Monitoring Coptic Masonry Affected by Clay Minerals and Microorganisms at the Church of Virgin Mary, Wadi El-Natrun (Egypt)
Abstract
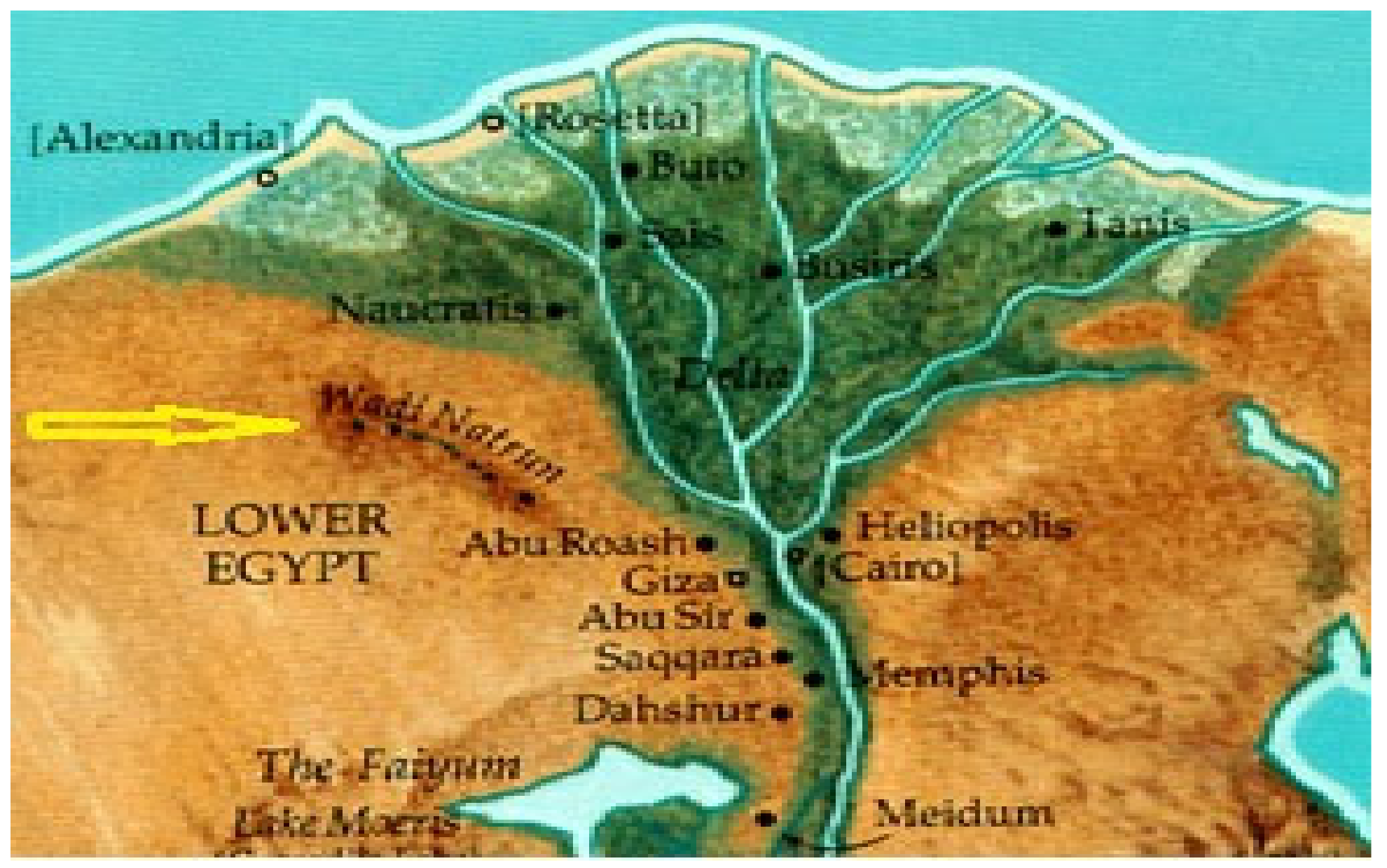
:1. Introduction
2. Materials and Methods
3. Results
3.1. Clay Mineralogy
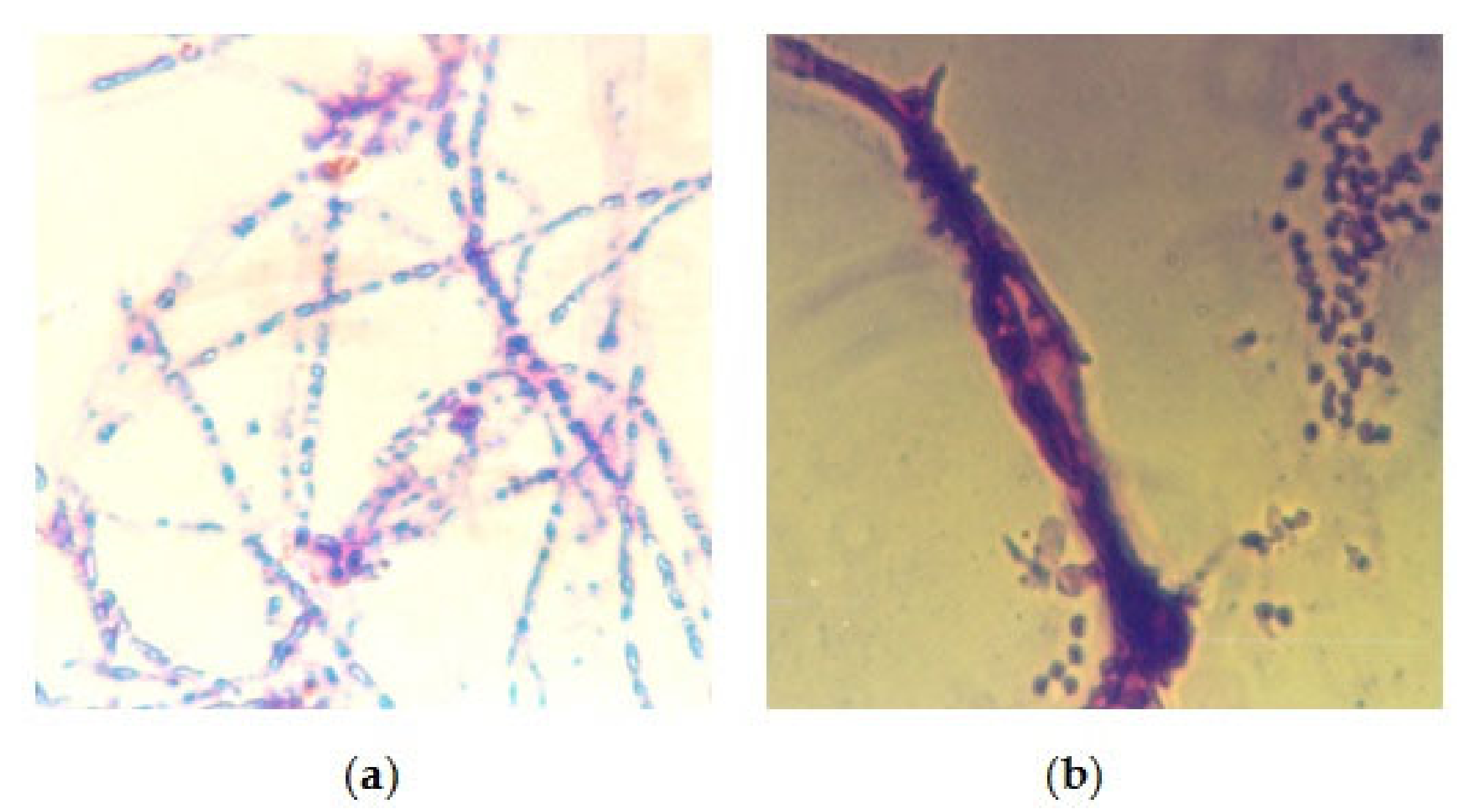
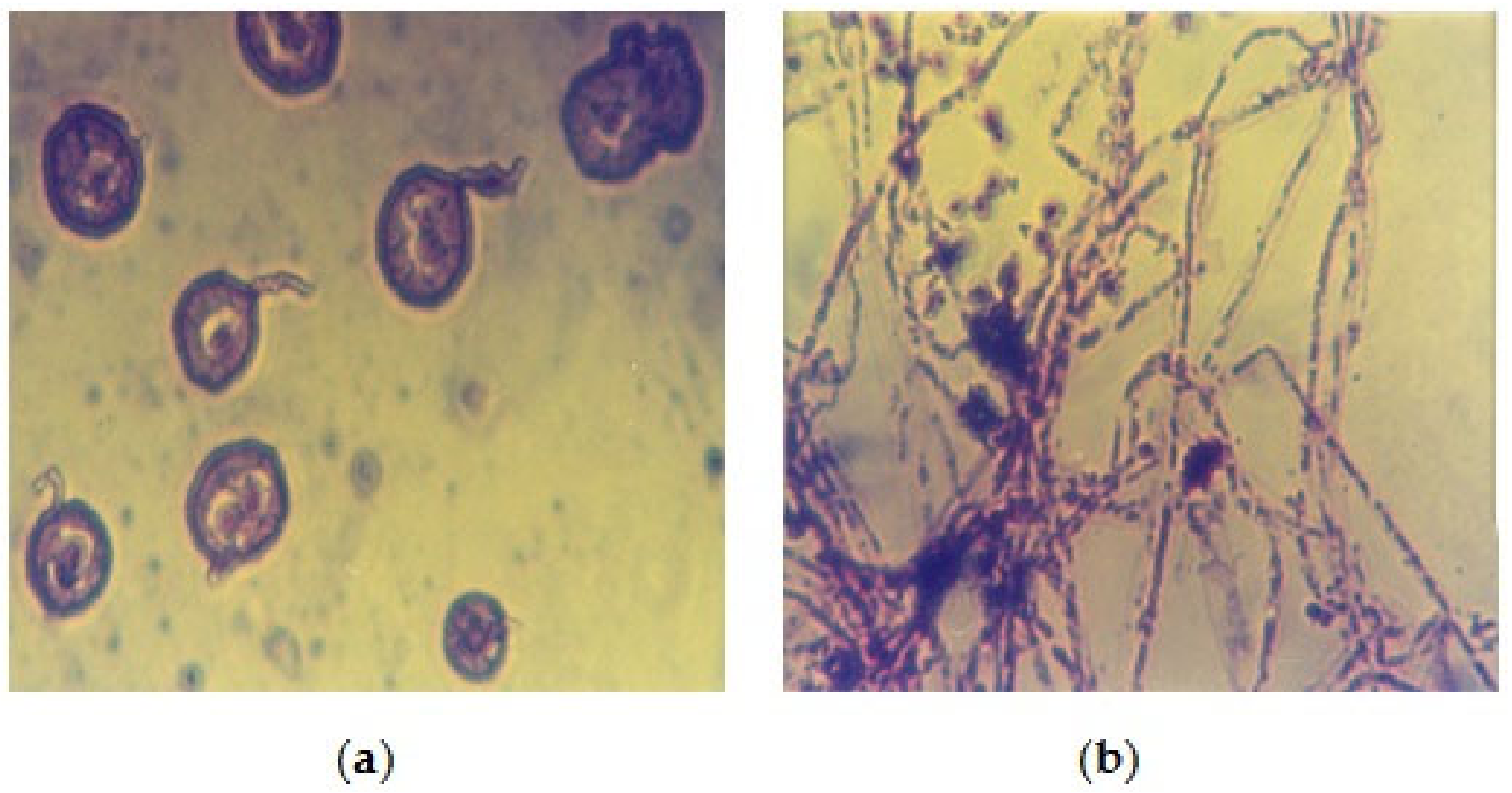
3.2. Biological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parandowska, E. Results of the Recent Restoration Campaigns (1995–2000) at Dayr al-Suryan. In Christianity and Monasticism in Wadi Al-Natrun: Essays from the 2002 International Symposium of the Saint Mark Foundation and the Saint Shenouda the Archimandrite Coptic Society; The American University in Cairo: Cairo, Egypt, 2009; Volume 3, p. 272. [Google Scholar]
- Innemie, K.C. Recent Discoveries of Wall-Paintings in Deir Al-Surian. Hugoye J. Syr. Stud. 1998, 1, 1–14. [Google Scholar]
- Innemie, K.C. Deir al-Surian (Egypt): Its Wall-paintings, Wall-texts, and Manuscripts. Hugoye J. Syr. Stud. 1999, 2, 7–15. [Google Scholar]
- Innemie, K.C. Deir al-Surian (Egypt): New Discoveries of January 2000. Hugoye J. Syr. Stud. 2000, 3, 1–7. [Google Scholar]
- Innemie, K.C. Deir al-Surian (Egypt): Conservation work of autumn 2000. Hugoye J. Syr. Stud. 2001, 4, 1–10. [Google Scholar]
- Innemie, K.C.; Van Rompay, L. Deir al-Surian (Egypt): New Discoveries of 2001–2002. Hugoye J. Syr. Stud. 2002, 5, 1–12. [Google Scholar]
- Riemer, W. Degrading Igneous Rock-Aspects and Identification of the Geotechnical Problem. In Proceedings of the International Symposium on Industrial Minerals and Building Stones, Istanbul, Turkey, 15–18 September 2003; pp. 841–848. [Google Scholar]
- Kondratyeva, I.A.; Gorbushina, A.A.; Boikova, A.I. Biodeterioration of Construction Materials. Glass Phys. Chem. 2006, 32, 254–256. [Google Scholar]
- JCPDS. Jaint Committee on Powder Diffraction Standards, Index to the Powder Diffraction File; American Society for Testing and Materials: West Conshohocken, PA, USA, 1967. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill’s Publishing Company: Austin, TX, USA, 1968. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis—Advanced Course; 11th print; Self-published: Madison, WI, USA, 1974. [Google Scholar]
- Pehlivanoglou, K.; Tsirambides, A.; Trontsios, G. Origin and Distribution of Clay Minerals in the Alexandroupolis Gulf, Aegean Sea, Greece, Estuarine. Coast. Shelf Sci. 2000, 51, 61–73. [Google Scholar] [CrossRef]
- Biscaye, P.E. Mineralogy and Sedimentation of Recent Deep Sea Clay in the Atlantic Ocean and Adjacent Seas and Oceans. Geol. Soc. Am. Bull. 1965, 76, 803–831. [Google Scholar] [CrossRef]
- Booth, C. Methods in Microbiology, 4th ed.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Stevens, R.B. Mycology Guide Book; Mycology Guide Book Committee, Mycology Society of American Universities, Washington Press: Seattle, WA, USA, 1981. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 3rd ed.; Burgess Publication Convention: Minneapolis, MN, USA, 1972. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; Academic Press: London, UK, 1980; Volumes 1 and 2. [Google Scholar]
- Ellis, D.; Davis, S.; Alexiou, R.; Bartely, R. Description of Medical Fungi, 2nd ed.; University of Adelaide: Adelaide, Australia, 2007; Volume 2. [Google Scholar]
- Lazzarini, L.; Laurenzi, T.M. Il Restauro della Pietra (The Restoration of Stone); No. 22; Cedam: Padua, Italy, 1986. [Google Scholar]
- Kamel, A.M.A. Dehydration of Gypsum Component of Plasters and Stuccos in Some Egyptian Archaeological Buildings and Evaluation of K2SO4 Activator as a Consolidant. Sci. Cult. 2019, 5, 49–59. [Google Scholar] [CrossRef]
- Theologitis, A.; Kapridaki, C.; Kallithrakas-Kontos, N.; Maravelaki-Kalaitzaki, P.; Fotiou, A. Mortar and Plaster Analysis as a Directive to the Design of Compatible Restoration Materials in Frangokastello (Crete). Mediterr. Archaeol. Archaeom. 2021, 21, 109–120. [Google Scholar] [CrossRef]
- Moussa, A. Assessing the Decay Agents of Wall Paintings in Al Qurna and Wadi El-Natrun Regions-Egypt. Ph.D. Thesis, Geology Department, Faculty of Science, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2007. [Google Scholar]
- Moussa, A.; Kantiranis, N.; Voudouris, K.; Stratis, J.; Ali, M.; Christaras, B. Diagnosis of Weathered Coptic Wall Paintings in the Wadi El Natrun Region, Egypt. J. Cult. Herit. 2009, 10, 152–157. [Google Scholar] [CrossRef]
- Robert, C.M.F.; Speweik, J.P. Repointing Mortar Joints in Historic Masonry Buildings. Preserv. Brief 1998, 2, 1–16. [Google Scholar]
- Toumbakari, E.E. Lime-Pozzolan-Cement Grouts and Their Structural Effects on Composite Masonry Walls. Ph.D. Thesis, Katholieke Universiteit Leuven, Faculteit Toegepaste Wetenschappen, Departement Burgerlijke Bouwkunde, Laboratorium Reyntjens, Heverlee, Belgium, 2002. [Google Scholar]
- Schulze, D.G. Clay Minerals. Encycl. Soils Environ. 2005, 2005, 246–254. [Google Scholar]
- Homburg, J.A. Archeology in Relation to Soils, Earth Systems and Environmental Sciences. Encycl. Soils Environ. 2005, 2005, 95–102. [Google Scholar]
- Carrillo, A.M.; Carriazo, J.G. Cu and Co Oxides Supported on Halloysite for the Total Oxidation of Toluene. Appl. Catal. B Environ. 2015, 164, 443–452. [Google Scholar] [CrossRef]
- Horváth, E.; Kristóf, J.; Frost, R.L.; Rédey, Á.; Vágvölgyi, V.; Cseh, T. Hydrazine-Hydrate Intercalated Halloysite under Controlled-Rate Thermal Analysis Conditions. J. Therm. Anal. Calorim. 2003, 71, 707–714. [Google Scholar] [CrossRef]
- West, S.L.; White, G.N.; Deng, Y.; McInnes, K.J.; Juo, A.S.R.; Dixon, J.B. Kaolinite, Halloysite, and Iron Oxide Influence on Physical Behavior of Formulated Soils. Soil Sci. Soc. Am. J. 2004, 68, 1452–1460. [Google Scholar] [CrossRef]
- Dayal, A.M.; Varma, A.K. Exploration Technique. Shale Gas Explor. Environ. Econ. Impacts 2017, 2017, 65–93. [Google Scholar]
- Eyssautier-Chuine, S.; Vaillant-Gaveau, N.; Charpentier, E.; Reffuveille, F. Comparison of Biofilm Development on Three Building and Restoration Stones Used in French Monuments. Int. Biodeterior. Biodegrad. 2021, 165, 1–10. [Google Scholar] [CrossRef]
- Fernandes, P. Applied Microbiology and Biotechnology in the Conservation of Stone Cultural Heritage Materials, Mini Review. Appl. Microbiol. Biotechnol. 2006, 73, 291–296. [Google Scholar] [CrossRef]
- Moussa, A.; Badawy, M.; Saber, N. Chromatic alteration of Egyptian Blue and Egyptian Green Pigments in Pharaonic Late Period Tempera Murals. Sci. Cult. 2021, 7, 1–15. [Google Scholar]
- Kiel, G.; Gaylarde, C.C. Diversity of Salt-Tolerant Culturable Aerobic Microorganisms on Historic Buildings in Southern Brazil. World J. Microbiol. Biotechnol. 2007, 23, 363–366. [Google Scholar] [CrossRef]
- Gettens, R.J.; Pease, M.; Stout, G.I. The Problem of Mold Growth in Paintings. Tech. Stud. Fine Arts 1941, 9, 127–143. [Google Scholar]
- Chaurasia, L.; Verma, R.K.; Bisht, V. Microbial Carbonate Precipitation by Urease Producing Bacteria in Cementitious Materials. Int. J. Adv. Biotechnol. Res. 2014, 5, 671–679. [Google Scholar]
- Warscheid, T.H.; Braams, J. Biodeterioration of Stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Bai, H.; Liu, D.; Zheng, W.; Ma, L.; Yang, S.; Cao, J.; Lu, X.; Wang, H.; Mehta, N. Microbially-Induced Calcium Carbonate Precipitation by a Halophilic Ureolytic Bacterium and its Potential for Remediation of Heavy Metal-Contaminated Saline Environments. Int. Biodeterior. Biodegrad. 2021, 165, 20–29. [Google Scholar] [CrossRef]
- Rolleke, S.; Muyzer, G.; Wawer, C.; Wanner, G.; Lubitz, W. Identification of Bacteria in a Biodegraded Wall Painting by Denaturing Gradient Gel Electrophoresis of PCR-Amplified Gene Fragments Coding for 16S rDNA. Appl. Environ. Microbiol. 1996, 62, 2059–2065. [Google Scholar] [CrossRef] [Green Version]
- Favero-Longo, S.E.; Matteucci, E.; Pinna, D.; Ruggiero, M.G.; Riminesi, C. Efficacy of the Environmentally Sustainable Microwave Heating Compared to Biocide Applications in the Devitalization of Phototrophic Communities Colonizing Rock Engravings of Valle Camonica, UNESCO World Heritage Site, Italy. Int. Biodeterior. Biodegrad. 2021, 165, 10–20. [Google Scholar] [CrossRef]





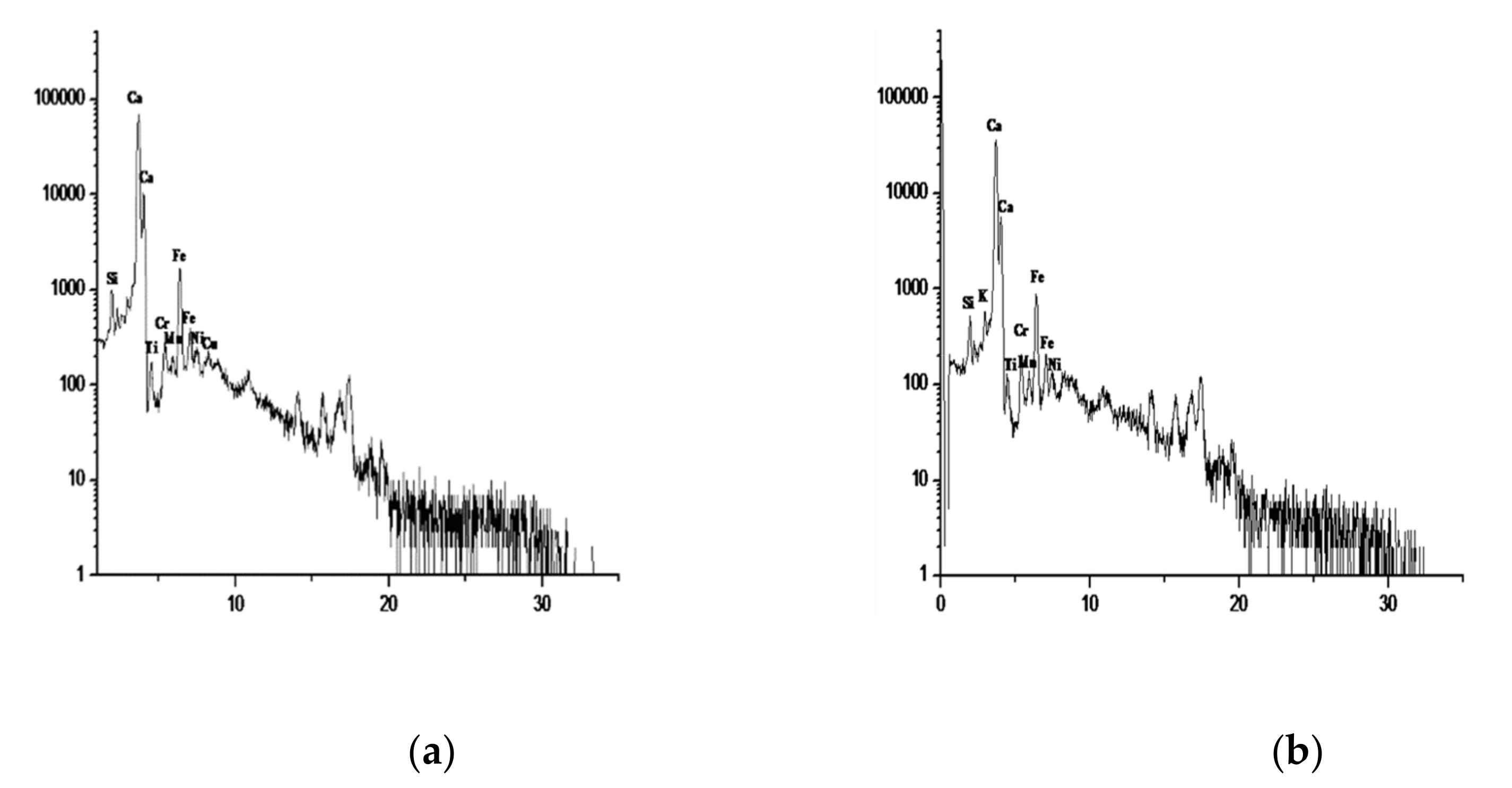


| Element % | Atomic % | Compound % | ||
|---|---|---|---|---|
| Na | 3.58 | 3.54 | Na2O | 4.83 |
| Mg | 5.75 | 5.37 | MgO | 9.54 |
| Al | 4.17 | 3.51 | Al2O3 | 7.89 |
| Si | 21.57 | 17.44 | SiO2 | 46.14 |
| S | 0.24 | 0.17 | SO3 | 0.60 |
| Cl | 12.32 | 7.89 | 0.00 | |
| K | 1.07 | 0.62 | K2O | 1.29 |
| Ca | 9.40 | 5.33 | CaO | 13.15 |
| Fe | 3.30 | 1.34 | FeO | 4.24 |
| O | 38.59 | 54.78 | - | - |
| Total | 100% | 100% | - | 87.68% |
| Element % | Atomic % | Compound % | ||
|---|---|---|---|---|
| Na | 39.09 | 43.51 | Na2O | 52.69 |
| Mg | 0.06 | 0.07 | MgO | 0.10 |
| Al | 0.11 | 0.11 | Al2O3 | 0.21 |
| Si | 0.26 | 0.24 | SiO2 | 0.56 |
| S | −0.06 | −0.05 | SO3 | −0.14 |
| Cl | 45.28 | 32.68 | 0.00 | |
| K | 0.12 | 0.08 | K2O | 0.14 |
| Ca | 0.66 | 0.42 | CaO | 0.92 |
| Fe | 0.18 | 0.08 | FeO | 0.23 |
| O | 14.29 | 22.86 | - | - |
| Total | 100% | 100% | - | 54.72% |
| Isolated Fungal Species | No. of Isolates | Percentage % |
|---|---|---|
| Aspergillus flavus group | 2 | 11.11 |
| Aspergillus flavus + Aspergillus niger | 3 | 16.66 |
| Aspergillus fumigatus | 3 | 16.66 |
| Aspergillus glaucus | 4 | 22.22 |
| Aspergillus occhraceus group | 1 | 5.55 |
| Mucor spp. | 1 | 5.55 |
| Aspergillus caudidus | 2 | 11.11 |
| Geotrichum spp. | 1 | 5.55 |
| Geotrichum spp. + Aspergillus fumigatus | 1 | 5.55 |
| Total isolates | 18 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, A.; Roshdy, M. Monitoring Coptic Masonry Affected by Clay Minerals and Microorganisms at the Church of Virgin Mary, Wadi El-Natrun (Egypt). Heritage 2021, 4, 4056-4067. https://doi.org/10.3390/heritage4040223
Moussa A, Roshdy M. Monitoring Coptic Masonry Affected by Clay Minerals and Microorganisms at the Church of Virgin Mary, Wadi El-Natrun (Egypt). Heritage. 2021; 4(4):4056-4067. https://doi.org/10.3390/heritage4040223
Chicago/Turabian StyleMoussa, Abubakr, and Mahmoud Roshdy. 2021. "Monitoring Coptic Masonry Affected by Clay Minerals and Microorganisms at the Church of Virgin Mary, Wadi El-Natrun (Egypt)" Heritage 4, no. 4: 4056-4067. https://doi.org/10.3390/heritage4040223
APA StyleMoussa, A., & Roshdy, M. (2021). Monitoring Coptic Masonry Affected by Clay Minerals and Microorganisms at the Church of Virgin Mary, Wadi El-Natrun (Egypt). Heritage, 4(4), 4056-4067. https://doi.org/10.3390/heritage4040223







