Abstract
Barium hydroxide was one of the most widely used inorganic materials to consolidate calcareous stones during the 19th and 20th. The consolidation process occurs through a carbonation reaction. Several researchers studied the consolidation mechanism; however, the results are sometimes in conflict. More experimental work using modern analytical techniques and a multi-analytical approach is necessary to shed light on the mechanisms involved. This research aims to validate the chemical composition of the developed secondary products and to evaluate the treatment’s effectiveness over time. Carrara marble and Vicenza white limestone were treated and subjected to natural, artificial, and biological weathering. Furthermore, only a few microsamples were collected from Venetian historical artifacts treated in the 1960s and 1970s. Microscopic observations, sponge tests, FTIR, SEM-EDX, and microbiological analyses investigated the stability of the treatment over time and ascertained the chemical composition of the acicular crystals developed from the carbonation reaction of barium hydroxide. The results prompted a number of considerations useful for future restorations and for developing innovative compounds for consolidation interventions.
1. Introduction
Barium hydroxide (Ba(OH)2) has been one of the most widely used inorganic consolidation agents in restoration in the 19th and 20th centuries. In recent years, the advance of organic polymers and nano-compounds has resulted in a reduction in barium application on calcareous stones [1,2]. However, it is still used to consolidate wall paintings and plasters [3,4,5,6,7]. In Italy, some examples of barium application are the consolidation of a little marble angel (XVI-XVII c.) in the cloister of the museum of St. Mark in Florence, the sculpted decorations and statues of Como cathedral, some marble statues of the main facade of the Lucca cathedral, and the fire-damaged stucco and marmorino decorations of the Foyer and sale Apollinee in “La Fenice” Theatre in Venice [8]. This conservation product is irreversible but potentially stable over time and can tolerate further treatments based on barium hydroxide as well as the application of other consolidating products years later [9]. Compared with polymeric products, its inorganic nature makes it chemically more compatible with the stone material. However, it induces fewer hardening effects since the consolidation mechanism provides only the precipitation of a reaction product in the stone capillaries, which is unable to solder fractures or completely fill cavities [10]. The application method can influence the potential effectiveness of the consolidation treatment. In fact, penetration into stone porosity can be difficult because of the pore-clogging phenomenon. Moreover, it has been demonstrated that an excess of barium hydroxide on the stone surface modifies the superficial properties of the treated stone due to the unacceptable color alteration [11,12,13]. Physically, the role of barium hydroxide is to partially fill the pores of calcareous rocks with an inert compound (witherite/barium carbonate) more insoluble in water than calcium carbonate or to immobilize sulfates already present in the porous stones. The chemical reaction mechanism of barium hydroxide with calcium carbonate has been widely discussed [1]. According to the known process, baryta water reacts with atmospheric carbon dioxide following the normal carbonation reaction (Scheme 1a). Lewin and Baer postulated the formation of a bonding material based on a multi-layered solid solution of barium–calcium carbonate having a variable amount of calcium and barium. In the outer layers of the crystalline structure, calcium ions are partially replaced by barium ions. For this reason, the calcium content would be high in the adjacent areas to calcite minerals, decreasing as the distance from them increases [14] (Scheme 1 b). When the distance between calcite grains is significant, the amount of pure barium carbonate in the pores of the stone is greater [9].
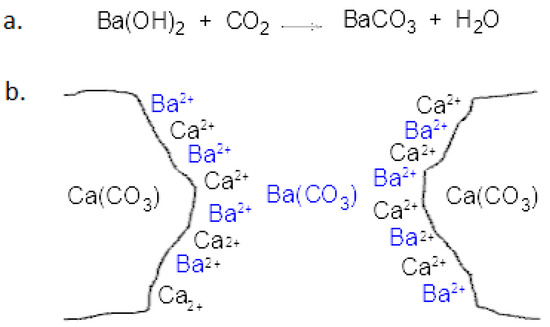
Scheme 1.
(a) Carbonation reaction of barium hydroxide; (b) structure of a bonding material based on a series of solid mixture of barium–calcium carbonate with a variable calcium and barium content between the calcite crystals, postulated by Lewin and Baer [14].
Some improvements to the treatment solution were suggested for enhancing the penetration of baryta water into the stone porosity. In particular, Lewin and Baer [15] formulated a mixed blend of a hot concentrated solution of barium hydroxide (≈20%), 10% of urea, and a certain amount of glycerin (unspecified quantity), which slowed down the growth of barium carbonate crystals. In fact, at ambient temperatures, urea generates both carbon dioxide, which is required for a carbonation reaction, and ammonia. Glycerin prevents the reaction too quickly and the drying of the solution at the stone surface (and the consequent whitening effect) [16,17]. Even though the effectiveness of the treatment was questioned, especially when applied by capillary absorption in situ [18], the solution was used with apparent success for restoring the main relief of the lunette of Scuola Grande San Giovanni Evangelista, for example [19], and one of the four fonts in the church of St. Fantin in Venice.
The mechanism of calcium substitution with barium ions at the surface of calcite crystals was first chemically explored on a large single crystal of Mexican spar calcite [14]. The chemical composition of the solid solutions that bridge the original calcite crystals was examined using an electron beam microprobe and X-ray diffraction analysis. The nature of barium–calcium carbonate was confirmed in crystals with epitaxial growth. Recent studies supported this theory through SEM-EDS trials on limestone samples treated with barium hydroxide by immersion. They also suggested a heterogeneous reaction that converts the outer shell of calcium carbonate grains into calcium hydroxide, which replaces barium hydroxide, and produces a second formation of calcite through interaction with carbon dioxide [1,20].
Few general comments can be made about this by considering the analytical parameters and methods used for studying the phenomenon. Using electron microprobes, the interaction between the electron beam and inhomogeneous samples, such as sub-micrometric particles, is complex. Since electron microprobes analyze the material’s elemental composition, it is relevant to know the precise volume of interaction between electrons and the surface of interest. Moreover, it is hard to obtain useful and reliable information about the presence and the content of a specific chemical element in a small sample of a solid mixture if the same element (calcium, in this case) is also contained in the substrate. In fact, in the high energy condition of the incident beam, the electrons can interact not only with the solid solution but also with the substrate, indicating a mixed composition of the two parts (solid solution and matrix). To avoid the excitation of the substrate, the calibration of the beam energy is often the only solution [21].
In order to shed light on the mechanism involved, this research aims to validate the chemical composition of secondary products developed by the application of barium hydroxide to stone materials as a consolidation material and to evaluate the stability and effectiveness of the treatment. Veined Carrara marble and Vicenza white limestone specimens were brushed with a saturated barium hydroxide solution and then subjected to natural and artificial weathering. Moreover, some microsamples were taken from Venetian monuments: (i) a font in St. Fantin church (Figure 1a) and (ii) a column in Scuola Grande San Giovanni Evangelista (Figure 1b) after a thorough study of the restoration reports in the archives of the Venetian Superintendency, (iii) the statue of St. Alvise on the main facade of the homonymous church (Figure 1c), and (iv) two laboratory’s specimens treated in the 1960s and 1970s with barium hydroxide solutions (Table 1). An analytical program was performed on the surfaces and cross-sectioned samples to detect the variation of stone texture after the product’s application and to investigate the chemical composition of the developed secondary products.
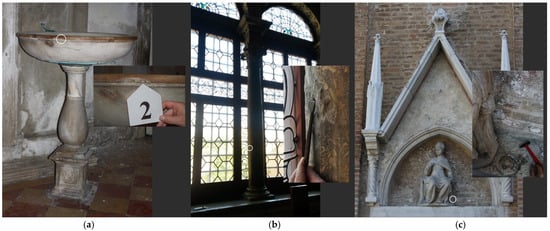
Figure 1.
The three Venetian artifacts examined and the related sampling points (see white circles). (a) The rim of a font in St. Fantin church; (b) a column made of Lumachella del Tasso in Scuola di San Giovanni Evangelista; (c) the lower right side of St. Alvise statue, on the main façade of the homonymous Church.

Table 1.
List of studied samples and description of related treatments and conservation conditions.
2. Materials and Methods
2.1. Experimental Samples and Related Weathering Methods
A saturated barium hydroxide solution prepared with distilled water and powder of barium hydroxide (Ba(OH)2 · 8 H2O), purchased by Carlo Erba, was applied by brushing onto specimens of veined Carrara marble [22,23] and Vicenza white limestone [24], measuring 5 cm × 5 cm × 2 cm. The brushing procedure was undergone carefully up to rejection. The choice of brushing method allowed us to compare different methods from capillary and immersion absorption used for historical samples (Table 1). Moreover, it permitted evaluation of the treatment’s effectiveness in comparison with organic consolidants in broader research [25,26].
The two carbonate lithotypes were selected because of their frequent and abundant use over the centuries as building and decorative materials. The specimens were first washed and dried in a ventilated oven, and then the solution was applied to three samples for each stone substrate. After treatment, the specimens were kept at room temperature until the surfaces were completely dry (15 days). Due to the different porosity (Carrara marble open porosity 0.4% and average pore radius 0.08 µm, Vicenza white limestone open porosity 29.9% and average pore radius 0.26 µm—experimental data related to mesoporosity [24,27]), the dry uptake observed in Carrara marble and Vicenza white limestone samples was dissimilar, in particular, 0.03 g and 2.60 g, respectively.
A couple of treated and untreated specimens were exposed to Venetian outdoor conditions for a year on a south-facing plastic support, inclined by 60°, on a balcony overlooking a garden far away from canals (Palazzo Badoer—S. Polo, Venice). The aging process was controlled periodically (monthly for the first three months, then every three months).
A SolarBox 3000 (CoFoMegra, Milan, Italy) provided by a xenon light source with one filter cutting both the wavelengths down to lower than 295 nm (outdoor simulation) and higher than 800 nm (IR radiation), was used in order to carry out artificial aging on two treated and untreated specimens. The test lasted 1200 h with irradiation equal to 500 W/m2 [28], and the photo-oxidative process was monitored every 200 h for the first 600 h and at the end (1200 h).
Two treated and untreated samples were exposed to outdoor conditions for a month, then placed for three months in a box with high humidity (about 95%) and a temperature of 24–26 °C to encourage the development and growth of biodeteriogens on the surfaces of specimens. The box was built taking into account the ASTM D 3273–76 standard [29].
Analytical Program for Experimental Samples
At point zero, several analytical methods were performed on treated and untreated specimens in order to record benchmarks useful for studying eventual chemical and physical variations of treatment during times. The same analytical protocol was repeated periodically during both natural and artificial deterioration.
The surface of all the specimens has been periodically observed under a Leica WILD M3Z stereomicroscope using increasing magnification (from 6.5× to 40×) to describe the macro/meso-physical and morphological transformation of the surfaces.
The chemical nature and stability of the product applied were verified by µFTIR analyses using a Jasco IRT-5000 Irtron infrared microscope. A standard KBr pellet was prepared, and by using a needle, a small amount of powder was sampled from the stone surface, deposited, and pressed onto the pellet to carry out transmittance measurements.
According to the UNI 11432 recommendation [30], sponge tests were performed to monitor the water absorption capacity during the aging procedures. The amount of water absorbed (Wa) by the material under test was expressed as
where Wa (g/(m2s)) is the water absorption per square meter and unit time, mi is the initial weight of the sponge inside the contact plate, mf is the final weight of the sponge inside the contact plate after the test, A is the sponge area, and t is the time contact. The contact time chosen for Carrara marble and Vicenza white limestone was 3 and 1 min, respectively, repeating the test 3 times for each sample.
Color changes (ΔE) induced by the application and degradation of Ba(OH)2 were evaluated by a portable spectrophotometer Konica Minolta CM-2600d, as stated in the ENI 15886:2010 standard and reported in the CIELab1976 system [31], and calculated as
Each measurement was performed using a polyethylene slide mask with 5 holes (considering 5 measures for each sample), always positioned in the same area of the sample to ensure repeatability.
The specimens were cut to obtain cross-sections and studied under a Philips XL 30 SERIES SEM in a high vacuum to detect the distribution and the maximum penetration depth of the treatment in the samples at the end of the aging processes [32]. An EDAX X-ray dispersive spectrometer equipped with a thin beryllium window was used for semi-quantitative assessments of element composition using accelerating voltage from 25 to 10 KeV. The analyses were enhanced using an SEM Zeiss EVO 15 in a high vacuum, equipped with lanthanum filament and a Bruker Quantax 6/60 detector EDAX X-ray dispersive spectrometer with a thin beryllium window (60 mm2).
For detecting the biological colonizations developed on the surfaces of the specimen after three months of exposure in an appropriate chamber, an optical microscope Leitz LABORLUX 12 POL S was used for mapping all the different typologies of fungi using a fixed frame for quantitative evaluations. In contrast, a Leitz Orthoplan optical microscope with increasing magnifications (50×, 81×, 138× and 275×) revealed the fungi in detail and allowed the capture of images using a Leica DC300 camera. A further study was performed using an SEM (Philips XL 30 SERIES), laying biodeteriogens on a carbon bi-adhesive disk using a needle. For the identification of the mycobiota, a mycological investigation was performed on the samples. The specimens were divided into four quadrants, and two of them in opposite squares were uniformly scraped using a sterile scalpel. The powder was dispersed in ultra-pure water in a total volume of 1 mL in an Eppendorf tube. The suspension was serially diluted (up to 10-3), and rates of each suspension were plated in duplicate on PDA (potato dextrose agar) containing 30 ppm streptomycin. Plates were incubated at 24 °C and exposed alternately (24 h/24 h) to light or darkness for one week. Prior to sporulation, colonies were removed from plates and placed in subculture on PDA. After one week, the colonies were imaged under an optical microscope to determine genus and species [33,34,35,36,37].
2.2. Monitoring Procedure for Evaluating Past Conservative Treatments
2.2.1. Old Laboratory Samples
In 1977, Professor L. Lazzarini (Iuav University of Venice) applied a warm solution of Ba(OH)2 by immersion on two specimens of Vicenza white limestone having a rectangular shape (10 cm × 10 cm × 1.7 cm). After drying, the surface of one of them was brushed with a silicone resin of unknown composition. The goal of such treatment was to test the possibility of using such soft limestones for paving the floors of houses. These specimens have been preserved in the laboratory of Iuav University until today.
2.2.2. Samples from Historical Buildings
In 1968, Professor S.Z. Lewin from New York University treated by capillary absorption a font in St. Fantin church (1507–1564) (Sestiere S. Marco), in Venice, with a cold mixture of barium hydroxide, urea, and glycerin. The font was sculpted in Carrara marble (Figure 1a). In the same year, he applied a similar solution with the same method to a column made of Lumachella del Tasso (a fossiliferous glauconitic sandstone with a carbonate cement [38]) in the Scuola Grande San Giovanni Evangelista (Sestiere S. Polo) (Figure 1b). Moreover, a fragment from the St. Alvise statue located on the main facade of the St. Alvise church in Venice, sampled for other purposes [25,39], revealed the presence of an unknown past barium treatment. For this reason, it was reconsidered in this work (Figure 1c).
2.2.3. Analytical Characterization of Past Treatments
According to the normal 3/80 recommendation [40], a micro fragment from each substrate mentioned in Section 3.2.1 and Section 3.2.2 was collected using a small chisel for verifying the alterations suffered by the treatment during times due to the interaction with indoor Venetian environment. All the samples were first examined under a Leica WILD M3Z stereomicroscope with increasing magnification (from 6.5× to 40×), then embedded in a cold setting epoxy resin for obtaining cross-sections. Then, the samples were studied using a Leitz LABORLUX 12 POL S optical microscope at 40× and 100× magnifications as well as under a Philips XL 30 SERIES SEM. An EDAX X-ray dispersive spectrometer (beryllium window) was used for the semi-quantitative determination of the elements using an accelerating voltage of 25 KeV. A JASCO IRT-5000 Irtron infrared microscope allowed the examination of the state of conservation of the treatment applied. The analyses were performed in transmittance mode on KBr pellets, on which micro-scales of the samples were deposited and pressed. The spectra were recorded with a spectral resolution of 1 cm−1 and 120 consecutive scans.
3. Results
3.1. Study of Ba(OH)2 Treatment Effects on Experimental Ad Hoc Samples
The application and drying of Ba(OH)2 solution on both Carrara marble and Vicenza limestone specimens caused a whitening of the surfaces visible to the naked eye (Figure 2), even if no surplus product was left on the treated specimens. This phenomenon seems to increase over time, forming a superficial white patina, especially in the case of Vicenza limestone, due to the spontaneous and continuous water evaporation, which induces the migration of barium salts from the inner part of the samples to the surface.
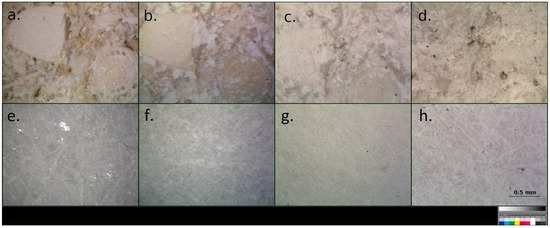
Figure 2.
(a–d) Whitening effect of Ba(OH)2 treatment on Vicenza limestone and (e–h) Carrara marble. (a,e) Untreated, (b,f) treated after 15 days from application, (c,g) treated after 6 months of natural weathering, and (d,h) treated after a year of natural weathering. The measurement bar in figure (h) can be considered for all images.
During outdoor exposure, both Carrara marble and Vicenza limestone suffered a variation of color data from zero time to the end of the aging processes, especially in the case of Vicenza limestone, in which a* and b* tended to return to untreated values. Variations of colorimetric parameters are reported in Table 2.

Table 2.
Variations of colorimetric parameters and water absorption values after the treatment application and at the end of the aging process (12 months for natural weathering and 1200 h for xenon test).
Colorimetric variations were more evident in the case of Vicenza limestone substrates, probably because of the higher porosity and the more quantity of product absorbed. As reported in Figure 3, during artificial aging, both Vicenza limestone and Carrara marble samples tended to increase whitening over time. A similar trend was also observed for Carrara marble exposed to natural deterioration, whereas Vicenza limestone gradually reduced whitening returning to similar values of untreated samples. This could be due to the development of biodeteriogens which conferred a darker color to the surfaces and/or to wind and rain erosion that partially removed the superficial patina from the samples.
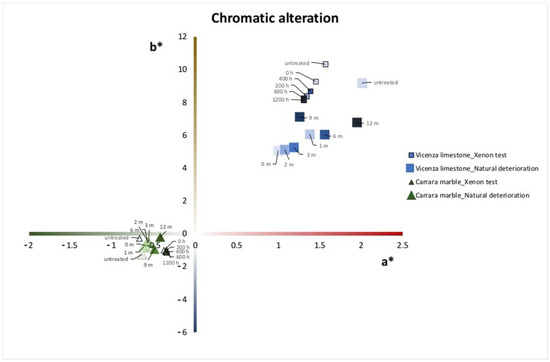
Figure 3.
Trend of color changes of Carrara marble and Vicenza limestone samples before and after the application of barium hydroxide, during natural deterioration, and xenon test.
From the morphological point of view, fifteen days after the application, secondary products in the form of acicular white crystals of witherite were observed in the superficial porosity of the stones by SEM due to the carbonation reaction of Ba(OH)2 with the carbon dioxide of the atmosphere.
The carbonation reaction was monitored by μFTIR after drying and during both natural (Figure 4) and artificial weathering. At zero time, the analysis of the superficial layer revealed the development of barium carbonate (BaCO3, conservation treatment) on calcite substrates (CaCO3, stone). In particular, the characteristic peaks of BaCO3 were readable at 1751, 1448, 858, 712, and 694 cm−1, whereas those of CaCO3 were at 1795, 1412, and 878 cm−1. After three months of natural weathering, the FTIR spectra showed an increase in peaks related to BaCO3 and a decrease in those related to CaCO3. Moreover, in samples exposed to weathering, the typical triplet of barium sulfate (BaSO4) at 1180, 1132, and 1080 cm−1 appeared in the spectra, increasing in intensity over time, whereas no gypsum was detected. This showed the higher reactivity of BaCO3 to air pollutants than CaCO3 and could also reflect on the Venetian air’s environmental healthiness and quality. Indeed, sulfurous anhydride from the atmosphere, mostly ascribable to fuel and biomass combustion [41], interacts with rainwater and humidity, developing sulfuric acid, which reacts with the primary and precipitated carbonates (CaCO3 and BaCO3, respectively) of the treated samples producing the related sulfates [42], as follows:
Ba(OH)2 + CO2 → BaCO3 + H2O
BaCO3 + H2SO4 → BaSO4 + CO2 + H2O
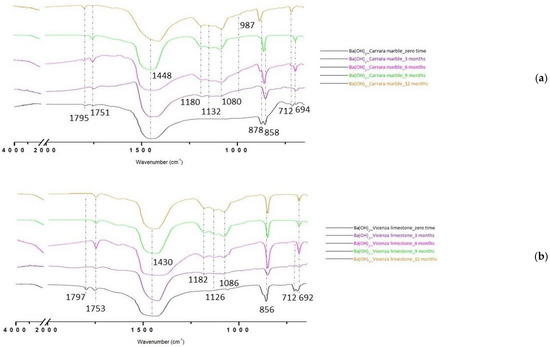
Figure 4.
μFTIR spectra of consolidating product applied to Carrara marble (a) and Vicenza white limestone (b) specimens and then exposed to outdoor conditions. Monitoring took place every three months from zero time (black line) to a year of natural weathering (orange line).
The authors also considered the possibility of a partial substitution of calcium ions with barium ions in calcium sulfate molecules already present in the sample:
CaSO4·2H2O + Ba(OH)2 → BaSO4 + Ca(OH)2 + 2H2O
Ca(OH)2 + CO2 → CaCO3 + H2O
However, in this specific case, the use of fresh samples for the experimentation and the total absence of peaks related to calcium sulfate (CaSO4 · 2H2O, gypsum) in FTIR spectra do not support the hypothesis.
The formation of BaSO4 did not cause damage to the stone structure or alter the performance acquired after applying the treatment solution.
The carbonation reaction and the consequent development of BaCO3 determined an interesting variation in water absorption values after applying the treatment to both lithotypes. The partial filling of the stones’ porosity by Ba(OH)2 and the consequent crystallization of BaCO3 in the pores and at the specimens’ surfaces physically limited the penetration of water in the stones’ structures (Wa variations from untreated and treated samples were: from 1.53 × 10−5 to 9.70 × 10−6 g/(m2s) for Carrara marble and from 1.36 × 10−3 to 3.17 × 10−4 g/(m2s) for Vicenza white limestone). During natural weathering, the developing of BaSO4 did not involve any significant variation in water absorption, even though BaSO4 salt (T = 298 K, Kps = 1.07·10−10) is more insoluble than BaCO3 (T = 298 K, Kps = 1.17·10−9), CaCO3 (T = 298 K, Kps = 1.07·10−10), or CaSO4 · 2H2O (T = 298 K, Kps = 7.10·10−5) [43]. Rather, the initial value of water absorption increased over time (Figure 5), probably because of the partial removal of the treatment from the surface by wind or biological growth, which colonized the specimens during the exposure to outdoor conditions.
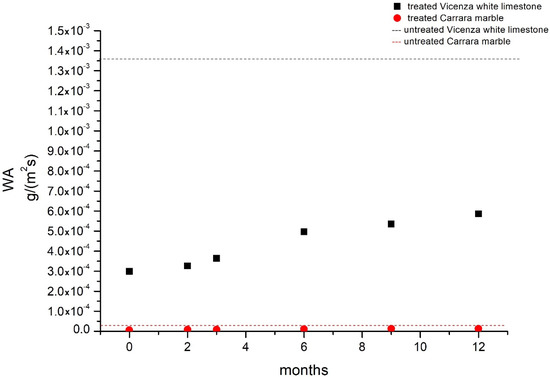
Figure 5.
Water absorption trend of surfaces treated with Ba(OH)2 during natural weathering. Considering the untreated values (1.53 × 10−5 g/(m2s) and 1.37 × 10−3 g/(m2s) for Carrara marble and Vicenza white limestone, respectively), the capacity of superficial water absorption tends gradually to return to untreated values.
The study of sample cross-sections using SEM allowed us to recognize barium particles as white matter due to the high atomic weight and to measure largely the penetration depth of the treatment into the stone substrate. According to the stones’ porosity, no penetration was detected in the case of Carrara marble, although a thin white layer was visible on the surface (Figure 6a). On the other hand, on Vicenza limestone, barium crystals were established quite homogeneously to a depth of 13 mm, decreasing in percentage with greater depth (Figure 6b).

Figure 6.
SEM micrographs using BSE of treated Carrara marble (a) and Vicenza limestone (b) cross-sections. The white areas represent barium distribution in the samples. The highest atomic weight of barium results in white dots in the images and allows the recognition of the element distribution (ascertained by EDS analysis).
Using high magnifications, SEM confirmed the morphological aspect of the developed secondary products in the form of acicular crystals having various grain sizes. However, the matrix effect made it impossible to detect their chemical compositions accurately by an EDX microprobe. In fact, the analysis of the acicular crystals showed the co-presence of calcium and barium in a stone matrix of CaCO3. Due to the small sizes of the minerals, the calcium signal in the EDX spectra of the treatment could be related to both the secondary crystals and primary CaCO3 of the stone (matrix).
Artificial aging seems not to alter the treated surfaces. UV light did not cause yellowing, craquelure, or micro-fractures to the superficial treatment layer. No peculiar differences in water absorption were found, or any significant changes in μFTIR spectra were spotted. Consequently, it can be supposed that the treatment is not sensitive to photo-oxidation.
Study of Biological Growth
Different resistance to mold growth occurred on treated and untreated specimens, even though they were exposed to the same conditions. The presence of fungi was more abundant on treated samples, and, in these, the percentage of biological activity on Vicenza limestone was double that on Carrara marble, even if the types of fungi were the same (Figure 7a). It is possible that the greater presence of fungi in treated samples was due to the different compactness of the superficial layer between untreated and treated samples. Indeed, the superficial carbonate matrix of treated samples consisting mainly of barium carbonate was developed by the re-precipitation process. As a result, it was less compact (Figure 2) and more porous, tending not to be comparable to the primary stone’s inherent carbonate matrix (especially in the case of Carrara marble). The structure of this superficial layer allowed the greater retention of fungal spores and promoted the development of a microhabitat suitable for their reproduction. Hence, the greater the thickness of the superficial layer, the greater the possibility of fungi development.
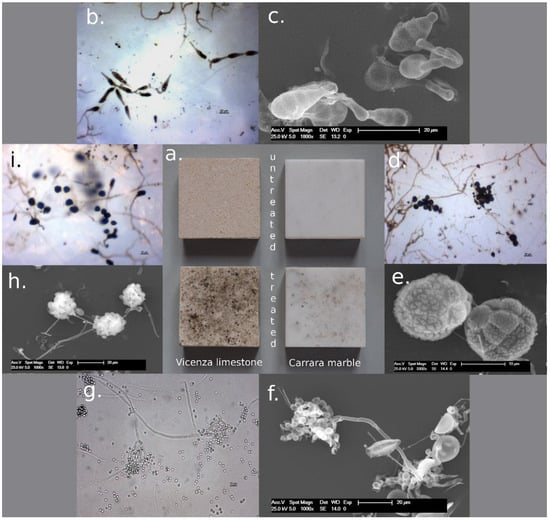
Figure 7.
Development of fungi on treated surfaces (a). The biological study under an optical microscope (b,d,g,i) and SEM (c,e,f,h) revealed mainly the presence of Alternaria sp. (b,c), Epicoccum sp. (d,e), Penicillium sp. (f,g), and Aspergillus sp. (h,i).
Alternaria sp. was identified as the dominant species on both treated and untreated specimens (Figure 7b,c). From the dilutions 10−1 and 10−2, the treated samples developed brown colonies identified through an optical microscope as Aspergillus. In fact, the samples showed a conidiophore with the typical shape of Aspergillum sp. (Figure 7h,i). Dark-brown colonies of Epicoccum sp. were also isolated from the samples: the production of pigments is yellow in the substrate, then becomes dark-orange, and the conidia were spherical, dark, and rough (Figure 7d,e). A dark-brown colony, having slow growth and clear edges, has been assumed to be Phymatotrichum sp. The optical microscope revealed spherical conidia, which developed from phialides and carried on the hyphae irregularly. More investigations are requested to confirm the identification. Finally, Penicillium sp. was isolated from the samples. The colony is dark-green with abundant dry spores on the surface. The microscopical study showed the typical brush-shaped conidiophore (Figure 7g,f).
The genera, which are composed of fungal colonies (Alternaria, Aspergillus, Epicoccus, and Penicillium), are ubiquitous, and their presence in the samples is not surprising. Due to the high allergological power of the Alternaria genus, more detailed information is available on the concentration of its spores in the air, which has been increasing in the atmosphere of Venice province in the last few years [44,45].
3.2. Evaluation of the Stability of Ba(OH)2 Treatments Applied in the Past Stone Materials
3.2.1. Old Laboratory Samples
The study of the fragments sampled from two specimens of Vicenza white limestone, treated in the past for experimental purposes and preserved in the laboratory of Iuav University, provided relevant data useful for enhancing previous theories. The application method by total immersion allowed the penetration of barium solution into the entire thickness of the specimens. SEM observations revealed crystals with acicular shape and epitaxial growth in the stone porosity. An elementary map made by the EDX microprobe showed the most barium amount in porous zones and at the interfaces between calcite and silico-aluminate areas. Even though the chemical analyses of acicular crystals suggested a mixed composition of calcium and barium, in this case, the matrix effect did not allow to confirm the certainty of Lewin’s theory of a partial substitution of Ca2+ ions from calcite with Ba2+ ions of barium hydroxide during the consolidation mechanism [15]. The specimen in which a silicone product was brushed on the surface after drying Ba(OH)2 confirmed that the product totally tolerated further treatments.
Some measurements were repeated in the middle of the needles, reducing the acceleration voltage from 20 to 10 keV to limit the propagation of the beam outside the acicular crystals. Moreover, the EDX spectra confirmed the mixed composition of barium and calcium. However, studying the EDX spectra, it was not possible to exclude secondary fluorescence effects related to X-rays emitted by barium atoms from 4.5 keV, which could beam elements of the matrix. Furthermore, to investigate further, a solution of pure barium hydroxide was left to carbonate on a petri dish and—exactly as observed in the case of the Venetian treated stones—pure barium carbonate salts with acicular shape crystallized (Figure 8a). The elemental composition of these crystals was ascertained by EDX using 10 keV (Figure 8c). Then, a part of the same crystals was mechanically distributed and deposited on a calcium carbonate surface (Figure 8b), and EDX analyses were repeated using the same analytical parameters (Figure 8d). In this case, the results showed a mixed composition of calcium and barium, confirming that a calcium peak is generated from secondary fluorescence effects caused by X-rays emitted by barium atoms.
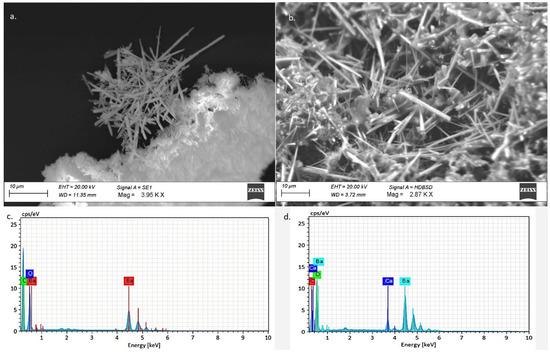
Figure 8.
SEM observations and EDX studies of the acicular crystals produced by barium hydroxide treatment: (a) SEM micrograph of BaCO3 crystallized on Petri dish; (c) EDX spectrum of the acicular crystals in figure (a) obtained at 10 keV; (b) SEM micrograph of BaCO3 crystallized on Petri dish and distributed on a CaCO3 substrate; (d) EDX spectrum of acicular crystals in figure (b) obtained at 10 keV.
3.2.2. Historical Venetian Surfaces
The surface of the font of St. Fantin church in Venice appeared macroscopically well-preserved with no new fractures. Some areas had whitened. The sampling was ineffective due to the homogeneity and compactness of the Carrara marble. The only fragment sampled from the rim of the font resulted microscopically homogeneous and compact, revealing only a superficial slight light brown deposit of dust. Microscopically, no superficial treatment was observed. The absence of any conservation product based on barium was confirmed by μFTIR and SEM-EDX analyses, probably due to both the sampling area and the marble’s compactness, which prevented the treatment solution’s penetration, as well as the ineffectiveness of the treatment method.
On the other hand, the column in the Scuola Grande San Giovanni Evangelista was generally well-preserved and compact, and no fractures or fissures were detectable. Nevertheless, the portion immediately adjoining a window appeared completely eroded and fragile. On this side, some flakes were lifted from the column shaft. However, no color alteration was observed. The FTIR analysis confirmed the permanence of the barium treatment in the stone (Figure 9). In particular, the spectra showed a main band at 1437 cm−1 related to a carbonate compound and the triplet related to BaSO4 (1180, 1126, and 1082 cm−1). The presence of BaSO4 could be related both to the sulfation of BaCO3 (Equations (3) and (4)) and the chemical reaction between Ba(OH)2, applied during the restoration treatment, and gypsum already present in the degraded stone (Equation (5)). The broadening of the band at 1437 cm−1 did not allow us to distinguish CaCO3 from BaCO3 [46]. SEM-EDX analyses revealed a non-homogeneous distribution of barium on the surface. Moreover, the penetration of the product into the stone fissures was effective, and the maximum depth measured was 250 μm. In some superficial areas, an alteration layer composed of sulfur, barium, and calcium was detected (Figure 10).
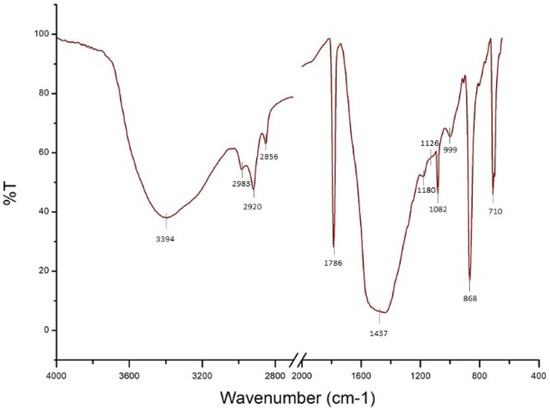
Figure 9.
FTIR spectrum of the deteriorated superficial layer of Scuola Grande San Giovanni Evangelista sample.
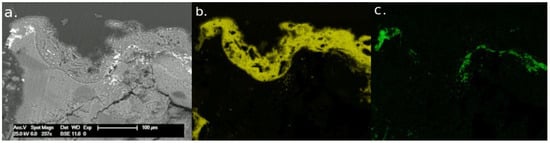
Figure 10.
SEM micrographs of the decayed superficial layer of sample from Scuola Grande San Giovanni Evangelista (a) and chemical analysis of degraded surface: (b) EDS map of sulfur distribution (yellow), (c) EDS map of barium distribution (green).
SEM-EDX observations and analyses of the marble micro fragments sampled from the St. Alvise statue in Venice [25,40] showed the permanence of a restoration treatment based on Ba(OH)2, applied before 1967, of which no archival documentation was found. The presence of barium and sulfur detected in the inter-grain spaces is probably linked to the sulfation of BaCO3. However, the high amount of sulfur in the stone could also be related to a previous sulfation of CaCO3. The presence of BaSO4 in the stone structure did not inhibit the subsequent penetration of a silicone resin into the separate spaces of the marble, which filled the spaces between the calcite grains and fixed barium treatment.
4. Discussion
The results obtained on carbonate stones treated with Ba(OH)2 produced interesting topics for discussion.
The use of barium hydroxide solution as a consolidant satisfies several conservation criteria, which a consolidation treatment theoretically requests. The consolidation process takes place as a consequence of the chemical reaction between Ba(OH)2 and CO2, giving rise to pure BaCO3 that precipitates into the stone in the form of acicular crystals. The chemical nature of the restoration product (BaCO3), ascertained by FTIR and EDX, guarantees good chemical compatibility with a calcium carbonate substrate (barium is vicarious of calcium). The crystals’ shape and the high atomic weight of barium make barium compounds well determined by SEM, allowing the possibility to assess the treatment over time through the study of a cross-section. In order to investigate the chemical composition of the crystals, it was fundamental to perform SEM-EDX analyses using the right acceleration voltage. Nevertheless, laboratory tests confirmed secondary fluorescence effects related to X-rays emitted by barium atoms that beam calcium of the matrix. For this reason, EDX spectra of the crystals showed the co-presence of barium and calcium, leading to the erroneous postulation of forming a mixed barium and calcium carbonate. The measured amount of calcium was higher in the analyzed areas next to calcite crystals because of a higher matrix effect, and it decreased as the distance from them increased.
The penetration depth is related to the stone porosity and state of conservation of the substrate: the greater the porosity, the greater the penetration depth, as demonstrated comparing Vicenza white limestone and Carrara marble or fresh samples and historic ones. The solution based on barium hydroxide, glycerin, and urea guaranteed a good distribution and penetration into Lumachella del Tasso substrate. In contrast, no trace of the treatment was observed in the case of Carrara marble, which has a lower porosity, using both a pure barium hydroxide solution and a mixture of barium hydroxide, urea, and glycerin. Better results could probably be obtained by improving the application method. Indeed, from the experimentation, brushing did not allow contact between the sample and solution for a long time, causing a less amount of treatment absorbed into stone porosity and a higher quantity of product on the stone surface. Among those investigated, only the immersion absorption procedure seems to guarantee the total impregnation of the sample; however, it is not applicable on site. It could be interesting to test under the vacuum method in order to improve the solution’s penetration into the substrate. However, comparing the penetration depth with the results obtained by applying organic polymers by brushing into the same carbonate rocks, the results obtained could be considered quite good. No penetration into Carrara marble resulted in the case of epoxy resin EP2101 [26], siloxane resins RC80, and RC90 [25]. In the case of Vicenza limestone, the penetration depth recorded by Ba(OH)2 was higher than the one obtained by applying EP2101 (250µm), or RC80 (7.5 mm) and RC90 (4.5 mm). Only EAS 40 (ethyl silicate) seems to penetrate almost the entire thickness of the specimen (2 cm).
The consolidation mechanism is due to the precipitation of barium carbonate and partial filling of stone porosity, without colmatating fractures or sticking to the internal surfaces of the pores as synthetic polymers usually do, but allowing future retreatment, as proved in the case study of the St. Alvise statue.
During the curing process, the moving of water from the inner part to the external part of the substrate, due to evaporation, causes the partial migration of salts and the formation of white crystals onto the stone surface. This generates a whitening effect over time, resulting in a barium carbonate patina that alters the esthetical features of the treated surface (Figure 2). For this reason, it could be recommended to maintain the surface wet after treatment to limit water evaporation from the inner part of the sample. Otherwise, it could be useful to mechanically clean the treated surface (i.e., by brushing with a soft bristle brush) after one month from the product’s application to remove the excess of the product from the surface. The partial filling of superficial stone porosity by barium carbonate crystals reduced the amount of water absorbed after treatment. However, the sponge test demonstrated that this value increased over time, probably due to wind and rain erosion that causes the gradual removal of the product from the surface.
The morphology of barium carbonate crystals and the formation of an incoherent superficial patina allow for retaining fungal spores settling on the surface, promoting biological activity.
Experimental data proved the high reactivity of barium carbonate in a sulfur-laden polluted atmosphere. Indeed, the periodic FTIR monitoring showed the appearance of barium sulfate on fresh treated samples after three months of natural weathering and the increase in the related peaks in the following months. No gypsum was revealed.
Photo-oxidation did not cause any chemical alteration to the barium salts, and during the xenon test, the treated stones were stable for the monitored parameters.
5. Conclusions
The conservation treatments’ assessment, performed on laboratory specimens and stones from Venetian monuments treated in the second half of the XX century with barium hydroxide, allowed for verifying the product’s stability and the chemical behavior over time.
Barium hydroxide, used as a strengthening agent for monumental stones, partially fills the porosity with an inorganic secondary product in the form of acicular crystals based on pure barium carbonate, avoiding the total closure of the pores and respecting the permeability of the material. SEM-EDX analyses confirmed the secondary fluorescence effect when an X-ray beam measured the needle crystals developed by the treatment. Therefore, the mixed composition of barium and calcium carbonate postulated in the past cannot be confirmed. Nonetheless, the chemical compatibility between the treatment and the stone is assured. Moreover, this treatment’s filling of the superficial stone porosity significantly reduces water absorption in the first year of exposure to outdoor conditions. The higher reactivity of barium carbonate with air pollutants ascertains the development of barium sulfate before gypsum, slowing down stone deterioration mechanisms. UV radiation, as expected, does not induce any variation to the chemical stability of the treatment or any esthetical alteration.
However, wind and rain erosion cause the gradual removal of the product from the surface, and the measured amount of water absorbed by the samples’ surfaces tends to progress to higher values. Moreover, during the curing process, water acts as a carrier for barium salts, which migrate to the surface and crystallize, causing a whitening effect visible to the naked eye. For these reasons, maintenance of the treated surface is strongly recommended.
The analyses of micro fragments sampled from historical Venetian monuments and specimens allowed us to confirm the results obtained from experimental samples. Moreover, they demonstrated that the application of the treatment allows the natural degradation of the stones to be slowed down, preserving the possibility of the retreatment of the surface and ensuring better conservation over time.
Author Contributions
Conceptualization, E.T.; methodology, E.T.; software, A.C.; validation, E.T.; formal analysis, E.T., F.M. and A.C.; investigation, E.T. data curation, E.T.; writing—original draft preparation, E.T.; writing—review and editing, E.T.; visualization, E.T.; supervision, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors wish to thank Lorenzo Lazzarini, Laboratory for Analyzing Materials of Ancient origin (LAMA)—Iuav University, for introducing us to this research and for his collaboration and shared expertise; Giovanni Vannacci, Department of Agriculture, Food and Environment (DAFE)—University of Pisa, and Ornella Salvadori, a biologist at the Soprintendenza Polo Museale Veneziano, for her collaboration in performing biological analyses; Soprintendenza per i beni architettonici e paesaggistici di Venezia e laguna and in particular the senior conservator and restorer Lucia Bassotto, and Scuola Grande di San Giovanni Evangelista for authorizing and supporting us in taking samples from Venetian monuments; Fabrizio Antonelli, Laboratory for Analyzing Materials of Ancient origin (LAMA), for critical reading and constructive suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansen, E.; Dohene, F.; Fidler, J.; Larson, J.; Martin, B.; Matteini, M.; Rodríguez-Navarro, C.; Sebastián Pardo, E.; Price, C.; de Tagle, A.; et al. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Rev. Conserv. 2003, 4, 13–25. [Google Scholar] [CrossRef]
- Bracci, S.; Sacchi, B.; Ferreira Pinto, A.P.; Delgado Rodrigues, J. Inorganic consolidants on stone artefacts: Optimisation of application procedures for marble and limestones. In Proceedings of the International Symposium, Stone Consolidation in Cultural Heritage, Research and Practice, Lisbon, Portugal, 6–7 May 2008; Rodrigues, J.D., Mimoso, J.M., Eds.; [Google Scholar]
- Ferroni, E.; Dini, D. Chemical-structural conservation of sulphatized marbles. In Proceedings of the Conservation of Stone II. Preprints of the Contribution to the International Symposium, Bologna, Italy, 27–30 October 1981; pp. 559–566. [Google Scholar]
- Matteini, M. In review: An assessment of Florentine methods of wall painting conservation based on the use of mineral treatments. In The Conservation of Wall Paintings, Proceedings of the Symposium Organized by the Courtauld Institute of Art and the Getty Conservation Institute, London, UK, 13–16 July 1987; Gather, S., Ed.; Getty Publications: Los Angeles, CA, USA, 1991; pp. 137–148. [Google Scholar]
- Matteini, M.; Moles, A. La “Metodologia del Bario” in relazione ai problemi di solfatazione e decoesione che interessano i dipinti murali. In Il Restauro Delle Opere D’arte; Accademia Nazionale Virgiliana: Mantova, Italy, 1987; pp. 33–41. [Google Scholar]
- Ferroni, E.; Malaguzzi, V.V.; Rovida, G. Experimental study by diffraction of heterogeneous systems as a preliminary to the proposal of a technique for the restoration of gypsum polpute murals. In Proceedings of the ICOM Conference, Amsterdam, Holland, 6–12 July 1969. [Google Scholar]
- Matteini, M.; Moles, A. Barium aluminates for the consolidation of mural paintings. In Proceedings of the ICOM Committee for Conservation, 5th Triennial Meeting, Zagreb, Croatia, 1–8 October 1978. [Google Scholar]
- Berlucchi, N.; Ginanni Corradini, R.; Bonomi, R.; Bemporad, E.; Tisato, M. “La fenice” theatre—Foyer and Apollinee rooms—Consolidation of fire-damaged stucco and marmorino decorations by means of combined applications of ion-exchange resins and Barium hydroxide. In Proceedings of the 9th International Congress on Deterioration and Conservation of Stone, Venice, Italy, 19–24 June 2000. [Google Scholar]
- Lazzarini, L.; Laurenzi Tabasso, M. Il Restauro Della Pietra; Cedam: Padova, Italy, 1986. [Google Scholar]
- Slížková, Z.; Drdácký, M.; Viani, A. Consolidation of weak lime mortars by means of saturated solution of calcium hydroxide or barium hydroxide. J. Cult. Herit. 2015, 16, 452–460. [Google Scholar] [CrossRef]
- Schaffer, R.J. Weathering of Natural Building Stones; His Majesty’s Stationery, Office: London, UK, 1932; Reprinted in Building Research Establishment: Watford, UK, 1972. [Google Scholar] [CrossRef]
- Toniolo, L.; Colombo, C.; Realini, M.; Peraio, A.; Positano, M. Evaluation of barium hydroxide treatment efficacy on a dolomitic marble. In Annali Di Chimica; Wiley-VCH: Weinheim, Germany, 2001; Volume 91, pp. 813–821. [Google Scholar]
- Matteini, M.; Moles, A. Aspetti critici del trattamento fondato sull’impiego di idrato di bario. In Pitture Murali; Danti, C., Matteini, M., Moles, M., Eds.; Centro Di della Edifimi s.r.l.: Firenze, Italy, 1990; pp. 297–302. [Google Scholar]
- Graf, D.L.; Lamar, L.E. Properties of calcium and magnesium carbonates and their bearing on some uses of carbonate rocks. Econ. Geol. 1955, 50, 696–713. [Google Scholar]
- Lewin, S.Z.; Baer, N.S. Rationale of the barium hydroxide-urea treatment of decayed stone. Stud. Conserv. 1974, 19, 24–35. [Google Scholar] [CrossRef]
- Lewin, S.Z. Method of Preserving Limestone Structure. U.S. Patent 529,213, 25 February 1966. [Google Scholar]
- Lewin, S.Z. Method of Preserving Limestone Structure. U.S. Patent 3,577,244, 5 May 1971. [Google Scholar]
- Schnabel, L. Evaluation of the barium hydroxide-urea consolidation. In Proceedings of the 7th International Congress on Deterioration and Conservation of Stone, Lisbon, Portugal, 15–18 June 1992; Rodrigues, J.D., Henriques, F., Jeremias, F.T., Eds.; Laboratório Nacional de Engenharia Civil: Lisboa, Portugal, 1992; pp. 1063–1072. [Google Scholar] [CrossRef]
- Favaro, M.; Naccari, A.; Crivellari, F.; Magris, D.; Pigo, M.; Burtet, B.; Fumo, G.; Fassina, V. New findings on past treatment’s effects on the lunette of San Giovanni Evangelista in Venice. In Proceedings of the 9th International Congress on Deterioration and Conservation of Stone, Venice, Italy, 19–24 June 2000. [Google Scholar]
- Delgado Rodrigues, J.; Ferreira Pinto, A.P. Laboratory and onsite study of barium hydroxide as a consolidant for high porosity limestones. J. Cult. Herit. 2016, 19, 467–476. [Google Scholar] [CrossRef]
- Joy, C.D. Monte Carlo Modelling for Electron Microscopy and Microanalysis; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Internazionale Marmi E Macchine. The Tuscan Marble Identities; Signa: Florence, Italy, 2010. [Google Scholar]
- Berto, L.; Favaretto, T.; Saetta, A.; Antonelli, F.; Lazzarini, L. Assessment of seismic vulnerability of art objects: The Galleria dei Prigioni sculptures at the Accademia Gallery in Florence. J. Cult. Herit. 2012, 13, 7–21. [Google Scholar] [CrossRef]
- Cattaneo, A.; De Vecchi, G.P.; Menegazzo Vitturi, L. Le pietre tenere dei Colli Berici. In Atti e Memorie Dell’accademia Patavina di Scienze, Lettere ed Arti, V.LXXXVIII, Parte II, Classe di Scienze Matematiche e Naturali; R. Accademia di Scienze, Lettere ed Arti: Padua, Italy, 1976; pp. 69–100. [Google Scholar]
- Tesser, E.; Lazzarini, L.; Bracci, S. Investigation on the chemical structure and ageing transformations of the cycloaliphatic epoxy resin EP2101 used as stone consolidant. J. Cult. Herit. 2018, 31, 72–82. [Google Scholar] [CrossRef]
- Tesser, E.; Antonelli, F. Evaluation of silicone based products used in the past as today for the consolidation of Venetian monumental stone surfaces. Mediterr. Archaeol. Archaeom. 2018, 18, 159–170. [Google Scholar] [CrossRef]
- Manganelli Del Fà, C. La Porosità Nei Materiali Lapidei Naturali e Artificiali–Problematiche di Determinazione Della Porosità. Correlazione Tra Caratteristiche Fisiche Dei Materiali, Porosità, Dinamica dei Fluidi, Degrado e Trattamenti Conservativi. Supplemento al n.; 10 di Fist Geoitalia: Modena, Italy, 2002. [Google Scholar]
- UNI 10925; Beni Culturali Materiali Lapidei Naturali ed Artificiali. Metodologia per L’irraggiamento Con Luce Solare Artificiale: Milan, Italy, 2001.
- ASTM D 3273-76; Standard Test Method For: Resistance to Growth of Mold on the Surface of Interior Coatings in an Environmental Chamber. ASTM International: West Conshohocken, PA, USA, 2000.
- UNI 11432; Beni Culturali. Materiali Lapidei Naturali ed Artificiali. Misura Della Capacità di Assorbimento di Acqua Mediante Spugna di Contatto: Milan, Italy, 2011.
- EN 15886; 2010 Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces. European Standard: Brussels, Belgium, 2010.
- Normal 8/81; Esame Delle Caratteristiche Morfologiche al Microscopio Elettronico a Scansione (SEM). CNR Milano e Roma—ICR: Rome, Italy, 1981.
- Caneva, G.; Nugari, M.P.; Salvadori, O. La Biologia Vegetale per i Beni Culturali; Nardini Ed.: Florence, Italy, 2007. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: London, UK, 1971. [Google Scholar]
- Hawksworth, D.L.; Sutton, B.C.; Ainsworth, G.C. Ainsworth and Bisby’s Dictionary of the Fungi, 7th ed.; Commonwealth Mycological Institute: London, UK; CAB Direct: Glasgow, UK, 1983. [Google Scholar]
- Ellis, M.B. More Dematiaceous Hyphomycetes; CABI Publishing: Wallingford, UK, 1976. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; Burgess Publishing, Co.: London, UK, 1969. [Google Scholar]
- Lazzarini, L.; Mariottini, M. A first study of some lumachelle (fossiliferous stones) used in Roman antiquity. In Proceedings of the Interdisciplinary Studies on Ancient Stone. Proceedings of the IX ASMOSIA Conference—Tarragona 2009; Garcia-M, A.G., Lapuente, P., Rodà., I., Eds.; Institut Català d’Arqueologia Classica: Tarragona, Spain, 2012; pp. 445–451. [Google Scholar]
- Tesser, E.; Lazzarini, L.; Ganzerla, R.; Antonelli, F. The decay of the polysiloxane resin Sogesil XR893 applied in the past century for consolidating monumental marble surfaces. J. Cult. Herit. 2017, 27, 107–115. [Google Scholar] [CrossRef]
- Normal 3/80, Materiali Lapidei: Campionamento, CNR Milano e Roma–ICR. 1980. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1296207416303442 (accessed on 15 September 2022).
- PCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- La Russa, M.F.; Comite, V.; Aly, N.; Barca, D.; Fermo, P.; Rovella, N.; Antonelli, F.; Tesser, E.; Aquino, M.; Ruffolo, S.A. Black crusts on Venetian built heritage, investigation on the impact of pollution sources on their composition. Eur. Phys. J. Plus 2018, 133, 370. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 82nd ed.; CRC Press LLC.: Boca Raton, FL, USA, 2001–2002. [Google Scholar]
- Ariano, R.; Bonifazi, F. Aerobiologia ed Allergeni Stagionali: Il Campionamento Aerobiologico Applicato Alla Pratica Clinica; ECIG: Genova, Switzerland, 2006; p. 240. [Google Scholar]
- ISPRA. Pollini Allergenici in Italia: Analisi dei Trend 2010–2019; Rapporti: Ispra, Italy, 2020; p. 335. [Google Scholar]
- Matteini, M.; Scuto, S. Consolidamento di manufatti lapidei con Idrossido di Bario. Test colorimetrici per la verifica della diffusione del consolidante. Arkos Sci. Restauro 2001, 1, 28–31. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).