Treatment of Acid Hydrolysis of a 1900 Large-Scale Composite Artwork by the Artist Roberto Sebastian Matta: Comparison between Traditional and Innovative Deacidifying Methodologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conservation Status of the Artwork
2.2. Graff-C Analysis
2.3. Pyrolysis Coupled with Mass Spectrometry (py-GC/MS)
2.4. pH Measurements
2.5. Deacidification Treatment: Test Planning on Samples
2.6. Samples: Preparation and Characteristics
2.7. Accelerated UV Aging
2.8. Tests of Solvents and Deacidifying Mixtures on Samples
- (1)
- First phase: identification of the organic solvent capable of conveying the deacidifying agent during application by spraying.
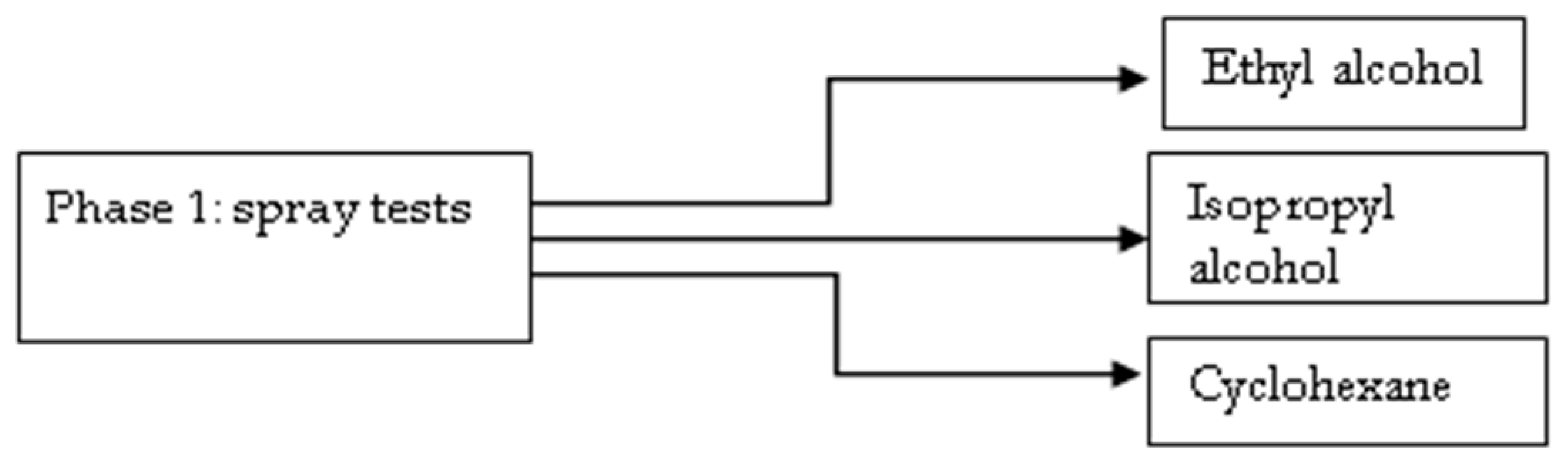
- (2)
- Second phase: the identification of the most effective deacidifying mixture.

2.9. Colorimetric Analysis
3. Results
3.1. Graff C Analysis
3.2. py-GC/MS Results
3.3. pH Data on the Artwork
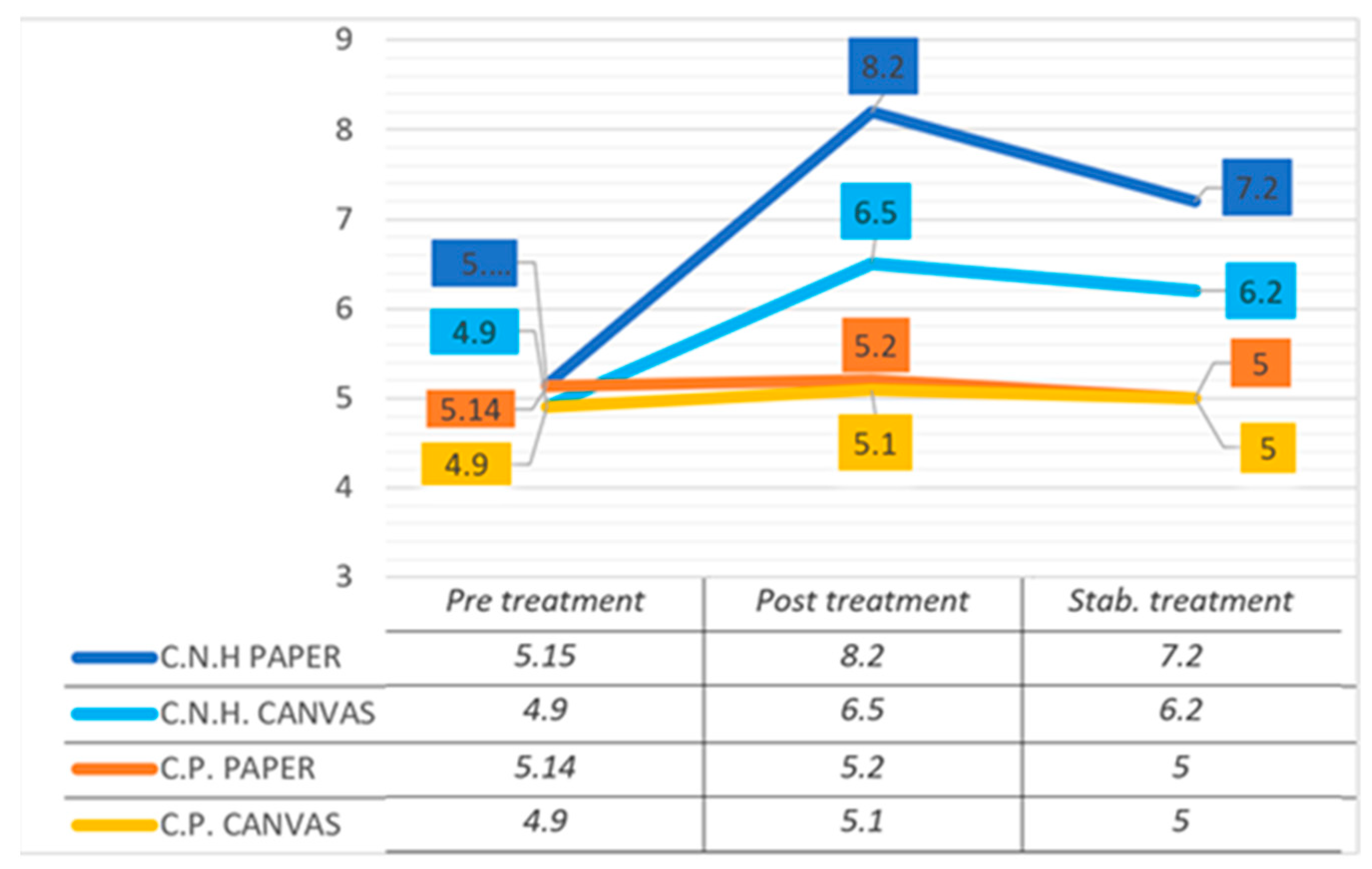
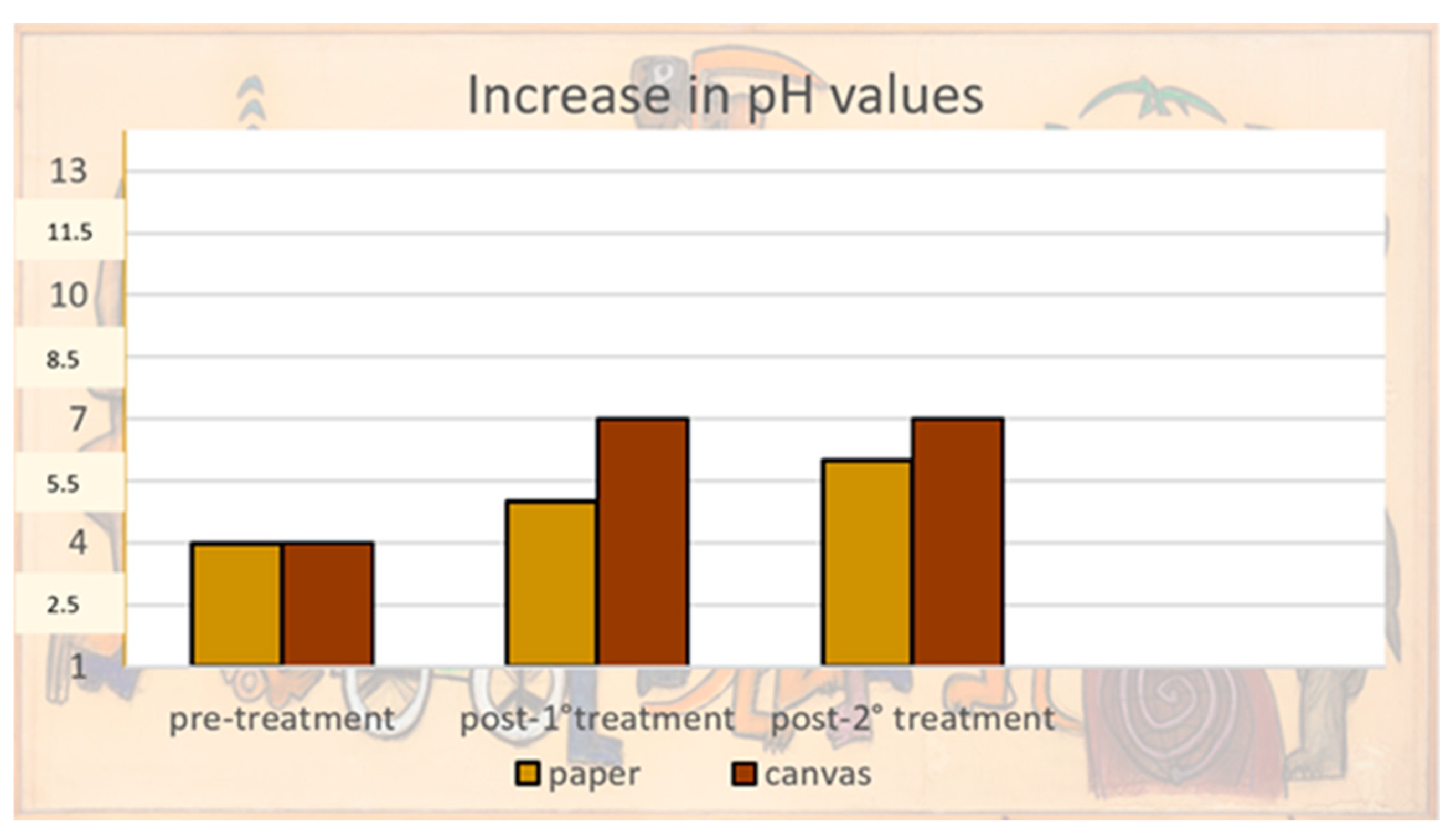
3.4. Deacidification Treatment: Evaluation of the Results by pH and Color Data
4. Discussion
- -
- The large dimensions of the work: one central panel 180.3 × 405.1 cm; two side panels 179.1 × 150.6 cm;
- -
- The different sensitivity to environmental thermo-hygrometric variations shown by the canvas and paper supports of the artwork and the water-solubility of the starch adhesive;
- -
- The limitations on the choice of the deacidifying intervention forced by the constraint existing between the secondary canvas support and the primary paper on which images are drawn in order to avoid structural trauma to the flat work;
- -
- The high level of acidity of the work, detected by pH measurements.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colantonio, C.; Pelosi, C.; Calabrò, G.; Spizzichino, V.; Partenzi, I.; Lanteri, L. Scientific investigation of contemporary pastel painting by Roberto Sebastian Matta: Characterization of original materials through multispectral imaging and spectroscopic techniques. Heritage 2023, 6, 2541–2558. [Google Scholar] [CrossRef]
- Fardi, T.; Pintus, V.; Kampasakali, E.; Pavlidou, E.; Schreiner, M.; Kyriacou, G. Analytical characterization of artist’s paint systems based on emulsion polymers and synthetic organic pigments. J. Anal. Appl. Pyrol. 2018, 135, 231–241. [Google Scholar] [CrossRef]
- Wei, H.; Pintus, V.; Schreiner, M. A comparison study of alkyd resin used in art works by Py-GC/MS and GC/MS: The influence of aging. J. Anal. Appl. Pyrol. 2013, 104, 441–447. [Google Scholar] [CrossRef]
- Product Data Sheet for Nanorestore Paper. Available online: https://www.csgi.unifi.it/products/paper_ita.html (accessed on 29 January 2023).
- EN 15886; Conservation of Cultural Property-Test Methods. Colour Measurement of Surfaces: Brussels, Belgium, 2010.
- Appunti del Corso di Metodologie Fisiche per i Beni Culturali; Archeometria; Dipartimento di Scienze di Base ed Applicate per l’Ingegneria, Laboratorio di Analisi non Distruttive ed Archeometria, Università degli Studi di Roma: Roma, Italy, 2009.
- Ferrari, G. Matta Cosmo Now-Verbo Dei Continenti; Nuova ERI: Torino, Italy, 1992; p. 14. [Google Scholar]
- Simkovic, I.; Francis, B.A.; Reeves, J.B. Pyrolysis-gas chromatography mass spectrometry analysis of starch-based index changers. J. Anal. Appl. Pyrol. 1997, 43, 145–155. [Google Scholar] [CrossRef]
- Lo Monaco, A.; Marabelli, M.; Pelosi, C.; Picchio, R. Colour measurements of surfaces to evaluate the restoration materials, O3A: Optics for Arts, Architecture, and Archaeology III, edited by Luca Pezzati and Renzo Salimbeni. In Proceedings of the SPIE 80840P, Munich, Germany, 23–26 May 2011; SPIE: Bellingham, WA, USA, 2011; Volume 8084, pp. 1–14. [Google Scholar] [CrossRef]
- Pelosi, C.; Capobianco, G.; Agresti, G.; Bonifazi, G.; Morresi, F.; Rossi, S.; Santamaria, U.; Serranti, S. A methodological approach to study the stability of selected watercolors for painting reintegration, through reflectance spectrophotometry, Fourier transform infrared spectroscopy and hyperspectral imaging, Spectroch. Acta A 2018, 198, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Genco, G.; Lo Monaco, A.; Pelosi, C.; Picchio, R.; Santamaria, U. A study of color change due to accelerated sunlight exposure in consolidated wood samples. Wood Res. 2011, 56, 511–524. [Google Scholar]
- Lorusso, S.; Natali, A.; Matteucci, C. Colorimetry applied to the field of cultural heritage: Examples of study cases. Conserv. Sci. Cult. Herit. 2007, 7, 187–220. [Google Scholar]
- Bernárdez-Vilaboa, R. The Implication of Vision and Colour in Cultural Heritage. Heritage 2020, 3, 1063–1068. [Google Scholar] [CrossRef]
- Elias, M.; Mas, N.; Cotte, P. Review of Several Optical Non-Destructive Analyses of an Easel Painting. Complementarity and Cross Checking of the Results. J. Cult. Herit. 2011, 124, 335–345. [Google Scholar] [CrossRef]
- Molada-Tebar, A.; Marqués-Mateu, Á.; Lerma, J.L. Correct use of color for cultural heritage documentation. ISPRS Ann. Photogramm. Remote Sens. Spatial Inf. Sci. 2019, IV-2/W6, 107–113. [Google Scholar]
- Fernandez-Balbuena, A.A.; Vázquez Moliní, D.; Gómez-Manzanares, Á.; Martínez-Antón, J.C.; Mayorga Pinilla, S. Reflectance Measurements on Cultural Heritage. In Heritage New Paradigm; Turcanu-Carutiu, D., Ed.; IntechOpen: London, UK, 2022; p. 149. [Google Scholar]
- Marchiafava, V.; Bartolozzi, G.; Cucci, C.; De Vita, M.; Picollo, M. Colour measurements for monitoring the conservation of contemporary artworks. JAIC J. Int. Colour Assoc. 2014, 13, 36–42. [Google Scholar]
- Bailão, A.; San Andrés, M.; Calvo, A. Colorimetric analysis of two watercolors used in retouching. Int. J. Conserv. Sci. 2014, 5, 329–342. [Google Scholar]
- Sequeira, S.; Casanova, C.; Cabrita, E.J. Deacidification of paper using dispersions of Ca(OH)2 nanoparticles in isopropanol. Study of efficiency. J. Cult. Herit. 2006, 7, 264–272. [Google Scholar] [CrossRef]
- Giorgi, R.; Dei, L.; Ceccato, M.; Schettino, C.; Baglioni, P. Nanotechnologies for Conservation of Cultural Heritage: Paper and Canvas Deacidification. Langmuir 2002, 18, 8198–8203. [Google Scholar] [CrossRef]
- Vascello, C. Produzione di Microparticelle a Base di Alginato Mediante Atomizzazione Assistita da Ultrasuoni. Ph.D. Thesis, Faculty of Engineering, University of Salerno, Salerno, Italy, 2010. [Google Scholar]
- Baglioni, P.; Giorgi, R. Soft and Hard Nanomaterials for Restoration and Conservation of Cultural Heritage. Soft Matter 2006, 2, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Amornkitbamrung, L.; Bračič, D.; Bračič, M.; Hribernik, S.; Malešič, J.; Hirn, U.; Vesel, A.; Kleinschek, K.S.; Kargl, R.; Mohan, T. Comparison of Trimethylsilyl Cellulose-Stabilized Carbonate and Hydroxide Nanoparticles for Deacidification and Strengthening of Cellulose-Based Cultural Heritage. ACS Omega 2020, 5, 29243–29256. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, G.; Merelli, C. Conservazione e restauro di opere d’arte su carta da lucido. Applicazione di nanotecnologie per la deacidificazione di disegni su carta da lucido impregnata. OPD Restauro 2017, 29, 276–283. [Google Scholar]
- Poggi, G.; Giorgi, R.; Mirabile, A.; Xing, H.; Baglioni, P. A stabilizer free non-polar dispersion for the deacidification of contemporary art on paper. J. Cult. Herit. 2017, 26, 44–52. [Google Scholar] [CrossRef]




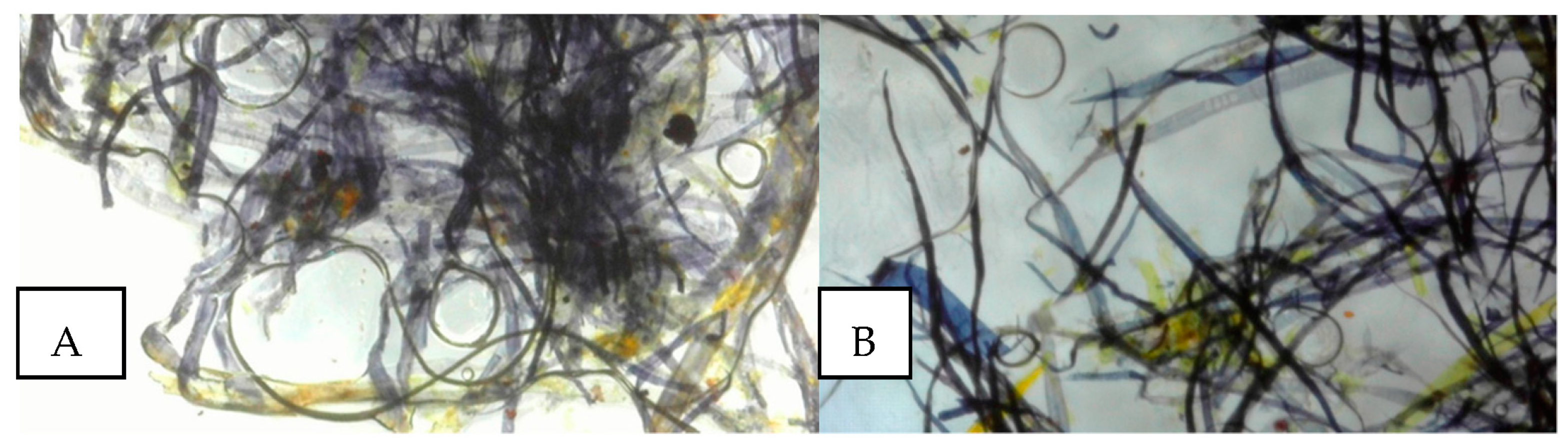
| Time of Retention (min) | Name | Origin | (%) Paper and Adhesive |
|---|---|---|---|
| 1.3 | Carbon dioxide | Cellulose | 9.2 |
| 1.5 | Pyruvic aldehyde | Cellulose | 8.3 |
| 4.5 | 2-hydroxy butanal | Cellulose | 1.7 |
| 5.8 | 2-furaldehyde | Cellulose/starch | 10.8 |
| 21.8 | 5-hydroxymethyl-2-furaldehyde | Cellulose/starch | 12.2 |
| 32.5 | Levoglucosan | Cellulose | 15.2 |
| pH Measurements on the Front | ||
|---|---|---|
 | ||
| Point of analysis | Area | Values |
| 1. spolvero paper | Bottom right | 4.47 |
| 2. spolvero paper | Top center | 5.37 |
| 3. spolvero paper | Bottom left | 4.99 |
| pH Measurements on the Back | ||
|---|---|---|
 | ||
| Point of analysis | Area | Values |
| 1. Canvas | Top right | 4.75 |
| 2. Canvas | Bottom left | 4.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Partenzi, I.; Marconi, M.; Vinciguerra, V.; Pelosi, C.; Fontani, N. Treatment of Acid Hydrolysis of a 1900 Large-Scale Composite Artwork by the Artist Roberto Sebastian Matta: Comparison between Traditional and Innovative Deacidifying Methodologies. Heritage 2023, 6, 2650-2663. https://doi.org/10.3390/heritage6030140
Partenzi I, Marconi M, Vinciguerra V, Pelosi C, Fontani N. Treatment of Acid Hydrolysis of a 1900 Large-Scale Composite Artwork by the Artist Roberto Sebastian Matta: Comparison between Traditional and Innovative Deacidifying Methodologies. Heritage. 2023; 6(3):2650-2663. https://doi.org/10.3390/heritage6030140
Chicago/Turabian StylePartenzi, Ilaria, Martina Marconi, Vittorio Vinciguerra, Claudia Pelosi, and Nicoletta Fontani. 2023. "Treatment of Acid Hydrolysis of a 1900 Large-Scale Composite Artwork by the Artist Roberto Sebastian Matta: Comparison between Traditional and Innovative Deacidifying Methodologies" Heritage 6, no. 3: 2650-2663. https://doi.org/10.3390/heritage6030140
APA StylePartenzi, I., Marconi, M., Vinciguerra, V., Pelosi, C., & Fontani, N. (2023). Treatment of Acid Hydrolysis of a 1900 Large-Scale Composite Artwork by the Artist Roberto Sebastian Matta: Comparison between Traditional and Innovative Deacidifying Methodologies. Heritage, 6(3), 2650-2663. https://doi.org/10.3390/heritage6030140










