Evaluation of the Suitability of Electrokinetic Treatment to Desalinate the Limestone of the Tomb of Cyrus, a UNESCO World Heritage Site in Iran
Abstract
:1. Introduction
- (1)
- Extreme pH changes around the electrodes, due to water hydrolysis (Equations (1) and (2)), which can cause new chemical alterations of the stone minerals. Especially worrisome is limestone, a material that is very sensitive to exposure to acidic pH, which can cause the dissolution of calcium carbonate.
- (2)
- The generation of micro-fractures, which can be caused by the mechanical stresses associated with the electric-field flow across the pores of the material [42]. This damage is higher in those materials containing piezoelectric minerals [43,44] and depends on the value of the current density applied.
2. Materials and Methods
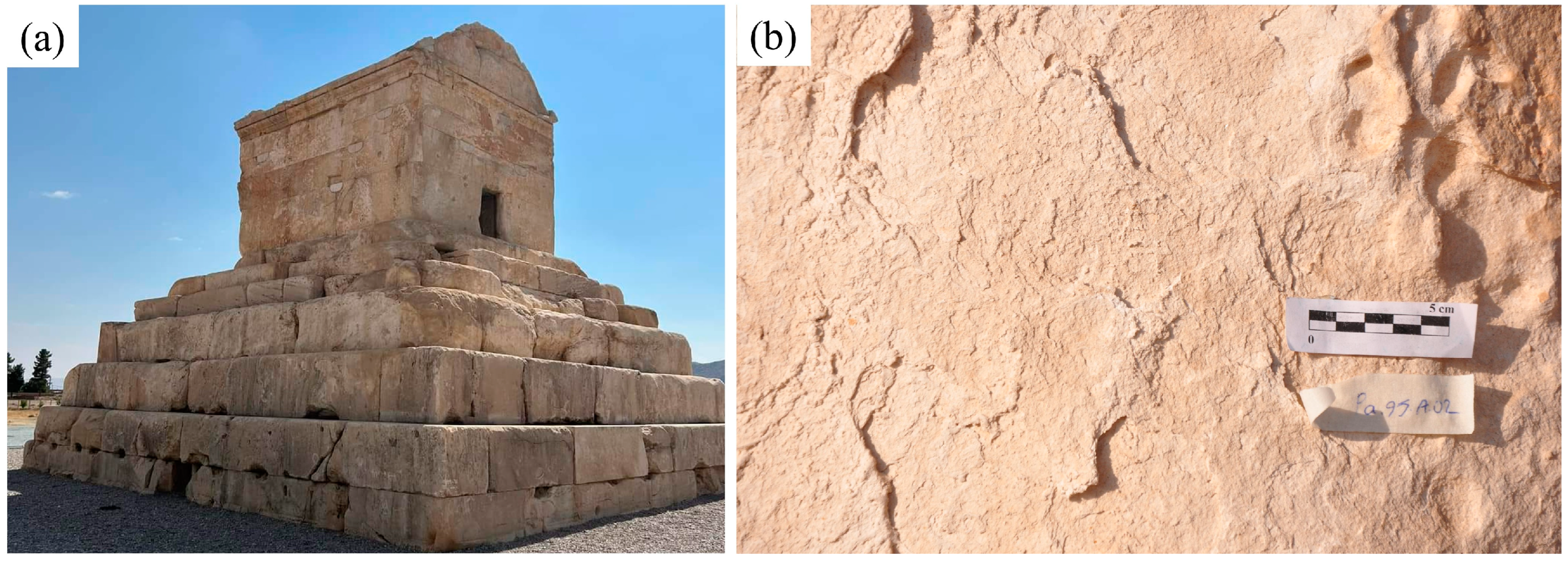
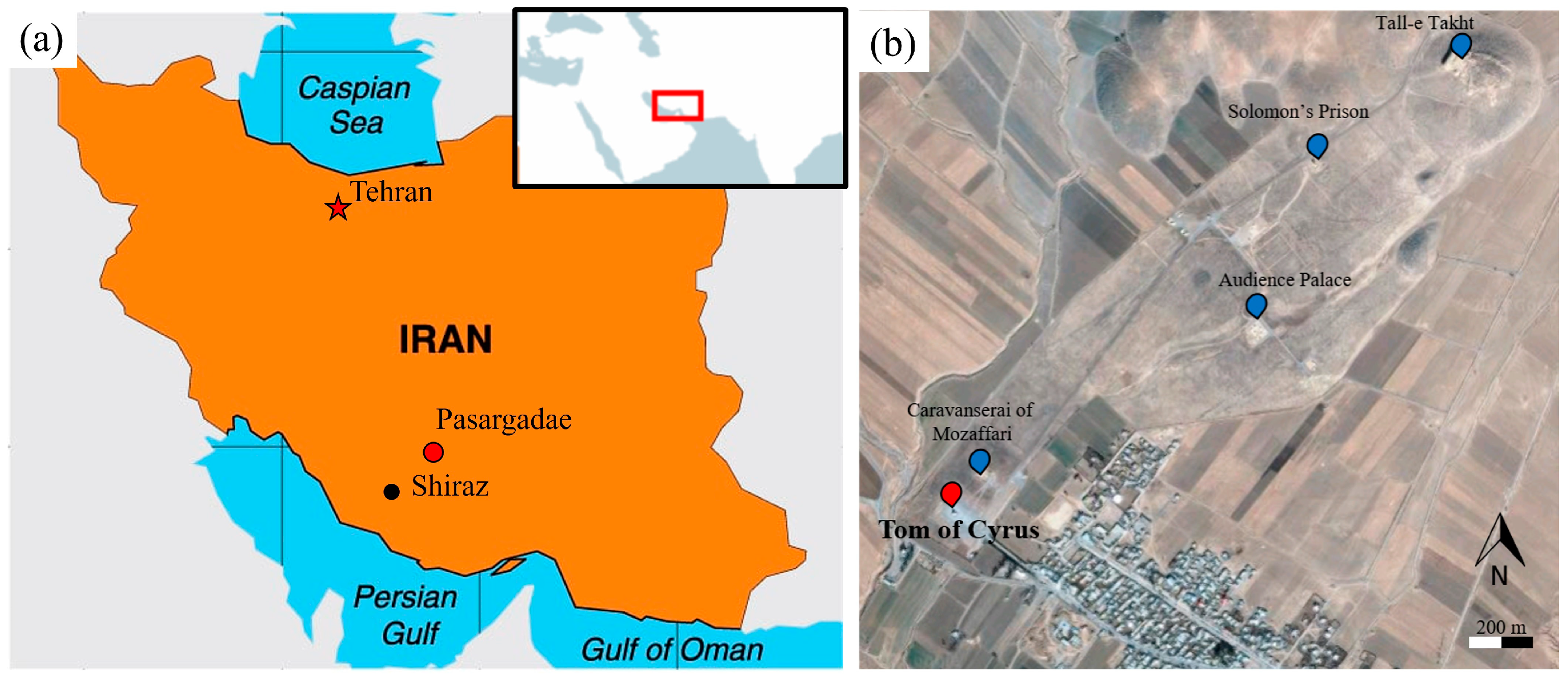
2.1. Description of the Archaeological Site
2.2. Previous Diagnosis and Sampling
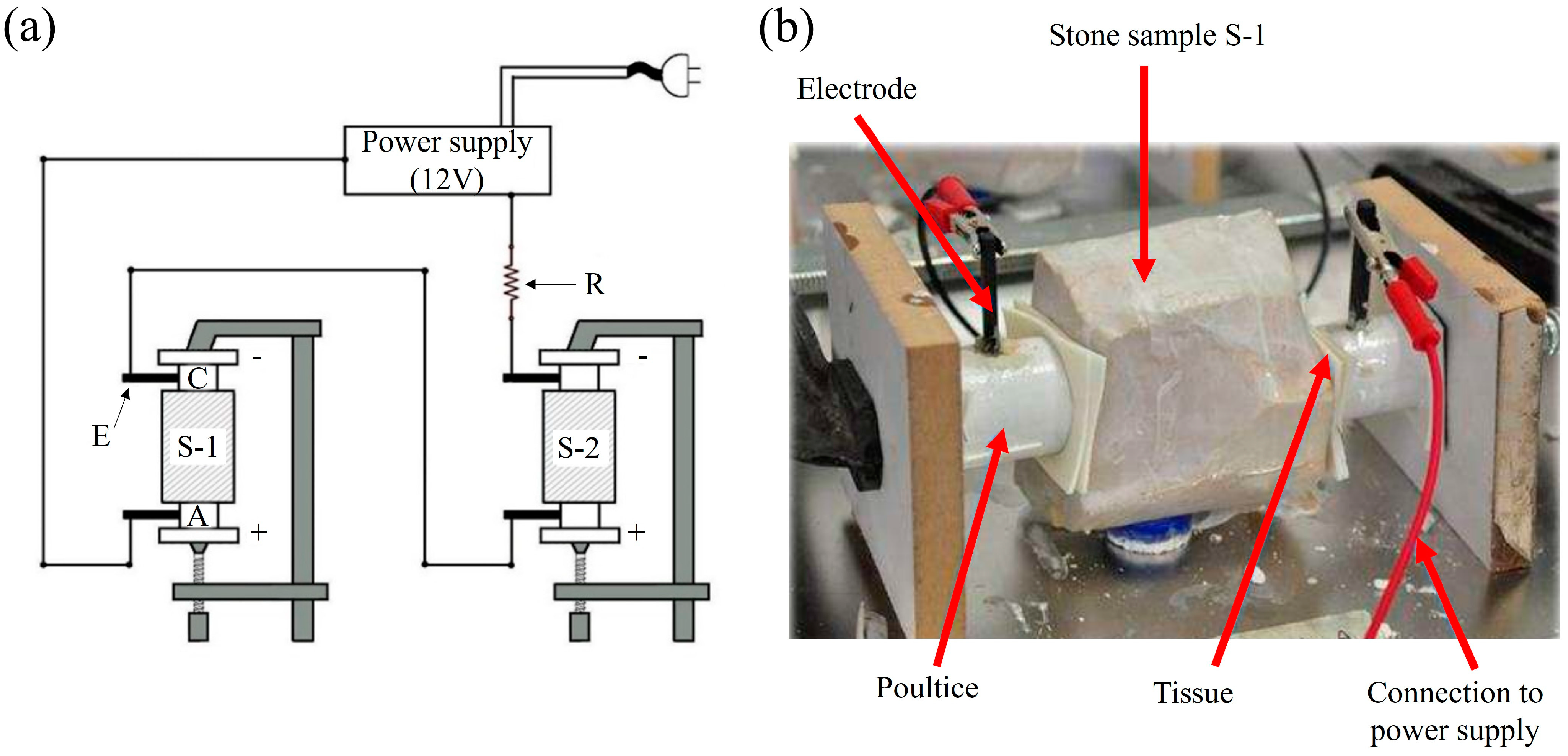
2.3. Electrokinetic Desalination Setup
3. Results and Discussion
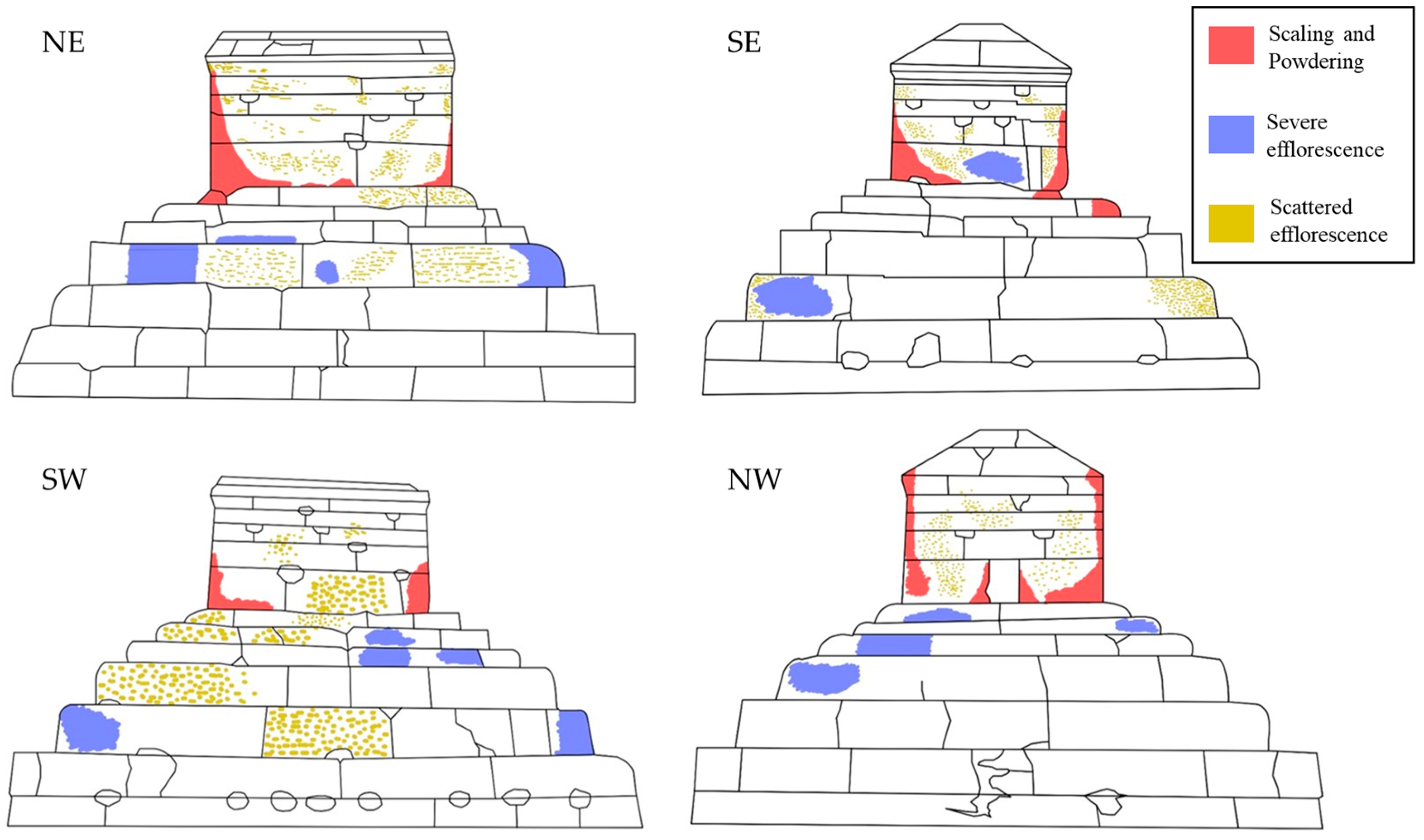
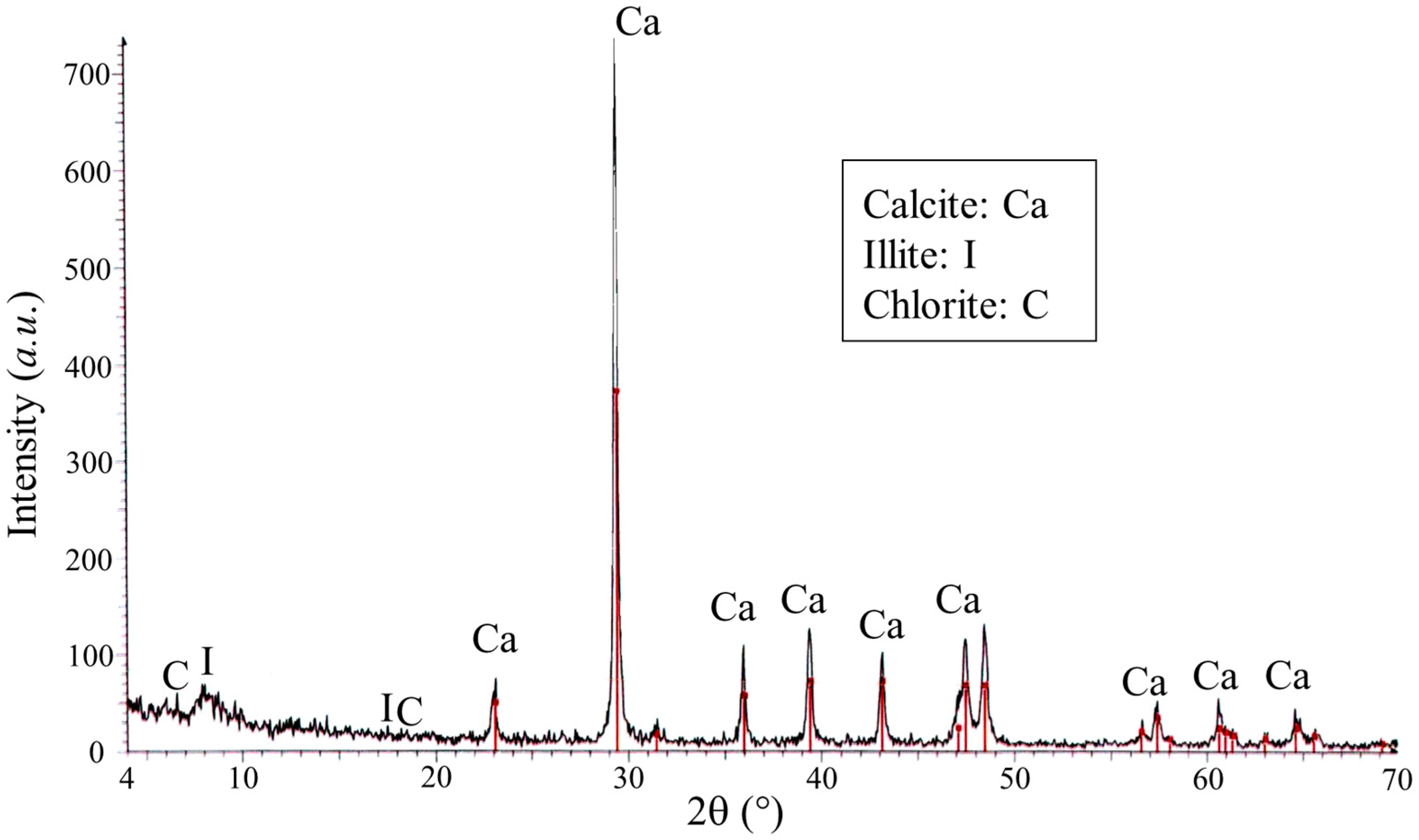
3.1. Previous Diagnosis of the Salt Problem
3.2. Stone Properties
3.3. Evolution of the Electrical Parameters: Current Density and Resistivity
3.4. pH Measurements
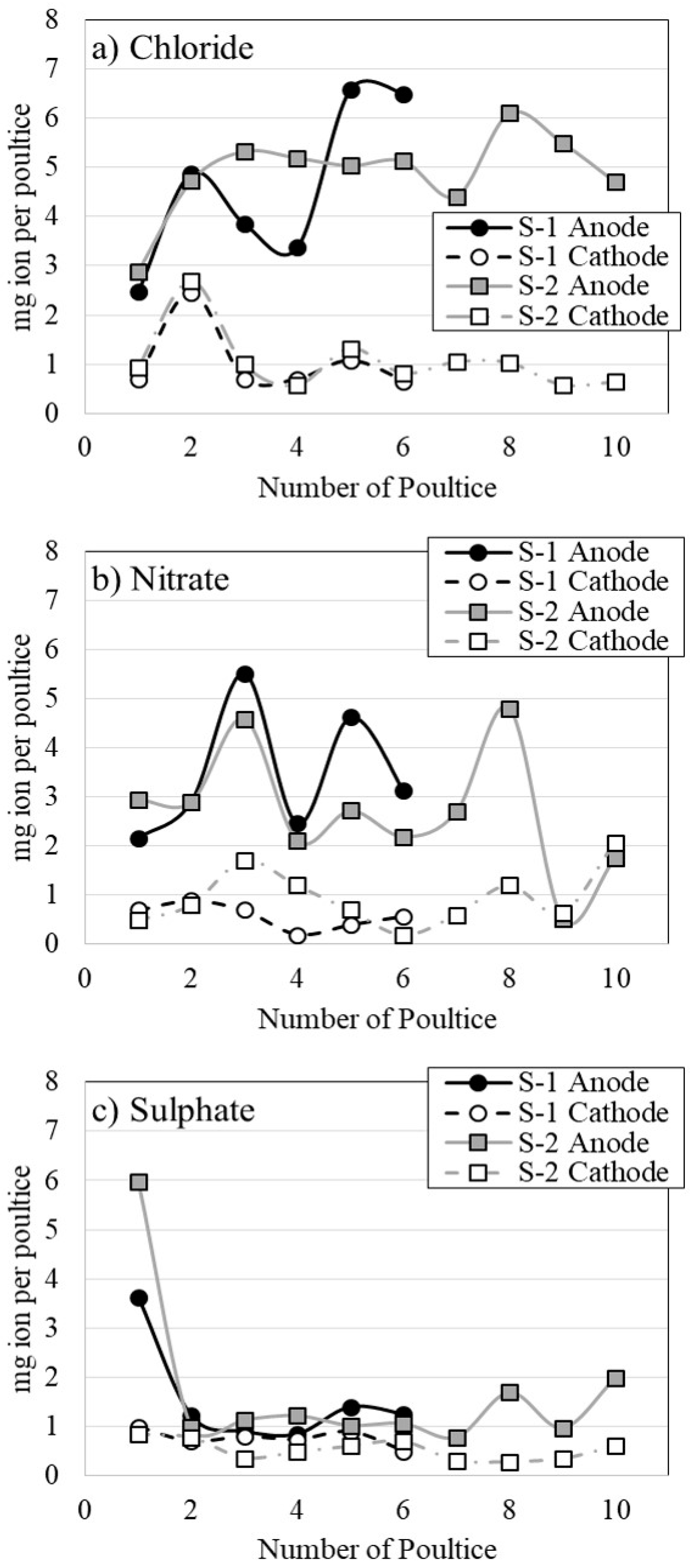
3.5. Anion Content Retained in the Poultices
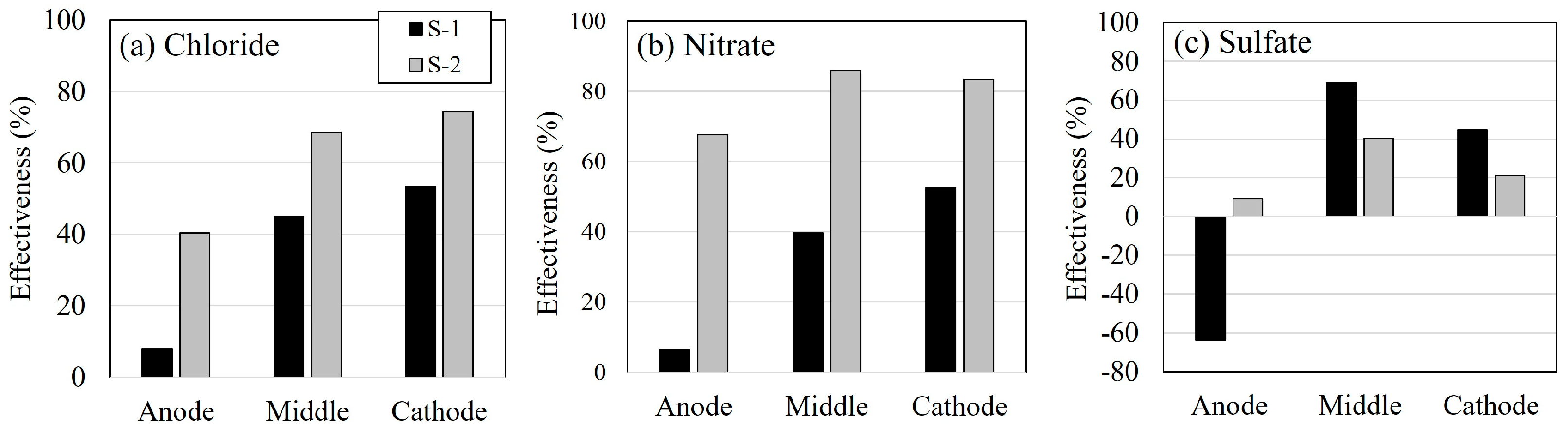
3.6. Desalination Effectiveness
3.7. Possible Changes in the Stone
4. Conclusions
- The hypothesis that the Tomb of Cyrus is affected by the action of soluble salts, especially by the presence of nitrates and sulfates, is reaffirmed. For this reason, in the future, it is possible that the concentration may increase, and it will be necessary to consider an intervention strategy;
- Electrokinetic techniques make it possible to achieve high desalination efficiency values in a short time. This efficiency can be increased by increasing the duration of the treatment;
- The buffer system is effective at protecting limestone against extreme pH environments that can cause chemical damage;
- The use of a calcium carbonate poultice at the anode allows for supplying calcium to the limestone, which can precipitate and evolve to calcium carbonate, causing a reduction in the accessible porosity of the stone.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charola, A.E. Salts in the Deterioration of Porous Materials: An Overview. J. Am. Inst. Conserv. 2000, 39, 327–343. [Google Scholar] [CrossRef]
- Charola, A.E.; Pühringer, J.; Steiger, M. Gypsum: A review of its role in the deterioration of building materials. Environ. Geol. 2007, 52, 339–352. [Google Scholar] [CrossRef]
- Oguchi, C.T.; Yu, S. A review of theoretical salt weathering studies for stone heritage. Prog. Earth Planet. Sci. 2021, 8, 32. [Google Scholar] [CrossRef]
- Arnold, A.; Zehnder, K. Salt weathering on monuments. In Proceedings of the 1st International Symposium on the Conservation of Monuments in the Mediterranean Basin, Bari, Italy, 7–10 June 1989. [Google Scholar]
- Alves, C.; Figueiredo, C.A.M.; Sanjurjo-Sánchez, J.; Hernández, A.C. Salt weathering of natural stone: A review of comparative laboratory studies. Heritage 2021, 4, 1554–1565. [Google Scholar] [CrossRef]
- La Iglesia, A.; González, V.; López-Acevedo, V.; Viedma, C. Salt crystallization in porous construction materials I Estimation of crystallization pressure. J. Cryst. Growth 1997, 177, 111–118. [Google Scholar] [CrossRef]
- Tsui, N.; Flatt, R.J.; Scherer, G.W. Crystallization damage by sodium sulfate. J. Cult. Herit. 2003, 4, 109–115. [Google Scholar] [CrossRef]
- Lubelli, B.; Van Hees, R.P.J.; Groot, C.J.W.P. The role of sea salts in the occurrence of different damage mechanisms and decay patterns on brick masonry. Constr. Build. Mater. 2004, 18, 119–124. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—II: Influence of crystal size on the crystallization pressure. J. Cryst. Growth 2005, 282, 470–481. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Lopez-Arce, P.; Doehne, E.; Greenshields, J.; Benavente, D.; Young, D. Treatment of rising damp and salt decay: The historic masonry buildings of Adelaide, South Australia. Mater. Struct. 2008, 42, 827–848. [Google Scholar] [CrossRef]
- Rivas, T.; Feijoo, J.; de Rosario, I.; Taboada, J. Use of Ferrocyanides on Granite Desalination by Immersion and Poultice-Based Methods. Int. J. Archit. Herit. 2017, 11, 588–606. [Google Scholar] [CrossRef]
- Unruh, J. A Revised Endpoint for Ceramics Desalination at the Archaeological Site of Gordion, Turkey. Stud. Conserv. 2007, 46, 81. [Google Scholar] [CrossRef]
- Husillos-Rodríguez, N.; Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Blanco-Varela, M.T.; Fort, R. Sacrificial mortars for surface desalination. Constr. Build. Mater. 2018, 173, 452–460. [Google Scholar] [CrossRef]
- Feijoo, J.; Ergenç, D.; Fort, R.; de Buergo, M.Á. Addition of ferrocyanide-based compounds to repairing joint lime mortars as a protective method for porous building materials against sodium chloride damage. Mater. Struct. Constr. 2021, 54, 14. [Google Scholar] [CrossRef]
- Lubelli, B.; van Hees, R.P.J. Desalination of masonry structures: Fine tuning of pore size distribution of poultices to substrate properties. J. Cult. Herit. 2010, 11, 10–18. [Google Scholar] [CrossRef]
- Pel, L.; Sawdy, A.; Voronina, V. Physical principles and efficiency of salt extraction by poulticing. J. Cult. Herit. 2010, 11, 59–67. [Google Scholar] [CrossRef]
- Petković, J.; Huinink, H.P.; Pel, L.; Kopinga, K.; van Hees, R.P.J. Salt transport in plaster/substrate layers. Mater. Struct. 2007, 40, 475–490. [Google Scholar] [CrossRef]
- Correia, J.; Matero, F. Calcium tartrate tetrahydrate preconsolidation of salt-contaminated limestone at mission San José y San Miguel de Aguayo. J. Am. Inst. Conserv. 2008, 47, 81–95. [Google Scholar] [CrossRef]
- Feijoo, J.; de Rosario, I.; Rivas, T.; Mosquera, M.J.; Benavides, R. Influence of a Pre-consolidation Treatment on the Desalination Effectiveness of a Highly Deteriorated Granite Façade of Medieval Age. Int. J. Archit. Herit. 2022, 1–19. [Google Scholar] [CrossRef]
- Sawdy, A.; Heritage, A.; Pel, L. A review of salt transport in porous media: Assessment methods and salt reduction treatments. In Proceedings of the Salt Weathering on Buildings and Stone Sculptures, Copenhagen, Denmark, 22–24 October 2008. [Google Scholar]
- Gupta, S.; Pel, L.; Steiger, M.; Kopinga, K. The effect of ferrocyanide ions on sodium chloride crystallization in salt mixtures. J. Cryst. Growth 2015, 410, 7–13. [Google Scholar] [CrossRef]
- Gupta, S.; Terheiden, K.; Pel, L.; Sawdy, A. Influence of ferrocyanide inhibitors on the transport and crystallization processes of sodium chloride in porous building materials. Cryst. Growth Des. 2012, 12, 3888–3898. [Google Scholar] [CrossRef]
- Granneman, S.J.C.; Lubelli, B.; van Hees, R.P.J. Mitigating salt damage in building materials by the use of crystallization modifiers—A review and outlook. J. Cult. Herit. 2019, 40, 183–194. [Google Scholar] [CrossRef]
- Bracciale, M.P.; Sammut, S.; Cassar, J.A.; Santarelli, M.L.; Marrocchi, A. Molecular crystallization inhibitors for salt damage control in porous materials: An overview. Molecules 2020, 25, 1873. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, L.M.; Rörig-Dalgård, I. Electrokinetic removal of Ca(NO3)2 from bricks to avoid salt-induced decay. Electrochim. Acta 2007, 52, 3454–3463. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Rörig-Dalgaard, I. Desalination of a brick by application of an electric DC field. Mater. Struct. 2009, 42, 961–971. [Google Scholar] [CrossRef]
- Skibsted, G.; Ottosen, L.M.; Jensen, P.E.; Paz-Garcia, J.M. Electrochemical desalination of bricks—Experimental and modeling. Electrochim. Acta 2015, 181, 24–30. [Google Scholar] [CrossRef]
- Marinho, J.N.; Salavessa, E.; Jalali, S.; Sousa, L.; Serôdio, C.; Carvalho, M.J.P. Evaluation of Electromigration Desalination of Granite Contaminated with Salts—A Contribution to the Conservation of Architectural Surfaces. In Proceedings of the 3rd RILEM Spring Convention and Conference (RSCC 2020), Guimarães, Portugal, 9–14 March 2020; Sena-Cruz, J., Correia, L., Azenha, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 217–228. [Google Scholar] [CrossRef]
- Feijoo, J.; Ottosen, L.M.; Pozo-Antonio, J.S. Influence of the properties of granite and sandstone in the desalination process by electrokinetic technique. Electrochim. Acta 2015, 181, 280–287. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Christensen, I.V. Electrokinetic desalination of sandstones for NaCl removal—Test of different clay poultices at the electrodes. Electrochim. Acta 2012, 86, 192–202. [Google Scholar] [CrossRef]
- Feijoo, J.; Matyščák, O.; Ottosen, L.M.; Rivas, T.; Nóvoa, X.R. Electrokinetic desalination of protruded areas of stone avoiding the direct contact with electrodes. Mater. Struct. Constr. 2017, 50, 82. [Google Scholar] [CrossRef]
- Ottosen, L.M. Electro-Desalination of Sandstones with Cracks. In Proceedings of the XV Edition of the International Conference on Durability of Building Materials and Components, Barcelona, Spain, 20–23 October 2020; International Center for Numerical Methods in Engineering: Barcelona, Spain, 2020; pp. 1173–1180. [Google Scholar] [CrossRef]
- Matyščák, O.; Ottosen, L.M.; Rörig-Dalgaard, I. Desalination of salt damaged Obernkirchen sandstone by an applied DC field. Comput. Chem. Eng. 2014, 71, 561–569. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Pedersen, A.J.; Rörig-Dalgaard, I. Salt-related problems in brick masonry and electrokinetic removal of salts. J. Build. Apprais. 2007, 3, 181–194. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Rörig-dalgaard, I.; Villumsen, A. Electrochemical removal of salts from masonry—Experiences from pilot scale. In Proceedings of the International Conference on Salt Weathering on Building and Stone Sculptures, Copenhagen, Denmark, 22 October 2008. [Google Scholar]
- Feijoo, J.; Ottosen, L.M.; Matyscak, O.; Fort, R. Electrokinetic desalination of a farmhouse applying a proton pump approach. First in situ experience. Constr. Build. Mater. 2020, 243, 118308. [Google Scholar] [CrossRef]
- Feijoo, J.; Rivas, T.; Nóvoa, X.R.; de Rosario, I.; Otero, J. In situ desalination of a granitic column by the electrokinetic method. Int. J. Archit. Herit. 2018, 12, 63–74. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Christensen, I.V.; Rörig-dalgaard, I. Electrochemical Desalination of Salt Infected Limestone Masonry of a Historic Warehouse. In Proceedings of the Structural Faults and Repair, Edinburg, UK, 3–5 July 2012. [Google Scholar]
- Kamran, K.; van Soestbergen, M.; Huinink, H.P.; Pel, L. Inhibition of electrokinetic ion transport in porous materials due to potential drops induced by electrolysis. Electrochim. Acta 2012, 78, 229–235. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X. Ionic transport in cementitious materials under an externally applied electric field: Finite element modeling. Constr. Build. Mater. 2012, 27, 450–460. [Google Scholar] [CrossRef]
- Li, C.; Man, H.; Song, C.; Gao, W. Fracture analysis of piezoelectric materials using the scaled boundary finite element method. Eng. Fract. Mech. 2013, 97, 52–71. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Gao, C.F. Fracture behaviors of piezoelectric materials. Theor. Appl. Fract. Mech. 2004, 41, 339–379. [Google Scholar] [CrossRef]
- Fanood, M.R.; Saradj, F.M. Learning from the past and planning for the future: Conditions and proposals for stone conservation of the mausoleum of Cyrus the Great in the World Heritage site of Pasargadae. Int. J. Archit. Herit. 2013, 7, 434–460. [Google Scholar] [CrossRef]
- Mohammadi, P.; Maghboli-Balasjin, N. Isolation and molecular identification of deteriorating fungi from cyrus the great tomb stones. Iran. J. Microbiol. 2014, 6, 361–370. [Google Scholar] [CrossRef]
- Shekofteh, A.; Molina Piernas, E.; Arizzi, A.; Cultrone, G.; Ahmadi, H.; Yazdi, M. Deterioration assessment of three types of limestones from Pasargadae world heritage site in Iran. In Proceedings of the Youth in Conservation of Cultural Heritage—YOCOCU, Madrid, Spain, 21–23 September 2016; pp. 72–76. [Google Scholar]
- Shekofteh, A.; Molina, E.; Arizzi, A.; Cultrone, G.; Ahmadi, H.; Yazdi, M. Characterization and damage assessment of stones used in the Pasargadae World Heritage Site, Achaemenian period. Int. J. Archit. Herit. 2019, 13, 521–536. [Google Scholar] [CrossRef]
- Gholipour-Shahraki, M.; Mohammadi, P. The Study of Growth of Calogaya sp. PLM8 on Cyrus the Great’s Tomb, UNESCO World Heritage Site in Iran. Int. J. Environ. Res. 2017, 11, 501–513. [Google Scholar] [CrossRef]
- Sohrabi, M.; Favero-Longo, S.E.; Pérez-Ortega, S.; Ascaso, C.; Haghighat, Z.; Talebian, M.H.; Fadaei, H.; de los Ríos, A. Lichen colonization and associated deterioration processes in Pasargadae, UNESCO world heritage site, Iran. Int. Biodeterior. Biodegrad. 2017, 117, 171–182. [Google Scholar] [CrossRef]
- Mozaffari, A. World Heritage in Iran: Perspectives on Pasargadae; Ashgate Publishing: Farnham, UK, 2014. [Google Scholar]
- UNE-EN 1936:2007; Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity. UNE: Madrid, Spain, 2007.
- Rörig-Dalgaard, I. Development of a poultice for electrochemical desalination of porous building materials: Desalination effect and pH changes. Mater. Struct. 2013, 46, 959–970. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Electrochemical removal of chlorides Modelling of the extraction, resulting profiles and determination of the efficient time of treatment. Cem. Concr. Res. 2000, 30, 615–621. [Google Scholar] [CrossRef]
- Grossi, C.M.; Esbert, R.M. Las sales solubles en el deterioro de rocas monumentales. Revisión bibliográfica. Mater. Construcción 1994, 44, 15–30. [Google Scholar] [CrossRef]
- Kloppmann, W.; Bromblet, P.; Vallet, J.M.; Vergès-Belmin, V.; Rolland, O.; Guerrot, C.; Gosselin, C. Building materials as intrinsic sources of sulphate: A hidden face of salt weathering of historical monuments investigated through multi-isotope tracing (B, O, S). Sci. Total Environ. 2011, 409, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Prieto, B.; Rivas, T.; Pereira, L. Gypsum-induced decay in granite monuments in Northwestern Spain. Mater. Constr. 2010, 60, 97–110. [Google Scholar] [CrossRef]
- Jiang, Y. Sources of sulfur in the Nandong underground river system, southwest China: A chemical and isotopic reconnaissance. Appl. Geochem. 2012, 27, 1463–1470. [Google Scholar] [CrossRef]
- C568-79; ASTM Anual Book of ASTM Standrads. Part 19. Soil and Rock. Building Stones. ASTM: West Conshohocken, PA, USA, 1981.
- Ottosen, L.M.; Christensen, I.V.; Rörig-Dalgård, I.; Jensen, P.E.; Hansen, H.K. Utilization of electromigration in civil and environmental engineering—Processes, transport rates and matrix changes. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2008, 43, 795–809. [Google Scholar] [CrossRef]
- Paz-García, J.M.; Johannesson, B.; Ottosen, L.M.; Alshawabkeh, A.N.; Ribeiro, A.B.; Rodríguez-Maroto, J.M. Modeling of electrokinetic desalination of bricks. Electrochim. Acta 2012, 86, 213–222. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Ferreira, C.M.D.; Christensen, I.V. Electrokinetic desalination of glazed ceramic tiles. J. Appl. Electrochem. 2010, 40, 1161–1171. [Google Scholar] [CrossRef]
- Cizer, Ö.; Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Elsen, J.; Van Gemert, D.; Van Balen, K. Phase and morphology evolution of calcium carbonate precipitated by carbonation of hydrated lime. J. Mater. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef]
- Beruto, D.T.; Botter, R. Liquid-like H2O adsorption layers to catalyze the Ca(OH)2/CO2 solid-gas reaction and to form a non-protective solid product layer at 20 °C. J. Eur. Ceram. Soc. 2000, 20, 497–503. [Google Scholar] [CrossRef]
- Gu, W.; Bousfield, D.W.; Tripp, C.P. Formation of calcium carbonate particles by direct contact of Ca(OH)2 powders with supercritical CO2. J. Mater. Chem. 2006, 16, 3312–3317. [Google Scholar] [CrossRef]
- Vance, K.; Falzone, G.; Pignatelli, I.; Bauchy, M.; Balonis, M.; Sant, G. Direct Carbonation of Ca(OH)2 Using Liquid and Supercritical CO2: Implications for Carbon-Neutral Cementation. Ind. Eng. Chem. Res. 2015, 54, 8908–8918. [Google Scholar] [CrossRef]
- Feijoo, J.; Fort, R.; Gomez-Villalba, L.S.; Rabanal, M.E.; Ottosen, L.M. Electroprecipitation of Magnesium and Calcium Compounds for Weathering Protection of Ornamental Rocks. Cryst. Growth Des. 2020, 20, 2337–2355. [Google Scholar] [CrossRef]
- Sassoni, E.; Masi, G.; Bignozzi, M.; Franzoni, E. Electrodeposition of Hydroxyapatite Coatings for Marble Protection: Preliminary Results. Coatings 2019, 9, 207. [Google Scholar] [CrossRef]
- Meloni, P.; Manca, F.; Carcangiu, G. Marble protection: An inorganic electrokinetic approach. Appl. Surf. Sci. 2013, 273, 377–385. [Google Scholar] [CrossRef]
- Feijoo, J.; Gomez-Villalba, L.S.; de los Ríos, A.; Fort, R. Electroprecipitation of inorganic borates, with different solubility, within monumental stones to avoid fungal colonization. Constr. Build. Mater. 2023, 368, 130435. [Google Scholar] [CrossRef]

| Physical Properties | Before Treatment | After Treatment | |
| S-1 | S-2 | ||
| Porosity (%) | 20.90 | 12.00 | 10.00 |
| Bulk density (g/cm3) | 1.74 | 2.05 | 2.14 |
| Average Anion Content (mg/kg) | Before Treatment | After Treatment | |
| S-1 | S-2 | ||
| NO3− | 72.65 | 48.72 | 15.18 |
| SO42− | 94.00 | 78.33 | 71.85 |
| Cl− | 98.25 | 63.37 | 38.24 |
| Anion | Anode | Cathode | Total | |||
|---|---|---|---|---|---|---|
| S-1 | S-2 | S-1 | S-2 | S-1 | S-2 | |
| Chloride | 27.7 | 49.0 | 6.3 | 10.6 | 34.0 | 59.6 |
| Nitrate | 20.8 | 27.2 | 3.5 | 9.6 | 24.3 | 36.8 |
| Sulfate | 9.3 | 16.9 | 4.6 | 5.3 | 13.9 | 22.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, N.; Feijoo, J.; Aly, N. Evaluation of the Suitability of Electrokinetic Treatment to Desalinate the Limestone of the Tomb of Cyrus, a UNESCO World Heritage Site in Iran. Heritage 2023, 6, 6993-7008. https://doi.org/10.3390/heritage6110365
Eslami N, Feijoo J, Aly N. Evaluation of the Suitability of Electrokinetic Treatment to Desalinate the Limestone of the Tomb of Cyrus, a UNESCO World Heritage Site in Iran. Heritage. 2023; 6(11):6993-7008. https://doi.org/10.3390/heritage6110365
Chicago/Turabian StyleEslami, Nasser, Jorge Feijoo, and Nevin Aly. 2023. "Evaluation of the Suitability of Electrokinetic Treatment to Desalinate the Limestone of the Tomb of Cyrus, a UNESCO World Heritage Site in Iran" Heritage 6, no. 11: 6993-7008. https://doi.org/10.3390/heritage6110365
APA StyleEslami, N., Feijoo, J., & Aly, N. (2023). Evaluation of the Suitability of Electrokinetic Treatment to Desalinate the Limestone of the Tomb of Cyrus, a UNESCO World Heritage Site in Iran. Heritage, 6(11), 6993-7008. https://doi.org/10.3390/heritage6110365







