Multianalytical Assessment of Armour Paints—The Ageing Characteristics of Historic Drying Oil Varnish Paints for Protection of Steel and Iron Surfaces in Sweden
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Paint Samples
2.1.1. Historical Paints
2.1.2. Replica Paints
2.1.3. Application of Replica Paints to the Steel Sheet Substrate
2.2. Ageing Conditions
2.2.1. Natural Ageing in Southern Sweden
2.2.2. Accelerated Ageing
2.3. Investigations and Analytical Methods
2.3.1. König Pendulum Hardness Rocker
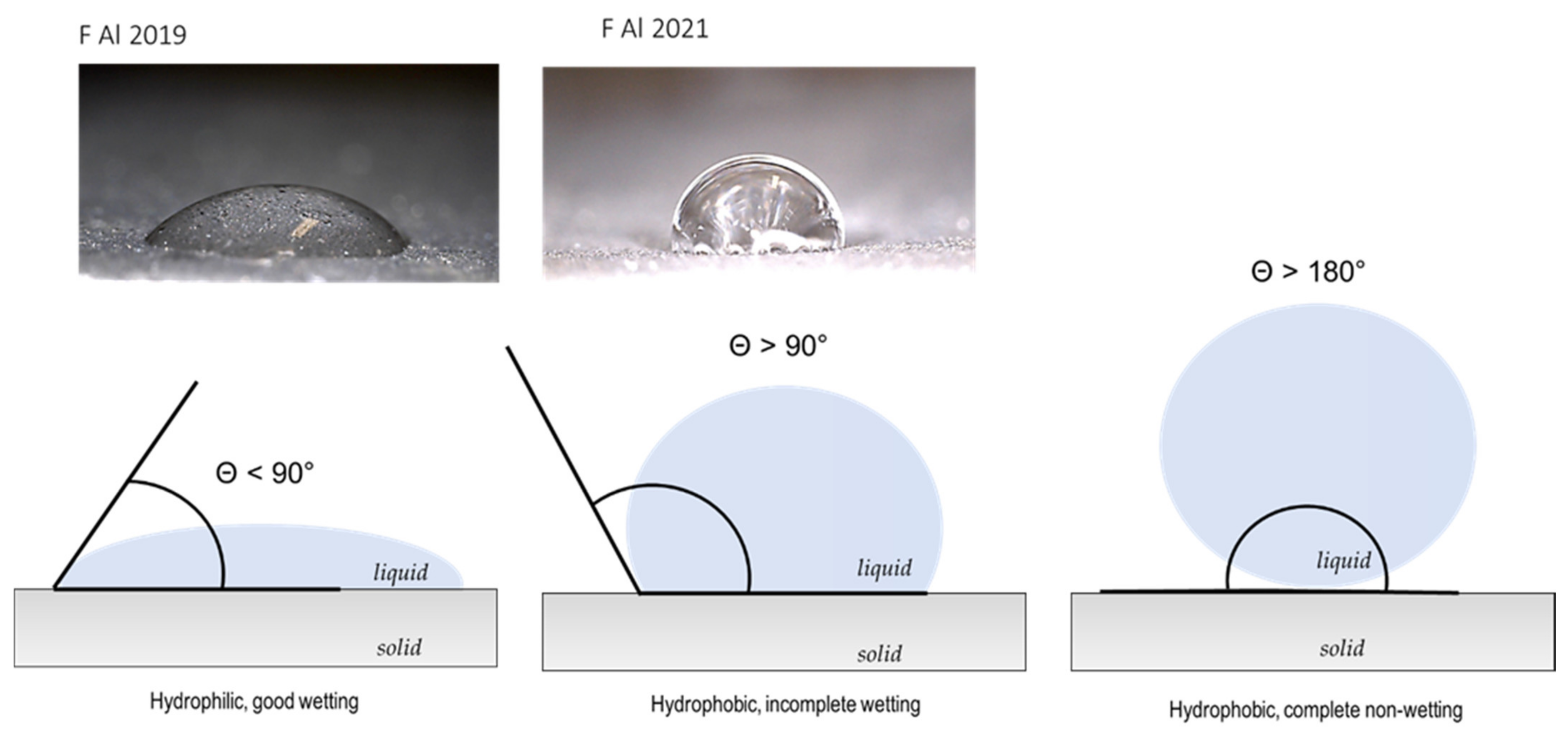
2.3.2. Contact Angle Measurements (Wettability)
2.3.3. Colourimetry
2.3.4. Fourier Transform Infrared Spectroscopy
2.3.5. Gas Chromotography–Mass Spectrometry
3. Results and Discussion
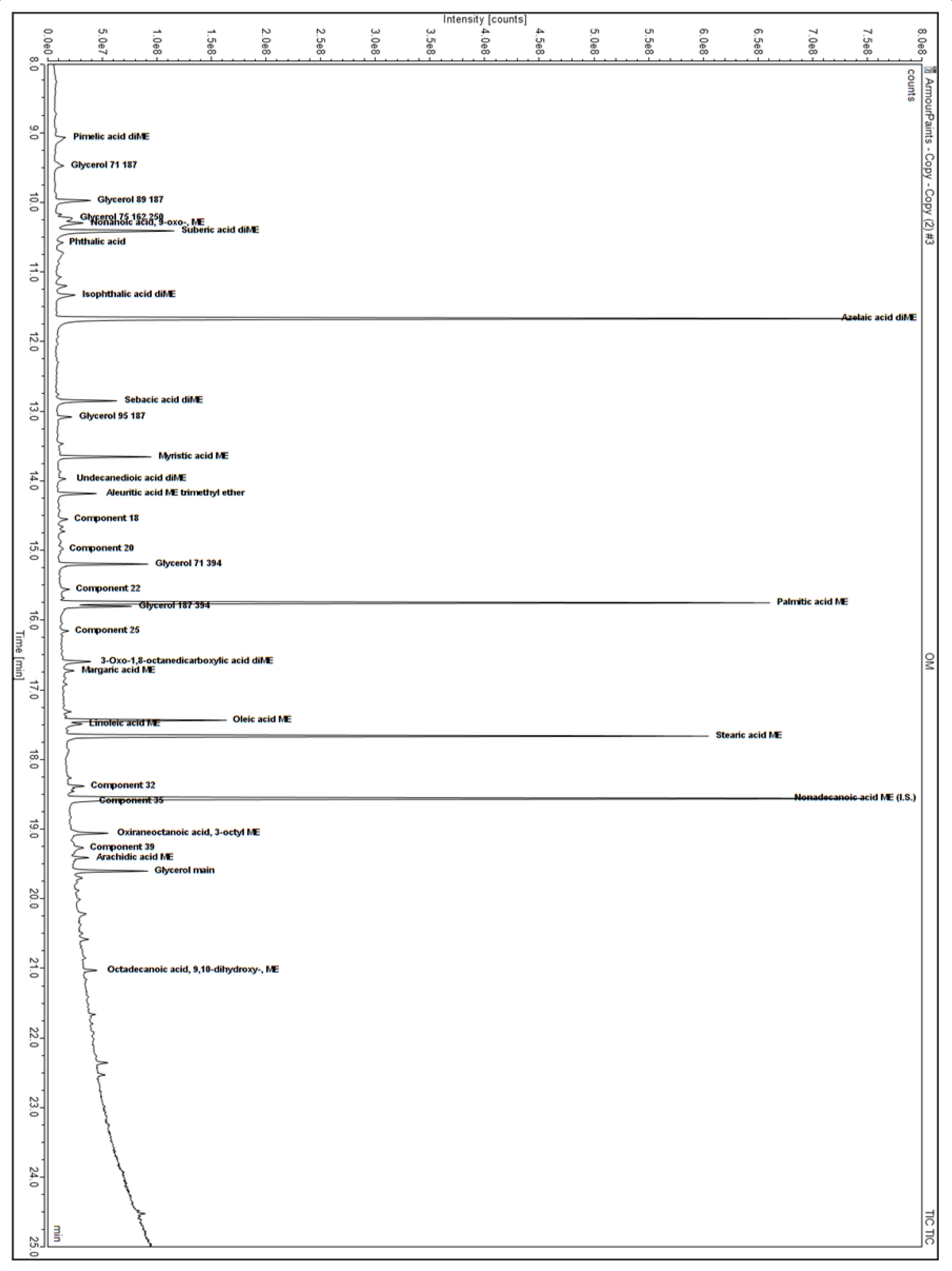
3.1. Organic Composition of Historical Samples
3.2. Replica Paints
3.2.1. Natural Ageing
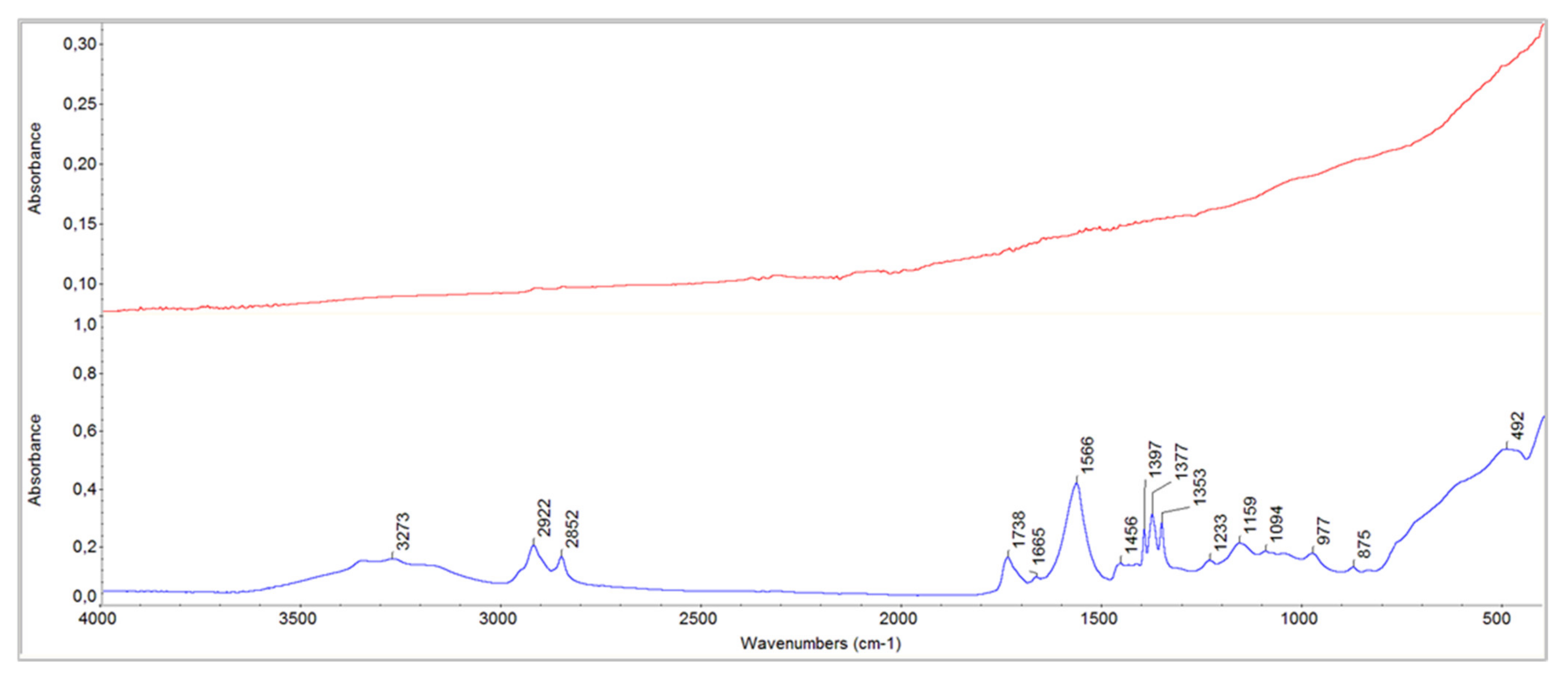
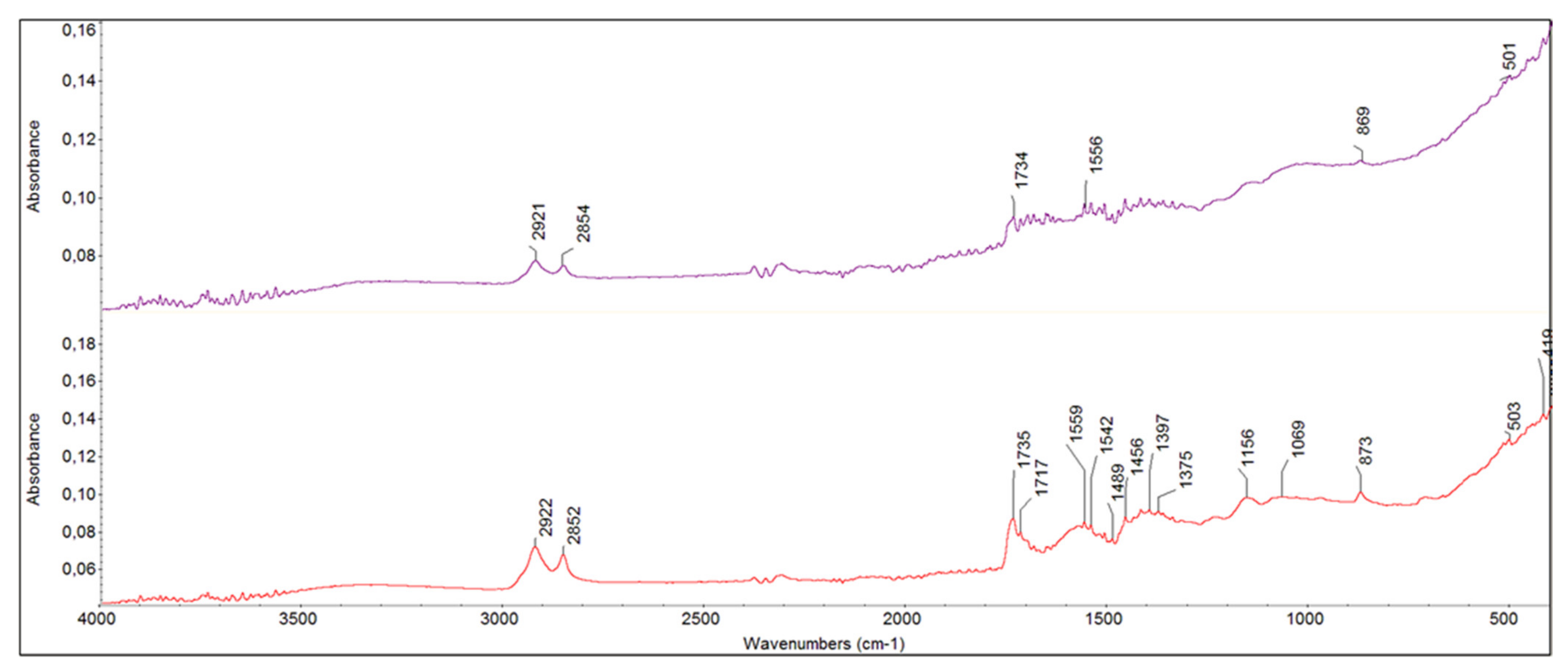
- In the interface between armour paint and lead-containing primer, instead, the organic component was present and visible in the IR spectra (shown in red in Figure 7 and Figure 8). In addition to the typical peaks related to the use of a lipidic binder, the absorptions due to metal carboxylates were clearly visible (in particular the peak very defined at 1566 cm−1).
3.2.2. Accelerated Ageing
- the increase of azelaic acid (especially compared to palmitic acid, A/P ratios) and dicarboxylic acids in general (%D);
- the decrease of unsaturated fatty acids, specifically oleic, linoleic and linolenic acids for P1 and oleic, linoleic and α-eleosteric acid for P2.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Material | Supplier | Specification | P1 | P2 | F Al | F AP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primer | Top Coat | Primer | Top Coat | Primer | Top Coat | Primer | Top Coat | |||
| Red-lead | Kremer Pigmente | 99.7 % lead oxides. | 80.0 | - | 80.0 | - | - | - | ||
| Aluminiumtri-phosphate | Grolman SAS | Rima Cor SWM. | - | - | - | - | 17.5 | 17.5 | ||
| Aluminum pigment | Carlfors Bruk | Leafing grade CB 180S 65. Particle size: 20 µm. Paste mixed with 25–40 wt. % white spirit. | - | 21.0 | - | 21.0 | - | 22.6 | - | 4.8 |
| Micaceous iron oxide (MIO) | Comptoire de mineraux & matieres premieres | Grade Ironor P. | - | 6.8 | - | 6.8 | - | - | 36.0 | |
| Talc | Mondo Minerals | Grade Finntalc M15. | - | 3.4 | - | 3.4 | - | - | ||
| Chalk | Omya | Grade Omya GU5. | - | - | - | - | 4.4 | 12.3 | 4.4 | 5.0 |
| Zinc oxide | Ever Zinc | Grade White Seal > 99% ZnO. | - | 10.2 | - | 10.2 | 14.8 | 10.20 | ||
| Black iron oxide | n.k | n.k | - | - | - | - | - | - | - | 4.6 |
| Pigment paste | n.k | Titanium white and black iron oxide, medium grey. | - | - | - | - | 50.0 | - | 50.0 | |
| Bentonite paste | Claytec | Mixed in n-paraffin and ethanol. | - | 3.95 | - | 3.95 | 5.1 | 2.3 | 5.1 | 2.0 |
| Siccative | Paint-maker | Sr, Ca, Co liquid salts in proportions 1:1:2 in n-paraffin to obtain a balanced drying from surface through the films. | - | 0.35 | - | 0.35 | 0.4 | 1.2 | 0.40 | 1.1 |
| Double boiled linseed oil (WHT) | German linseed oil | High temperature boiled (280 °C), manganese compound as drier. | 20.0 | - | 20.0 | 22.0 | 14.6 | 35.1 | 14.6 | 30.6 |
| Stand Oil (SW) | German linseed oil | Viscosity 20 dPa. Consists of a 1:1 mixture of stand oil 50 dPa and double boiled linseed oil. | - | - | - | 18.6 | ||||
| Tung oil (TW) | German tung oil | - | - | - | - | 13.5 | 8.0 | 11.7 | 8.0 | 10.2 |
| Air blown cold pressed linseed oil (OH) | Modern Swedish linseed oil | In Paint 1 only. Heated to 130–150 °C, drier: Co-Zr ethyl hexane/octate/proprionate | - | 54.3 | - | - | ||||
Appendix B
| Paintings and Paint Samples | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Historical Paints | Paint Recipes | Field Exposed | |||||||||||||
| OM | GMW | BJ Grey | BJ Red | BWP1 | BWP3 | F AI2019 | F AI2021 | F AP2019 | F AP2021 | ||||||
| S | A1 | A2 | S | A1 | A2 | ||||||||||
| Fatty acids and other compounds | Glycerol | 8.6 | 6.2 | 11.5 | 10.5 | 16.8 | 14.8 | 6.6 | 10.0 | 9.2 | 10.5 | 12.1 | 9.2 | 11 | 11.2 |
| Pimelic acid | 0.8 | 1.8 | 1 | 0.5 | 1.0 | 0.5 | 0.8 | 0.9 | 0.4 | 0.8 | 0.4 | 1.0 | 0.6 | 0.9 | |
| Suberic acid | 5.6 | 8.8 | 4.4 | 5.9 | 3.5 | 5.7 | 6.1 | 3.1 | 4.2 | 6.2 | 4.6 | 6.6 | 5.4 | 6.7 | |
| Azelaic acid | 27.9 | 26 | 26.6 | 38.8 | 24.3 | 32.5 | 31.3 | 23.5 | 26.8 | 34.9 | 22.9 | 25.0 | 21.0 | 26.9 | |
| Sebacic acid | 2.5 | 3.4 | 3 | 3 | 1.6 | 2.9 | 3.4 | 1.6 | 2.0 | 2.9 | 2.9 | 4.1 | 4.0 | 5.5 | |
| Myristic acid | 3.2 | 3.1 | 1.4 | 1.1 | 5.3 | 6.4 | 4.6 | 6.2 | 5.0 | 4.2 | 2.2 | 2.0 | 2.0 | 2.2 | |
| Palmitic acid | 23.8 | 16.9 | 24.9 | 22.2 | 19.9 | 23.9 | 21.9 | 22.8 | 20.9 | 17.7 | 17.7 | 18.6 | 16.8 | 22.0 | |
| Stearic acid | 21.3 | 32.9 | 18.6 | 15.1 | 16.7 | 18.5 | 16.7 | 19.8 | 18.9 | 15.8 | 16.4 | 15.4 | 14.3 | 17.3 | |
| Oleic acid | 5.9 | 0.4 | 7.5 | 1.6 | 9.9 | 9.1 | 8.2 | 10.9 | 11.0 | 6.5 | 17.9 | 15.3 | 20.1 | 19.6 | |
| Linoleic acid | 0.5 | 0.6 | 1.1 | 1.3 | 1.0 | 0.4 | 0.4 | 1.2 | 1.7 | 0.5 | 0.3 | 0.1 | 0.4 | 0.3 | |
| Linolenic acid | x | ||||||||||||||
| Alpha-eleostearic acid | x | ? | ? | ? | ? | ||||||||||
| Nonanoic acid, 9-(o-propylphenyl)- | x | x | x | x | x | x | x | ||||||||
| Heptanoic acid 7-(o-pentylphenyl)- | x | x | x | x | x | x | x | ||||||||
| Phthalic acid | x | ||||||||||||||
| Pentaerytritol | x | ||||||||||||||
| Benzoic acid | x | ||||||||||||||
| Arachidic acid | x | x | x | x | |||||||||||
| Behenic acid | x | x | x | x | |||||||||||
| Dehydroabietic acid | x | x | |||||||||||||
| Octadecanoic acid, 9,10-dihydroxy-, | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| hydroxy-and methoxy- octadecanoic acids | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Molar ratios among fatty acids | P/S | 1.1 | 0.5 | 1.3 | 1.5 | 1.3 | 1.3 | 1.3 | 1.2 | 1.1 | 1.1 | 1.2 | 1.2 | 1.2 | 1.2 |
| A/P | 1.2 | 1.5 | 1.1 | 1.7 | 1.2 | 1.4 | 1.4 | 1.0 | 1.3 | 2.0 | 1.3 | 1.3 | 1.2 | 1.2 | |
| D/P | 1.5 | 2.3 | 1.4 | 2.1 | 1.5 | 1.7 | 1.9 | 1.2 | 1.6 | 2.5 | 1.7 | 1.9 | 1.8 | 1.8 | |
| %D | 36.7 | 39.9 | 35 | 48.2 | 30.4 | 41.7 | 41.6 | 29.1 | 33.4 | 44.8 | 30.9 | 36.7 | 31.0 | 40.0 | |
| O/S | 0.3 | <<0.1 | 0.4 | 0.1 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.4 | 1.1 | 1.0 | 1.4 | 1.1 | |
References
- Källbom, A.; Almevik, G. Maintenance of Painted Steel Sheet Roofs on Historic Buildings in Sweden. Int. J. Archit. Herit. 2020, 14, 1–16. [Google Scholar] [CrossRef]
- Reuterswärd, P. Optimal Skötsel av Stålbroar. [Optimal Care of Steel Bridges]; Research Report 2010–130; Swerea KIMAB: Stockholm, Sweden, 2011. [Google Scholar]
- Reuterswärd, P. Ommålning av Kvarnsilos på Kvarnholmen 2013. [Repainting of Mill Silos at Kvarnholmen 2013]; Research Report; Swerea KIMAB: Stockholm, Sweden, 2013. [Google Scholar]
- IVA (The Royal Swedish Academy of Engineering Sciences, Corrosion Committee). Målning av Järnkonstruktioner Utsatta för Atmosfärens Inverkan. Meddelande nr 1. [Painting of Steel Constructions Exposed to Atmospheric Impact. Message no 1.]; Ingenjörsvetenskapakademien: Stockholm, Sweden, 1935. [Google Scholar]
- IVA (The Royal Swedish Academy of Engineering Sciences, Corrosion Committee). Handbok I Rostskyddsmålning av Stålkonstruktioner Utsatta för Atmosfärens Inverkan. [Handbook in Anticorrosive Painting of Steel Constructions Exposed to the Atmosphere]; Ingenjörsvetenskapsakademien: Stockholm, Sweden, 1961. [Google Scholar]
- Edwards, J.D. Aluminum Paint and Powders, 1st ed.; Reinhold Publishing Corporation: New York, NY, USA, 1936. [Google Scholar]
- Holland, A. Decorative and functional metallic effect pigments. Paint Coat. Ind. 2016, 32, 82–84. [Google Scholar]
- Jordan, L. Tung oil. Particularly referring to the possibilities of production within the British Empire, with a bibliography of the literature. J. Soc. Chem. Ind. 1929, 48, 847–859. [Google Scholar] [CrossRef]
- Standeven, H. House Paints, 1900–1960: History and Use; Getty Conservation Institute: Los Angeles, CA, USA, 2011. [Google Scholar]
- Ling, T.T.; Rhodes, F.H. The Oxidation of Chinese Wood Oil. Ind. Eng. Chem. 1925, 17, 508–512. [Google Scholar]
- Hilditch, T.P.; Mendelowitz, A. The component fatty acids and glycerides of tung oil. J. Sci. Food Agric. 1951, 2, 548–556. [Google Scholar] [CrossRef]
- Schönemann, A.; Frenzel, W.; Unger, A.; Kenndler, E. An Investigation of the Fatty Acid Composition of New and Aged Tung Oil. Stud. Conserv. 2006, 51, 99–110. [Google Scholar] [CrossRef]
- Suida, H.; Salvaterra, H. Rostschutz Und Rostschutzanstrich; Technisch-Gewerbliche, B., Wien, J., Eds.; Springer: Berlin, Germany, 1931; Volume 6. [Google Scholar]
- Singer, E. Fundamentals of Paints, Varnish and Lacquer Technology; The American Paint Journal Company: Washington, DC, USA, 1957. [Google Scholar]
- Sabin, A.H. Industrial and Artistic Technology of Paint and Varnish; John Wiley & Sons: New York, NY, USA, 1927. [Google Scholar]
- Nylén, P. Färger, lacker och fernissor. [Paints, lacquers and varnishes]. In Handbok i Kemisk Teknologi. Band III. Red; Angel, G., Ed.; Natur och Kultur: Stockholm, Sweden, 1948. [Google Scholar]
- Crebert, T.H. Eigenschaften und Eigenarten von Lyftoxydiertem Leinöl, eine Übersicht. Fette Und Seife 1938, 45, 676. [Google Scholar] [CrossRef]
- Stenberg, C. Influence of the Fatty Acid Pattern on the Drying of Linseed Oils. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2004. [Google Scholar]
- Kalpers, H. Der Anstrich aus Aluminium. Dingler Polytech. J. 1930, 345, 64–67. [Google Scholar]
- Bayliss, D.; Deacon, D. Steelwork Corrosion Control, 2nd ed.; Spon Press: London, UK, 2002. [Google Scholar]
- Brock, T.; Groteklaes, M.; Mischke, P. European Coatings Handbook; Curt R Vincent Verlag: Hanover, Germany, 2002. [Google Scholar]
- Forsgren, M. Corrosion Control Through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Sørensen, P.; Kiil, A.; Dam-Johansen, S.; Weinell, K. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Talbert, R. Paint Technology Handbook; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Malshe, V.; Waghoo, G. Chalk resistant epoxy resins. Prog. Org. Coat. 2004, 51, 172–180. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Farzaneh, S.; Abdallah-Elhirtsi, S.; Esmaeillou, B.; Nony, F.; Baron, A. Thermal Aging Effect on Mechanical Properties of Polyurethane. Int. J. Polym. Anal. Charact. 2014, 19, 571–584. [Google Scholar] [CrossRef] [Green Version]
- International Council on Monuments and Sites. The Nara Document on Authenticity; ICOMOS: Nara, Japan, 1994. [Google Scholar]
- International Council on Monuments and Sites. ICOMOS Charter—Principles for the Analysis, Conservation and Structural Restoration of Architectural Heritage; ICOMOS: Victoria Falls, Zambia, 2003. [Google Scholar]
- International Council on Monuments and Sites. European Quality Principles for EU-Funded Interventions with Potential Impact Upon Cultural Heritage; ICOMOS: Paris, France, 2019. [Google Scholar]
- Araujo, W.S.; Margarit, I.C.P.; Mattos, O.R.; Fragata, F.L.; de Lima-Neto, P. Corrosion Aspects of Alkyd Paints Modified with Linseed and Soy Oils. Electrochim. Acta 2010, 55, 6204–6211. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Esfandeh, M.; Farnood, R.R. Evaluation of Corrosion Performance of a Self-healing Epoxy-based Coating Containing Linseed Oil-filled Microcapsules via Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 2017, 105, 212–224. [Google Scholar] [CrossRef]
- Ahmad, S.; Ashraf, S.; Zafar, F. Development of linseed oil based polyesteramide without organic solvent at lower temperature. J. Appl. Polym. Sci. 2007, 104, 1143–1148. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Wissling, P. Metallic Effect Pigments. In European Coatings Literature; Vincentz Network: Hannover, Germany, 2006. [Google Scholar]
- Wei, L.; Haiping, Z.; Yuanyuan, S.; Hui, Z.; Jesse, Z. Preparation of aluminium metallic pigmented powder coatings with high color stability using a novel method: Microwave bonding. Prog. Org. Coat. 2020, 147, 105787. [Google Scholar]
- Gao, A.; Pi, P.; Wen, X.; Zheng, D.; Cai, Z.; Cheng, J.; Yang, Z. Preparation and characterisation of aluminium pigments encapsulated by composite layer containing organic silane acrylate resin and SiO2. Pigment Resin Technol. 2012, 41, 149–155. [Google Scholar] [CrossRef]
- Babcock, G.M.; Painfield, N.J.; Retwish, F.B.; Woolsey, W.P. Stabilised Vehicles for Leafing Aluminium Coatings. U.S. Patent 2904525, 15 September 1959. [Google Scholar]
- Kotlík, P.; Doubravová, K.; Horálek, J.; Kubáč, L.; Akrman, J. Acrylic copolymer coatings for protection against UV rays. J. Cult. Herit. 2014, 15, 44–48. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Chiantore, O. Thermally assisted hydrolysis and methylation-pyrolysis-gas chromatography/mass spectrometry of light-aged linseed oil. J. Anal. Appl. Pyrolysis 2001, 58, 503–512. [Google Scholar] [CrossRef]
- Izzo, F.C.; Balliana, E.; Pinton, F.; Zendri, E. A preliminary study of the composition of commercial oil, acrylic and vinyl paints and their behaviour after accelerated ageing conditions. Conserv. Sci. Cult. Herit. 2014, 14, 353–369. [Google Scholar]
- Berg, J.; Van den Berg, K.J.; Boon, J. Chemical changes in curing and ageing oil paints. In Proceedings of the 12th Triennial Meeting, Lyon, France, 29 August–3 September 1999; pp. 248–253. [Google Scholar]
- van den Berg, J.D.; Van den Berg, K.J.; Boon, J.J. Identification of non-cross-linked compounds in methanolic extracts of cured and aged linseed oil-based paint films using gas chromatography–mass spectrometry. J. Chromatogr. A 2002, 950, 195–211. [Google Scholar] [CrossRef]
- Erhardt, D.; Tumosa, C.S.; Mecklenburg, M.F. Long-Term Chemical and Physical Processes in Oil Paint Films. Stud. Conserv. 2005, 50, 143–150. [Google Scholar] [CrossRef]
- Erhardt, D.; Tumosa, C.S.; Mecklenburg, M. Natural and accelerated thermal aging of oil paint films. Stud. Conserv. 2000, 45, 65–69. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, Q. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Fuster-López, L.; Izzo, F.C.; Piovesan, M.; Sperni, L.; Zendri, E. Study of the chemical composition and the mechanical behaviour of 20th century commercial artists’ oil paints containing manganese-based pigments. Microchem. J. 2016, 124, 962–973. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. ISO 2178:2016: Non-Magnetic Coatings on Magnetic Substrates—Measurement of Coating Thickness—Magnetic Method; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- International Organization for Standardization. ISO 4287:1997: Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters; International Organization for Standardization: Genève, Switzerland, 1997. [Google Scholar]
- International Organization for Standardization. ISO 12944-2:1998: Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 2: Classification of Environments; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Atlas. Weathering Testing Guidebook; Atlas Material Testing Solutions: Chicago, IL, USA, 2001. [Google Scholar]
- Wernståhl, K. Jämförelser Mellan Ljusdoser Vid Naturlig Och Accelererad Väderexponering. [Comparison between Light Doses at Natural and Accelerated Weather Exposure]; SP Ytskydd och korrosion (RISE): Borås, Sweden, 1993. [Google Scholar]
- International Organization for Standardization. ISO 4628-6:2011: Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 6: Assessment of Degree of Chalking by Tape Method; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Irigoyen, M.; Bartolomeo, P.; Perrin, F.X.; Aragon, E.; Vernet, J.L. UV ageing characterisation of organic anticorrosion coatings by dynamic mechanical analysis, Vickers microhardness, and infra-red analysis. Polym. Degrad. Stab. 2001, 74, 59–67. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, Z.; Huang, Z.; Jing, X. The dependence of pendulum hardness on the thickness of acrylic coating. J. Coat. Technol. Res. 2013, 10, 433–439. [Google Scholar] [CrossRef]
- Izzo, F. 20th Century Artists’ Oil Paints: A Chemical–Physical Survey. Ph.D. Thesis, University “Ca’ Foscari”, Venice, Italy, 2011. [Google Scholar]
- Izzo, F.C.; van den Berg, K.J.; van Keulen, H.; Ferriani, B.; Zendri, E. Modern Oil Paints—Formulations, Organic Additives and Degradation: Some Case Studies. In Issues in Contemporary Oil Paint; van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., Tagle, A., Heydenreich, d., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Izzo, F.C.; Zanin, C.; van Keulen, H.; Da Roit, C. From pigments to paints: Studying original materials from the atelier of the artist Mariano Fortuny y Madrazo. Int. J. Conserv. Sci. 2017, 8, 547–564. [Google Scholar]
- Caravá, S.; García, C.R.; de Agredos-Pascual, M.L.V.; Mascarós, S.M.; Izzo, F. Investigation of modern oil paints through a physico-chemical integrated approach. Emblematic cases from Valencia, Spain. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2020, 240, 118633. [Google Scholar] [CrossRef]
- Fuster-López, L.; Izzo, F.; Damato, V.; Yusà-Marco, D.; Zendri, E. An insight into the mechanical properties of selected commercial oil and alkyd paint films containing cobalt blue. J. Cult. Herit. 2019, 35, 225–234. [Google Scholar] [CrossRef]
- Fuster-López, L.; Izzo, F.C.; Andersen, C.K.; Murray, A.; Vila, A.; Picollo, M.; Stefani, L.; Jiménez, R.; Aguado-Guardiola, E. Picasso’s 1917 paint materials and their influence on the condition of four paintings. SN Appl. Sci. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Iedema, P.D. Toward a complete molecular model for the formation of metal soaps in oil paints. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Boon, J.J.; Hoogland, F.; Keune, K. Chemical processes in aged oil paints affecting metal soap migration and aggregation. In Proceedings of the AIC Annual Meeting, Providence, RI, USA, 16–19 June 2006. [Google Scholar]
- Keune, K.; Boon, J.J. Analytical Imaging studies of cross-sections of paintings affected by lead soap aggregate formation. Stud. Conserv. 2007, 52, 161–176. [Google Scholar] [CrossRef]
- Ploeger, R.; Scalarone, D.; Chiantore, O. he characterization of commercial artists’ alkyd paints. J. Cult. Herit. 2008, 9, 412–419. [Google Scholar] [CrossRef]
- Arminger, B.; Jaxel, J.; Bacher, M.; Gindl-Altmutter, W.; Hansmann, C. On the drying behavior of natural oils used for solid wood finishing. Prog. Org. Coat. 2020, 148, 105831. [Google Scholar] [CrossRef]
- Grundke, K.; Pöschel, K.; Synytska, A.; Frenzel, R.; Drechsler, A.; Nitschke, M.; Welzel, P.B. Experimental studies of contact angle hysteresis phenomena on polymer surfaces—Toward the understanding and control of wettability for different applications. Adv. Colloid Interface Sci. 2015, 222, 350–376. [Google Scholar] [CrossRef]
- Hansen, D. Fakta om Linoljefärg [Facts about Linseed Oil Paints]. Wibo Färg AB. 2021. Available online: http://www.wibofarg.se/knappar/varfor-linoljefarg/fakta-om-linoljefarg.html (accessed on 9 June 2021).
- Wexler, H. Polymerization of drying oils. Chem. Rev. 1964, 64, 591–611. [Google Scholar] [CrossRef]
- Rogala, D.; Lake, S.; Maines, C.; Mecklenburg, M. Condition Problems Related to Zinc Oxide Underlayers: Examination of Selected Abstract Expressionist Paintings from the Collection of the Hirshhorn Museum and Sculpture Garden, Smithsonian Institution. J. Am. Inst. Conserv. 2010, 49, 96–113. [Google Scholar] [CrossRef]
- Osmond, G. Zinc white: A review of zinc oxide pigment properties and implications for stability in oil-based paintings. AICCM Bull. 2012, 33, 20–29. [Google Scholar] [CrossRef]
- Sebedio, J.L.; Grandgirard, A. Cyclic fatty acids: Natural sources, formation during heat treatment, synthesis and bio logical properties. Prog. Lipid Res. 1989, 28, 303–336. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 1522:1998. Paints and Varnishes—Pendulum Damping Test; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- International Organization for Standardization. ISO 16474-2:2013. Paints and Varnishes—Methods of Exposure to Laboratory Light Sources. Part 2: Xenon-Arc Lamps; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
Short Biography of Authors






| Typology | Name | Object Description | Comment |
|---|---|---|---|
| Historical paints | OM | National listed building, The Oat Mill in Nacka, Stockholm. | Aluminum pigmented armour paint from a structural part that has been protected from atmospheric exposure since 1934. The silos were painted with original armour paints in 1928. |
| GMW | A cast iron sign for an industry; Göteborgs Mekaniska Werkstad | Aluminium pigmented armour paint ca. 1920–1940. | |
| BJ-red | Bridge in Björneborg (built 1870) | The original paints have been overpainted (sparkling top paint beneath, indicating scale armour paint). | |
| BJ-grey | Overpainting. | ||
| Replica paints | F-Al | Field (outdoor) exposed armour paints on steel sheet, in Mariestad, Sweden. | Replica aluminium pigmented armour paint, field tests started April 2017, outtake in December 2019 (33 months) and March 2021 (49 months). |
| F-AP | Replica armour paint, field tests started April 2017, outtake in December 2019 (33 months) and March 2021 (49 months). Higher content of MIO, greyish. | ||
| P1-R | Artificially aged. Paint 1—solid reference. | Replica paints. Primer of red lead and top layer of aluminium pigmented armour paint. Blown linseed varnish as binder. | |
| P1-A1 | Artificially aged. Paint 1—Aged 1 | As above. First outtake 1000 h. | |
| P1-A2 | Artificially aged. Paint 1—Aged 2 | As above. Second outtake 5500 h. | |
| P2-R | Artificially aged. Paint 2—solid reference | Replica paints. Primer of red lead and top layer of aluminium pigmented armour paint. Heat-bodied linseed oil varnish, stand oil and tung oil mixture as binder. | |
| P2-A1 | Artificially aged. Paint 2—Aged 1 | As above. First outtake 1000 h | |
| P2-A2 | Artificially aged. Paint 2—Aged 2 | As above. Second outtake 5500 h. |
| Variant | Reference (S) | A1 (1000 h) | A2 (5500 h) | |
|---|---|---|---|---|
| Tape tests (ISO4628-6:2011) | ||||
| Paint 1 | 0 | ≤2 | ≤2 | |
| Paint 2 | 0 | ≤2 | ≤2 | |
| Contact angle | ||||
| Paint 1 | 67.9 ± 1.0° | 89. 9 ± 0.7° | 89.3 ± 0.6° | |
| Paint 2 | 77.1 ± 0.8° | 86.6 ± 0.6° | 85.7 ± 0.5° | |
| König pendulum rocker counter (Sd), cumulative increase in percentage (ISO 1522:1998) [73] | ||||
| Paint 1 | 16.3 (0.6) | 33.8 (6.5) 96.8% | 48.4 (6.7) 196.9% | |
| Paint 2 | 14.3 (1.2) | 37.5 (3.0) 162.2% | 48.8 (6.7) 241.2% | |
| Colour changes with respect to reference | ||||
| Paint 1 | ΔL | - | 10 | −7 |
| Δa | - | 0.3 | 0.9 | |
| Δb | - | 0.7 | 2.7 | |
| ΔE | - | 10.0 | 7.6 | |
| Paint 2 | ΔL | - | 12.3 | −8.2 |
| Δa | - | 0.3 | 1.0 | |
| Δb | - | 0.3 | 3.9 | |
| ΔE | - | 12.3 | 9.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Källbom, A.; Nevin, A.; Izzo, F.C. Multianalytical Assessment of Armour Paints—The Ageing Characteristics of Historic Drying Oil Varnish Paints for Protection of Steel and Iron Surfaces in Sweden. Heritage 2021, 4, 1141-1164. https://doi.org/10.3390/heritage4030063
Källbom A, Nevin A, Izzo FC. Multianalytical Assessment of Armour Paints—The Ageing Characteristics of Historic Drying Oil Varnish Paints for Protection of Steel and Iron Surfaces in Sweden. Heritage. 2021; 4(3):1141-1164. https://doi.org/10.3390/heritage4030063
Chicago/Turabian StyleKällbom, Arja, Austin Nevin, and Francesca C. Izzo. 2021. "Multianalytical Assessment of Armour Paints—The Ageing Characteristics of Historic Drying Oil Varnish Paints for Protection of Steel and Iron Surfaces in Sweden" Heritage 4, no. 3: 1141-1164. https://doi.org/10.3390/heritage4030063
APA StyleKällbom, A., Nevin, A., & Izzo, F. C. (2021). Multianalytical Assessment of Armour Paints—The Ageing Characteristics of Historic Drying Oil Varnish Paints for Protection of Steel and Iron Surfaces in Sweden. Heritage, 4(3), 1141-1164. https://doi.org/10.3390/heritage4030063








