Glass Beads, Markers of Ancient Trade in Sub-Saharan Africa: Methodology, State of the Art and Perspectives
Abstract
1. Introduction: Overview of Glass Production History
2. Glass Bead Research
3. Analytical Methods Used to Analyse the Glassy Matrix and Colouring Agents
3.1. Optical Microscopy
3.2. Scanning Electron Microscopy (SEM)
3.3. X-ray Fluorescence (XRF)
3.4. Neutron Activation Analysis (NAA)
3.5. Laser Ablation Inductively Coupled Plasma Mass Spectrometry
3.6. Laser Induced Breakdown Spectroscopy (LIBS)
3.7. Raman Spectroscopy
4. Bead Research in Sub-Saharan Africa
4.1. Brief Overview on Methods of Classification
4.2. Bead Type Identification for Sub-Saharan Africa
4.3. Matrix and Colouring Agent Composition as Chrono-Technological Markers
4.4. Recycled Glass and/or Heterogeneous Reprocessed Beads
4.5. Discerning between European Replicas and Earlier Ancient Beads
5. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poupeau, G.; Le Bourdonnec, F.X.; Carter, T.; Delerue, S.; Shackley, M.S.; Barrat, J.A.; Dubernet, S.; Moretto, P.; Calligaro, T.; Milic, M.; et al. The use of SEM-EDS, PIXE and EDXRF for obsidian provenance studies in the Near East: A case study from Neolithic Çatalhöyük (Central Anatolia). J. Archaeol. Sci. 2010, 37, 2705–2720. [Google Scholar] [CrossRef]
- Tykot, R.H. Chemical fingerprinting and source tracing of obsidian: The Central Mediterranean trade in black gold. Acc. Chem. Res. 2002, 35, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Beaujard, P. Un seul système-monde avant le 16e siècle? L’océan Indien au cœur de l’intégration de l’hémisphère afro-eurasien. In Histoire Globale, Mondialisations et Capitalisme; Beaujard, P., Berger, L., Norel, P., Eds.; La Découverte-Recherches: Paris, France, 2009; pp. 82–148. [Google Scholar]
- Beaujard, P. Les Mondes de L’océan INDIEN, Vol. 1, De la Formation de l’État au Premier Système Monde Afro-eurasien, Vol. 2. L’océan Indien, au Cœur des Globalisations de l’Ancien Monde (7e-15e siècles); Armand Colin: Paris, France, 2012. [Google Scholar]
- Francis, P., Jr. Asia’s Maritime Bead Trade, 300BC to the Present; University of Hawaii Press: Honolulu, HI, USA, 2002. [Google Scholar]
- Bonneau, A.; Moreau, J.F.; Hancock, R.G.V.; Karklins, K. Archaeometrical analysis of glass beads: Potential, limitations, and results. Beads J. Soc. Beads Res. 2014, 26. Available online: http://surface.syr.edu/beads/vol26/iss1/7 (accessed on 20 April 2019).
- Hancock, R.G.V.; McKechnie, J.; Aufreiter, S.; Karklins, K.; Kapches, M.; Sempowski, M.; Moreau, J.F.; Kenyon, I. Non-destructive analysis of European cobalt blue glass trade beads. J. Radioanal. Nucl. Chem. 2000, 244, 567–573. [Google Scholar] [CrossRef]
- Saitowitz, S.J. Classification of glass trade beads. S. Afr. Mus. Bull. 1988, 18, 41–45. [Google Scholar]
- Janssens, K. (Ed.) Modern Methods for Analysing Archaeological and Historical Glass, 1st ed.; J. Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Brill, R.H. The Chemical interpretation of the texts. In Glass and Glassmaking in Ancient Mesopotamia; Oppenheim, L., Brill, R.H., Barag, D., von Saldern, A., Eds.; The Corning Museum of Glass: New York, NY, USA, 1970; pp. 105–108. [Google Scholar]
- Henderson, J. Ancient Glasses, an Interdisciplinary Exploration; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Shortland, A.J. Lapis Lazuli from the Kiln: Glass and Glassmaking in the Late Bronze Age; Leuven University Press: Leuven, Belgium, 2012. [Google Scholar]
- Tite, M.S.; Shortland, A.J. Production Technology of Faience and Related Early Vitreous Materials; Monograph 72; School of Archaeology, University of Oxford: Oxford, UK, 2008. [Google Scholar]
- Jackson, C.M. Making colourless glass in Roman Period. Archaeometry 2005, 47, 763–780. [Google Scholar] [CrossRef]
- McGray, P. (Ed.) Prehistory and History of Glassmaking Technology; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1998; Volume VIII. [Google Scholar]
- Schuler, F. Ancient glassmaking techniques: The molding process. Archaeology 1959, 12, 47–52. [Google Scholar]
- Degryse, P.; Schneider, J. Pliny the Elder and Sr-Nd isotopes: Tracing the provenance of raw materials for Roman glass production. J. Archaeol. Sci. 2008, 35, 1993–2000. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Colomban, P.; Mangone, A.; Valloteau, C.; Cambon, P. Mobile Raman spectroscopy analysis of ancient enamelled glass masterpieces. Anal. Methods 2013, 5, 4345–4354. [Google Scholar] [CrossRef]
- Greiff, S.; Schuster, J. Technological study of enamelling on Roman glass: The nature of opacyfing, decolourising and fining agents used with the glass beakers from Lübsow (Lubieszewo, Poland). J. Cult. Herit. 2008, 9, e27–e32. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Valloteau, C.; Colomban, P. Inside the glassmaker technology: Search of Raman criteria to discriminate between Emile Gallé and Philippe-Joseph Brocard Enamels and Pigment Signatures. J. Raman Spectrosc. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Caggiani, M.C.; Paris, C. Pigments and enamelling/gilding technology of Mamluk mosque lamps and bottle. J. Raman Spectrosc. 2012, 43, 1975–1984. [Google Scholar] [CrossRef]
- Henderson, J. Tradition and experiment in first millennium A.D. glass production. The emergence of early Islamic glass technology in late antiquity. Acc. Chem. Res. 2002, 35, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Newton, R.G. Conservation and Restoration of Glass; Routledge: Abingdon, UK, 2008. [Google Scholar]
- Ricciardi, P.; Colomban, P.; Tournié, A.; Milande, V. Non-destructive on-site identification of ancient glasses: Genuine artefacts, embellished pieces or forgeries? J. Raman Spectrosc. 2009, 40, 604–617. [Google Scholar] [CrossRef]
- Cothren, M.W. Picturing the Celestial City: The Medieval Stained Glass of Beauvais Cathedral; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Cummings, K. A History of Glass Forming; University of Pennsylvania Press: Philadelphia, PA, USA, 2002. [Google Scholar]
- Sayre, E.V.; Smith, R.W. Compositional categories of ancient glass. Science 1961, 133, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Galuska, L.; Machacek, J.; Pieta, K.; Sedlackova, H. The glass of great Moravia: Vessel and Window glass, and small Objects. J. Glass Stud. 2012, 54, 61–65. [Google Scholar]
- Armitage, E.L. Stained Glass: History, Technology and Practice; Leonard Hill: London, UK, 1959. [Google Scholar]
- Glover, I.C.; Henderson, J. Early glass in South and South-East Asia, China and South-East Asia. In Art Commercial Interaction; Percival David Foundation of Chinese Art: London, UK, 1995; pp. 141–169. [Google Scholar]
- Henderson, J.; Chenery, S.; Kröger, J.; Faber, E.W. Glass provenance along the Silk Road: The use of trace element analysis. In Recent Advances in the Scientific Research on Ancient Glass and Glaze; Gan, F., Li, Q., Henderson, J., Eds.; Series on Archaeology and History of Science in China; World Scientific: Singapore, 2016; Volume 2, pp. 17–42. [Google Scholar]
- Tamura, T.; Oga, K. Archaeometrical investigation of natron glass excavated in Japan. Microchem. J. 2016, 126, 7–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Q. Faience beads of the western Zhou Dynasty excavated in Gansu province, China: A technical study. In Ancient Glass Research along the Silk Road; Gan, F.X., Brill, R., Tian, S., Eds.; World Scientific: Singapore, 2009; pp. 275–289. [Google Scholar]
- Dussubieux, L.; Kusimba, C.M.; Gogte, V.; Kusimba, S.B.; Gratuze, B.; Oka, R. The trading of ancient glass beads: New analytical data from South Asian and East African soda-alumina glass beads. Archaeometry 2008, 50, 797–821. [Google Scholar] [CrossRef]
- Gan, F.; Li, Q.; Henderson, J. (Eds.) Recent Advances in the Scientific Research on Ancient Glass and Glaze; Series on Archaeology and History of Science in China; World Scientific: Singapore, 2016; Volume 2. [Google Scholar]
- Colomban, P.; Khoi, D.N.; Liem, N.Q.; Roche, C.; Sagon, G. Sa Huynh and Cham potteries: Microstructure and likely processing. J. Cult. Herit. 2004, 5, 149–155. [Google Scholar] [CrossRef]
- Saminpanya, S.; Bavornyospiwat, N.; Homklin, S.; Danyutthapolchai, S.; Bupparenoo, P. Physical and chemical properties of the ancient glass beads from the highland log-coffin culture and the lowland areas, Thailand: Considerations on their colors and technology. J. Archaeol. Sci. Rep. 2016, 8, 366–380. [Google Scholar] [CrossRef]
- Bouzougar, A.; Barton, N.; Vanhaeren, M.; D’Errico, F. 82,000-year-old shell beads from North Africa and implications for the origins of modern Human behaviour. Proc. Natl. Acad. Sci. USA 2007, 104, 9964–9969. [Google Scholar] [CrossRef] [PubMed]
- Holden, C. Oldest beads suggest early symbolic behaviour. Science 2004, 304, 369. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.A. Glass beads and glass production in early south India: Contextualizing Indo-Pacific bead manufacture. Archaeol. Res. Asia 2016, 6, 4–15. [Google Scholar] [CrossRef]
- Carter, A.K.; Abraham, S.A.; Kelly, G.O. Updating Asia’s Maritime Bead Trade: An Introduction. Archaeol. Res. Asia 2016, 6, 1–3. [Google Scholar] [CrossRef]
- Kelly, G.O. Heterodoxy, orthodoxy and communities of practice: Stone bead and ornament production in Early Historic South India (c. 400 BCE–400 CE). Archaeol. Res. Asia 2016, 6, 30–50. [Google Scholar] [CrossRef]
- Wood, M. Glass beads from pre-European contact sub-Saharan Africa: Peter Francis’s work revisited and updated. Archaeol. Res. Asia 2016, 6, 65–80. [Google Scholar] [CrossRef]
- Wood, M.; Panighello, S.; Orsega, E.F.; Robertshaw, P.; van Elteren, J.T.; Crowther, A.; Horton, M.; Boivin, N. Zanzibar and Indian Ocean trade in the first millennium Ce: The glass bead evidence. Archaeol. Anthropol. Sci. 2017, 9, 879–901. [Google Scholar] [CrossRef]
- Van der Sleen, W.G.N. A Handbook on Beads; Liberty Cap Books: York, PA, USA, 1973. [Google Scholar]
- Freestone, I.C. The provenance of ancient glass through compositional analysis. Mater. Res. Soc. Symp. Proc. 2005, 852, 1–14. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A. On-site Raman Identification and Dating of Ancient/Modern Stained Glasses at the Sainte-Chapelle, Paris. J. Cult. Herit. 2007, 8, 242–256. [Google Scholar] [CrossRef]
- Degryse, P. Glass Making in the Graeco-Roman World; Leuven University Press: Leuven, Belgium, 2014. [Google Scholar]
- Foy, D.; Nenna, M.D. Echanges et commerce du verre dans le monde antique. In Monographies Instrumentum 24, Actes du Colloque de L’Association Française pour l’Archéologie du Verre (AFAV), Aix-en-Provence-Marseille, 7–9 juin 2001; CNRS-mmsh-Université: Aix-Marseille, France, 2003; ISBN 2-907303-72-4. [Google Scholar]
- Picon, M.; Vichy, M. D’Orient en Occident: l’origine du verre à l’époque romaine et durant le haut Moyen Age. In Échanges et Commerce du verre dans le Monde Antique Monographies Instrumentum 24, Actes du Colloque de L’Association Française pour l’Archéologie du Verre (AFAV), Aix-en-Provence-Marseille, 7–9 juin 2001; CNRS-mmsh-Université: Aix-Marseille, France, 2003; pp. 17–31. ISBN 2-907303-72-4. [Google Scholar]
- Valooto, M.; Verità, M. Glasses from Pompei, Herculaneum, and the sand of the rivers Belus and Volturno. Studi della Soprintend. Archeol. di Pompei 2002, 6, 63–73. [Google Scholar]
- Freestone, I. Pliny on Roman glassmaking. In Archaeology, History and Science: Integrating Approaches to Ancient Materials; Martinon-Torres, M., Rehren, T., Eds.; Left Coast Press: Walnut Creek, CA, USA, 2008; pp. 77–100. [Google Scholar]
- Verità, M.; Zecchin, S. Thousand years of Venetian glass: The evolution of chemical composition from the origins to the 18th century. In Annales du 17e Congrès de l’Association Internationale pour l’Histoire Du Verre (AIHV); Janssens, K., Degryse, P., Cosyns, P., Caen, J., Van’t dack, L., Eds.; Antwerp, 4th to 8th September 2006; Aspeditions: Antwerp, Belgium, 2009; pp. 602–613. [Google Scholar]
- De Raedt, I.; Janssens, K.; Veekman, J.; Vincze, J.; Vekemans, B.; Jeffries, T.E. Trace analysis for distinguishing between Venetian and façon-de-Venise glass vessels of the 16th and 17th century. J. Anal. At. Spectrosc. 2001, 16, 1012–1017. [Google Scholar] [CrossRef]
- Šmit, Ž.; Janssens, K.; Schalm, O.; Kos, M. Spread of Façon-de-Venise Glassmaking through Central and Western Europe. Nucl. Instrum. Methods Phys. Res. B 2004, 213, 717–722. [Google Scholar] [CrossRef]
- Francis, P., Jr. Glass Beads in Asia: Part I. Introduction, Asian Perspectives; University of Hawai’i Press: Honolulu, HI, USA, 1989; Volume 28, pp. 1–21. [Google Scholar]
- Dungworth, D. Three and a half centuries of bottle manufacture. Ind. Archaeol. Rev. 2012, 34, 37–50. [Google Scholar] [CrossRef]
- Bertran, H. Nouveau Manuel Complet de la Peinture sur Verre, sur Porcelaine et sur Email; Encyclopédie-Roret-Ed; Mulo, L.: Paris, France, 1913. [Google Scholar]
- Brisac, C. A Thousand Years of Stained Glass; Book Sales: Macdonald, CA, USA, 1986. [Google Scholar]
- Cappa, G. Le génie verrier de l’Europe. Témoignages de l’Historicisme à la Modernité (1840–1998), 2nd ed.; Mardaga: Liège, Belgium, 1991. [Google Scholar]
- Bontemps, G. Guide du Verrier—Traité historique et pratique de la fabrication des verres, cristaux, vitraux; Librairie du Dictionnaire des Arts Manufacturés: Paris, France, 1868. [Google Scholar]
- Colomban, P. The destructive/non-destructive identification of enamels, pottery, glass artifacts and associated pigments—A brief overview. Arts 2013, 2, 77–110. [Google Scholar] [CrossRef]
- Eppler, R.A.; Eppler, D.F. Glazes and Glass Coatings; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Nassau, K. The Physics and Chemistry of Color, 2nd ed.; Wiley-VCH: New York, NY, USA, 2001. [Google Scholar]
- Tilley, R. Colour and the Optical Properties of Materials; Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Neri, E.; Morvan, C.; Colomban, P.; Guerra, M.F.; Prigent, V. Late Roman and Byzantine Mosaic opaque “Glass-ceramics” Tesserae (5th-9th century). Ceram. Int. 2016, 42, 18859–18869. [Google Scholar] [CrossRef]
- Colomban, P. The use of metal nanoparticles to produce yellow, red and iridescent colour, from Bronze Age to present times in lustre pottery and glass: Solid State Chemistry, Spectroscopy and Nanostructure. J. Nano Res. 2009, 8, 109–132. [Google Scholar] [CrossRef]
- Berke, H.; Wiedemann, H.G. The chemistry and fabrication of anthropogenic pigments Chinese blue and purple in ancient China. East Asian Sci. Technol. Med. 2000, 17, 94–120. [Google Scholar]
- Colomban, P.; Tournié, A.; Ricciardi, P. Raman spectroscopy of copper nanoparticle-containing glass matrices: Ancient red stained-glass windows. J. Raman Spectrosc. 2009, 40, 1949–1955. [Google Scholar] [CrossRef]
- Fornacelli, C.; Colomban, P.; Turbanti Memmi, I. Toward a Raman/FORS discrimination between Art Nouveau and contemporary stained glasses from CdSxSe1-x nanoparticles signatures. J. Raman Spectrosc. 2015, 46, 1129–1139. [Google Scholar] [CrossRef]
- Pollard, A.M.; Heron, C. Archaeological Chemistry; Royal Society of Chemistry: Cambridge, UK, 1996. [Google Scholar]
- Fouché, L. Mapungubwe: Ancient Bantu Civilization on the Limpopo. Reports on excavations at Mapungubwe; Cambridge University Press: Cambridge, UK, 1937. [Google Scholar]
- Bonneau, A.; Moreau, J.F.; Auger, R.; Hancock, R.G.V.; Émard, B. Analyses physico-chimiques des perles de traite en verre de facture européenne: Quelles instrumentations pour quels résultats ? Archéologiques 2014, 26, 109–132. [Google Scholar]
- Colomban, P. Analyse non destructive des objets d’art par méthodes spectroscopiques portables. Tech. de l’Ingénieur 2012, 217, 1–12. [Google Scholar]
- Colomban, P. The on-site/remote Raman analysis with portable instruments—A review of drawbacks and success in Cultural Heritage studies and other associated fields. J. Raman Spectrosc. 2012, 43, 1529–1535. [Google Scholar] [CrossRef]
- Bellina, B. Maritime Silk Roads’ Ornament Industries: Socio-political Practices and Cultural Transfers in the South China Sea. Camb. Archaeol. J. 2014, 24, 345–377. [Google Scholar] [CrossRef]
- Koleini, F.; Prinsloo, L.C.; Biemond, W.M.; Colomban, P.; Ngo, A.; Boeyens, J.; van der Ryst, M. Towards refining the classification of glass trade beads imported into southern Africa from the 8th to the 16th century AD. J. Cult. Herit. 2016, 19, 435–444. [Google Scholar] [CrossRef]
- Fischbach, N.; Ngo, A.T.; Colomban, P.; Pauly, M. Beads excavated from Antsiraka Boira necropolis (Mayotte Island, 12th–13th century); Colouring agents and glass matrix composition comparison with contemporary Southern Africa sites. Revue d’Archéomètrie-Archéosciences 2016, 40, 83–102. [Google Scholar] [CrossRef]
- Vicenzi, E.P.; Eggins, S.; Logan, A.; Wysoczanski, R. Microbeam characterization of Corning archaeological references glasses. New additions to the Smithsonian microbeam standard collection. J. Res. Natl. Inst. Stand. Technol. 2002, 107, 719–727. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Casadio, F.; Bellot-Gurlet, L.; Faber, K.; Zelleke, G.; Milande, V.; Tilliard, L. On-site identification of early Böttger red stonewares using portable XRF/Raman instruments: 2 glaze and gilding analysis. J. Am. Ceram. Soc. 2015, 98, 3006–3013. [Google Scholar] [CrossRef]
- Koleini, F.; Colomban, P.; Antonites, A.; Pikirayi, I. Raman and XRF classification of Asian and European glass beads recovered at Mutamba, a southern African Middle Iron Age site. J. Archaeol. Sci. Rep. 2017, 13, 333–340. [Google Scholar] [CrossRef]
- Koleini, F.; Machiridza, L.H.; Pikirayi, I.; Colomban, P. The chronology of Insiza cluster Khami-phase sites in south-western Zimbabwe: Compositional insights from pXRF and Raman analysis of excavated exotic glass finds. Archaeometry 2019, 61, 874–890. [Google Scholar] [CrossRef]
- Costa, M.; Barrulas, P.; Dias, L.; da Conceição Lopes, M.; Barreira, J.; Clist, B.; Karklins, K.; da Piedade de Jesus, M.; da Silva Domingos, S.; Vandenabeele, P.; et al. Multi-analytical approach to the study of the European glass beads found in the tombs of Kulumbimbi (Mbanza Kongo, Angola). Microchem. J. 2019, 149, 103390. [Google Scholar] [CrossRef]
- Dussubieux, L.; Gratuze, B.; Blet-Lemarquand, M. Mineral soda alumina glass: Occurrence and meaning. J. Archaeol. Sci. 2010, 37, 1646–1655. [Google Scholar] [CrossRef]
- Yuan, H.L.; Gao, S.; Liu, X.M. Accurate U-Pb age and trace element determinations of zircon by laser ablation-inductively coupled plasma-mass spectrometry. Geostand. Geoanal. Res. 2004, 28, 353–370. [Google Scholar] [CrossRef]
- Degryse, P.; Henderson, J.; Hodgins, G. Isotopes in Vitreous Materials; Leuven University Press: Leuven, Belgium, 2009. [Google Scholar]
- Koch, J.; Günther, D. Review of the State-of-the-Art of Laser Ablation Inductively Coupled Plasmarometry. Appl. Spectrosc. 2011, 65, 155A–162A. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Anglos, D.; Zafiropulos, V.; Georgiou, S.; Tornar, V. Lasers in the Preservation of Cultural Heritage: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Miziolek, A.W.; Palleschi, V.; Schechte, I. Laser Induced Breakdown Spectroscopy; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Gaudiuso, R.; Dell’Aglio, M.; De Pascale, O.; Senesi, G.S.; De Giacomo, A. Laser Induced Breakdown Spectroscopy for elemental analysis in environmental, cultural heritage and space applications: A review of methods and results. Sensors 2010, 10, 7434–7468. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P. Polymerisation degree and Raman identification of ancient glasses used for jewellery, ceramics enamels and mosaics. J. Non-Cryst. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Bead Researcher Society. (Compiled by K. Karklins). Available online: https://beadresearch.org/wp-content/uploads/2016/08/Africa.pdf (accessed on 7 November 2016).
- Koleini, F.; Prinsloo, L.C.; Biemond, W.; Colomban, P.; Ngo, A.T.; Boeyens, J.; Van der Ryst, M.M.; van Brakel, K. Glass trade on Magoro Hill, an archaeological site in southern Africa: Glass types and pigments. Herit. Sci. 2016, 4, 1–20. [Google Scholar]
- Koleini, F.; Pikirayi, I.; Colomban, P. Revisiting Baranda: A multi-analytical approach in classifying sixteenth/seventeenth-century glass beads from northern Zimbabwe. Antiquity 2017, 91, 751–764. [Google Scholar] [CrossRef]
- Beck, H.C. The beads of Mapungubwe District. In Mapungubwe I; Fouché, L., Ed.; Cambridge University Press: Cambridge, UK, 1937; pp. 104–113. [Google Scholar]
- Malleret, L. Classification et nomenclature des « perles » archéologiques en fonction de la symétrie minérale. Bull. de l’Ecole française d’Extrême Orient 1949, 51, 117–124. [Google Scholar] [CrossRef]
- Van Riet Lowe, C. The Glass Beads of Mapungubwe; Archaeological Series 9; Archaeological Survey: Pretoria, South Africa, 1955. [Google Scholar]
- Kidd, K.E.; Kidd, M.A. A classification system for glass beads for the use of field archaeologists. In Proceedings of the 1982 Glass Trade Bead Conference; Hayes, C.F., III, Ed.; Rochester Research Note 16; Rochester Museum and Science Center: New York, NY, USA, 1983; pp. 219–257. [Google Scholar]
- Karklins, K. Glass Beads. Studies in Archaeology, Architecture and History; Parks Canada: Ottawa, ON, Canada, 1985. [Google Scholar]
- Davidson, C.C.; Clark, J.D. Trade wind beads: An interim report of chemical studies. Azania 1974, 9, 75–86. [Google Scholar] [CrossRef]
- Buratti, M. Le symbolisme des couleurs dans les productions perlées du Cameroun. In Les Couleurs dans les Arts D’afrique. De la Préhistoire à nos Jours; Gutierrez, M., Buratti, M., Valentin, M., Ballinger, M., Eds.; Editions des Archives Contemporaines: Paris, France, 2016; pp. 67–82. [Google Scholar]
- DAACS. Cataloging Manual Beads. Available online: http://www.daacs.org/wp-content/uploads/2016/10/DAACSBeadManual.pdf (accessed on 20 May 2019).
- Decorse, C.R. Beads as chronological indicators in West African archaeology: A re-examination. BEADS J. Soc. Beads Res. 1989, 1, 41–53. [Google Scholar]
- Saitowitz, S.J.; Reid, D.L.; van der Merwe, N.J. Bead trade from Islamic Egypt to South Africa c. AD 900–1250. S. Afr. J. Sci. 1996, 92, 101–104. [Google Scholar]
- Saitowitz, S.J.; Sampson, C.G. Glass trade beads from rock shelters in the upper Karoo. S. Afr. Archaeol. Bull. 1992, 47, 94–103. [Google Scholar] [CrossRef]
- Robertshaw, P.; Wood, M.; Melchiorre, E.; Popelka-Filcoff, R.S.; Glascock, M.D. Southern African glass beads: Chemistry, glass sources and patterns of trade. J. Archaeol. Sci. 2010, 37, 1898–1912. [Google Scholar] [CrossRef]
- Wood, M.; Dussubieux, L.; Robertshaw, P. Glass finds from Chibuene, a 6th to 17th century AD port in southern Mozambique. S. Afr. Archaeol. Bull. 2012, 67, 59–74. [Google Scholar]
- Rousaki, A.; Coccato, A.; Verhaeghe, C.; Clist, B.O.; Bostoen, K.; Vandenabeele, P.; Moens, L. Combined spectroscopic analysis of beads from the tombs of Kindoki, Lower Congo Province (Democratic Republic of the Congo). Appl. Spectrosc. 2016, 70, 76–93. [Google Scholar] [CrossRef][Green Version]
- Coccato, A.; Costa, M.; Rousaki, A.; Clist, B.O.; Karklins, K.; Bostoen, K.; Manhita, A.; Cardoso, A.; Dias, C.B.; Candeias, A.; et al. Micro-Raman spectroscopy and complementary techniques (hXRF, VP-SEM-EDS, μ-FTIR and Py-GC/MS) applied to the study of beads from the Kongo Kingdom (Democratic Republic of the Congo). J. Raman Spectrosc. 2017, 48, 1458–1478. [Google Scholar] [CrossRef]
- Babalola, A.B. Ancient history of technology in West Africa: The Indigenous. Glass/Glass Bead Industry and the Society in Early Ile-Ife, Southwest Nigeria. J. Black Stud. 2017, 48, 501–527. [Google Scholar] [CrossRef]
- Prinsloo, L.C.; Colomban, P. A Raman spectroscopic study of the Mapungubwe oblates: Glass trade beads excavated at an Iron Age archaeological site in South Africa. J. Raman Spectrosc. 2008, 39, 79–90. [Google Scholar] [CrossRef]
- Davison, C.C. Chemical resemblance of garden roller and M1 glass beads. Afr. Stud. 1973, 32, 247–257. [Google Scholar] [CrossRef]
- Gardner, G.A. Mapungubwe II; J.L. van Schaik: Pretoria, South Africa, 1963. [Google Scholar]
- Carey, M. Powder-glass beads in Africa. In Ornaments from the Past: Beads Studies after Beck; Glover, I., Hughes-Brock, H., Henderson, J., Eds.; The Bead Study Trust: London, UK, 2003; pp. 108–114. [Google Scholar]
- Simak, E. Traditional Mauritanian Powder-glass Kiffa beads. ORNAMENT 2006, 29, 50–54. [Google Scholar]
- Haigh, J. Present-day bead-making in Ghana. In Ornaments from the Past: Bead Studies after Beck; Glover, I., Hughes-Brock, H., Henderson, J., Eds.; The bead Study Trust: Cambridge, UK, 2003; pp. 115–117. [Google Scholar]
- Euba, O. Of blue beads and red; the role of Ife in the West African trade in Kori beads. J. Hist. Soc. Niger. 1982, 11, 109–127. [Google Scholar]
- Lankton, J.W.; Ige, O.A.; Rehren, T. Early primary glass production in Southern Nigeria. J. Afr. Archaeol. 2006, 4, 111–138. [Google Scholar] [CrossRef]
- Wood, M. Glass beads and Pre-European trade in the Shashe-Limpopo-region. In Master of Arts Dissertation; University of the Witwatersrand: Johannesburg, South Africa, 2005. [Google Scholar]
- Wood, M. A glass bead sequence for Southern Africa from the 8th to the 16th century AD. J. Afr. Archaeol. 2011, 9, 67–84. [Google Scholar] [CrossRef]
- Wood, M. Interconnections; Glass Beads and Trade in Southern and Eastern Africa and the Indian Ocean—7th to 16th Centuries AD. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2012. [Google Scholar]
- Wilmsen, E.; Dussubieux, L.; Huffmann, T.; Wood, M. Chemical analyses of glass beads from two Early Iron Age sites in Zimbabwe: Zhizo Hill and Makuru. Aziana Archaeol. Res. Afr. 2018, 53, 369–382. [Google Scholar] [CrossRef]
- Huffman, T. Handbook to the Iron Age; University of KwaZulu-Natal Press: Scotville, VT, USA, 2007. [Google Scholar]
- Robertshaw, P.; Rasoarifetra, B.; Wood, M.; Melchiorre, E.; Popelka-Filcoff, R.S.; Glascock, M.D. Chemical analysis of glass beads from Madagascar. J. Afr. Archaeol. 2006, 4, 91–109. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P. New investigation on glass beads from the necropolis of Vohemar, Northern Madagascar. 2019; Unpublished work. [Google Scholar]
- Robertshaw, P.; Wood, M.; Haour, A. Chemical analysis, chronology, and context of a European glass bead assemblage from Garumele, Niger. J. Archaeol. Sci. 2014, 41, 591–604. [Google Scholar] [CrossRef]
- Koleini, F.; Colomban, P.; Pikirayi, I. Post-15th century European glass beads in southern Africa: Identification, composition and classification using pXRF and Raman spectroscopy. 2019; Submitted for publication. [Google Scholar]
- Cagno, S.; Brondi Badano, M.; Mathis, F.; Strivay, D.; Janssen, K. Study of medieval glass from Savona (Italy) and their relation with the glass produced in Altare. J. Archaeol. Sci. 2012, 39, 2191–2197. [Google Scholar] [CrossRef]
- Dussubieux, L.; Karklins, K. Glass bead production in Europe during the 17th century/Elemental analysis of glass materials found in London and Amsterdam. J. Archaeol. Sci. Rep. 2016, 5, 574–579. [Google Scholar] [CrossRef]
- Robertshaw, P.; Magnavita, S.; Wood, M.; Melchiorre, E.; Popelka-Filcoff, R.; Glascock, M.D. Glass beads from Kissi (Burkina Faso): Chemical analysis and archaeological interpretation. In Crossroads: Cultural and Technological Developments in First Millennium BC/AD West Africa; Magnavita, S., Koté, L., Breunig, P., Idé, O.A., Eds.; Journal of African Archaeology Monograph Series 2; Africa Magna Verlag: Frankfurt, Germany, 2009; pp. 105–118. [Google Scholar]
- Prinsloo, L.C.; Tournié, A.; Colomban, P. A Raman spectroscopic study of glass trade beads excavated at Mapungubwe hill and K2, two archaeological sites in southern Africa, raises questions about the last occupation date of the hill. J. Archaeol. Sci. 2011, 38, 3264–3277. [Google Scholar] [CrossRef]
- Colomban, P.; Truong, C. Non-destructive Raman study of the glazing technique in lustre potteries and faience (9–14th centuries): Silver ions, nanoclusters, microstructure and processing. J. Raman Spectrosc. 2004, 35, 195–207. [Google Scholar] [CrossRef]
- Tite, M.; Pradell, T.; Shortland, A. Discovery, production and use of tin-based opacifiers in glasses, enamels and glazes from the late iron age onwards: A reassessment. Archaeometry 2008, 50, 67–84. [Google Scholar] [CrossRef]
- Pages-Camagna, S.; Colinart, S.; Coupry, C. Fabrication process of archaeological Egyptian blue and green pigments enlightened by Raman microscopy and scanning electron microscopy. J. Raman Spectrosc. 1999, 30, 313–317. [Google Scholar] [CrossRef]
- Cheng, X.; Yin, X.; Ma, Y.; Lei, Y. Three fabricated pigments (Han purple, indigo and emerald green) in ancient Chinese artifacts studied by Raman microscopy, energy-dispersive X-ray spectrometry and polarized light microscopy. J. Raman Spectrosc. 2007, 38, 1274–1280. [Google Scholar] [CrossRef]
- Lei, Y.; Xia, Y. Study on production techniques and provenance of faience beads excavated in China. J. Archaeol. Sci. 2015, 53, 32–42. [Google Scholar] [CrossRef]
- Prinsloo, L.C.; Boeyens, J.C.A.; Van der Ryst, M.M.; Webb, G. Raman signatures of the modern pigment (Zn,Cd)S1-χSe χ and glass matrix of a red bead from Magoro Hill, an archaeological site in Limpopo Province, South Africa, recalibrate the settlement chronology. J. Mol. Struct. 2012, 1023, 123–127. [Google Scholar] [CrossRef]
- Tournié, A.; Prinsloo, L.C.; Colomban, P. Raman classification of glass beads excavated on Mapungubwe hill and K2, two archaeological sites in South Africa. J. Raman Spectrosc. 2011, 43, 532–542. [Google Scholar] [CrossRef]
- Kirmizi, B.; Gokturk, H.; Colomban, P. Colouring Agents in the Pottery Glazes of Western Anatolia: A New Evidence for the Use of Naples Yellow Pigment Variations during the Late Byzantine Period. Achaeometry 2015, 57, 476–496. [Google Scholar] [CrossRef]
- Rosi, F.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Grygar, T.; Bezdicka, P.; Brunetti, B.G.; Sgamellotti, A.; Burgio, L.; Seccaronif, C.; Miliani, C. Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situ characterisation of Renaissance plates by portable micro-Raman and XRF studies. J. Raman Spectrosc. 2011, 42, 407–414. [Google Scholar] [CrossRef]
- Faurel, X.; Vanderperre, A.; Colomban, P. Pink Pigment optimisation by resonance Raman Spectroscopy. J. Raman Spectrosc. 2003, 34, 290–294. [Google Scholar] [CrossRef]
- Opper, M.J.; Opper, H. Powdered-glass beads and beads trade in Mauritania. BEADS J. Soc. Bead Res. 1993, 5, 37–54. [Google Scholar]
- Babalola, A.B.; McIntosh, S.; Dussubieux, L.; Rehren, T. Ile-Ife and Igbo Olokun in the history of glass in West Africa. Antiquity 2017, 91, 732–750. [Google Scholar] [CrossRef]
- Brill, R.H.; Stapleton, C.P. Chemical Analyses of Early Glasses; Corning Museum: New York, NY, USA, 2012; Volume 3. [Google Scholar]
- Wedepohl, K.H.; Simon, K.; Kronz, A. Data on 61 chemical elements for the characterization of three major glass compositions in late antiquity and the Middle Ages. Archaeometry 2011, 53, 81–102. [Google Scholar] [CrossRef]
- Babalola, A.B.; Rehren, T.; Ige, A.; McIntosh, S. The Glass Making Crucibles from Ile-Ife, SW Nigeria. J. Afr. Archaeol. 2018, 16, 31–59. [Google Scholar] [CrossRef]
- Koleini, F.; Colomban, P.; Pikirayi, I. Chronology of Danamonde, Naletale, Gomoremhiko and Zinjanja based on the composition of traded glass beads by pXRF and Raman Spectroscopy. 2019; Submitted for publication. [Google Scholar]
- Colomban, P. Rocks as blue, green and black pigments/dyes of glazed pottery and enamelled glass artefacts—A review. Eur. J. Mineral. 2013, 25, 863–879. [Google Scholar] [CrossRef]
- Gratuze, B.; Soulier, I.; Barrandon, J.N.; Foy, D. De l’origine du cobalt dans les verres. Revue d’archéométrie 1992, 16, 97–108. [Google Scholar] [CrossRef]
- Gratuze, B.; Soulier, I.; Blet, M.; Vallauri, L. De l’origine du cobalt: Du verre à la céramique. Revue D’archéométrie 1996, 20, 77–104. [Google Scholar] [CrossRef]
- Gronenborn, D. Beads and the emergence of the Islamic slave trade in the southern Chad basin (Nigeria). BEADS J. Soc. Beads Res. 2009, 21, 47–51. [Google Scholar]
- Guerrero, S. Venetian glass beads and the slave trade from Liverpool, 1750–1800. BEADS J. Soc. Bead Res. 2010, 22, 52–70. [Google Scholar]
- Pallaver, K. A recognized currency in beads: Glass beads as money in 19th century East Africa: The Central Caravan road. In Money in Africa; Catherine, E., Fuller, H., Perkins, J., Eds.; The British Museum: London, UK, 2009; pp. 20–29. [Google Scholar]
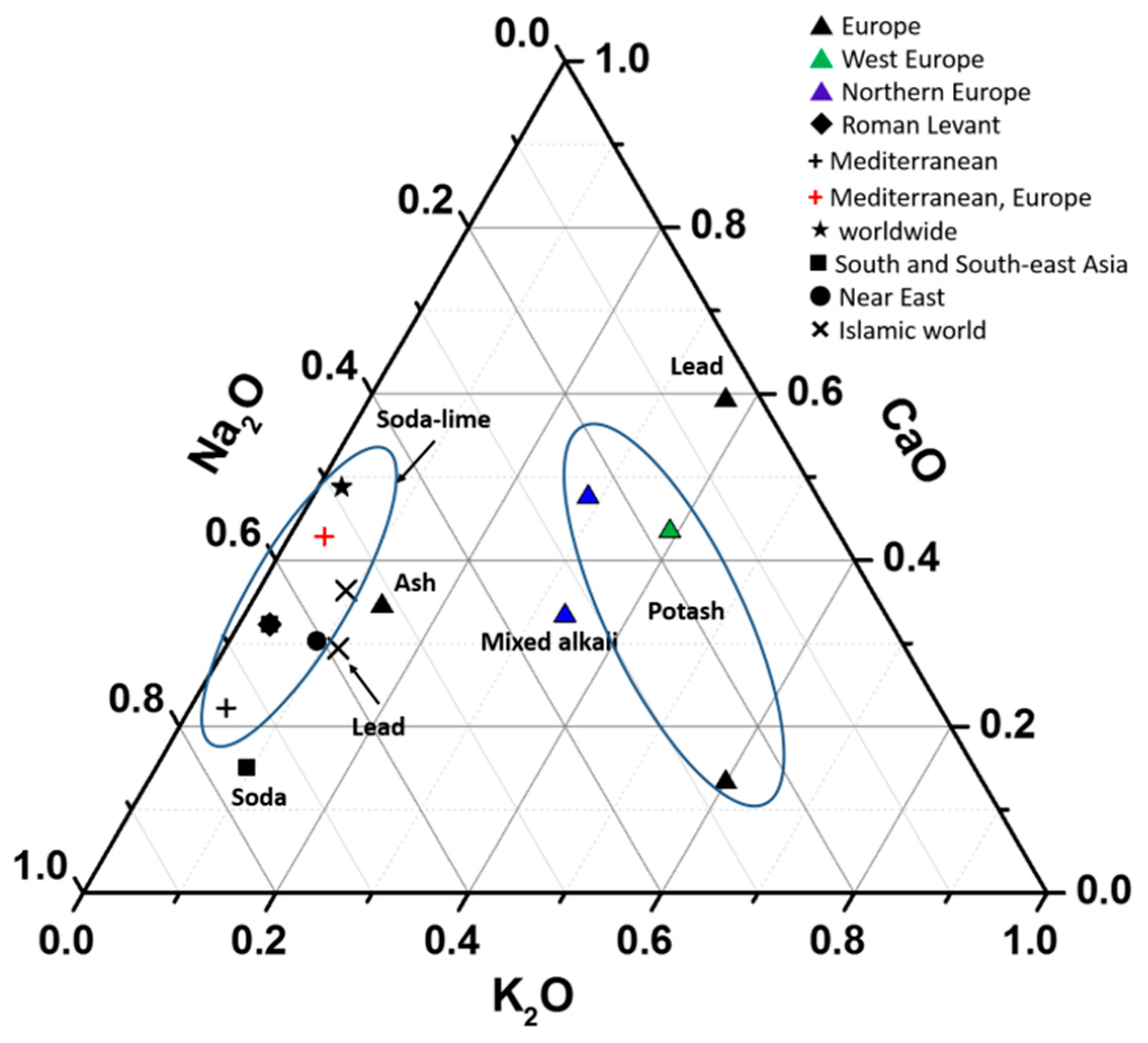

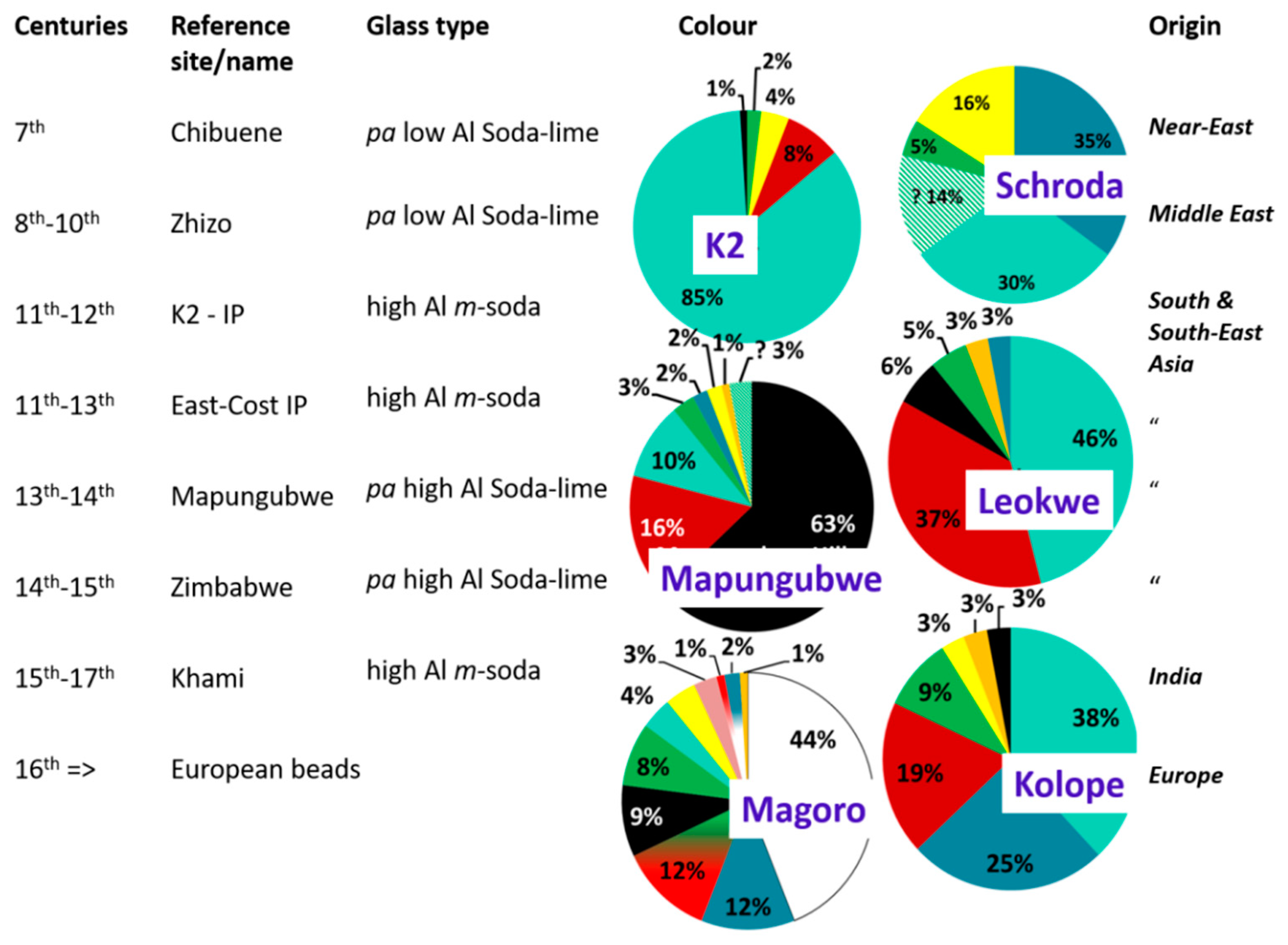
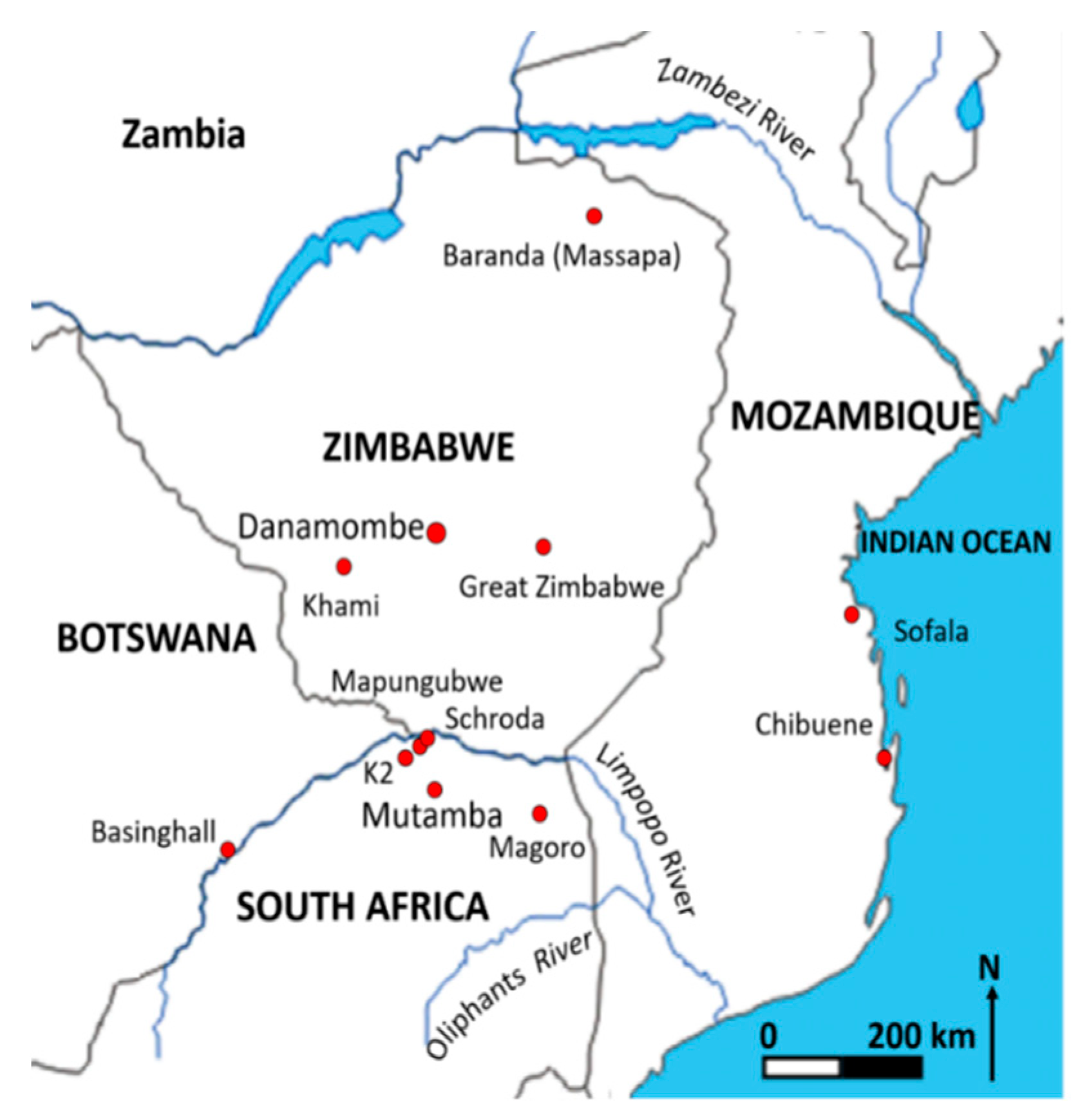
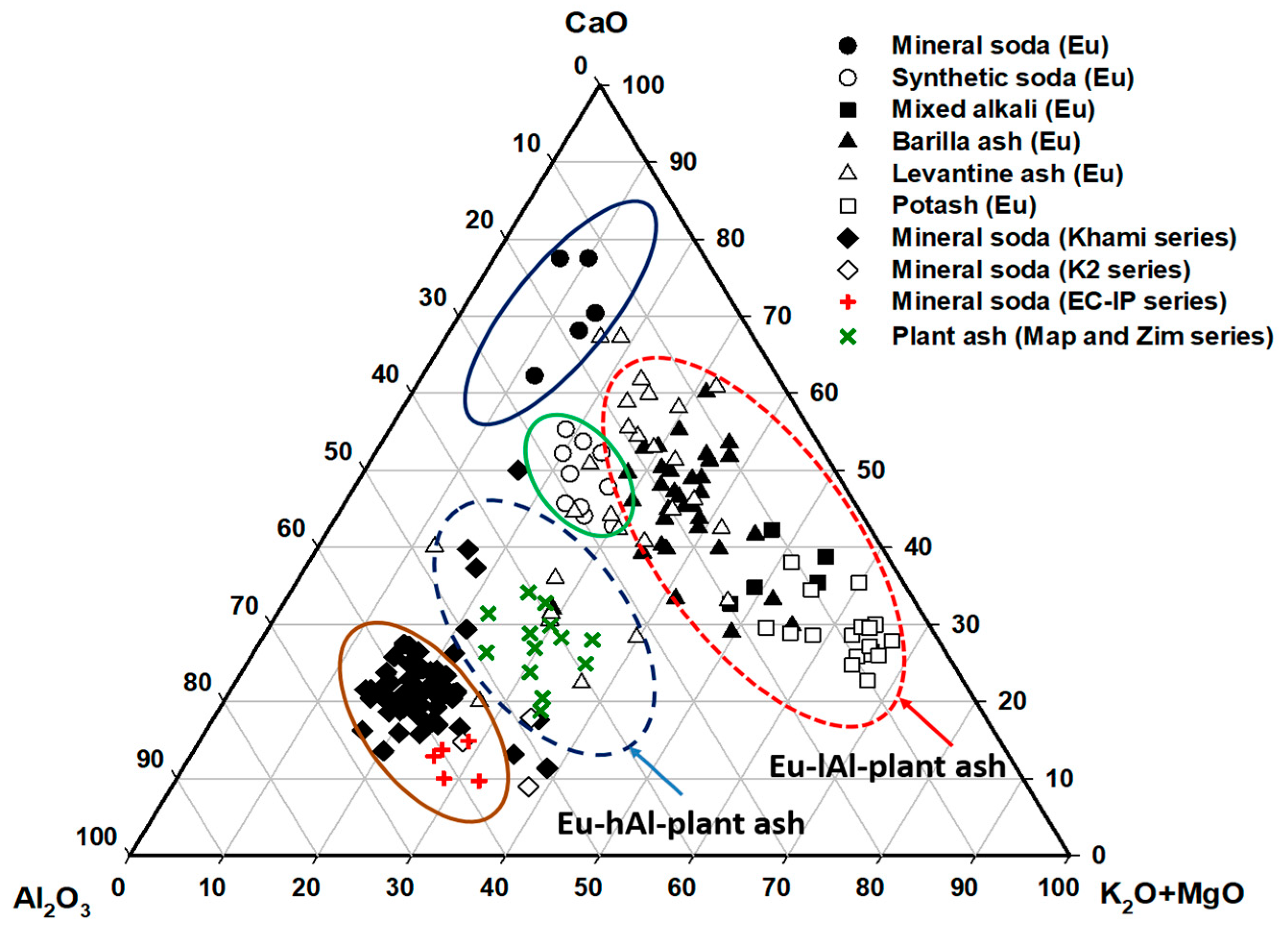
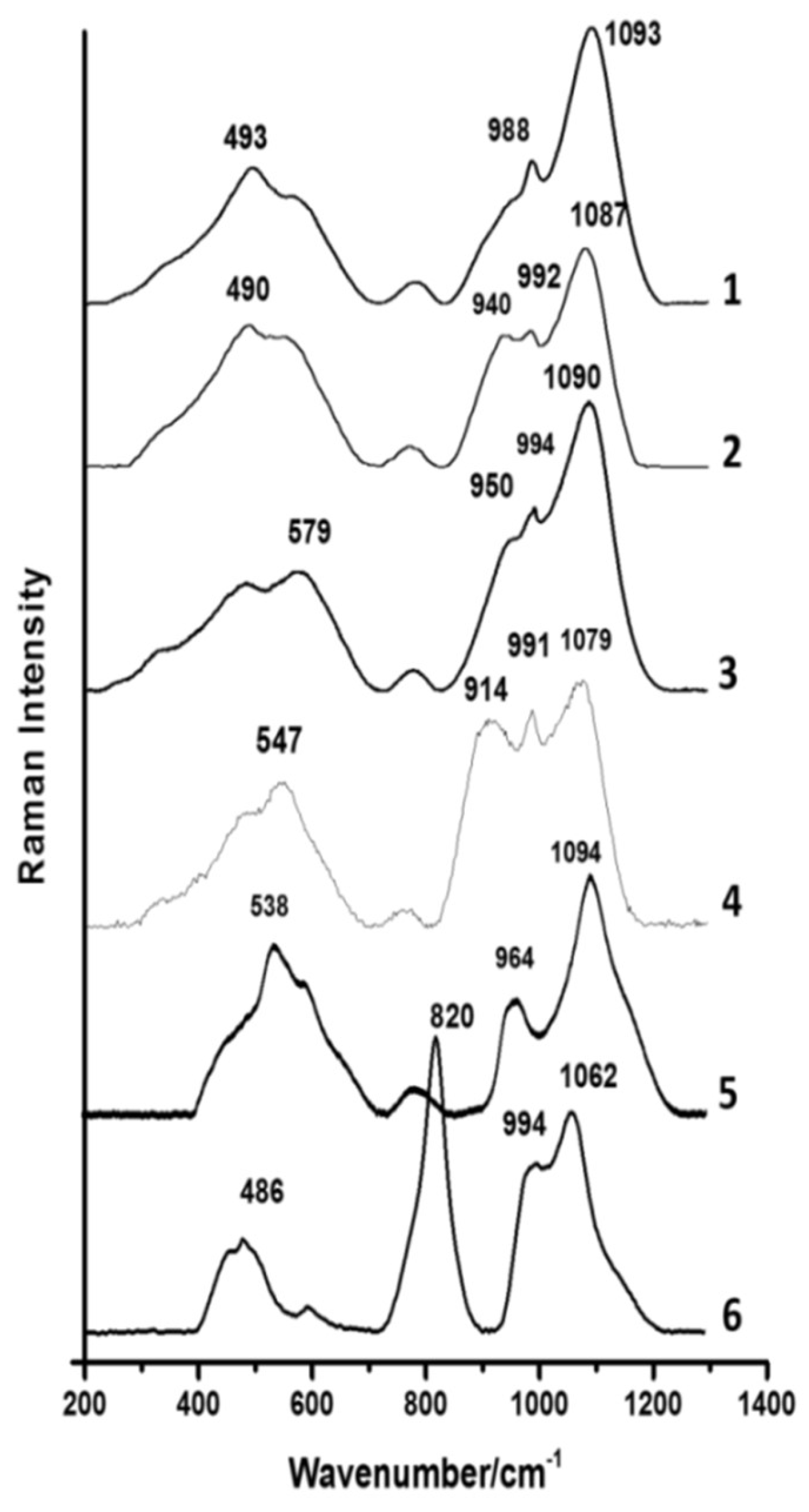
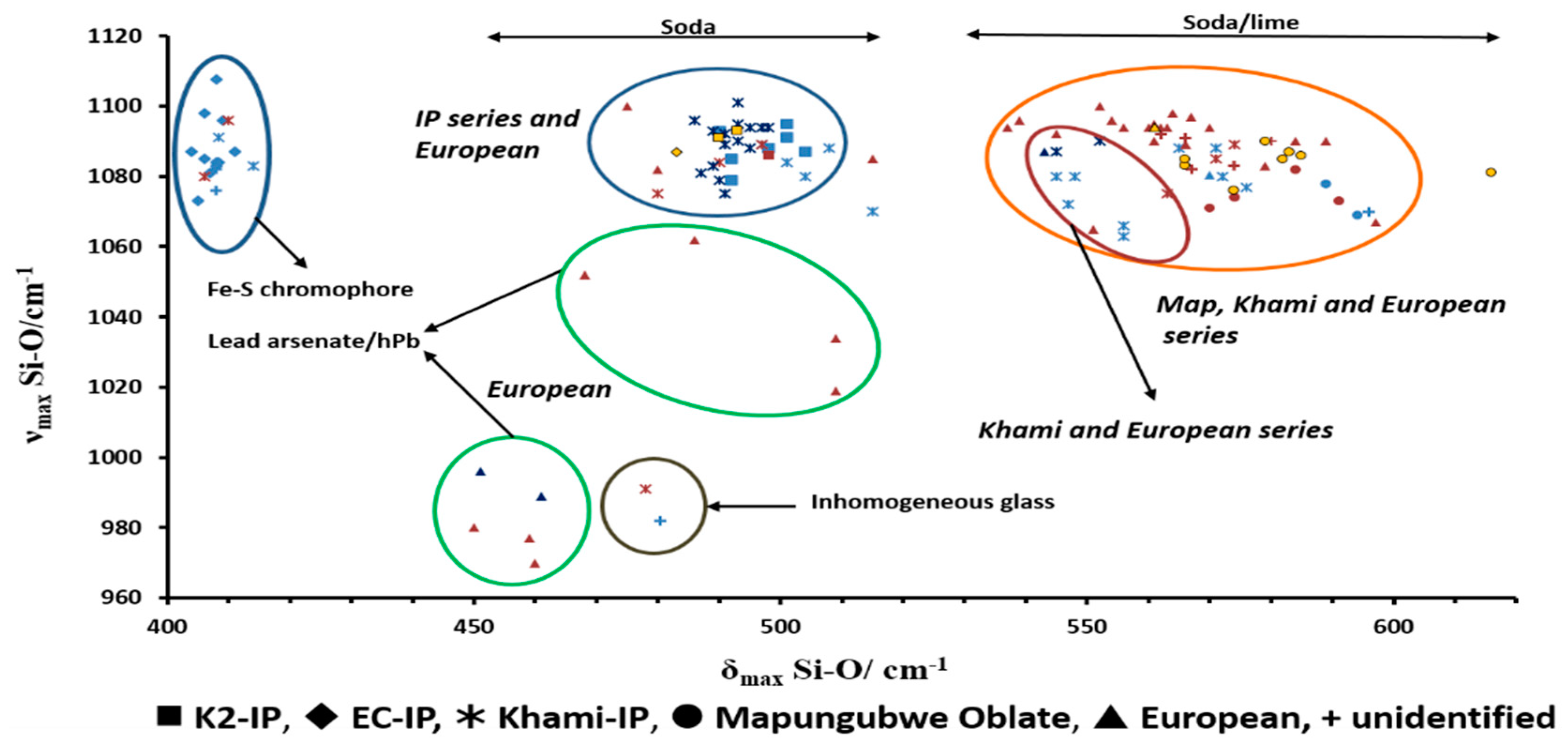
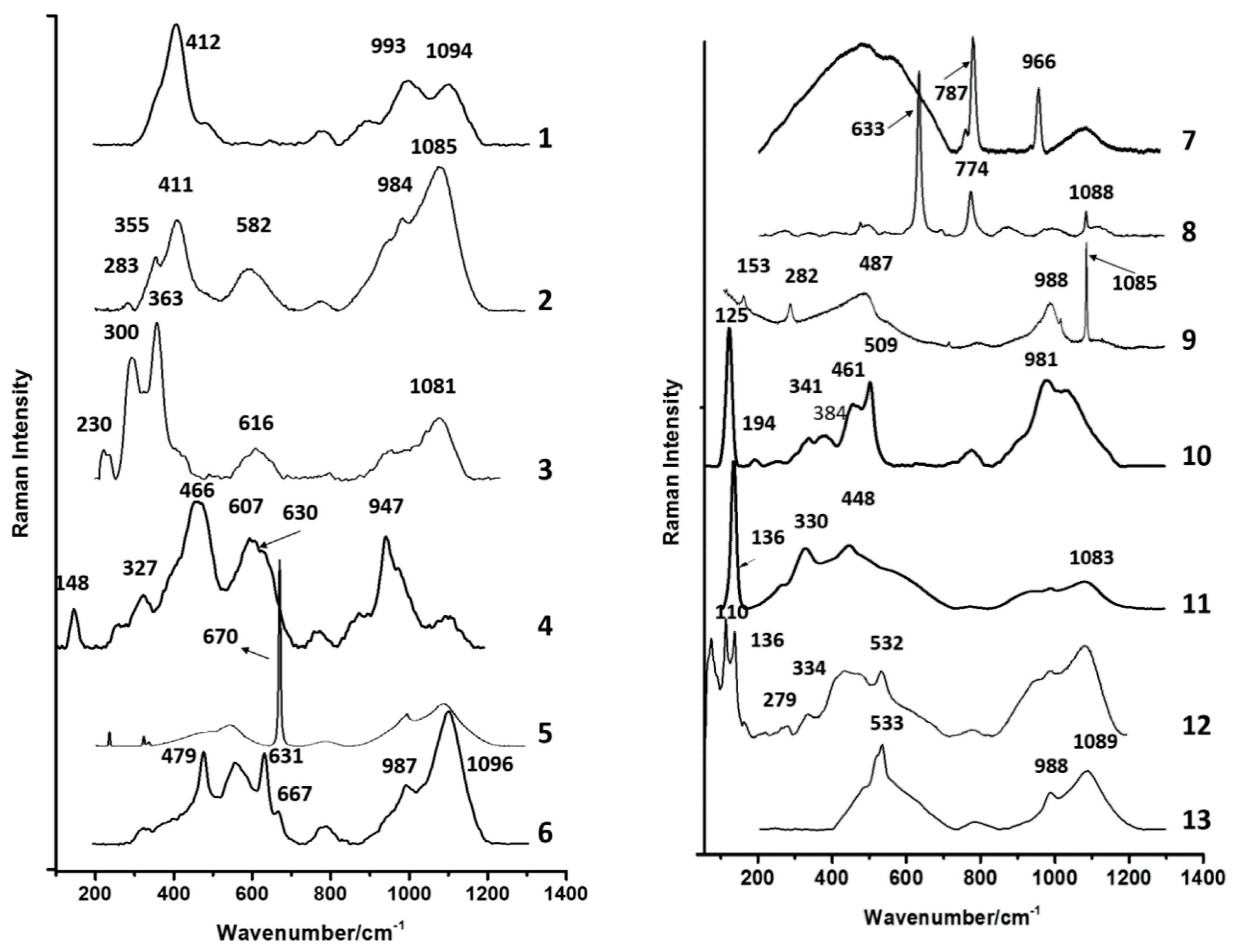
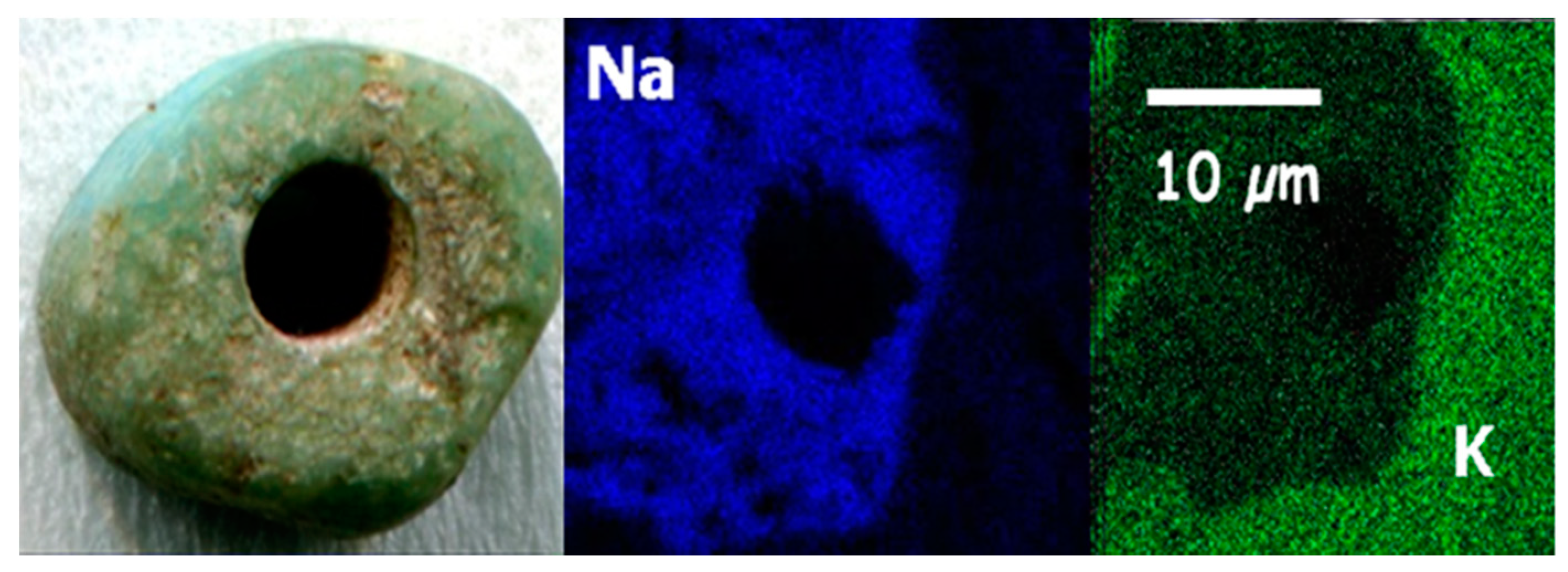
| Glass Type | Sub-Groups | Average Oxide Content (wt%) | Expected Alkali Source | Period (Centuries) | Expected Origin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Na2O | K2O | CaO | MgO | PbO | |||||
| Soda | Low Al | 13–20 | 0.4–1.5 | 2–9 | 0.4–2 | from 2nd c. AD | Middle East, South Asia, South-East Asia [5,56] | ||
| High Al (Al2O3: 4–10%) | 15–20 | 1.5–2.5 | 2–4 | 0.2–1 | ? | from 1st c. BC–6th c. AD | |||
| 9th–19th c. AD | South Asia [34,56] | ||||||||
| Venetian cristallo, Façon de Venise | 12–15 | 2–4 | 4–10 | 1–3 | Ashes | 15th–18th c. AD | Europe [54] | ||
| Soda Lime | Low Al, high Mg | 8–20 | 0–3 | 3–10 | 2–10 | Plant ashes | from 15th–8th c. BC | Near East [22] | |
| Low Al, low Mg | 13–20 | 0–1 | 5–10 | 0–1 | Natron | from 8th c. BC–3rd c. AD | Roman Levant (no Sb2O3) [48] | ||
| Low Al, low Mg, high Sb | 15–20 | 0–1 | 4–6 | 0–1 | Natron | 1st–3rd c. AD | Mediterranean area [46] | ||
| High Fe/Mg | 16–20 | 0–1 | 5–10 | 1–2 | Natron | 3rd–5th c. AD | Mediterranean area, Europe [46] | ||
| Levantine glass | 10–15 | 0–1 | 8–12 | 0–1 | Natron | 5th–8th c. AD | |||
| High Mg early Islamic glass | 10–18 | 1–3 | 6–12 | 3–7 | Natron and Plant ashes | 9th–10th c. AD | Islamic world [22] | ||
| Modern | 10–20 | 0–1 | 10–20 | 0–1 | Synthetic soda | 19th c. AD | Worldwide [59] | ||
| potash | Low Mg, high K | 0–8 | 8–18 | 0–4 | 0–1 | Plant ashes | Bronze Age | Europe [9] | |
| High Al | from 1st c. BC | Vietnam, South China [30,33] | |||||||
| Medium Al | South Asia [30] | ||||||||
| High K European Glass | 0–8 | 8–18 | 6–20 | 0–5 | Plant ashes | from 8th c. AD | West Europe [57] | ||
| High lime, low Alkali | <10 | <12 | 15–20 | 0–1 | Plant ashes (oak) | 15th–17th c. AD | Northern Europe [57] | ||
| Mixed | Mixed Alkali Glass | 5–10 | 5–10 | 10 | 2–6 | Plant ashes (seaweed) | 16th–17th c. AD | Northern Europe [57] | |
| Lead | High Ba | from 3rd c. BC | China [35] | ||||||
| High Na | from 1st c. AD | China [35] | |||||||
| High Na | from 2nd c. BC | Roman [22] | |||||||
| High–lead Islamic glass | 8–10 | 0–2 | 4–5 | 0–1 | 30–40 | Natron and Plant ashes | 10th c. AD–14th c. AD | Islamic world [22] | |
| High-lead medieval glass | 0–1 | 3–10 | 4–16 | 1–3 | 20–65 | Plant ashes | 8th–14th c. AD | Europe [26,27] | |
| Colour | Elements | Phase | Raman Detection | First Use | Remarks |
|---|---|---|---|---|---|
| White | bubbles | yes | |||
| Si | quartz (SiO2) | yes | |||
| Ti | rutile, anatase (TiO2) | yes | |||
| Zr | zircon (ZrSiO4), zirconia (ZrO2) | yes | 20th c. | ||
| P | apatite (Ca3(PO4)2) | yes | Antiquity | bones | |
| Ca | calcite (CaCO3) | yes | |||
| Sb | antimonate (CaSb2O7) | yes | Antiquity | ||
| Sb | antimonate (CaSb2O6) | yes | Antiquity | ||
| Sn | cassiterite (SnO2) | yes | 5th c. AD | ||
| As | arsenate (Ca, Pb)1.5AsO4 | yes | 17th c. AD | ||
| Blue | Cu | Egyptian blue (CaCuSi4O10) | yes | 3000 BC | |
| Ba, Cu | Han blue (BaCuSi4O10) | yes | 5th c. BC | ||
| Ba, Cu | Han violet (BaCuSi2O6) | yes | 2nd c. BC | ||
| Cu | dissolved Cu2+ | no | Turquoise in alkali glass matrix | ||
| S | lazurite (Na8 [Al6Si6O24] Sn) | yes | 1st c. BC | ||
| ultramarine | yes | 19th c. | |||
| Co | dissolved Co2+ | indirectly | |||
| spinels (Co, Cr, X) AlO4 | yes | 19th c. | |||
| olivine (CoSiO2) | yes | 17th c. | |||
| cobalt oxide (Co3O4) | yes | 19th c. | |||
| V | zircon (V: ZrSiO4) | yes | 20th c. | ||
| Yellow | Fe | dissolved Fe3+ | no | ||
| Sb | pyrochlore (PbSb2−xMxO7−d) | yes | 15th c. BC | Naples yellow | |
| Sn | Pb2Sn1−yMyO4 | yes | Antiquity | ||
| U | dissolved UO2+ | yes | |||
| Pb | Pb oxides | yes | Antiquity | ||
| Sn | sphene (CaSnSiO5) | yes | malayite | ||
| Zn, Cr | ZnCrO4 | yes | 19th c. | ||
| Green | Cu | Cu2+ dissolved | no | Antiquity | |
| Cr | Cr3+ dissolved | no | |||
| Cr | Cr2O3 | yes | 19th c. | ||
| 3CaO. Cr2O3. 3SiO2 | yes | 19th c. | Victoria green, malayite | ||
| Cr, Co | spinels: CoCr2O4, CoTiO4 | yes | 19th c. | ||
| olivine (NiSiO4) | yes | ||||
| Red | Cu | Cu° | indirect | Bronze age | |
| Fe | hematite | yes | 15th c. | ||
| Au | Au° | indirect | 16th c. |
| Location | Country (Number of Sites) | Percentage |
|---|---|---|
| West Africa | Ghana (13), Mauritania (9), Mali (7), Senegal (3), Burkina Faso (2), Niger, Sierra Leone; Benin (2), Nigeria, (12), Guinea | 46% |
| North Central Africa | Congo (4), Cameroon, Angola (4) | 7% |
| Southern Africa | South Africa, Botswana & Namibia (17), Zimbabwe (6) | 21% |
| East Africa | Kenya, Uganda and Zanzibar (12), Ethiopia (7), Madagascar (3), Sudan (5), Tanzania (2) | 26% |
| Type | Place | Date | Remarks | References |
|---|---|---|---|---|
| Garden Roller | K2 Limpopo Valley, SA | K2 (1100–1220) | Moulds found with beads. Big beads were made by sintering/melting smaller imported ones | [107,112,113,114] |
| Kiffa beads | Kiffa, Mauritania | 19th c. | Made from glass powder | [115,116] |
| Bodom | Ghana Western Africa | 19th c. | Powder-glass | [5,117] |
| Iyun, Segi | Ife, Nigeria | 12th–14th c. 18th c. | Grinding, powder-glass beads | [118] |
| Ile-Ife, Nigeria | 11th–15th c. | Glass cake, melting beads, high lime–high alumina, high lime–low alumina, soda-lime. | [119] | |
| Igbo Olokun, Nigeria | 11th–15th c. | Crucible production of high lime–high alumina and low lime–high alumina glass from raw local materials. | [111] | |
| Light green drawn bead | Basinghall (Botswana) | 1592–1648 | Inhomogeneous glass made by sintering soda, mixed alkali and potash glass. | [78] |
| Blue beads | Carthage/Utica | 1st c. | Glass powder + glassy cement | [78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koleini, F.; Colomban, P.; Pikirayi, I.; Prinsloo, L.C. Glass Beads, Markers of Ancient Trade in Sub-Saharan Africa: Methodology, State of the Art and Perspectives. Heritage 2019, 2, 2343-2369. https://doi.org/10.3390/heritage2030144
Koleini F, Colomban P, Pikirayi I, Prinsloo LC. Glass Beads, Markers of Ancient Trade in Sub-Saharan Africa: Methodology, State of the Art and Perspectives. Heritage. 2019; 2(3):2343-2369. https://doi.org/10.3390/heritage2030144
Chicago/Turabian StyleKoleini, Farahnaz, Philippe Colomban, Innocent Pikirayi, and Linda C. Prinsloo. 2019. "Glass Beads, Markers of Ancient Trade in Sub-Saharan Africa: Methodology, State of the Art and Perspectives" Heritage 2, no. 3: 2343-2369. https://doi.org/10.3390/heritage2030144
APA StyleKoleini, F., Colomban, P., Pikirayi, I., & Prinsloo, L. C. (2019). Glass Beads, Markers of Ancient Trade in Sub-Saharan Africa: Methodology, State of the Art and Perspectives. Heritage, 2(3), 2343-2369. https://doi.org/10.3390/heritage2030144






