The Colors of the Butterfly Wings: Non-Invasive Microanalytical Studies of Hand-Coloring Materials in 19th-Century Daguerreotypes
Abstract
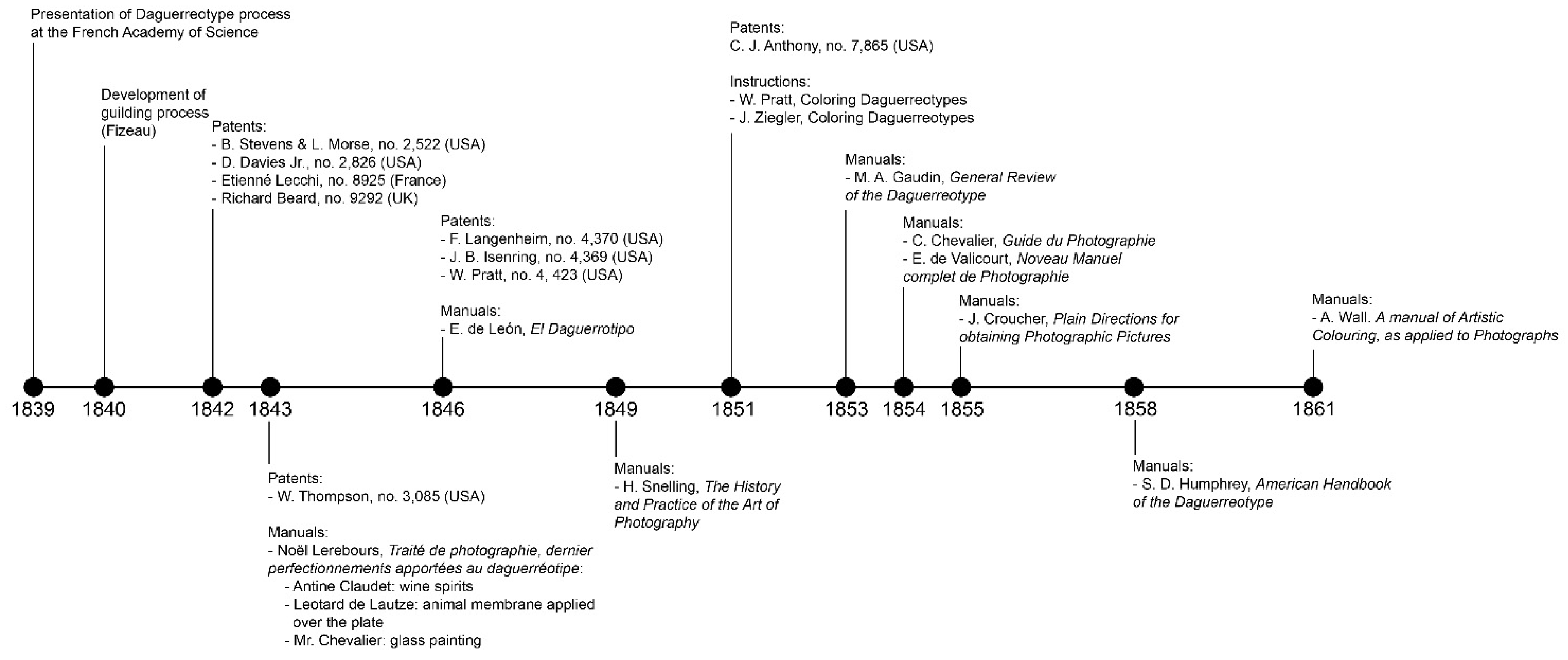
1. Introduction
2. Materials and Methods
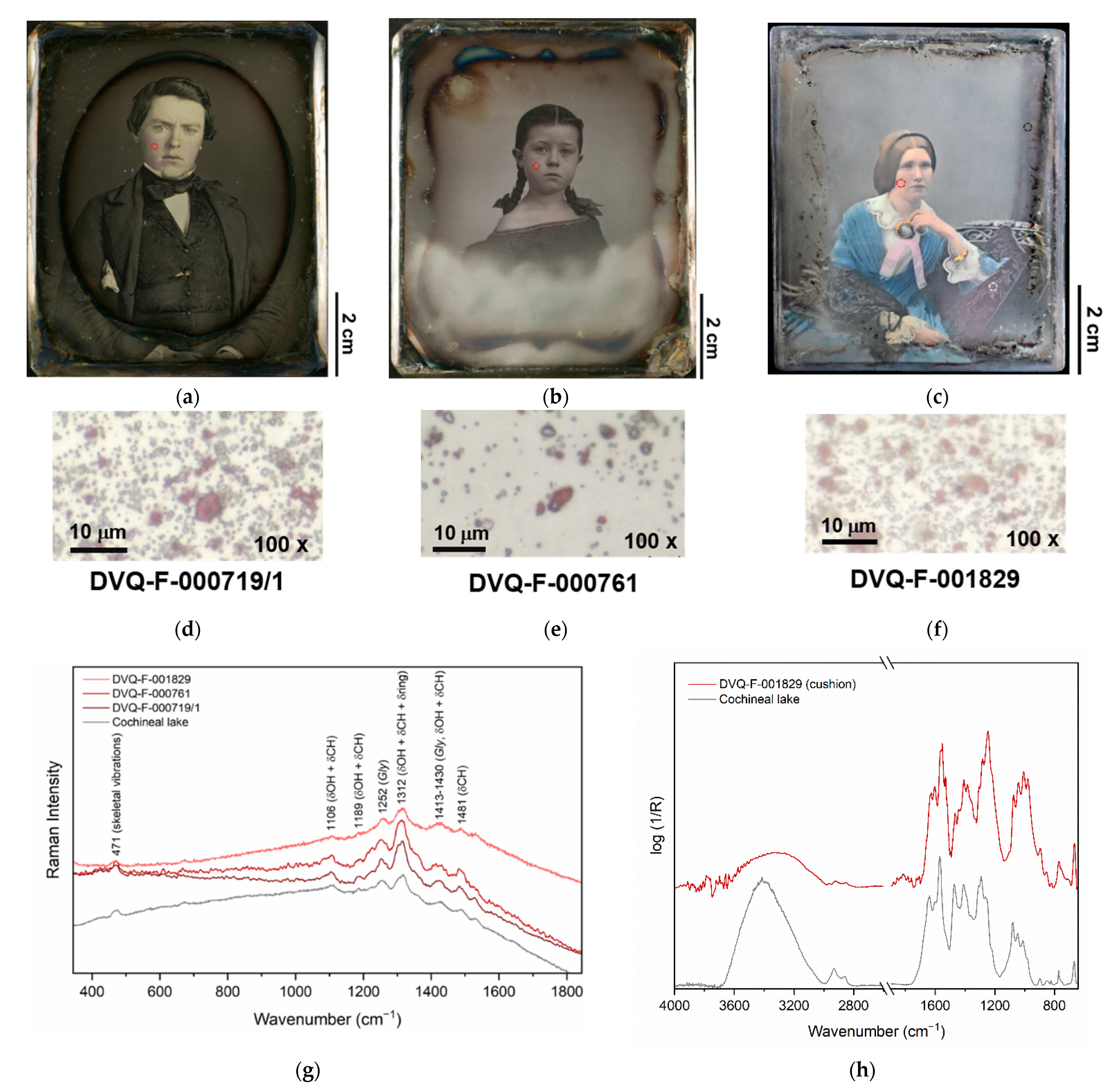
2.1. Daguerreotypes Analyzed
2.2. Stereomicroscopy
2.3. Raman Microspectroscopy
2.4. X-ray Fluorescence Spectroscopy
2.5. Micro Reflection Fourier-Transform Infrared Spectroscopy
3. Results
3.1. Single Pigments
3.2. Mixtures of Pigments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

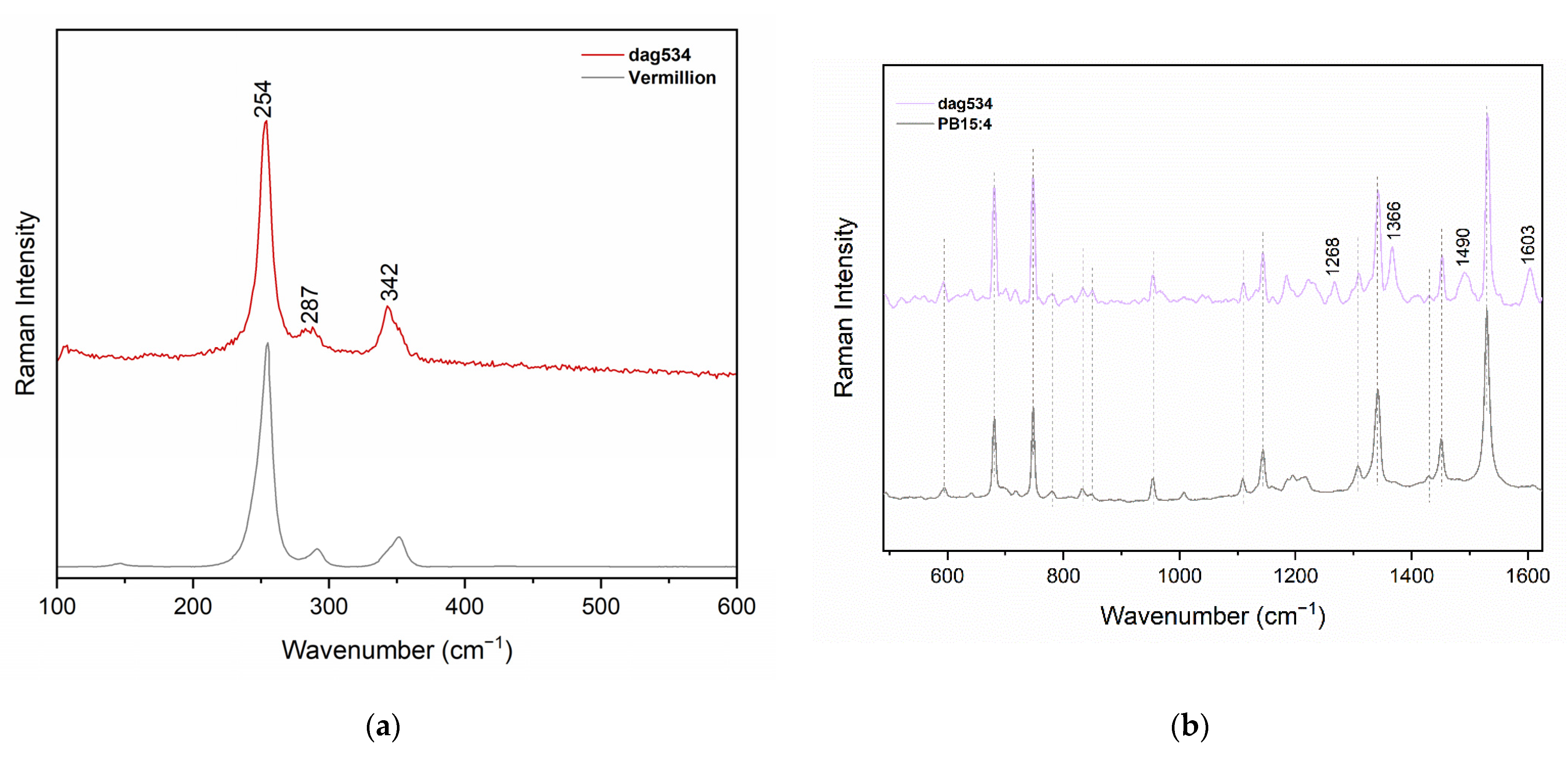
Appendix B
| This Work (cm−1) | Literature (cm−1) [31,32,43] | Assignment |
|---|---|---|
| 1627 | 1633 | νC-C |
| 1604 | 1615 | - |
| 1552 | 1565 | νIC-C |
| 1467 | 1466 | νI–IIC-C |
| 1407 | 1409 | δCH + δOH |
| 1384 | 1379 | - |
| 1282 | 1285 | δOH |
| 1246 | 1252–1255 | νC-O catechol functions |
| 1072 | 1070–1080 | ρCH3 |
| 1041 | 1046 | ν-CC in a glucose unit |
| 1006 | 1005 | ρCH3 |
| 977 | 983 | - |
| 895 | 885–890 | ωCH2 + δOH |
| 768 | 750 | - |
| 664 | 667 | δCH |
References
- Cattaneo, B. (Ed.) Elvira Tonelli I Dagherrotipi. In Il Restauro Della Fotografia. Materiali Fotografici e Cinematografici, Analogici e Digitali; Nardini Editore: Florence, Italy, 2012; pp. 63–78. [Google Scholar]
- Barger, M.S.; White, W.B. The Daguerreotype: Nineteenth-Century Technology and Modern Science; Johns Hopkins, pbk., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2000; ISBN 978-0-8018-6458-2. [Google Scholar]
- Barger, M.S. Daguerreotype Research at the Materials Research Laboratory, The Pennsylvania State University: 1979–1984. Top. Photogr. Preserv. 2009, 13, 137–146. [Google Scholar]
- Ravines, P.; Li, L.; McElroy, R. An Electron Microscopy Study of the Image Making Process of the Daguerreotype, the 19th Century’s First Commercially Viable Photographic Process. J. Imaging Sci. Technol. 2016, 60, 30504-1. [Google Scholar] [CrossRef]
- Lehmann, A.-S. The Transparency of Color: Aesthetics, Materials, and Practices of Hand Coloring Photographs between Rochester and Yokohama. Getty Res. J. 2015, 7, 81–96. [Google Scholar] [CrossRef]
- de Seauve, V.; Languille, M.-A.; Vanpeene, S.; Andraud, C.; Garnier, C.; Lavédrine, B. Replication and Study of the Colouration of Edmond Becquerel’s Photochromatic Images. J. Cult. Herit. 2020, 45, 114–121. [Google Scholar] [CrossRef]
- Smith, L. Color and Victorian Photography; Bloomsbury Visual Arts: London, UK, 2020; ISBN 978-1-00-308497-6. [Google Scholar]
- Jacob, M.G. Il Dagherrotipo a Colori. Tecniche e Conservazione; Nardini Editore: Florence, Italy, 1992. [Google Scholar]
- Buerger, J.E. French Daguerreotypes; University of Chicago Press: London, UK, 1989. [Google Scholar]
- Nareid, H. A Review of the Lippmann Colour Process. J. Photogr. Sci. 1988, 36, 140–147. [Google Scholar] [CrossRef]
- Seauve, V.; Languille, M.; Kociak, M.; Belin, S.; Ablett, J.; Andraud, C.; Stéphan, O.; Rueff, J.; Fonda, E.; Lavédrine, B. Spectroscopies and Electron Microscopies Unravel the Origin of the First Colour Photographs. Angew. Chem. Int. Ed. 2020, 59, 9113–9119. [Google Scholar] [CrossRef]
- Romer, G.; Delamoir, J. The First Color Photographs. Sci. Am. 1989, 261, 88–99. [Google Scholar] [CrossRef]
- Snelling, H.H. The History and Practice of the Art of Photography; Books on Demand: Norderstedt, Germany, 1849; ISBN 978-3-7481-8239-9. [Google Scholar]
- Jacob, M.G. Colour and the Daguerreotype. Daguerreotype J. 2014, 1, 12–19. [Google Scholar]
- Ruggles, M. Paintings on a Photographic Base. J. Am. Inst. Conserv. 1985, 24, 92–103. [Google Scholar] [CrossRef]
- Ferguson, S.H. In Living Color: Process and Materials of the Hand Colored Daguerreotype. Daguerreian Annu. 2008, 13–18. [Google Scholar]
- Swan, A. Appendix II. Coloriage Des Epreuves: French Methods and Materials for Coloring Daguerreotypes. In French Daguerreotypes; University of Chicago Press: London, UK, 1989; pp. 150–163. [Google Scholar]
- Ogayar Oroz, P. To Colour a Mirror. Identification of Hand-Colouring Techniques in Daguerreotypes by Studying and Reconstructing. Master’s Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2022. [Google Scholar]
- Archives, T.N. The National Archives-Currency Converter: 1270–2017. Available online: https://www.nationalarchives.gov.uk/currency-converter/ (accessed on 18 August 2022).
- Wall, A.H. A Manual of Artistic Colouring, as Applied to Photographs. In Color and Victorian Photography; Smith, L., Ed.; Routledge: London, UK, 2020; ISBN 978-1-4742-6420-4. [Google Scholar]
- Kozachuk, M.S.; Avilés, M.O.; Martin, R.R.; Potts, B.; Sham, T.-K.; Lagugné-Labarthet, F. Imaging the Surface of a Hand-Colored 19th Century Daguerreotype. Appl. Spectrosc. 2018, 72, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Anglos, D.; Melesanaki, K.; Zafiropulos, V.; Gresalfi, M.J.; Miller, J.C. Laser-Induced Breakdown Spectroscopy for the Analysis of 150-Year-Old Daguerreotypes. Appl. Spectrosc. 2002, 56, 423–432. [Google Scholar] [CrossRef]
- Golovlev, V.V.; Gresalfi, M.J.; Miller, J.C.; Anglos, D.; Melesanaki, K.; Zafiropulos, V.; Romer, G.; Messier, P. Laser Characterization and Cleaning of 19th Century Daguerreotypes II. J. Cult. Herit. 2003, 4, 134–139. [Google Scholar] [CrossRef]
- Catalogo Immagini Archivio Fotografico-FAF Toscana. Available online: https://www.alinari.it/en/explore-unicum-objects (accessed on 24 October 2022).
- Pozzi, F.; Lombardi, J.R.; Leona, M. Winsor & Newton Original Handbooks: A Surface-Enhanced Raman Scattering (SERS) and Raman Spectral Database of Dyes from Modern Watercolor Pigments. Herit Sci 2013, 1, 23. [Google Scholar] [CrossRef]
- Osticioli, I.; Pagliai, M.; Comelli, D.; Schettino, V.; Nevin, A. Red Lakes from Leonardo’s Last Supper and Other Old Master Paintings: Micro-Raman Spectroscopy of Anthraquinone Pigments in Paint Cross-Sections. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117273. [Google Scholar] [CrossRef]
- Innocenti, S.; Ricci, M.; Lanterna, G.; Fontana, R.; Striova, J.; Becucci, M. Direct Microextraction for Red Lakes Detection in Painting Layers by Raman Spectroscopy. Eur. Phys. J. Plus 2021, 136, 1081. [Google Scholar] [CrossRef]
- Berrie, B.H.; Strumfels, Y. Change Is Permanent: Thoughts on the Fading of Cochineal-Based Watercolor Pigments. Herit. Sci. 2017, 5, 30. [Google Scholar] [CrossRef]
- Kirby, J. The Reconstruction of Late 19th-Century French Red Lake Pigments. In Proceedings of the Art of the Past. Sources and Reconstructions; Clarke, M., Townsend, J.H., Stijnman, A., Eds.; Archetype: London, UK, 2005; pp. 69–77. [Google Scholar]
- Trenary, M. Reflection Absorption Infrared Spectroscopy and the Structure of Molecular Adsorbates on Metal Surfaces. Annu. Rev. Phys. Chem. 2000, 51, 381–403. [Google Scholar] [CrossRef]
- Pagliai, M.; Osticioli, I.; Nevin, A.; Siano, S.; Cardini, G.; Schettino, V. DFT Calculations of the IR and Raman Spectra of Anthraquinone Dyes and Lakes. J. Raman Spectrosc. 2018, 49, 668–683. [Google Scholar] [CrossRef]
- Harris, M.; Stein, B.K.; Tyman, J.H.P.; Williams, C.M. The Structure of the Colourant/Pigment, Carmine Derived from Carminic Acid. J. Chem. Res. 2009, 2009, 407–409. [Google Scholar] [CrossRef]
- Schlather, A.E.; Gieri, P.; Robison, M.; Centeno, S.A.; Manjavacas, A. Nineteenth-Century Nanotechnology: The Plasmonic Properties of Daguerreotypes. Proc. Natl. Acad. Sci. USA 2019, 116, 13791–13798. [Google Scholar] [CrossRef] [PubMed]
- Hrnjić, M.; Hagen-Peter, G.A.; Birch, T.; Barfod, G.H.; Sindbæk, S.M.; Lesher, C.E. Non-Destructive Identification of Surface Enrichment and Trace Element Fractionation in Ancient Silver Coins. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 478, 11–20. [Google Scholar] [CrossRef]
- Brocchieri, J.; Scialla, E.; Sabbarese, C. Estimation of Ag Coating Thickness by Different Methods Using a Handheld XRF Instrument. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 486, 73–84. [Google Scholar] [CrossRef]
- Lomax, S.Q. Phthalocyanine and Quinacridone Pigments: Their History, Properties and Use. Stud. Conserv. 2005, 50, 19–29. [Google Scholar] [CrossRef]
- Angelin, E.M.; Babo, S.; Ferreira, J.L.; Melo, M.J. Raman Microscopy for the Identification of Pearlescent Pigments in Acrylic Works of Art. J. Raman Spectrosc. 2019, 50, 232–241. [Google Scholar] [CrossRef]
- Samain, L.; Gilbert, B.; Grandjean, F.; Long, G.J.; Strivay, D. Redox Reactions in Prussian Blue Containing Paint Layers as a Result of Light Exposure. J. Anal. At. Spectrom. 2013, 28, 524. [Google Scholar] [CrossRef]
- Moretti, G.; Gervais, C. Raman Spectroscopy of the Photosensitive Pigment Prussian Blue. J. Raman Spectrosc. 2018, 49, 1198–1204. [Google Scholar] [CrossRef]
- Saunders, D.; Kirby, J. Light-Induced Colour Changes in Red and Yellow Lake Pigments. Natl. Gallery Tech. Bull. 1994, 15, 79–97. [Google Scholar]
- Radepont, M.; Coquinot, Y.; Janssens, K.; Ezrati, J.-J.; de Nolf, W.; Cotte, M. Thermodynamic and Experimental Study of the Degradation of the Red Pigment Mercury Sulfide. J. Anal. At. Spectrom. 2015, 30, 599–612. [Google Scholar] [CrossRef]
- Kang, S.; Pawar, R.C.; Pyo, Y.; Khare, V.; Lee, C.S. Size-Controlled BiOCl–RGO Composites Having Enhanced Photodegradative Properties. J. Exp. Nanosci. 2016, 11, 259–275. [Google Scholar] [CrossRef]
- Haberová, K.; Jančovičová, V.; Veselá, D.; Machatová, Z.; Oravec, M. Impact of Organic Binders on the Carminic-Colorants Stability Studied by: ATR-FTIR, VIS and Colorimetry. Dye. Pigment. 2021, 186, 108971. [Google Scholar] [CrossRef]
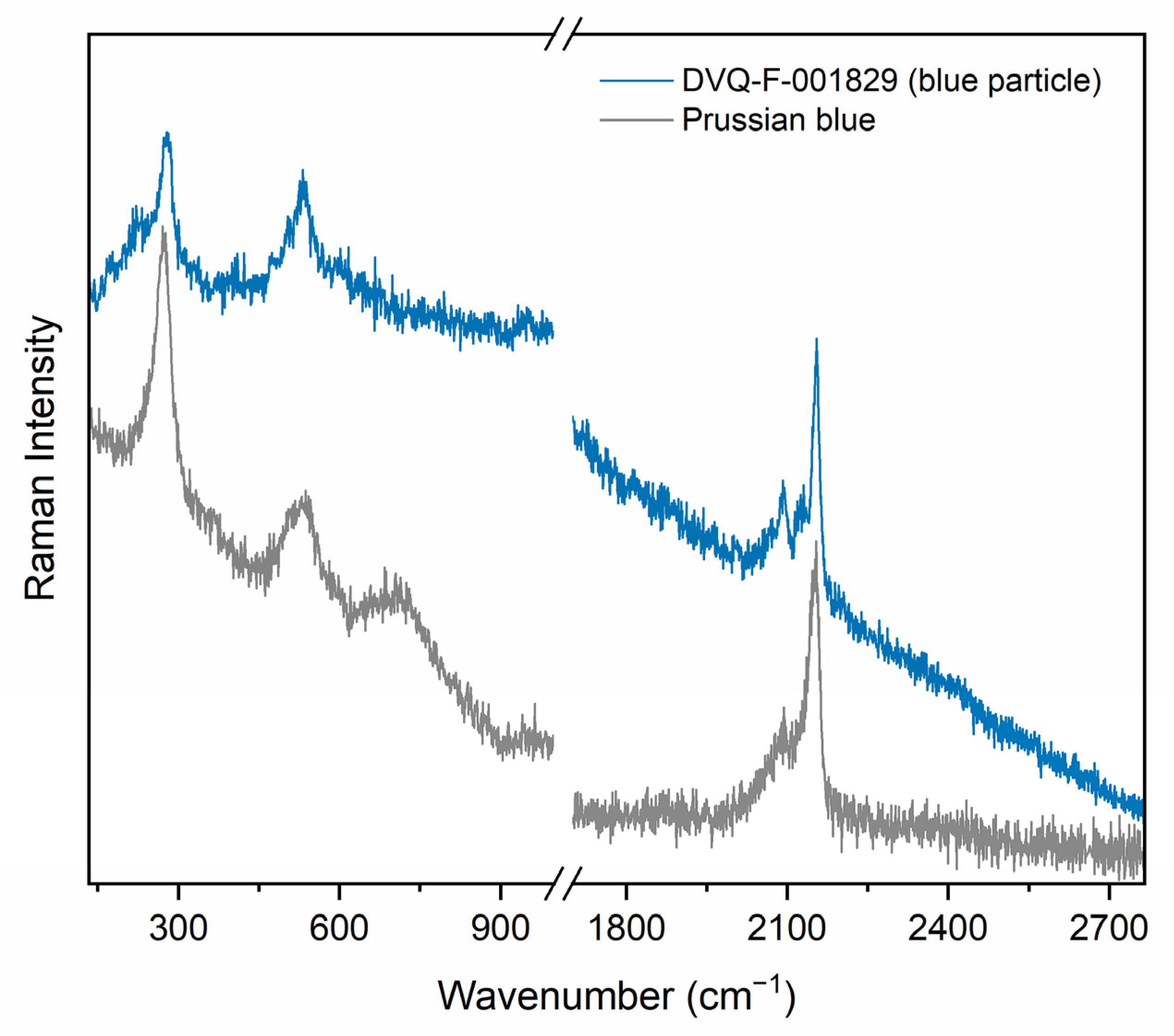
| Pigment | Chemical Composition | Historical Records | Scientific Literature |
|---|---|---|---|
| Cochineal lake | Main component is carminic acid | Gaudin (1844), Snelling (1849), Vaillat (1850), Ziegler (1851), Croucher (1855), Baird (1855), Legros (1856), Humphrey (1858), Wall (1861) | Kozachuk, et al. [21], probably in a coloring box studied by Swan [17] |
| Red oxide | Fe2O3 | Snelling (1849), Vaillat (1850), Baird (1855), Humphrey (1858) | Swan [17], Anglos et al. [22], |
| Vermillion/Cinnabar | HgS | Gaudin (1844), Vaillat (1850), Ziegler (1851) | N.D. |
| Chrome red | Pb2O(CrO4) | Ziegler (1851) | N.D. |
| Madder lake | Purpurin, alizarin, and other components. | Ziegler (1851), Legros (1856) | N.D. |
| Prussian blue | Fe4[Fe(CN)6]3 | Gaudin (1844), Snelling (1849), Vaillat (1850), Ziegler (1851), Legros (1856) | Swan [17], Anglos et al. [22], Golovlev, et al. [23] |
| Ultramarine | Na8-x [SiAlO4]6·[S2,S3,SO4,Cl]2−x | Ziegler (1851), Croucher (1855), Baird (1855), Humphrey (1858) | N.D. |
| Antwerp blue | Fe4[Fe3(CN)6]nH2O + CoO·Al2O3 | Snelling (1849) | N.D. |
| Indigo | Main component indigotin | Gaudin (1844), Snelling (1849), Ziegler (1851) | N.D. |
| Indigo carmine | 5,5′-indigodisulfonic acid Na salt | Legros (1856) | N.D. |
| English smalt/Cobalt blue | CoO·Al2O3 | Ziegler (1851) | N.D. |
| Gamboge | Resin from Garcinia tree | Snelling (1849) | N.D. |
| Indian yellow | Magnesium euxanthate | Snelling (1849) | N.D. |
| Yellow ochre | FeO(OH) + Clay | Snelling (1849), Vaillat (1850), Ziegler (1851), Wall (1861) | N.D. |
| Cadmium yellow | CdS | Gaudin (1844), Ziegler (1851), Humphrey (1858) | N.D. |
| Chrome yellow | PbCrO4 | Snelling (1849), Ziegler (1851), Croucher (1855), Baird (1855) | Swan [17] |
| Chrome orange | PbCrO4·PbO | Ziegler (1851) | N.D. |
| Naples yellow | Pb3(SbO4)2 | Ziegler (1851), Wall (1861) | N.D. |
| Turbith (Turpeth mineral) | Hg3O2SO4 | Ziegler (1851) | N.D. |
| Shell gold | Au-Ag-Cu alloy | Croucher (1855), Baird (1855), Wall (1861) | Anglos, et al. [22] |
| Shell silver | Ag mixed with gum Arabic | Baird (1855), Wall (1861) | N.D. |
| Emerald green | Cu(C2H3O2)2·3Cu(AsO2)2 could also refer to Viridian green (Cr2O3·2H2O) | Legros (1856) | N.D. |
| Burnt Sienna | Fe oxide | Snelling (1849), Vaillant (1850), Croucher (1855), Legros (1856), Humphrey (1858), Wall (1861) | N.D. |
| Burnt Umber | Fe oxide + Mn oxide | Snelling (1849), Baird (1855), Humphrey (1858) | N.D. |
| Bistre | Extract from tarry soot of burnt resinous wood | Gaudin (1844), Snelling (1849), Wall (1861) | N.D. |
| Tripoli/rotten-stone | Diatoms and weathered chert | Humphrey (1858) | N.D. |
| Barium white | BaSO4 | N.D. | Anglos et al. [22], Golovlev et al. [23] |
| Lead white | Pb(OH)2·PbCO3 | Ziegler (1851), Legros (1856) | Anglos et al. [22] |
| Bismuth white | 4BiNO3(OH)2-BiO(OH) or BiOCl | Ziegler (1851) | Swan [17] (variety not fully identified) |
| White clay | Kaolin | Ziegler (1851) | N.D. |
| Spanish white | CaCO3 | Ziegler (1851) | N.D. |
| Zinc white | ZnO | Ziegler (1851) | N.D. |
| Lamp black | Amorphous carbon (soot) | Snelling (1849), Baird (1855), Humphrey (1858) | N.D. |
| Collection | Code | Daguerreotypist | Date | Subject | Plate Size (cm) | Housing |
|---|---|---|---|---|---|---|
| FAF | DVQ-F-000535 | Unknown | Before 1845 | Portrait of a couple | 7.6 × 6.4 | Hinged case |
| DVQ-F-000761 | Unknown | After 1850 | Portrait of a girl (crayon daguerreotype) | 8.4 × 7.2 | Hinged case | |
| DVQ-F-001667 | Désiré François Millet (active 1840–1868, Paris, France) | 1855 ca. | Portrait of a young woman | 11.0 × 8.5 | Colored glass | |
| DVQ-F-001829 | Antoine Claudet (1797–1867), active in London | - | Portrait of a young woman | 7.7 × 6.5 | Stereoscopic case | |
| DVQ-F-002339 | James E. McClees and Washington Lafayette Germon(actives 1846–1855,Philadelphia, USA) | 1847–1860 | Portrait of a seated man | 14.0 × 10.7 | Hinged case | |
| DVQ-F-002694 | Unknown | After 1850 | Portrait of a seated man with a cane | 8.2 × 7.1 | Passe-partout | |
| DVQ-F-000719/1 | Unknown | - | Portrait of a young man | 8.4 × 7.1 | Hinged case | |
| Chiesa-Gosio | Dag504-3 | Unknown | - | Portrait of a young woman | 8.0 × 9.4 | Hinged case |
| Dag534 | Unknown | After 1844 | Portrait of a woman in a bonnet | 8.0 × 7.0 | Unknown |
| Point of Analysis | XRF Results (cps) | Ratio AgKα/AgL3-shell | ||||
|---|---|---|---|---|---|---|
| CuKα | AgL3-shell | AgKα | HgL3-shell | AuL3-shell | ||
| 01 | 38.8 | 1603.1 | 7048.5 | 119.1 | 219.2 | 4.39 |
| 02 | 36.9 | 1523.5 | 10948.6 | 119.2 | 230.9 | 7.18 * |
| 03 | 30.2 | 1414.2 | 7515.6 | 145.5 | 325.0 | 5.31 |
| 04 | 32.7 | 1439.7 | 7351.8 | 154.7 | 337.2 | 5.10 |
| 05—jewelry | 36.5 | 947.7 | 7202.5 | 148.0 | 879.4 | 7.59 |
| 06 | 30.2 | 1757.7 | 7354.5 | 162.6 | 275.6 | 4.18 |
| This Work | Other Names in the Historical Records | Daguerreotype | Area | Scientific Lit. |
|---|---|---|---|---|
| Cochineal lake | Carmine, carmine lake | DVQ-F-001829 by Claudet, DVQ-F-000761, DVQ-F000719/1 | Flesh tones, cushion | [17,21] |
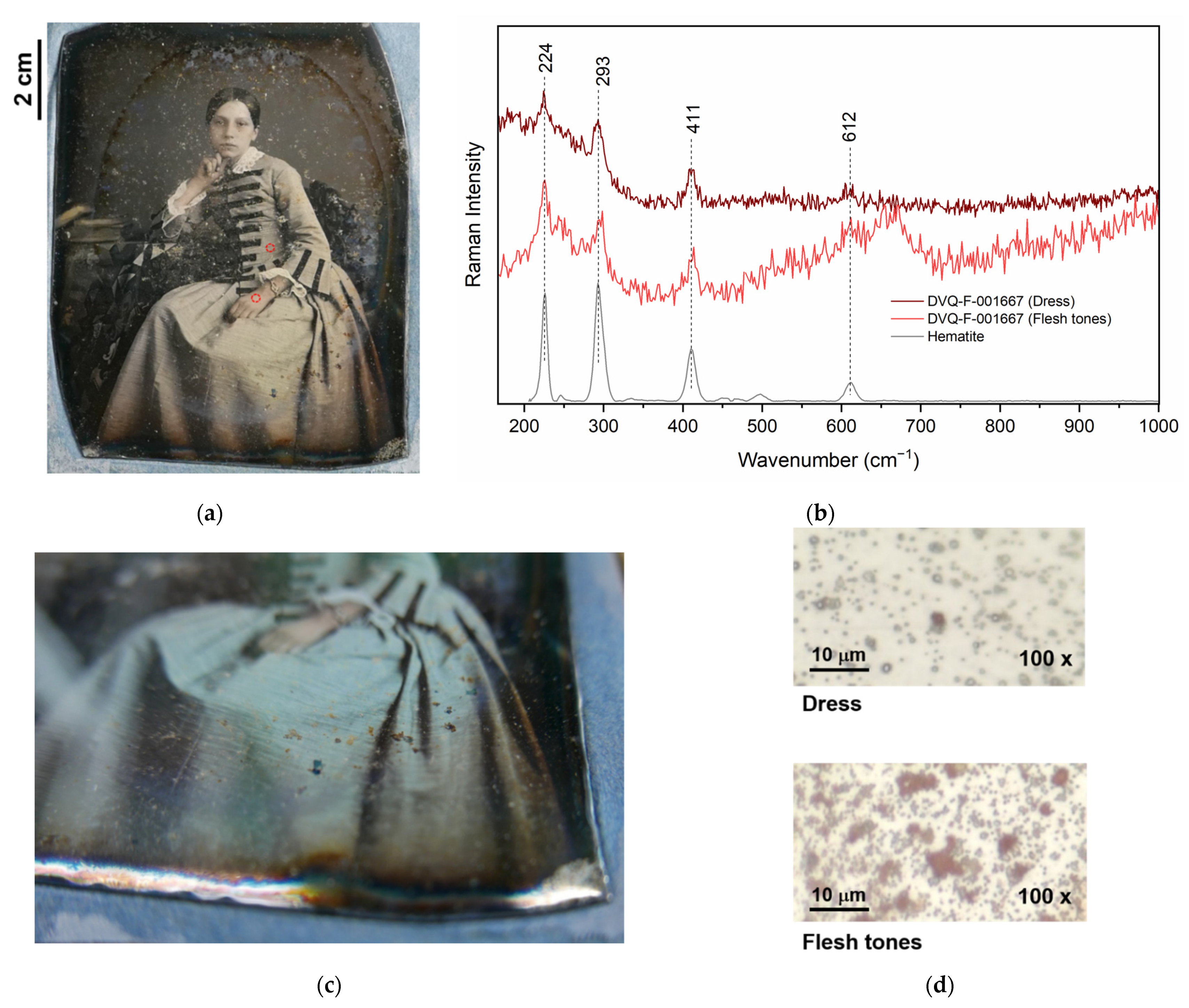
| Iron oxide | Light red | DVQ-F-001667 by Millet, DVQ-F-002339 by McClees & Germon | Flesh tones, dress | [17,23] |
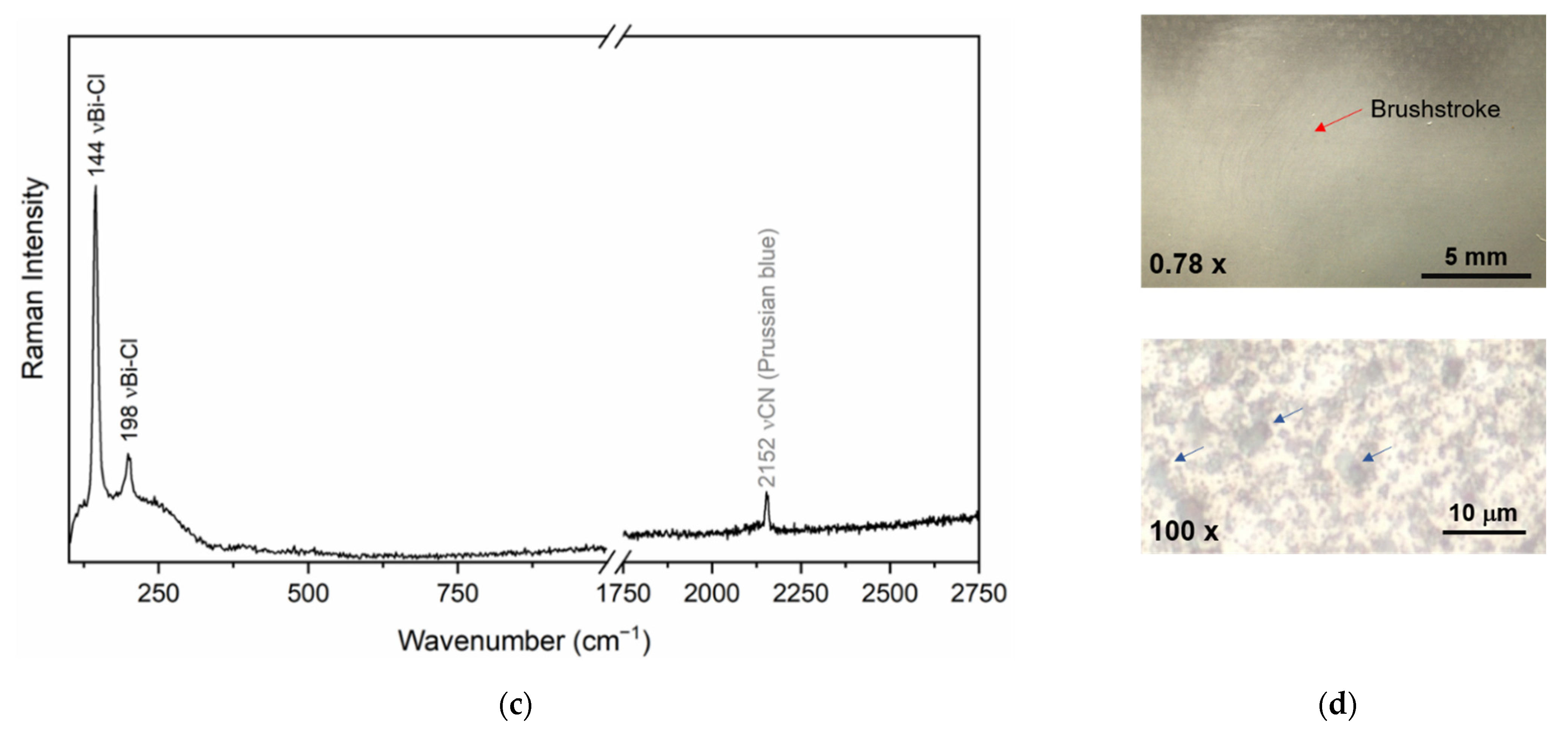
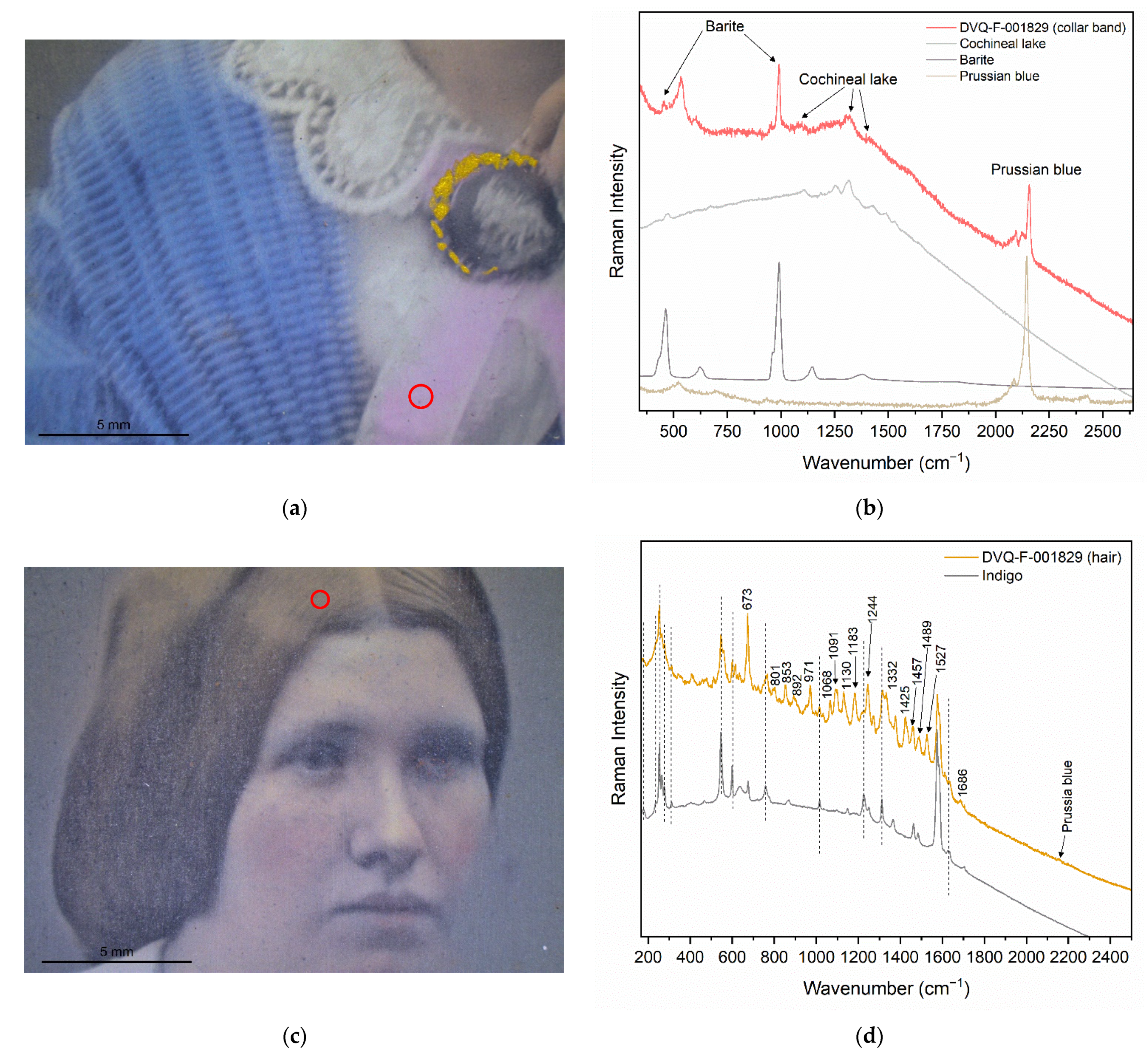
| Prussian blue | - | DVQ-F-001829 by Claudet | Dress, flesh tones, hair | [17,22,23] |
| Barite | Not mentioned (white?) | DVQ-F-001829 by Claudet | Dress | [17,22,23] |
| Vermilion | - | Dag534 | Flesh tones | - |
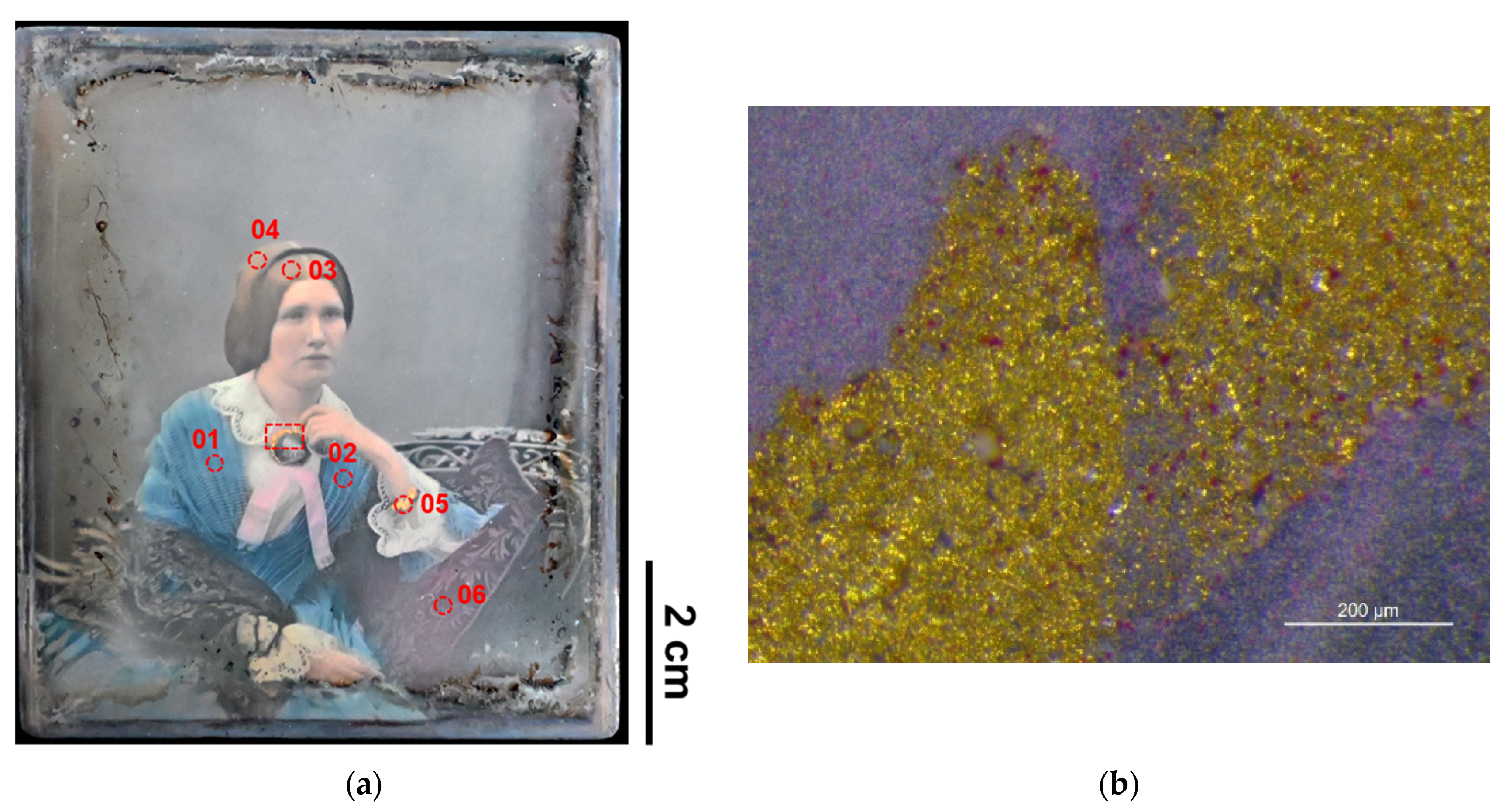
| Shell gold (in one case mixed with Prussian blue) | - | DVQ-F-001829 by Claudet, DVQ-F-000535 | Jewelry | [22] |
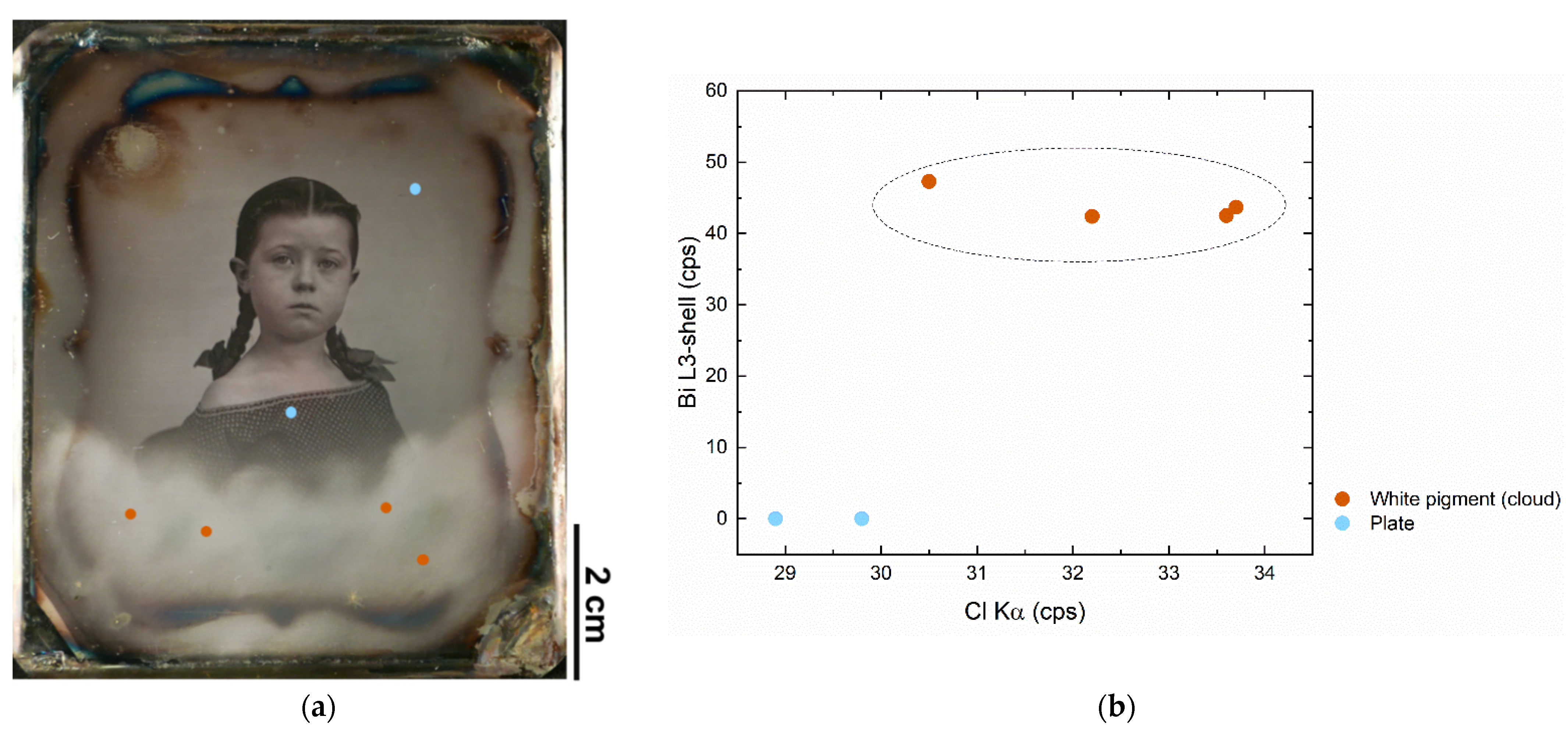
| Bi white (Bi oxychloride) + Prussian blue | - | DVQ-F-000761 | Crayon effect | [17] |
| Cochineal lake and Barite | Crimson | DVQ-F-001829 by Claudet | Dress (collar) | - |
| Cochineal and Prussian blue | The mixture suggested to obtain the richest purple color | DVQ-F-001829 by Claudet | Curtain | - |
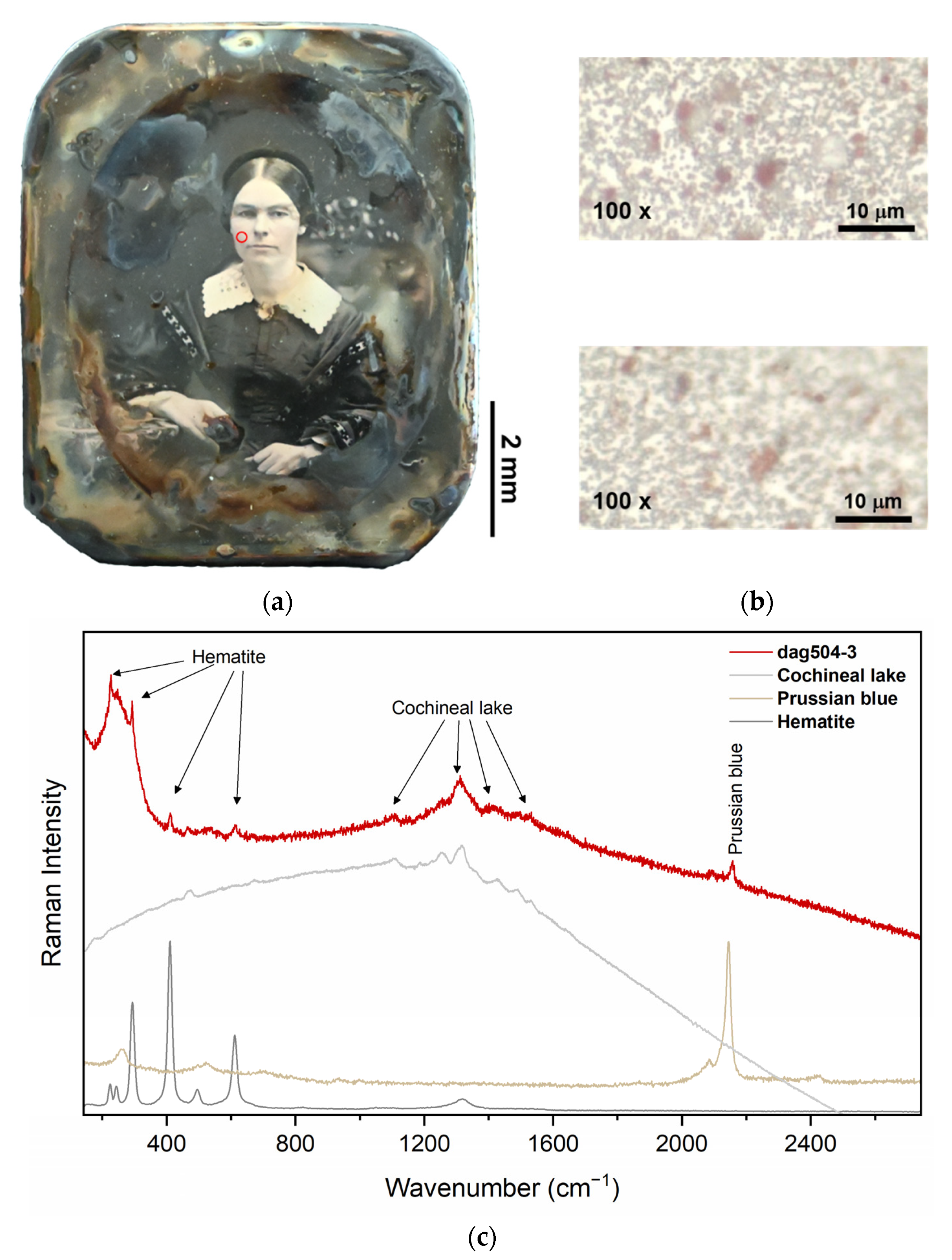
| Iron oxide + Cochineal lake + Prussian blue | The mixture of light red and carmine is called “scarlet.” | Dag504-3 | Flesh tones | - |
| Indigo + Prussian blue + natural yellow pigment | - | DVQ-F-001829 by Claudet | Hair | - |
| Phthalocyanine and red SOP | Anachronistic, possible modification | Dag534 | Flesh tones | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero Balbas, D.; Cattaneo, B.; Cagnini, A.; Belluzzo, P.; Innocenti, S.; Rossi, S.; Fontana, R.; Striova, J. The Colors of the Butterfly Wings: Non-Invasive Microanalytical Studies of Hand-Coloring Materials in 19th-Century Daguerreotypes. Heritage 2022, 5, 4306-4324. https://doi.org/10.3390/heritage5040221
Quintero Balbas D, Cattaneo B, Cagnini A, Belluzzo P, Innocenti S, Rossi S, Fontana R, Striova J. The Colors of the Butterfly Wings: Non-Invasive Microanalytical Studies of Hand-Coloring Materials in 19th-Century Daguerreotypes. Heritage. 2022; 5(4):4306-4324. https://doi.org/10.3390/heritage5040221
Chicago/Turabian StyleQuintero Balbas, Diego, Barbara Cattaneo, Andrea Cagnini, Paolo Belluzzo, Silvia Innocenti, Sandra Rossi, Raffaella Fontana, and Jana Striova. 2022. "The Colors of the Butterfly Wings: Non-Invasive Microanalytical Studies of Hand-Coloring Materials in 19th-Century Daguerreotypes" Heritage 5, no. 4: 4306-4324. https://doi.org/10.3390/heritage5040221
APA StyleQuintero Balbas, D., Cattaneo, B., Cagnini, A., Belluzzo, P., Innocenti, S., Rossi, S., Fontana, R., & Striova, J. (2022). The Colors of the Butterfly Wings: Non-Invasive Microanalytical Studies of Hand-Coloring Materials in 19th-Century Daguerreotypes. Heritage, 5(4), 4306-4324. https://doi.org/10.3390/heritage5040221








