, 47–53. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.-H.; Park, S.S.; Kim, B.J.; Ryu, W.-S.; Kim, C.K.; Oh, M.-Y.; Chung, J.-W.; Yoon, B.-W. A Quantitative Comparison of the Vertebral Artery and Transverse Foramen Using CT Angiography. J. Clin. Neurol. 2012, 8, 259–264. [Google Scholar] [CrossRef]
- Kotil, K.; Kilincer, C. Sizes of the Transverse Foramina Correlate with Blood Flow and Dominance of Vertebral Arteries. Spine J. 2014, 14, 933–937. [Google Scholar] [CrossRef]
- Zimmerman, H.B.; Farrell, W.J. Cervical Vertebral Erosion Caused by Vertebral Artery Tortuosity. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1970, 108, 767–770. [Google Scholar] [CrossRef]
- Waldron, T.; Antoine, D. Tortuosity or Aneurysm? The Palaeopathology of Some Abnormalities of the Vertebral Artery. Int. J. Osteoarchaeol. 2002, 12, 79–88. [Google Scholar] [CrossRef]
- Cagnie, B.; Petrovic, M.; Voet, D.; Barbaix, E.; Cambier, D. Vertebral Artery Dominance and Hand Preference: Is There a Correlation? Man. Ther. 2006, 11, 153–156. [Google Scholar] [CrossRef]
- Vural, A.; Çiçek, E.D. Is the Asymmetry between the Vertebral Arteries Related to Cerebral Dominance? Turk. J. Med. Sci. 2019, 49, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.T.; Lee, S.W.; Son, B.C.; Sung, J.H.; Yang, S.H.; Kim, I.S.; Park, C.K. Analysis of Anatomical Variations of Bone and Vascular Structures around the Posterior Atlantal Arch Using Three-Dimensional Computed Tomography Angiography. J. Neurosurg. Spine 2008, 8, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.S.; Yip, P.K. Evaluation of Vertebral Artery Hypoplasia and Asymmetry by Color-Coded Duplex Ultrasonography. Ultrasound. Med. Biol. 2004, 30, 605–609. [Google Scholar] [CrossRef]
- Bruneau, M.; Cornelius, J.F.; Marneffe, V.; Triffaux, M.; George, B. Anatomical Variations of the V2 Segment of the Vertebral Artery. Neurosurgery 2006, 59, ONS20–ONS24. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Liu, Y.S.; Chen, Y.C.; Shih, Y.H.; Chang, C.C.; Chuang, M.T. Variations in the Origin and Course of the Extracranial Vertebral Artery on Multidetector Computed Tomography Angiography. Iran. J. Radiol. 2018, 15, e61623. [Google Scholar] [CrossRef]
- Shin, H.Y.; Park, J.K.; Park, S.K.; Jung, G.S.; Choi, Y.S. Variations in Entrance of Vertebral Artery in Korean Cervical Spine: MDCT-Based Analysis. Korean J. Pain 2014, 27, 266–270. [Google Scholar] [CrossRef]
- Sassoli Fazan, V.P.S.; Gonçalves Caetano, A.; Rodrigues Filho, O.A. Anomalous Origin and Cervical Course of the Vertebral Artery in the Presence of a Retroesophageal Right Subclavian Artery. Clin. Anat. 2004, 17, 354–357. [Google Scholar] [CrossRef]
- Kośla, K.N.; Majos, M.; Podgórski, M.; Polguj, M.; Topol, M.; Stefańczyk, L.; Majos, A. Anomalous Course and Diameter of Left-Sided Vertebral Arteries - Significance and Predisposing Factors in Clinical Practice. Ann. Anat. 2014, 196, 360–364. [Google Scholar] [CrossRef]
- Cavdar, S.; Dalcik, H.; Ercan, F.; Arbak, S.; Arifoglu, Y. A Morphological Study on the V2 Segment of the Vertebral Artery. Okajimas Folia Anat. Jpn. 1996, 73, 133–138. [Google Scholar] [CrossRef]
- Choi, K.D.; Choi, J.H.; Kim, J.S.; Kim, H.J.; Kim, M.J.; Lee, T.H.; Lee, H.; Moon, I.S.; Oh, H.J.; Kim, J. Il Rotational Vertebral Artery Occlusion: Mechanisms and Long-Term Outcome. Stroke 2013, 44, 1817–1824. [Google Scholar] [CrossRef]
- Go, G.; Hwang, S.H.; Park, I.S.; Park, H. Rotational Vertebral Artery Compression: Bow Hunter’s Syndrome. J. Korean Neurosurg. Soc. 2013, 54, 243–245. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, M.; Potvliege, R.; Roles, F.; De Smedt, E. The Accessory Costrotransverse Foramen: A Radioanatomical Study. J. Comput. Assist. Tomogr. 1984, 8, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Podgõrski, M.; Jȩdrzejewski, K.; Topol, M.; Majos, A. Fenestration and Duplication of the Vertebral Artery: The Anatomical and Clinical Points of View. Clin. Anat. 2013, 26, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Sim, E.; Vaccaro, A.R.; Berzlanovich, A.; Thaler, H.; Ullrich, C.G. Fenestration of the Extracranial Vertebral Artery: Review of the Literature. Spine 2001, 26, E139–E142. [Google Scholar] [CrossRef] [PubMed]
- Tran-Dinh, H.D.; Soo, Y.S.; Jayasinghe, L.S. Duplication of the Vertebro-Basilar System. Australas. Radiol. 1991, 35, 220–224. [Google Scholar] [CrossRef]
- Zhang, W.; Xing, W.; Zhong, G.; He, J. Acute Cerebral Infarction of Posterior Circulation in a Patient with Vertebral Artery Fenestration Deformity: A Case Report. Heliyon 2022, 8, e11210. [Google Scholar] [CrossRef]
- Ionete, C.; Omojola, M.F. MR Angiographic Demonstration of Bilateral Duplication of the Extracranial Vertebral Artery: Unusual Course and Review of the Literature. Am. J. Neuroradiol. 2006, 27, 1304–1306. [Google Scholar]
- Cacciola, F.; Phalke, U.; Goel, A. Vertebral Artery in Relationship to C1-C2 Vertebrae: An Anatomical Study. Neurol. India 2004, 52, 178–184. [Google Scholar]
- Lanier, R.R.J. The Presacral Vertebrae of American White and Negro Males. Am. J. Phys. Anthropol. 1939, 25, 341–420. [Google Scholar] [CrossRef]
- Lee, M.J.; Cassinelli, E.; Riew, K.D. The Feasibility of Inserting Atlas Lateral Mass Screws via the Posterior Arch. Spine 2006, 31, 2798–2801. [Google Scholar] [CrossRef]
- Ossenfort, W.F. The Atlas in Whites and Negroes. Am. J. Phys. Anthropol. 1926, 9, 439–443. [Google Scholar] [CrossRef]
- Awadalla, A.M.; Fetouh, F.A. Morphometric Analysis of the Vertebral Artery Groov of the First Cervical Vertebra (Atlas). Pan. Arab. J. Neurosurg. 2009, 13, 66–71. [Google Scholar]
- Lamberty, B.G.H.; Zivanovic, S. The Retro-Articular Vertebral Artery Ring of the Atlas and Its Significance. Acta Anat. 1973, 85, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Le Double, A.F. Traité Des Variations de La Colonne Vertébrale de l’Homme et de Leur Signification Au Point de Vue de l’Anthropologie Zoologique; Vigot Frères: Paris, France, 1912. [Google Scholar]
- Le Minor, J.M. The Retrotransverse Foramen of the Human Atlas Vertebra: A Distinctive Variant within Primates. Acta Anat. 1997, 160, 208–212. [Google Scholar] [CrossRef]
- Le Minor, J.M.; Trost, O. Bony Ponticles of the Atlas (C1) over the Groove for the Vertebral Artery in Humans and Primates: Polymorphism and Evolutionary Trends. Am. J. Phys. Anthropol. 2004, 125, 16–29. [Google Scholar] [CrossRef]
- Lyrtzis, C.; Tsakotos, G.; Kostares, M.; Piagkou, M.; Mariorakis, C.; Natsis, K. The Prevalence and Morphometry of the Atlas Vertebra Retrotransverse Foramen. Acta Med. Acad. 2022, 51, 189–198. [Google Scholar] [CrossRef]
- Agrawal, R.; Suba Ananthi, K.; Agrawal, S.; Usha, K. Posterior Arch of Atlas with Abnormal Foramina in South Indians. J. Anat. Soc. India 2012, 61, 30–32. [Google Scholar] [CrossRef]
- Bilodi, A.K.; Gupta, S.C. Presence of Retro Transverse Groove or Canal in Atlas Vertebrae. J. Anat. Soc. India 2005, 54, 16–18. [Google Scholar]
- Gupta, S.C.; Gupta, C.D.; Arora, A.K.; Maheshwari, B.B. The Retrotransverse Groove (Canal) in Indian Atlas Vertebrae. Anat. Anz. 1979, 145, 514–516. [Google Scholar]
- Hasan, M.; Shukla, S.; Shakil Siddiqui, M.; Simgh, D. Posterolateral Tunnels and Ponticuli in Human Atlas Vertebrae. J. Anat. 2001, 199, 339–343. [Google Scholar] [CrossRef]
- Krishnamurty, A.; Nayak, S.R.; Khan, S.; Prabhu, L.V.; Ramanathan, L.A.; Ganesh Kumar, C.; Prasad Sinha, A. Arcuate Foramen of Atlas: Incidence, Phylogenetic and Clinical Significance. Rom. J. Morphol. Embryol. 2007, 48, 263–266. [Google Scholar]
- Lalit, M.; Piplani, S.; Arora, A.K.; Kullar, J.S.; Sharma, T. Incidence of Atlas Bridges and Tunnels - Their Phylogeny, Ontogeny and Clinical Implications. Rev. Argent. Anatomía Clínica 2014, 6, 26–34. [Google Scholar] [CrossRef][Green Version]
- Patel, N.P.; Gupta, D.S.; Parmar, N.D. Incidence of Ponticles in Human Atlas Vertebrae - A Study from South Gujarat Population. Indian J. Clin. Anat. Physiol. 2015, 2, 135–139. [Google Scholar] [CrossRef]
- Rekha, B.S.; Rajeshwari, T. Study of Ponticuli in Human Atlas Vertebrae. J. Evol. Med. Dent. Sci. 2013, 2, 8849–8855. [Google Scholar]
- Venkatachalam, N. Ponticuli in Human Atlas Vertebrae and Its Significance. Int. J. Health Sci. Res. 2015, 5, 246–350. [Google Scholar]
- Gunness-Hey, M. The Koniag Eskimo Presacral Vertebral Column: Variations, Anomalies and Pathologies. Ossa 1982, 7, 99–118. [Google Scholar]
- Karau, P.B.; Ogengo, J.A.; Hassanali, J.; Odula, P. Anatomy and Prevalence of Atlas Vertebrae Bridges in a Kenyan Population: An Osteological Study. Clin. Anat. 2010, 23, 649–653. [Google Scholar] [CrossRef]
- Maqbool, A.; Athar, Z.; Hameed, O. Prevalence and Morphometry of Arcuate Foramen in Atlas Vertebrae in Pakistanis. Med. Forum Mon. 2014, 25, 35–39. [Google Scholar]
- Veleanu, C.; Barzu, S.; Panescu, S.; Udroiu, C. The Retrotransverse Groove or Canal of the Atlas and Its Significance. Acta Anat. 1977, 97, 400–402. [Google Scholar] [CrossRef]
- Radojevic, S.; Negovanovic, B. La Gouttière et Les Anneaux Osseux de l’artère Vertébrale de l’atlas (Etude Anatomique et Radiologique). Acta Anat. 1963, 55, 186–194. [Google Scholar] [CrossRef]
- Mitchell, J. The Incidence and Dimensions of the Retroarticular Canal of the Atlas Vertebra. Acta Anat. 1998, 163, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gimeno, J.A.; Llido, S.; Nalla, S. Double Retrotransverse Foramen of Atlas (C1). World Neurosurg. 2018, 114, e869–e872. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gimeno, J.A.; Llido, S.; Perez-Bermejo, M.; Nalla, S. Prevalence of Anatomic Variations of the Atlas Vertebra. Spine J. 2018, 18, 2102–2111. [Google Scholar] [CrossRef]
- Sanchis-Gimeno, J.A.; Blanco-Perez, E.; Perez-Bermejo, M.; Llido, S.; Nalla, S. Retrotransverse Foramen of the Atlas: Prevalence and Bony Variations. Eur. Spine J. 2018, 27, 1272–1277. [Google Scholar] [CrossRef]
- Sanchis-Gimeno, J.A.; Llido, S.; Miquel-Feutch, M.; Quiles-Guinau, L.; Rios, L.; Murillo-Llorente, M.; Perez-Bermejo, M.; Nalla, S. The Decreasing Prevalence of the Arcuate Foramen. World Neurosurg. 2018, 110, 521–525. [Google Scholar] [CrossRef]
- Cakmak, O.; Gurdal, E.; Ekinci, G.; Yildiz, E.; Cavdar, S. Arcuate Foramen and Its Clinical Significance. Saudi Med. J. 2005, 26, 1409–1413. [Google Scholar]
- Cirpan, S.; Yonguc, G.N.; Edizer, M.; Mas, N.G.; Magden, A.O. Foramen Arcuale: A Rare Morphological Variation Located in Atlas Vertebrae. Surg. Radiol. Anat. 2017, 39, 877–884. [Google Scholar] [CrossRef]
- Kavakli, A.; Aydinlioglu, A.; Yesilyurt, H.; Kus, I.; Diyarbakirli, S.; Erdem, S. Variants and Deformities of Atlas Vertebrae in Eastern Anatolian People. Saudi Med. J. 2004, 25, 322–325. [Google Scholar] [PubMed]
- Senoglu, M.; Gümüsalan, Y.; Yüksel, Z.Z.; Uzel, M.; Çelik, M.; Özbag, D. The Effect of Posterior Bridging of C-1 on Craniovertebral Surgery. J. Neurosurg. Spine 2006, 5, 50–52. [Google Scholar] [CrossRef]
- Simsek, S.; Yigitkanli, K.; Comert, A.; Acar, H.I.; Seckin, H.; Er, U.; Belen, D.; Tekdemir, I.; Elhan, A. Posterior Osseous Bridging of C1. J. Clin. Neurosci. 2008, 15, 686–688. [Google Scholar] [CrossRef]
- Taylor, K.M. Assessing Population Variation Using Heritable Nonmetric Traits: A Bronze Age Assemblage from Tell Abraq, United Arab Emirates. Master of Arts Dissertation, Boise State University, Boise, ID, USA, 2021. [Google Scholar]
- Saccheri, P.; Travan, L. The Craniovertebral Junction, between Osseous Variants and Abnormalities: Insight from a Paleo-Osteological Study. Anat. Sci. Int. 2022, 97, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Éry, K. Anthropological Studies on an Early Avar Period Population at Backo Petrovo Selo (Yugoslavia) Part 2: Analysis of the Data. Anthropol. Hung. 1990, 21, 33–53. [Google Scholar]
- Quiles-Guiñau, L.; Gómez-Cabrero, A.; Miquel-Feucht, M.; Aparicio-Bellver, L. Retrotransverse Foramen in Atlas Vertebrae of the Late 17th and 18th Centuries. Ital. J. Anat. Embryol. 2016, 121, 123–132. [Google Scholar] [CrossRef]
- Sanchis-Gimeno, J.A.; Llido, S.; Nalla, S. The Retrotransverse Foramen of the Atlas Is Not a Modern Anatomic Variation. World Neurosurg. 2019, 123, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Allen, W. On the Varieties of the Atlas in the Human Subject, and the Homologies of Its Transverse Processes. J. Anat. Physiol. 1879, 14, 18–27. [Google Scholar]
- Cleland, M. On the Serial Homologies of the Articular Surfaces of the Mammalian Atlas, Axis and Occipital Bone. Proc. R. Soc. Edin. 1860, 2, 221. [Google Scholar]
- Saunders, S.R.; Popovich, F. A Family Study of Two Skeletal Variants: Atlas Bridging and Clinoid Bridging. Am. J. Phys. Anthropol. 1978, 49, 193–203. [Google Scholar] [CrossRef]
- Selby, S.; Garn, S.M.; Kanareff, V. The Incidence and Familial Nature of a Bony Bridge on the First Cervical Vertebra. Am. J. Phys. Anthropol. 1955, 13, 129–141. [Google Scholar] [CrossRef]
- Cvrček, J.; Velemínský, P.; Dupej, J.; Vostrý, L.; Brůžek, J. Kinship and Morphological Similarity in the Skeletal Remains of Individuals with Known Genealogical Data (Bohemia, 19th to 20th Centuries): A New Methodological Approach. Am. J. Phys. Anthropol. 2018, 167, 541–556. [Google Scholar] [CrossRef]
- Cvrček, J.; Velemínský, P.; Dupej, J.; Jor, T.; Brůžek, J. Kinship and the Familial Occurrence of Skeletal Developmental Anomalies in the Noble Swéerts-Sporck Family (Bohemia, 17th to 20th Centuries). Int. J. Paleopathol. 2021, 34, 163–167. [Google Scholar] [CrossRef]
- Velemínský, P.; Dobisíková, M. Morphological Likeness of the Skeletal Remains in a Central European Family from 17th to 19th Century. Homo 2005, 56, 173–196. [Google Scholar] [CrossRef]
- Ríos, L.; Ovejero, J.I.C.; Prieto, J.P. Identification Process in Mass Graves from the Spanish Civil War I. Forensic Sci. Int. 2010, 199, e27–e36. [Google Scholar] [CrossRef]
- Pyo, J.; Lowman, R.M. The “Ponticulus Posticus” of the First Cervical Vertebra. Radiology 1959, 72, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.; Schilling, A.; Suazo Galdames, I. Ponticulus Posticus on the Posterior Arch of Atlas, Prevalence Analysis in Asymptomatic Patients. Int. J. Morphol. 2010, 28, 317–322. [Google Scholar] [CrossRef]
- Bodon, G.; Grimm, A.; Hirt, B.; Seifarth, H.; Barsa, P. Applied Anatomy of Screw Placement via the Posterior Arch of the Atlas and Anatomy-Based Refinements of the Technique. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Huang, M.; Adu, I.K.; Wang, J.; Cui, G. Retrotransverse Foramen and Retrotransverse Groove Anatomic Variations of the Atlas Vertebra in the Chinese Population. World Neurosurg. 2021, 152, e193–e200. [Google Scholar] [CrossRef]
- Falk, D. Evolution of Cranial Blood Drainage in Hominids: Enlarged Occipital/Marginal Dinuses and Emissary Foramina. Am. J. Phys. Anthropol. 1986, 70, 311–324. [Google Scholar] [CrossRef]
- Valdueza, J.M.; von Münster, T.; Hoffman, O.; Schereiber, S.; Einhäupl, K.M. Postural Dependency of the Cerebral Venous Outflow. Lancet 2000, 355, 200–201. [Google Scholar] [CrossRef]
- Pękala, J.R.; Tempski, J.; Krager, E.; Johansen, J.; Łazarz, D.P.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A. Systematic Review and Meta-analysis of the Prevalence of the Retrotransverse Foramen of the Atlas. J. Anat. 2023, 243, 570–578. [Google Scholar] [CrossRef]
| Males | Females | Unknown Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cervical Level | YA | MAA | SA | YA | MAA | SA | YA | MAA | SA | Total |
| C1 | 3 | 6 | 0 | 1 | 6 | 1 | 0 | 0 | 0 | 17 |
| C2 | 4 | 5 | 0 | 2 | 7 | 1 | 0 | 1 | 0 | 20 |
| C3 | 3 | 5 | 0 | 1 | 7 | 2 | 0 | 0 | 0 | 18 |
| C4 | 3 | 5 | 0 | 1 | 7 | 2 | 0 | 1 | 0 | 19 |
| C5 | 4 | 5 | 0 | 1 | 7 | 2 | 0 | 1 | 0 | 20 |
| C6 | 4 | 5 | 0 | 1 | 7 | 2 | 0 | 1 | 0 | 20 |
| C7 | 4 | 7 | 0 | 1 | 5 | 2 | 2 | 1 | 0 | 22 |
| Total | 25 | 38 | 0 | 8 | 46 | 12 | 2 | 5 | 0 | 136 |
| Cervical Level | Excluded Vertebrae due to Limiting Factors | Normal Vertebrae [n (%)] | Osseous Variants of the Foramen Transversarium [n (%)] | Total [n (%)] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absence | Hypoplastic | Complete Double | Incomplete Double | Multiple | Complete + Incomplete Double | |||||||||||||
| UL | BL | UL | BL | UL | BL | UL | BL | UL | BL | |||||||||
| C1 | 3 | 9 (8.33) | 0 (0) | 0 (0) | 1 (0.93) | 0 (0) | 3 (2.78) | 0 (0) | 1 (0.93) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (12.97) | ||||
| C2 | 4 | 16 (14.81) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (14.81) | ||||
| C3 | 2 | 15 (13.89) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.93) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (14.81) | ||||
| C4 | 3 | 14 (12.96) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.93) | 1 (0.93) | 0 (0) | 0 (0) | 0 (0) | 16 (14.81) | ||||
| C5 | 4 | 8 (7.41) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.85) | 5 (4.63) | 0 (0) | 1 (0.93) | 0 (0) | 0 (0) | 16 (14.81) | ||||
| C6 | 7 | 3 (2.78) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.78) | 0 (0) | 6 (5.56) | 0 (0) | 0 (0) | 0 (0) | 1 (0.93) | 13 (12.04) | ||||
| C7 | 5 | 11 (10.19) | 0 (0) | 0 (0) | 1 (0.93) | 0 (0) | 1 (0.93) | 0 (0) | 2 (1.85) | 1 (0.93) | 0 (0) | 0 (0) | 1 (0.93) | 17 (15.75) | ||||
| Total | 28 | 76 (70.37) | 0 (0) | 0 (0) | 2 (1.85) | 0 (0) | 7 (6.48) | 2 (1.85) | 16 (14.81) | 2 (1.85) | 1 (0.93) | 0 (0) | 2 (1.85) | 108 (100) | ||||
| Males | Females | Comparison of Differences | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cervical Level | Right | Left | Right | Left | By Sex | By Laterality | |||||||
| Diameter | n | Measure | n | Measure | n | Measure | n | Measure | U | p | U | p | |
| C1 | A–P | 5 | 7.55 ± 1.09 | 6 | 7.17 ± 1.07 | 5 | 6.56 ± 0.96 | 3 | 6.63 ± 0.21 | 33.00 | 0.364 | 34.00 | 0.369 |
| M–L | 5 | 6.14 ± 1.48 | 6 | 6.48 ± 0.90 | 5 | 6.44 ± 1.09 | 3 | 6.03 ± 0.54 | 39.00 | 0.680 | 43.00 | 0.870 | |
| R | 5 | 80.27 ± 9.92 | 6 | 91.01 ± 12.12 | 5 | 98.91 ± 14.19 | 3 | 90.92 ± 7.77 | 23.00 | 0.083 | 41.00 | 0.744 | |
| C2 | A–P | 7 | 5.18 ± 0.81 | 6 | 6.02 ± 1.04 | 8 | 6.63 ± 1.27 | 7 | 5.68 ± 0.71 | 63.00 | 0.112 | 108.50 | 0.884 |
| M–L | 7 | 5.85 ± 1.30 | 6 | 6.38 ± 0.91 | 8 | 6.39 ± 0.83 | 7 | 6.07 ± 2.04 | 88.00 | 0.662 | 107.50 | 0.852 | |
| R | 7 | 112.94 ± 21.63 | 6 | 106.88 ± 12.03 | 8 | 98.42 ± 16.52 | 7 | 106.91 ± 33.96 | 82.00 | 0.475 | 92.00 | 0.406 | |
| C3 | A–P | 7 | 4.98 ± 0.87 | 5 | 5.63 ± 0.54 | 4 | 5.36 ± 0.81 | 6 | 4.92 ± 0.54 | 54.00 | 0.692 | 53.00 | 0.622 |
| M–L | 7 | 6.18 ± 0.91 | 5 | 6.92 ± 0.75 | 4 | 6.71 ± 0.56 | 5 | 6.60 ± 0.36 | 47.50 | 0.644 | 37.50 | 0.218 | |
| R | 7 | 124.82 ± 8.92 | 5 | 123.01 ± 6.72 | 4 | 126.77 ± 16.59 | 5 | 139.21 ± 16.92 | 35.00 | 0.177 | 46.00 | 0.526 | |
| C4 | A–P | 5 | 4.96 ± 0.78 | 6 | 5.78 ± 1.04 | 8 | 5.54 ± 0.77 | 5 | 5.35 ± 1.07 | 70.00 | 0.931 | 66.00 | 0.514 |
| M–L | 5 | 6.49 ± 1.03 | 6 | 7.05 ± 1.11 | 7 | 6.46 ± 0.71 | 5 | 6.55 ± 0.63 | 52.00 | 0.389 | 56.00 | 0.356 | |
| R | 5 | 131.30 ± 13.56 | 6 | 122.72 ± 12.20 | 7 | 120.42 ± 19.36 | 5 | 127.16 ± 34.91 | 57.00 | 0.580 | 55.00 | 0.326 | |
| C5 | A–P | 4 | 5.26 ± 0.85 | 4 | 6.14 ± 0.75 | 1 | 5.65 | 2 | 5.27 ± 0.46 | 8.00 | 0.414 | 9.00 | 0.273 |
| M–L | 5 | 6.23 ± 1.07 | 4 | 6.97 ± 0.57 | 1 | 6.55 | 2 | 5.50 ± 1.22 | 8.00 | 0.309 | 16.00 | 0.749 | |
| R | 4 | 114.00 ± 13.63 | 4 | 114.34 ± 11.08 | 1 | 115.93 | 2 | 105.88 ± 32.34 | 11.00 | 0.838 | 13.00 | 0.715 | |
| C6 | A–P | 3 | 5.80 ± 1.69 | 2 | 6.10 ± 1.50 | 0 | — | 2 | 5.76 ± 1.32 | 4.00 | 0.699 | 7.00 | 0.773 |
| M–L | 3 | 6.06 ± 0.99 | 2 | 6.76 ± 1.85 | 1 | 4.35 | 2 | 6.10 ± 0.82 | 5.00 | 0.456 | 8.00 | 0.624 | |
| R | 3 | 106.60 ± 12.74 | 2 | 110.42 ± 3.23 | 0 | — | 2 | 107.07 ± 10.21 | 5.00 | 1.000 | 6.00 | 0.564 | |
| C7 | A–P | 3 | 3.25 ± 0.27 | 6 | 3.73 ± 0.93 | 4 | 3.94 ± 0.79 | 3 | 3.59 ± 1.05 | 24.00 | 0.427 | 34.00 | 0.922 |
| M–L | 3 | 3.95 ± 0.64 | 6 | 4.51 ± 1.02 | 4 | 4.80 ± 1.03 | 3 | 4.61 ± 1.41 | 23.00 | 0.368 | 31.00 | 0.696 | |
| R | 3 | 122.32 ± 22.88 | 6 | 123.01 ± 20.16 | 4 | 122.29 ± 15.74 | 3 | 128.48 ± 18.53 | 29.00 | 0.791 | 26.00 | 0.380 | |
| Presence of Osseous Variants in Vertebra C1 [n (%)] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Normal Vertebra C1 [n (%)] | Complete | Incomplete | ||||||
| Osseous Variant | Unilateral | Bilateral | Unilateral | Bilateral | Complete + Incomplete | Total | ||
| Ponticulus posticus | 8 (47.06) | 3 (17.65) | 0 (0) | 2 (11.76) | 2 (11.76) | 2 (11.76) | 17 (100.0) | |
| Ponticulus lateralis | 16 (94.12) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.88) | 17 (100.0) | |
| Retrotransverse foramen | 6 (35.29) | 0 (0) | 1 (5.88) | 7 (41.18) | 2 (11.76) | 1 (5.88) | 17 (100.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amores, A.; Tanga, C.; Somma, M.C.; La Salvia, V.; Antonelli, S.; Viciano, J. Osseous Variants of the Cervical Spine with Potential Pathological Significance: Possible Evidence of Vertebrobasilar Insufficiency in a Skeletal Sample from the Post-Classical Cemetery of Corfinio (12th–15th Centuries CE, L’Aquila, Italy). Heritage 2025, 8, 178. https://doi.org/10.3390/heritage8050178
Amores A, Tanga C, Somma MC, La Salvia V, Antonelli S, Viciano J. Osseous Variants of the Cervical Spine with Potential Pathological Significance: Possible Evidence of Vertebrobasilar Insufficiency in a Skeletal Sample from the Post-Classical Cemetery of Corfinio (12th–15th Centuries CE, L’Aquila, Italy). Heritage. 2025; 8(5):178. https://doi.org/10.3390/heritage8050178
Chicago/Turabian StyleAmores, Anabel, Carmen Tanga, Maria Carla Somma, Vasco La Salvia, Sonia Antonelli, and Joan Viciano. 2025. "Osseous Variants of the Cervical Spine with Potential Pathological Significance: Possible Evidence of Vertebrobasilar Insufficiency in a Skeletal Sample from the Post-Classical Cemetery of Corfinio (12th–15th Centuries CE, L’Aquila, Italy)" Heritage 8, no. 5: 178. https://doi.org/10.3390/heritage8050178
APA StyleAmores, A., Tanga, C., Somma, M. C., La Salvia, V., Antonelli, S., & Viciano, J. (2025). Osseous Variants of the Cervical Spine with Potential Pathological Significance: Possible Evidence of Vertebrobasilar Insufficiency in a Skeletal Sample from the Post-Classical Cemetery of Corfinio (12th–15th Centuries CE, L’Aquila, Italy). Heritage, 8(5), 178. https://doi.org/10.3390/heritage8050178





