Yellow Lake Pigments from Weld in Art: Investigating the Winsor & Newton 19th Century Archive
Abstract
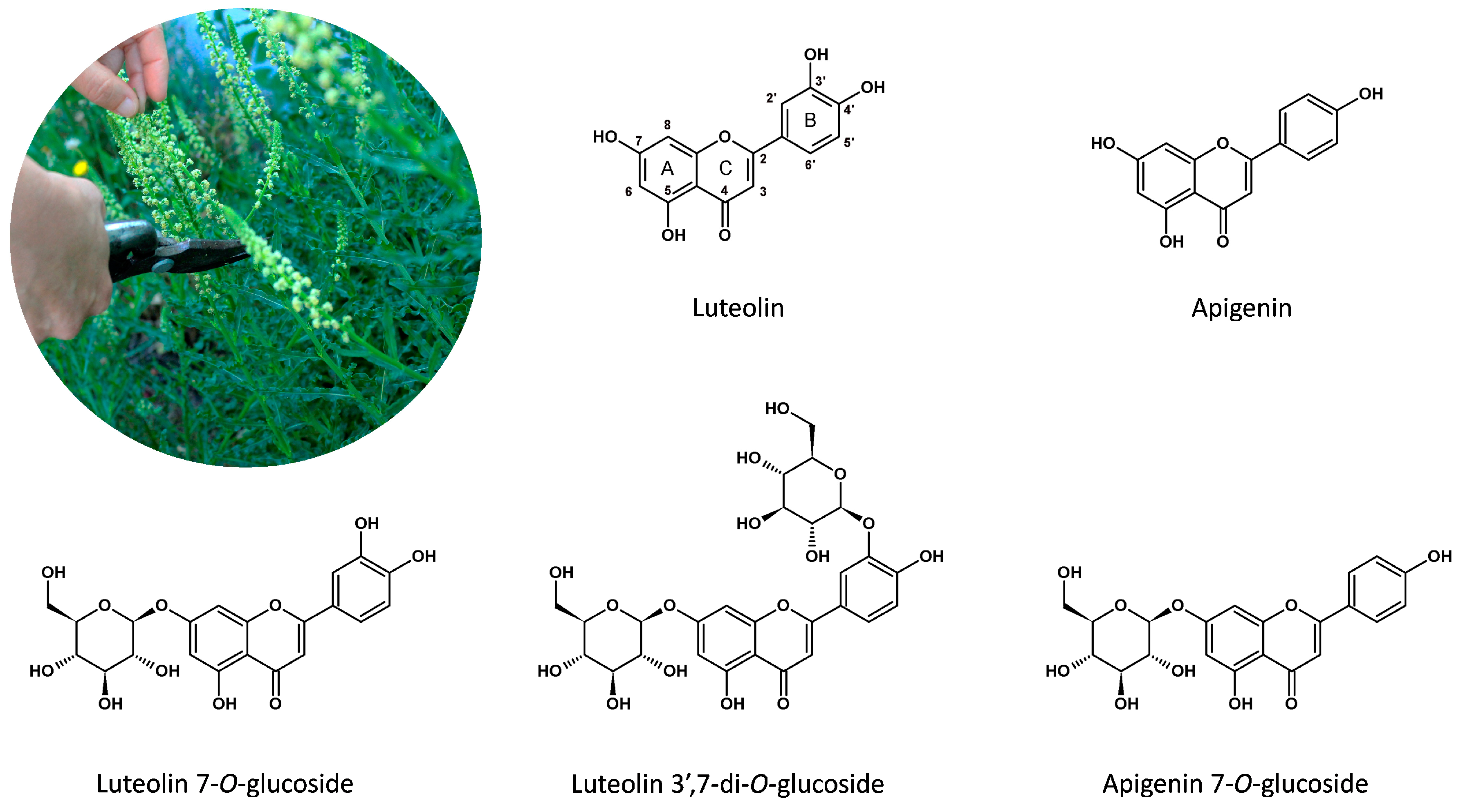
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods for Weld Lake Pigments
2.3. Paint References
2.4. Equipment and Characterization Methods
2.4.1. Colorimetry
2.4.2. High-Performance Liquid Chromatography with a Diode Array Detector (HPLC-DAD)
2.4.3. Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry (UHPLC-DAD-HRMS)
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Weld Lake Pigment Recipes in the Winsor & Newton 19th Century Archive Database
3.2. Extraction Method
3.3. Characterization of the Weld Lake Pigments
3.3.1. HPLC-DAD Analysis
3.3.2. FTIR Analysis
3.4. Infrared Markers of Weld Lake Pigments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype Publications: London, UK, 2007. [Google Scholar]
- Kirby, J.; van Bommel, M.; Verhecken, A. Natural Colorants for Dyeing and Lake Pigments: Practical Recipes and Their Historical Sources; Archetype Publications: London, UK, 2014. [Google Scholar]
- Clarke, M. Colours versus colorants in art history: Evaluating lost manuscript yellows. Rev. História Arte 2011, 1, 138–151. [Google Scholar]
- Zhang, X.; Good, I.; Laursen, R. Characterization of dyestuffs in ancient textiles from Xinjiang. J. Archaeol. Sci. 2008, 35, 1095–1103. [Google Scholar] [CrossRef]
- Liu, J.; Guo, D.; Zhou, Y.; Wu, Z.; Li, W.; Zhao, F.; Zheng, X. Identification of ancient textiles from Yingpan, Xinjiang, by multiple analytical techniques. J. Archaeol. Sci. 2011, 38, 1763–1770. [Google Scholar] [CrossRef]
- Marques, R.; Sousa, M.M.; Oliveira, M.C.; Melo, M.J. Characterization of Weld (Reseda luteola L.) and spurge flax (Daphne gnidium L.) by high-performance liquid chromatography-diode array detection-mass spectrometry in Arraiolos historical textiles. J. Chromatog. A 2009, 1216, 1395–1402. [Google Scholar] [CrossRef]
- Nyström, I. Spectroscopic analysis of artists’ pigments and materials used in southern Swedish painted wall hangings from the eighteenth and nineteenth centuries. Stud. Conservat. 2015, 60, 353–367. [Google Scholar] [CrossRef]
- Moiteiro, C.; Gaspar, H.; Rodrigues, A.I.; Lopes, J.F.; Carnide, V. HPLC quantification of dye flavonoids in Reseda luteola L. from Portugal. J. Sep. Sci. 2008, 31, 3683–3687. [Google Scholar] [CrossRef] [PubMed]
- Cristea, D.; Bareau, I.; Vilarem, G. Identification and quantitative HPLC analysis of the main flavonoids present in Weld (Reseda luteola L.). Dyes Pigment. 2003, 57, 267–272. [Google Scholar] [CrossRef]
- Ferreira, E. New Approaches Towards the Identification of Yellow Dyes in Ancient Textiles. Ph.D. Thesis, University of Edinburgh, Edinburgh, Scotland, 2002. [Google Scholar]
- Amat, A.; Clementi, C.; Miliani, C.; Romani, A. Complexation of apigenin and luteolin in weld lake: A DFT/TDDFT investigation. Phys. Chem. 2010, 12, 6672–6684. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yang, X.; He, J.; Cai, S.; Xiao, K.; Zhu, L. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese (II) and its inhibition kinetics on xanthine oxidase. RSC Adv. 2017, 7, 53385–53395. [Google Scholar] [CrossRef]
- Gao, L.G.; Wang, H.; Song, X.L.; Cao, W. Research on the chelation between luteolin and Cr (III) ion through infrared spectroscopy, UV-vis spectrum and theoretical calculations. J. Mol. Struct. 2013, 1034, 386–391. [Google Scholar] [CrossRef]
- Smith, G.J.; Thomsen, S.J.; Markham, K.R.; Andary, C.; Cardon, D. The photostabilities of naturally occurring 5-hydroxyflavones, flavonols, their glycosides and their aluminium complexes. J. Photoch. Photobio. A 2000, 136, 87–91. [Google Scholar] [CrossRef]
- Castro, R.; Miranda, A.; Melo, M.J. Interpreting lac dye in medieval written sources: New knowledge from the reconstruction of recipes relating to illuminations in Portuguese manuscripts. In Sources in Art Technology: Back to Basics; Clarke, M., Townsend, J., Stijnman, A., Eds.; Archetype Publications: London, UK, 2016; pp. 88–99. [Google Scholar]
- Vitorino, T.; Melo, M.J.; Carlyle, L.; Otero, V. New insights into brazilwood manufacture through the use of historically accurate reconstructions. Stud. Conserv. 2015, 61, 255–273. [Google Scholar] [CrossRef]
- Melo, M.J.; Castro, R.; Nabais, P.; Vitorino, T. The book on how to make all the colour paints for illuminating books: Unravelling a Portuguese Hebrew illuminators’ manual. Herit. Sci. 2018, 6, 44. [Google Scholar] [CrossRef]
- Vitorino, T.; Otero, V.; Carlyle, L.; Melo, M.J.; Parola, A.J.; Picollo, M. Nineteenth-century cochineal lake pigments from Winsor & Newton: Insight into their methodology through reconstructions. In Proceedings of the ICOM-CC 18th Triennial Conference Preprints, Copenhagen, Denmark, 4–8 September 2017; Bridgland, J., Ed.; Paper 0107. [Google Scholar]
- Nabais, P.; Oliveira, J.; Pina, F.; Teixeira, N.; de Freitas, V.; Brás, N.F.; Clemente, A.; Rangel, M.; Silva, A.M.S.; Melo, M.J. A 1000-year-old mystery solved: Unlocking the molecular structure for the medieval blue from Chrozophora tinctoria, also known as folium. Sci. Adv. 2020, 6, eaaz7772. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Carlyle, L. Page-image recipe databases, a new approach for accessing art technological manuscripts and rare printed sources: The Winsor & Newton archive prototype. In Proceedings of the ICOM-CC 14th Triennial Meeting, The Hague, The Netherlands, 12–16 September 2005; Bridgland, J., Ed.; James & James: London, UK, 2005; Volume 1, pp. 24–29. [Google Scholar]
- Clarke, M.; Carlyle, L. Page-image recipe databases: A new approach to making art technological manuscripts and rare printed sources accessible. In Art of the Past—Sources and Reconstructions; Clarke, M., Townsend, J., Stijnman, A., Eds.; Archetype Publications: London, UK, 2005; pp. 49–52. [Google Scholar]
- Carlyle, L.; Alves, P.; Otero, V.; Melo, M.J.; Vilarigues, M. A question of scale and terminology, extrapolating from past practices in commercial manufacture to current laboratory experience: The Winsor & Newton 19th century artists’ materials archive database. In Proceedings of the ICOM-CC 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011; Bridgland, J., Ed.; Paper 0102. [Google Scholar]
- Carlyle, L. The Artist’s Assistant: Oil Painting Instruction Manuals and Handbooks in Britain, 1800–1900, with Reference to Selected Eighteenth-Century Sources; Archetype Publications: London, UK, 2001. [Google Scholar]
- Otero, V.; Pinto, J.V.; Carlyle, L.; Vilarigues, M.; Cotte, M.; Melo, M.J. Nineteenth century chrome yellow and chrome deep from Winsor & NewtonTM. Stud. Conservat. 2017, 62, 123–149. [Google Scholar]
- Otero, V.; Campos, M.F.; Pinto, J.V.; Vilarigues, M.; Carlyle, L.; Melo, M.J. Barium, zinc & strontium yellows in late 19th-early 20th century oil paintings. Herit. Sci. 2017, 5, 46. [Google Scholar] [CrossRef]
- Mouri, C.; Mozaffarian, V.; Zhang, X.; Laursen, R. Characterization of flavonols in plants used for textile dyeing and the significance of flavonol conjugates. Dyes Pigment. 2014, 100, 135–141. [Google Scholar] [CrossRef]
- Zhang, X.; Laursen, R.A. Development of mild extraction methods for the analysis of natural dyes in textiles of historical interest using LC-diode array detector-MS. Anal. Chem. 2005, 77, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Favaro, G.; Clementi, C.; Romani, A.; Vickackaite, V. Acidichromism and ionochromism of luteolin and apigenin, the main components of the naturally occurring yellow weld: A spectrophotometric and fluorimetric study. J. Fluoresc. 2007, 17, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Villela, A.; van der Klift, E.J.C.; Mattheussens, E.S.G.M.; Derksen, G.C.H.; Zuilhof, H.; van Beek, T.A. Fast chromatographic separation for the quantitation of the main flavone dyes in Reseda luteola (weld). J. Chromatogr. A 2011, 1218, 8544–8550. [Google Scholar] [CrossRef] [PubMed]
- Villela, A. Textile Dyeing with a Flavonoid Dye: Photo-Stability and Analytical Chemistry Methods. Ph.D. Thesis, Wageningen University, Wageningen, The Netherland, 2020. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Machado, N.F.L.; de Batista Carvalho, L.A.E.; Otero, J.C.; Marques, M.P.M. A conformational study of hydroxyflavones by vibrational spectroscopy coupled to DFT calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 109, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Baranović, G.; Šegota, S. Infrared spectroscopy of flavones and flavonols. Reexamination of the hydroxyl and carbonyl vibrations in relation to the interactions of flavonoids with membrane lipids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Heneczkowski, M.; Kopacz, M.; Nowak, D.; Kuzniar, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. Drug Res. 2001, 58, 415–420. [Google Scholar]
- Sanches, D.; Ramos, A.M.; Coelho, J.F.J.; Costa, C.S.M.F.; Vilarigues, M.; Melo, M.J. Correlating thermophysical properties with the molecular composition of 19th century chrome yellow oil paints. Polym. Degrad. Stab. 2017, 138, 201–211. [Google Scholar] [CrossRef]


| Production Name | Unique Recipe Code § | Pigment Code | Synthesis Methods |
|---|---|---|---|
| Yellow from Weld | 4PP148AL01 (copy in P4P088L01) | WL1 | A. To 10 mL of boiling water add 0.2 g of CaCO3 and then slowly add 0.86 g of KAl(SO4)2·12H2O, always stirring. Leave it to rest and decant the solution; keep the precipitate. B. To 50 mL of boiling water add 2 g of weld flowers. Then add 0.014 g of KHCO3 and leave it to boil during 20 min. Filter it and keep the solution. Put the solution B to boil. When boiling, add the precipitate A and leave it boiling for 1 h, always stirring. Leave it to rest for 1 day and filter the yellow lake pigment. |
| 4PP148AL14 (copy in P4P089L14) | WL2 | A. To 10 mL of boiling water add 0.86 g of KAl(SO4)2·12H2O and then slowly add 0.2 g of CaCO3, always stirring. Leave it to rest and decant the solution; keep the precipitate. B. To 50 mL of boiling water add 2 g of weld flowers. Then add 0.014 g of KHCO3 and leave it to boil during 20 min. Filter it and keep the solution. Put the solution B to boil. When boiling, add the precipitate A and leave it boiling for 1 h, always stirring. Leave it to rest for 1 day and filter the yellow lake pigment. | |
| Yellow Lake. Cool tint. | P1P348AL01 (copy in X6P228L01 ¥) | WL3 | To 50 mL of boiling water add 0.43 g of K2CO3. When dissolved add 2 g of weld flowers and leave it to boil during 20 min. Filter it and keep the solution. To the yellow solution add 0.86 g of KAl(SO4)2·12H2O, always stirring. Leave it to rest and filter the yellow lake pigment. |
| Weld Yellow | P4P100L10 | WL4 | To 50 mL of boiling water add 0.018 g of K2CO3 and then 4 g of weld flowers. Boil 10 min. Filter it and keep the solution. To the yellow solution add 0.107 g of KAl(SO4)2·12H2O and then 0.07 g of Na2B4O7.10H2O, always stirring. Leave it to rest and filter the yellow lake pigment. |
| WL5 | To 50 mL of boiling water add 0.018 g of K2CO3 and then 2 g of weld flowers. Boil 10 min. Filter it and keep the solution. To the yellow solution, add 0.177 g of Al(OH)3, always stirring. Leave it to rest and filter the yellow lake pigment. |
| Dye Source | Extraction Method | Complexing Agent | Additives | |||
|---|---|---|---|---|---|---|
| Weld | Potassium bicarbonate KHCO3 | Potassium carbonate K2CO3 | Alum KAl(SO4) 2 | Hydrated alumina Al(OH)3 | Calcium carbonate CaCO3 | Sodium Borate Na2B4O7 |
 |  |  |  |  |  |  |
| Production name | Code | Synthesis | pH extraction 1 | pH final | η (%) 2 | |
| Yellow from Weld | WL1 |      | 6.18 | 4.05 | ηW&N = 25% ηEXP = 31% | |
| WL2 |      | 6.27 | 3.36 | ηW&N = 18% ηEXP = 32% | ||
| Yellow Lake. Cool tint. | WL3 |     | 9.46 | 3.63 | ηW&N = 12.5% ηEXP = 12% | |
| Weld Yellow | WL4 |      | 6.02 | 5.07 | ηW&N = n.a. ηEXP = 6% | |
| WL5 |     | 6.35 | 6.15 | ηW&N = n.a. ηEXP = 9% | ||
| Recipe | HPLC | Infrared |
|---|---|---|
WL1 L* = 86.25 ± 0.06 a* = 1.56 ± 0.19 b* = 81.82 ± 1.00 gypsum (●) |  |  |
WL2 L* = 82.28 ± 0.21 a* = 5.78 ± 0.29 b* = 82.91 ± 0.57 gypsum (●) |  |  |
WL3 L* = 86.49 ± 0.27 a* = 2.78 ± 0.27 b* = 80.2 ± 0.24 |  |  |
WL4 L* = 76.93 ± 0.69 a* = 8.81 ± 0.58 b* = 74.93 ± 0.92 |  |  |
WL5 L* = 85.66 ± 0.13 a* = 0.96 ± 0.15 b* = 75.75 ± 0.06 Al(OH)3 (◆) |  |  |
| Lut | Lut-7-O-glu | WL3 | WL4 | Literature [32] | |
|---|---|---|---|---|---|
| Lut | Assignments | ||||
| 3398 | 3451 | 3390 | 3375 | ~3400 | ν(O3’—H), ν(O7—H), ν(O4′—H) |
| 3227 | - | - | - | ν(O5—H) | |
| - | - | 2931 | 2924 | ||
| 1666 | 1657 | 1632 | 1632 | 1656 | ν(C=O), ν(C2—C3), δ(O3—H) |
| 1613 | 1608 | 1589 | 1589 | 1612 | ν(C=O), δ(O—H), δ(O4′—H) |
| 1575 | - | - | - | 1575 | ν(C=O), δ(O—H), δ(O—H) |
| - | 1561 | - | - | 1561 | ν(C2=C3), δ(O5—H), δ(O4′—H), δ(O7—H) |
| - | - | 1535 | 1535 | 1518 | δ(O7—H), δ(O—H)A |
| 1509 | 1499 | 1484 | 1489 | 1507 | δ(O5—H) |
| 1446 | 1445 | 1456 | δ(O—H)B | ||
| - | - | 1438 | 1438 | 1439 | δ(O—H)B, δ(O3—H) |
| 1383 | 1385 | 1364 | 1360 | 1367 | νs(C—O1—C2), δ(O4′—H) |
| 1341 | 1346 | - | - | - | n.a. |
| - | 1314 | 1313 | δ(O—H), ν(C4′—O) | ||
| 1307 | - | - | - | 1303 | ν(C4′—O) |
| - | 1297 | - | - | 1284 | ν(C4′—O) |
| 1262 | 1272 | 1263 | 1263 | 1263 | δ(C2—H), ν(C2—O1), ν(C—C), δ(O5—H) |
| - | 1226 | - | - | - | n.a. |
| 1210 | 1208 | - | 1206 | 1210 | δ(O—H)A, ν(C2—O1) |
| 1186 | 1179 | 1178 | 1173 | 1194 | δ(C6—H), δ(O7—H) |
| 1160 | - | -- | - | 1162 | δ(O—H)B, δ(O7—H) |
| 1117 | 1128 | 1106 | 1109 | 1120 | δ(C2—H), δ(O—H)B |
| 1096 | 1089 | 1077 | 1068 | 1094 | νs(C—O1—C2), ν(C—O1), ν(C3—C4), ϕipA,B, δ(O—H), δ(O7—H) |
| 1034 | 1030 | 1049 | 1043 | 1031 | νs(C—O1—C2), ϕipA,B, δ(C2—H) |
| 1003 | - | - | - | 999 | ϕA + ϕipB |
| 945 | - | - | - | 946 | ϕA,B, Δ(C3—C4=O), ν(O1—C2) |
| 848 | 840 | 823 | 843 | 839 | γ(C2—H) |
| 805 | 790 | - | - | n.a. | |
| 768 | 755 | 765 | 765 | 766 | ϕA |
| 725 | - | - | - | 728 | ϕopA, Γ(C3—C4=O), γ(O5—H) |
| 685 | 688 | - | - | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veneno, M.; Nabais, P.; Otero, V.; Clemente, A.; Oliveira, M.C.; Melo, M.J. Yellow Lake Pigments from Weld in Art: Investigating the Winsor & Newton 19th Century Archive. Heritage 2021, 4, 422-436. https://doi.org/10.3390/heritage4010026
Veneno M, Nabais P, Otero V, Clemente A, Oliveira MC, Melo MJ. Yellow Lake Pigments from Weld in Art: Investigating the Winsor & Newton 19th Century Archive. Heritage. 2021; 4(1):422-436. https://doi.org/10.3390/heritage4010026
Chicago/Turabian StyleVeneno, Maria, Paula Nabais, Vanessa Otero, Adelaide Clemente, M. Conceição Oliveira, and Maria João Melo. 2021. "Yellow Lake Pigments from Weld in Art: Investigating the Winsor & Newton 19th Century Archive" Heritage 4, no. 1: 422-436. https://doi.org/10.3390/heritage4010026
APA StyleVeneno, M., Nabais, P., Otero, V., Clemente, A., Oliveira, M. C., & Melo, M. J. (2021). Yellow Lake Pigments from Weld in Art: Investigating the Winsor & Newton 19th Century Archive. Heritage, 4(1), 422-436. https://doi.org/10.3390/heritage4010026






