Chemistry for Audio Heritage Preservation: A Review of Analytical Techniques for Audio Magnetic Tapes
Abstract
1. Introduction
1.1. Challenges
- Original formulations were and are considered trade secrets
- Audio tapes are rarely marked on the tape as to manufacturer and type
- Audio tapes often are placed in different boxes than they came in
- There were running changes during production
- There were process control variations
- There were different storage conditions
Benoît Thiébaut, in his presentation to the 2005 AMIA conference, indicated that he had found a range of video cassettes with the same type designation comprised of four clearly different chemical formulations [6]. In discussing this result with Bob Perry5, he stated that one would never see this much variation in a particular type number during the time he was at Ampex (1969–1992). Scotch/3M was open about the variations in type 1116. Bradshaw indicated7 that aging could possibly create some of the differences found by Thiébaut and that additional analysis would be beneficial. He also indicated the potential for seasonal changes and the difficulties of moving a successful tape line from one climatic location to another. Outsourcing further complicates this analysis, as the box may have one brand on it and the tape may have been manufactured at another facility.
1.2. Goals
1.3. Related Fields
1.3.1. Data Tape
1.3.2. Instrumentation Tape
1.3.3. Video Tape
1.3.4. Logging Tape
1.3.5. Beyond Reels
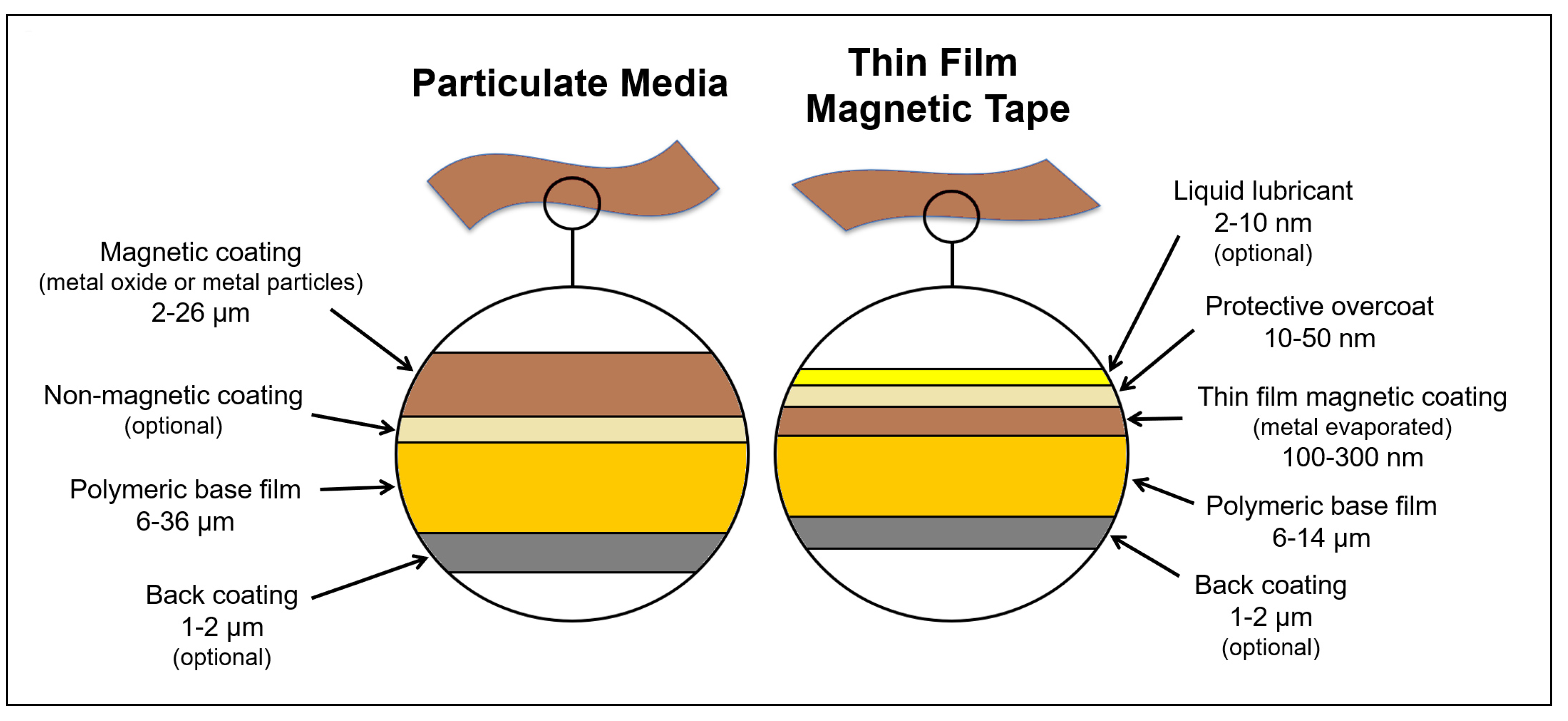
2. Composition of Magnetic Tapes for Audio Data Storage
3. Tape Degradation Problems
- Chemical nature of materials in the tape
- Manufacturing defects
- Temperature and humidity conditions over the life of the tape
- Exposure to liquids, dust, debris, or corrosive gases
- Handling history including frequent access or playback without proper conditioning or on defective equipment
- Exposure to strong magnetic fields
3.1. (i) Base Film Degradation
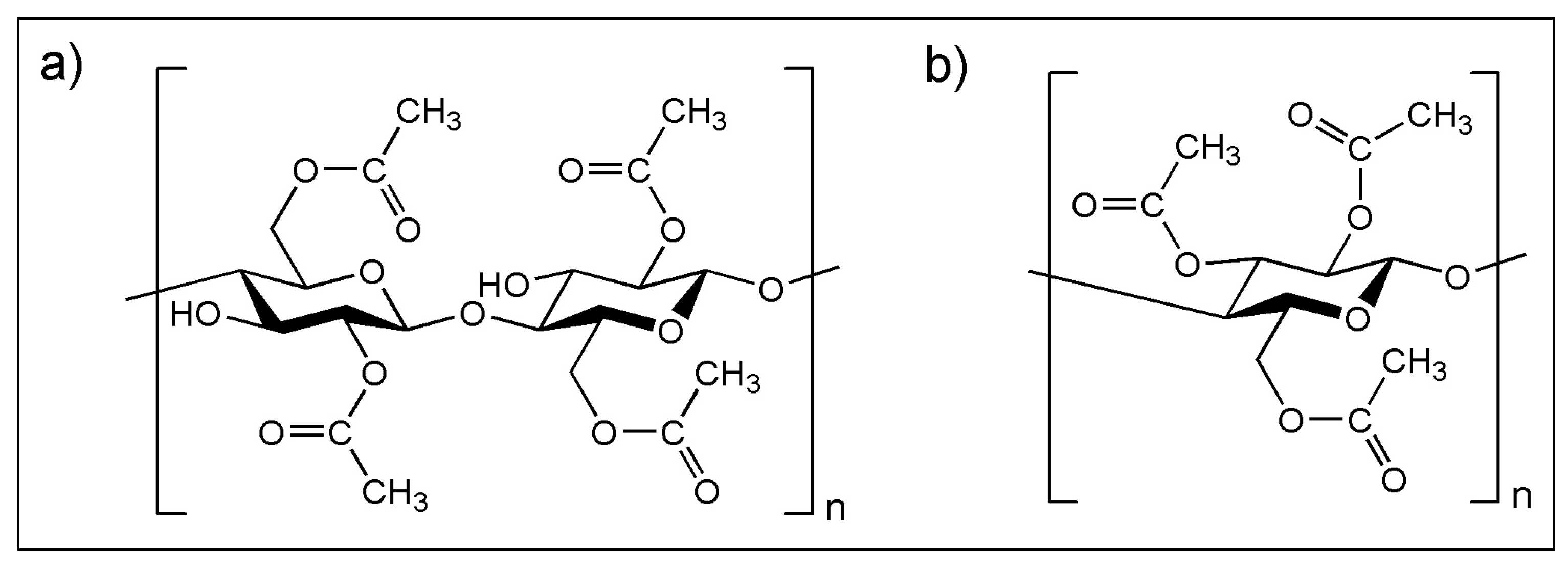
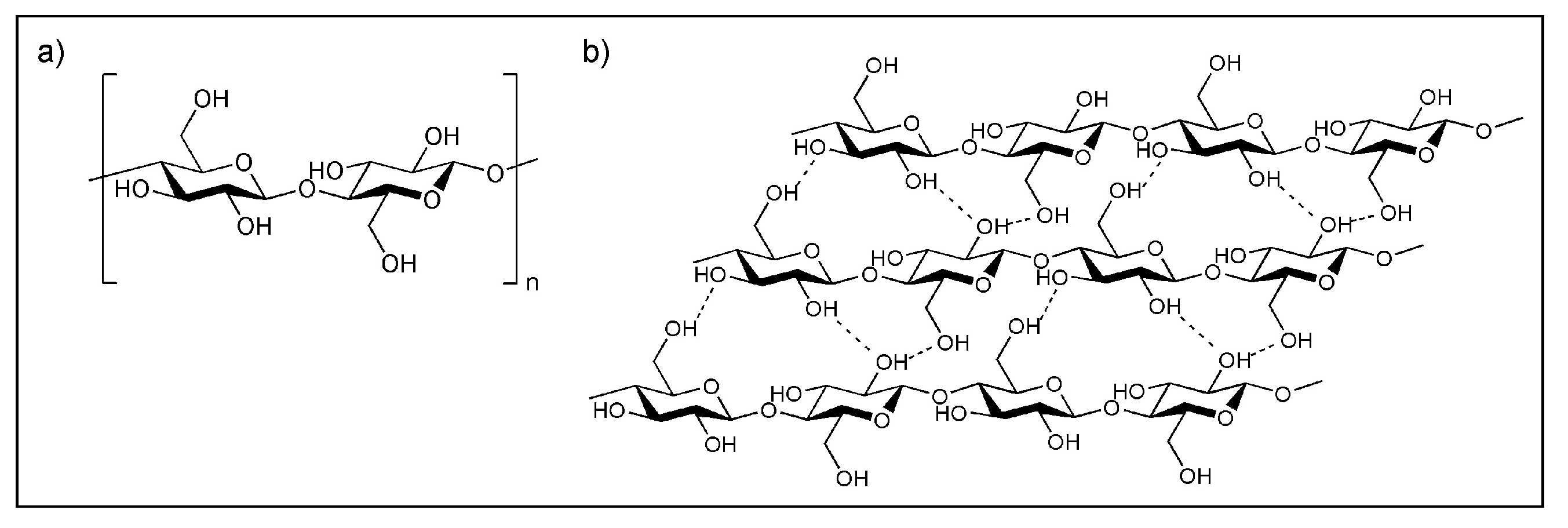
- Acetate tapes, degrade overall by chemical decomposition with formation of acetic acid (vinegar syndrome) promoted by high temperature and humidity, but catalyzed by iron oxide. Acetic acid is able to break the bonds between the polymeric chain, thus the material becomes brittle and shrinkage is observed [3,71,72]. Consequently, the binder containing the magnetic particles separates. In addition, the components of the emulsion can separate and crystalline deposits or liquid-filled bubbles can appear. Heating is especially damaging to acetate tapes, too [3]. The thermal behavior of cellulose acetate was studied: the thermal degradation started with acetic acid formation followed by dehydration. The acid formed catalyzes the further degradation and the FTIR spectra showed that at the original absorptions at 1725 (>C=O stretching), 1375 (C–H deformation) and 1250 (–C–O– stretching) cm decreased and a new band at 1580 cm (−C=C−) appeared, indicating the complete removal of the ester groups at [73]. Acetate tapes should be considered unstable and at high priority for copying.
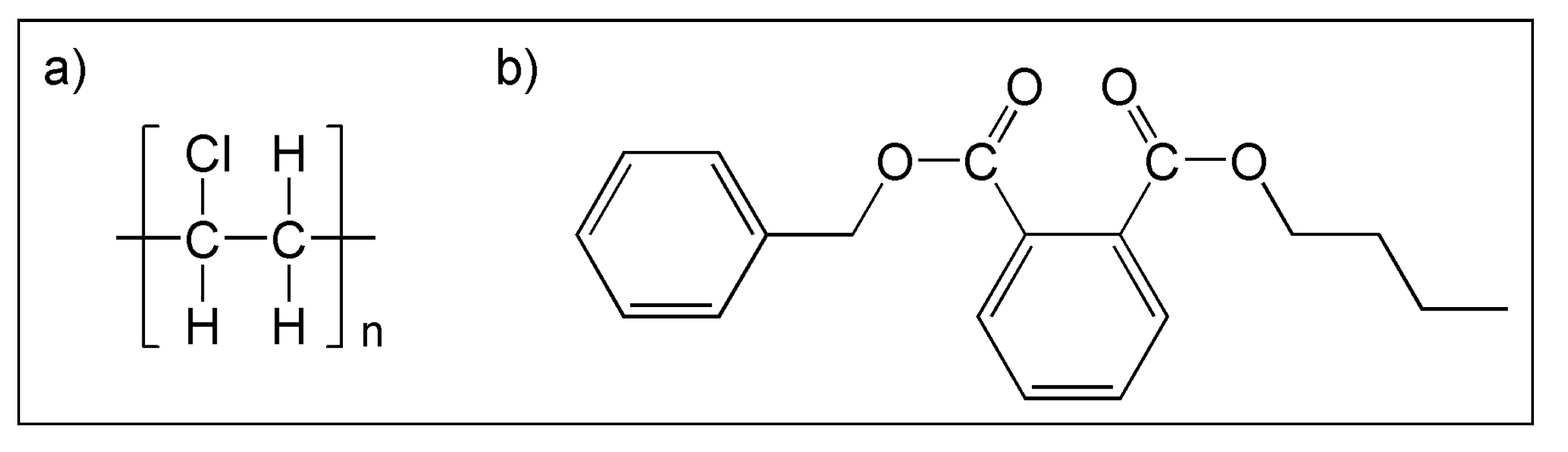
- PVC: The main way of degradation consists in the elimination of HCl with the formation of double bonds, which in turn can be oxidized to –C=O or –C–OH moieties, in the presence of oxygen and humidity. The polymer undergoes chain scission leading to a gradual deterioration of mechanical properties and chemical resistance. The entity of the HCl loss is depending on the presence of defects in the polymer structure (such as branching or allylic and tertiary labile chlorines).
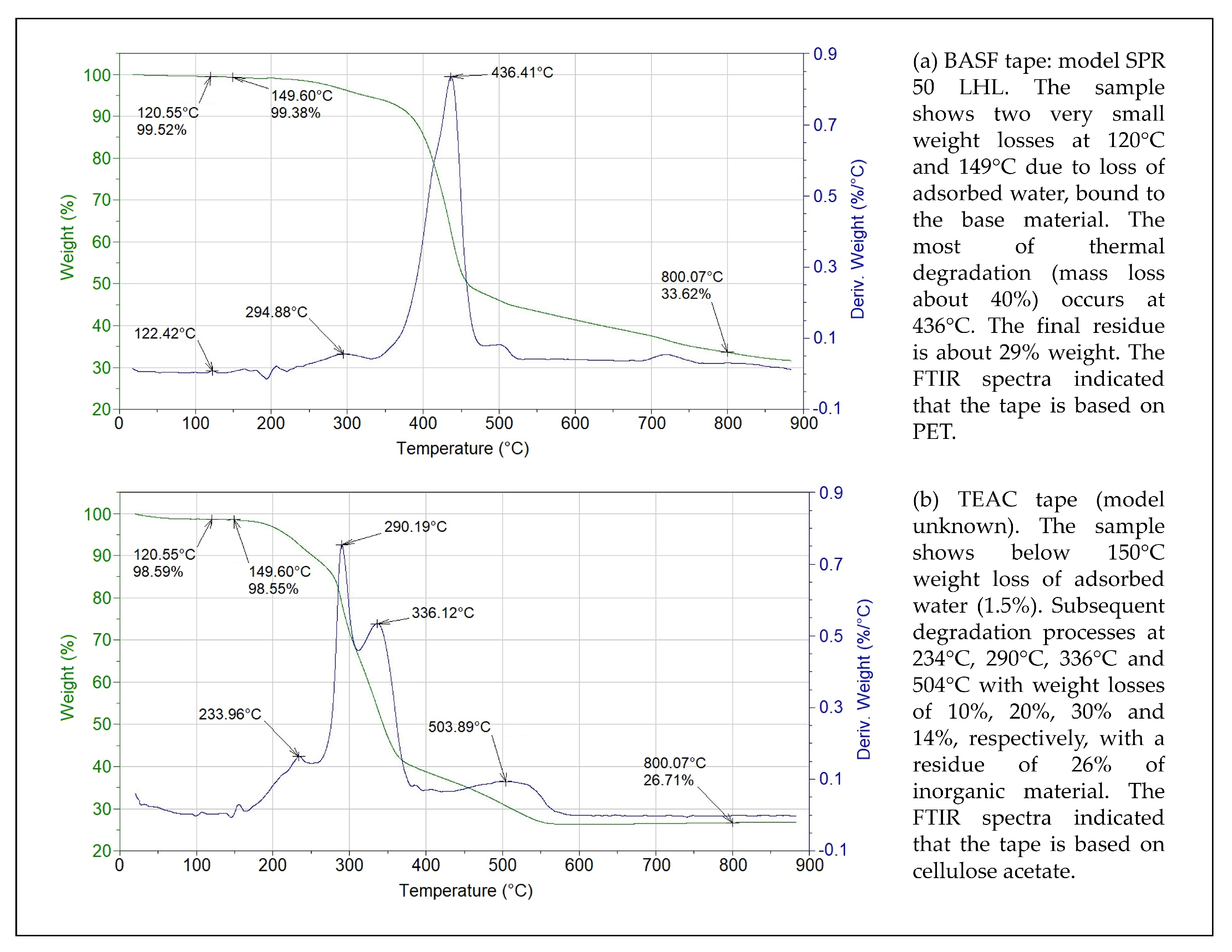
- PET: Although the thermal stability is high, its sensitivity to humidity and the presence of trace metal ions play a significant role in the rate of degradation [74]. Thermal degradation can occur during processing into film or molded products (at 200–300 ): it can start with a random breaking of the in-chain ester linkage, with formation of vinyl ester and carboxylic end groups. The transesterification of vinyl esters produces vinyl alcohol and acetaldehyde. Extraction of hydrogen can also occur promoted by metal traces giving rise to macroradicals from which hydroperoxides can be formed by reaction with oxygen with further breakdown of the polymer. In fact, even if PET is essentially a hydrophobic polymer, the ester functionality is known to undergo significant hydrolysis in moist, wet or humid conditions; under basic conditions; under UV light; or at temperature above glass transition temperature, resulting in an increase of carboxyl end groups and reduction in the molecular weight [75]. It is assumed that water diffuses into the amorphous region of the polymer where hydrolysis occurs at a rate which depends upon the shape, morphology, degree of crystallinity, relative humidity, and temperature. Experiments concerning the heat and moisture diffusion in magnetic tape packs indicated that they can be described by the heat diffusion equation for a hollow cylinder and thermal and moisture diffusivity coefficients have been shown to be anisotropic and significantly higher in the axial direction compared with the radial one [76].The evolution of molecular orientation and microstructure during and following the deformation of amorphous PET above and below Tg was deeply investigated by URS-FTIR spectroscopy and PM-IRLD, furnishing interesting data concerning the modifications of thin films under stress [77].
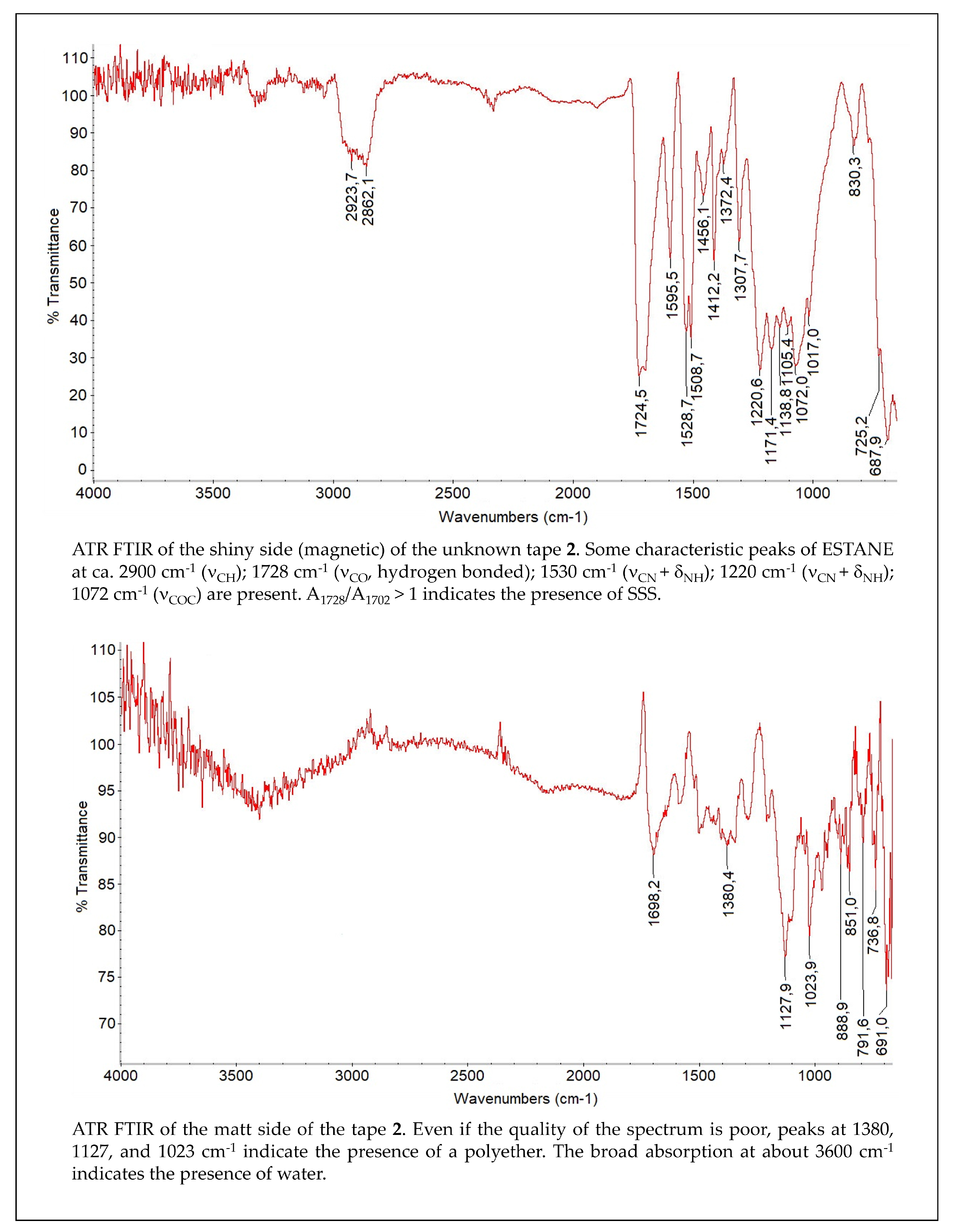
3.2. (ii) Binder Degradation
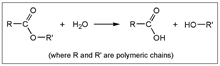
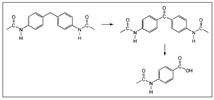
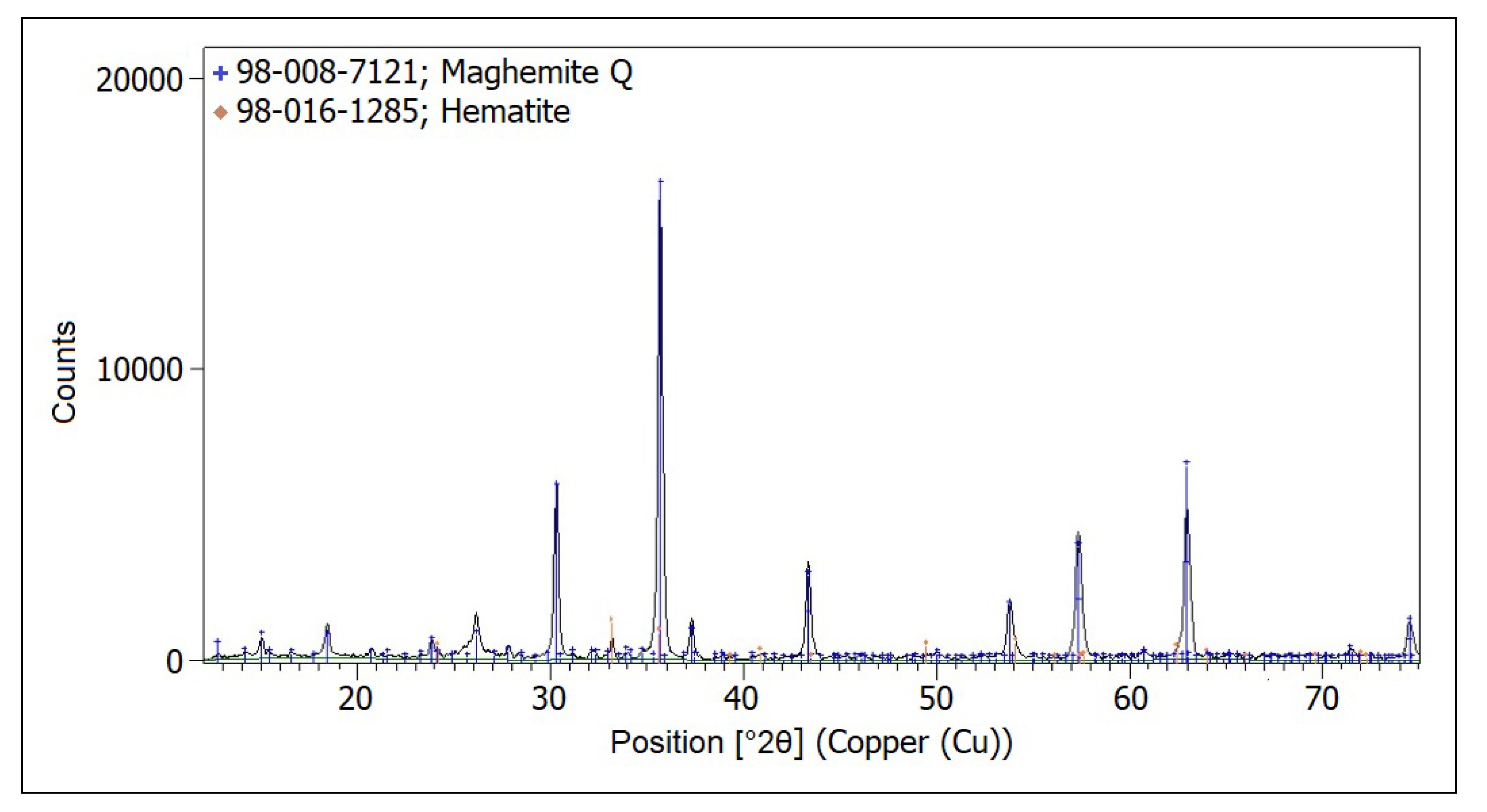
3.3. (iii) Stability of Magnetic Material
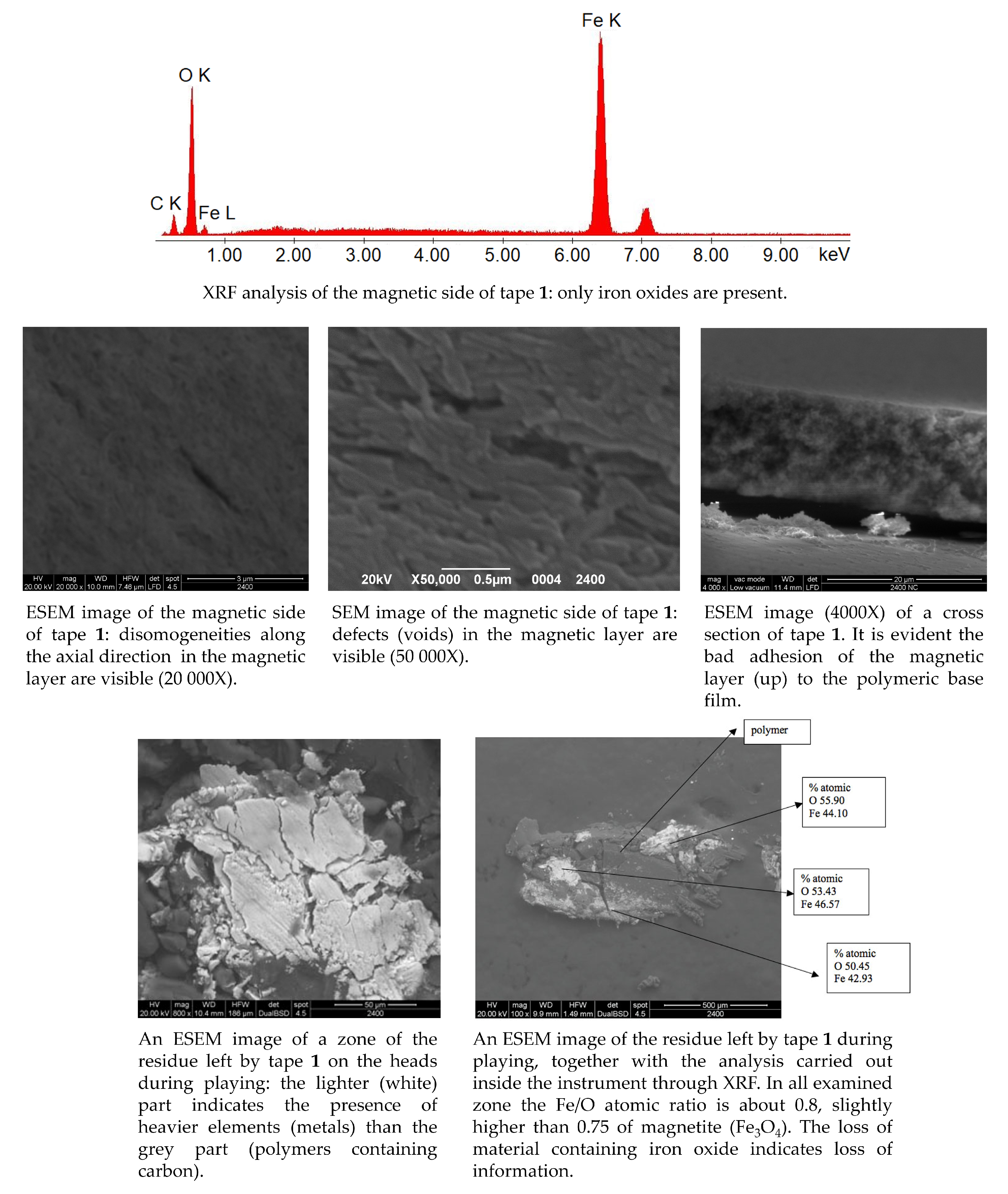
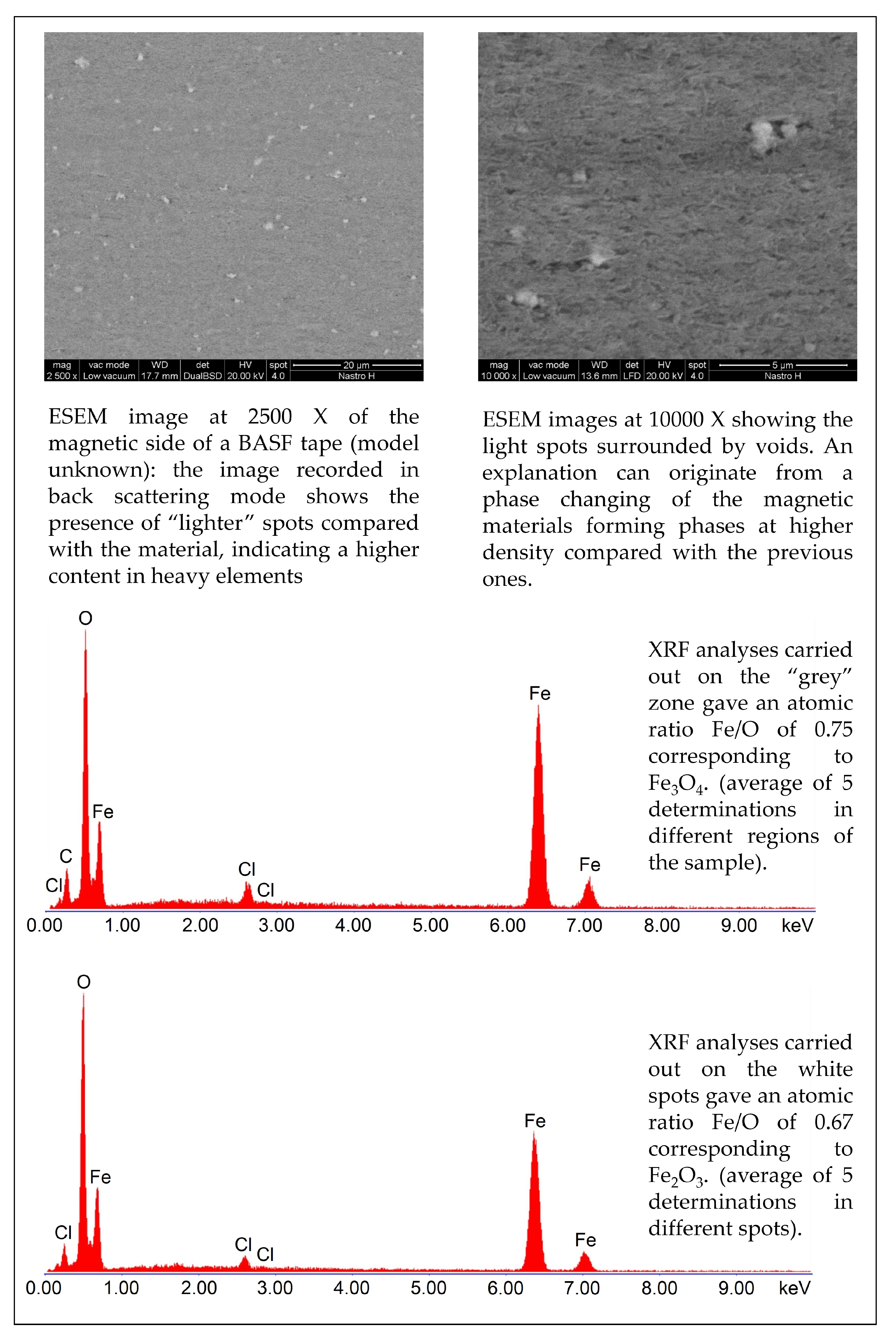
4. Analytical Methods to Characterize the Tapes Components and to Study the Degraded Tapes
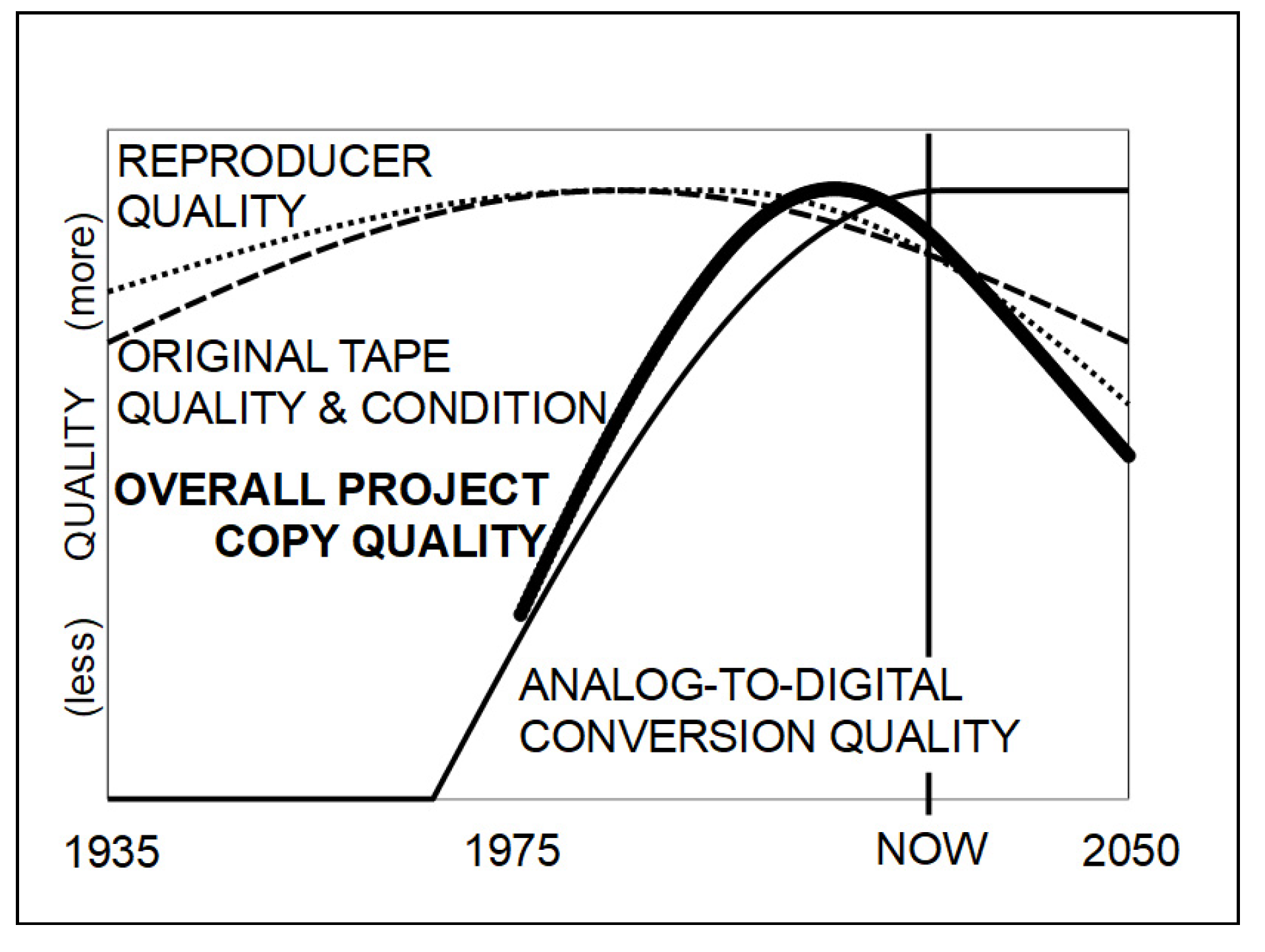
5. Restoration
6. Preservation, Handling, Storage
- Tapes must be wound end-to-end, onto a rigid hub, before being put into storage to avoid pack tension distortions.
- Any residue from degradation should be removed as long as there is no further damage to the tape.
- A current outline of storage recommendations for tape can be found in [124] (Section 3.3). There are some caveats to consider when analyzing these limits and this is an area of further research as film historians are freezing film to preserve it longer.
- ○
- It is likely that tapes coated on an acetate base film do not perform as well at the lower range of the recommended RH.
- ○
- The “do not freeze” warning about tape came from concerns over early tapes that used a variety of naturally occurring lubricants, often extracted from marine creatures. What is interesting is that some sample lubricants that Hess has in his possession appear to congeal below about , thus these would be congealed at the minimum recommended temperature of recommended in [124].
- The above two sub-points suggest further research.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AES | Audio Engineering Society (New York, NY, USA) |
| ATR FTIR | Attenuated total reflectance Fourier transform infrared spectroscopy |
| ESEM | Environmental scanning electron microscopy |
| FT-IR | Fourier transform infrared spectroscopy |
| GC MS | Gas chromatography mass spectrometry |
| IASA | International Association of Sound and Audiovisual Archives (Amsterdam, The Netherlands) |
| LD | linear dichroism |
| NAB | National Association of Broadcasters (Washington, DC, USA) |
| NMR | Nuclear magnetic resonance |
| PEN | Polyethylene naphthalate (used as a base film) |
| PE-PU | Polyester polyurethane (used as a binder) |
| PET | Polyethylene terephthalate (common base film) |
| PM-IRLD | polarization modulation infrared linear dichroism |
| PVC | Polyvinyl chloride (historic base film) |
| RH | Relative humidity |
| SEM | Scanning electron microscopy |
| SIMS | Secondary ion mass spectrometry |
| SMPTE | Society of Motion Picture and Television Engineers (White Plains, NY, USA) |
| SPME | Solid phase micro extraction |
| Tg | Glass transition temperature |
| TGA | Thermogravimetric analysis |
| UHV | Ultra high vacuum |
| URS-FTIR | Ultra-rapid scanning spectroscopy |
| VOC | Volatile organic compounds |
| XRD | X-ray powder diffraction |
References
- Engel, F. Magnetic Tape from the Early Days to the Present. J. Audio Eng. Soc. 1988, 36, 606–616. [Google Scholar]
- Schüller, D. Preserving the Facts for the Future: Principles and Practices for the Transfer of Analog Audio Documents into the Digital Domain. J. Audio Eng. Soc. 2001, 49, 618–621. [Google Scholar]
- Hess, R. Tape Degradation Factors and Challenges in Predicting Tape Life. ARSC J. 2008, 39, 240–274. [Google Scholar]
- Richardson, C.A. Solving the Sticky Shed Problem in Magnetic Recording Tapes: New Laboratory Research and Analysis Provides a Safe and Effective Remedy; Audio Engineering Society: New York, NY, USA, 2006. [Google Scholar]
- Richardson, C. Process for Restoring Magnetic Recording tape Damaged by Sticky Shed Syndrome. U.S. Patent US6797072, 28 September 2004. [Google Scholar]
- Thiébaut, B. Particulate magnetic tape materials characterisation and degradation study. In Proceedings of the Association of Moving Image Archivists (AMIA) Conference, Austin, TX, USA, 30 November–1 December 2005. [Google Scholar]
- Hollerith, H. Art of Compiling Statistics. U.S. Patent US395782A, 8 January 1884. [Google Scholar]
- Bradshaw, R.; Schroeder, C. Fifty years of IBM innovation with information storage on magnetic tape. IBM J. Res. Dev. 2003, 47, 373–383. [Google Scholar] [CrossRef][Green Version]
- Braun, W.L. The NAB Recording and Reproducing Standards. J. Audio Eng. Soc. 1968, 16, 168–173. [Google Scholar]
- Telemetry Group; Range Commander Council. Telemetry Standards. IRIG STANDARD 106-04; US Army: Arlington County, VA, USA, 2004. [Google Scholar]
- Hauttekeete, L.; Evens, T.; Moor, K.D.; Schuurman, D.; Mannens, E.; de Walle, R.V. Archives in motion: Concrete steps towards the digital disclosure of audiovisual content. J. Cult. Herit. 2011, 12, 459–465. [Google Scholar] [CrossRef]
- Casey, M. FACET (Field Audio Collection Evaluation Tool)–Procedures Manual Version 1.0; Technical Report; Indiana University: Bloomington, IN, USA, 2008. [Google Scholar]
- Cassidy, B.M.; Breitung, E.M. Magnetic Tapes, Playable or Not? Against Grain 2015, 27, 1. [Google Scholar] [CrossRef]
- Casey, M. Why media preservation can’t wait: The gathering storm. IASA J. 2015, 44, 14–22. [Google Scholar]
- Allen-Robertson, J. The materiality of digital media: The hard disk drive, phonograph, magnetic tape and optical media in technical close-up. New Media Soc. 2017, 19, 455–470. [Google Scholar] [CrossRef]
- Heller, M.C. Orpheus unglued: sticky shed syndrome and tape’s archival anxieties. Sound Stud. 2017, 3, 64–70. [Google Scholar] [CrossRef]
- Green, C.V. Chemical Analysis of Magnetic Recording Tape. J. Audio Eng. Soc. 1960, 8, 156–158. [Google Scholar]
- Scheiman, J.; Schwartz, R. An Old-School Tape Evaluator. U.S. Patent US3359783, 1967. [Google Scholar]
- Cinadr, B. Details on Coating Systems. U.S. Patent US3650828, 1972. [Google Scholar]
- Kaempf, G.; Loewer, H.; Witman, M. Polymers as substrates and media for data storage. Polym. Eng. Sci. 1987, 27, 1421–1425. [Google Scholar] [CrossRef]
- Kim, K.; Farkas, J.; Hasman, D.; Miller, T.; Zellia, J.; Jacobs, P. Recent developments in binder design for advanced media. J. Magn. Magn. Mater. 1999, 193, 265–275. [Google Scholar] [CrossRef]
- Yilgör, E.; Yurtsever, E.; Yilgör, I. Hydrogen bonding and polyurethane morphology. II. Spectroscopic, thermal and crystallization behavior of polyether blends with 1,3-dimethylurea and a model urethane compound. Polymer 2002, 43, 6561–6568. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Garrett, J.T.; Xu, R.; Cho, J.; Runt, J. Phase separation of diamine chain-extended poly(urethane) copolymers: FTIR spectroscopy and phase transitions. Polymer 2003, 44, 2711–2719. [Google Scholar] [CrossRef]
- Tu, R.S. Polymers in Information Storage Technology; Chapter Mechanism of Chemical Reactions Involved in Magnetic Coatings; Springer: New York, NY, USA, 1989; pp. 345–350. [Google Scholar]
- Nakamae, K.; Tanigawa, S.; Sumiya, K.; Matsumoto, T. Polymers in Information Storage Technology; Chapter Role of Active Functional Groups and Conformation of Adsorbed Polymers in the Dispersibility of Magnetic Particles; Springer: New York, NY, USA, 1989; pp. 407–420. [Google Scholar]
- Boynton, G.; Ingersoll, H. Magnetic Tape Binder from a Polyurethane, a Polyol and an Isocyanate. U.S. Patent US3926826, 16 Decmeber 1975. [Google Scholar]
- Deffeyes, R. Magnetic Tape. U.S. Patent US4020227, 1976. [Google Scholar]
- Johnson, R. Process of Making High-Temperature Magnetic Tape. U.S. Patent US4189514, 19 February 1980. [Google Scholar]
- Ninomiya, Y.; Hashimoto, A. Magnetic Recording Medium. U.S. Patent US4529661, 1985. [Google Scholar]
- Sharrock, M.P. Particulate Magnetic Recording Media: A Review. IEEE Trans. Magn. 1989, 25, 4374–4389. [Google Scholar] [CrossRef]
- Potgiesser, J.A.L.; Koorneef, J. Mechanical wear and degeneration of the magnetic properties of magnetic heads caused by the tape. Radio Electron. Eng. 1974, 44, 313–318. [Google Scholar] [CrossRef]
- Hisano, S.; Saito, K. Research and development of metal powder for magnetic recording. J. Magn. Magn. Mater. 1998, 190, 371–381. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Guo, H.; Barnard, A.S. Iron Oxides: From Nature to Applications; Chapter Thermodynamics of Iron Oxides and Oxyhydroxides in Different Environments; Wiley: Hoboken, NJ, USA, 2016; pp. 269–292. [Google Scholar]
- Vereda, F. Iron Oxides: From Nature to Applications; Chapter Introduction to Standard Spectroscopic Methods: XRD, IR/Raman, and Mössbauer; Wiley: Hoboken, NJ, USA, 2016; pp. 295–324. [Google Scholar]
- Faivre, D. (Ed.) Iron Oxides: From Nature to Applications; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Dismukes, J.P.; Martin, D.F.; Ekstrom, L.; Wang, C.C.; Coutts, M.D. Ferromagnetic Chromium Dioxide for Magnetic Tape. Prod. R D 1971, 10, 319–329. [Google Scholar] [CrossRef]
- Nishida, Y.; Hisamichi, Y.; Kondo, H. Behavior of perfluoropolyether in particulate magnetic recording media. IEEE Trans. Magn. 1996, 32, 3738–3740. [Google Scholar] [CrossRef]
- Bhushan, B.; Hahn, F.W. Stains on magnetic tape heads. Wear 1995, 184, 193–202. [Google Scholar] [CrossRef]
- Bhushan, B.; Patton, S.T. Tribology in ultra-high density tape drive systems: State of the art and future challenges. IEEE Trans. Magn. 1998, 34, 1883–1888. [Google Scholar] [CrossRef]
- Bhushan, B. Tribology and Mechanics of Magnetic Storage Devices; Springer: New York, NY, USA, 1996. [Google Scholar]
- Bhushan, B.; Cichomski, M.; Tao, Z.; Tran, N.T.; Ethen, T.; Merton, C.; Jewett, R.E. Nanotribological Characterization and Lubricant Degradation Studies of Metal-Film Magnetic Tapes Using Novel Lubricants. J. Tribol.-Trans. ASME 2007, 129, 621–627. [Google Scholar] [CrossRef]
- van Groenou, A.B. Tribology of magnetic storage systems, a short review. J. Magn. Magn. Mater. 1991, 95, 289–312. [Google Scholar] [CrossRef]
- Wierenga, P.; Schaake, R. The effect of mechanical and chemical surface properties on the friction of magnetic tapes. Wear 1987, 119, 29–50. [Google Scholar] [CrossRef]
- Raeymaekers, B.; Etsion, I.; Talke, F.E. Enhancing tribological performance of the magnetic tape/guide interface by laser surface texturing. Tribol. Lett. 2007, 27, 89. [Google Scholar] [CrossRef]
- Bhushan, B.; Khatavkar, D.V. Role of tape abrasivity on friction, wear, staining and signal degradation in audio tapes. Wear 1995, 190, 16–27. [Google Scholar] [CrossRef]
- Bhushan, B. (Ed.) Nanotribology and Nanomechanics. An Introduction; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Bhushan, B. Mechanics and Reliability of Flexible Magnetic Media; Springer: New York, NY, USA, 2012. [Google Scholar]
- Bradshaw, R.L.; Bhushan, B. Friction in Magnetic Tapes III: Role of Chemical Properties. ASLE Trans. 1984, 27, 207–219. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. Nanotribological properties of novel lubricants for magnetic tapes. Ultramicroscopy 2009, 109, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Tribology and Mechanics of Magnetic Storage Devices; Chapter Interface Temperature of Sliding Surfaces; Springer Science & Business Media: Berlin, Germany, 1996; pp. 366–411. [Google Scholar]
- Raymond Engineering Inc. newblock OSO Head and Tape Studies; Final Report No. 705-8; Technical Report; NASA/GSFC: Washington, DC, USA, 1968. [Google Scholar]
- Bressan, F.; Bertani, R.; Furlan, C.; Simionato, F.; Canazza, S. An ATR FTIR And ESEM Study On Magnetic Tapes For The Assessment Of The Degradation Of Historical Audio Recordings. J. Cult. Herit. 2016, 18, 313–320. [Google Scholar] [CrossRef]
- Judge, J.; Schmidt, R.; Weiss, R.; Miller, G. Media stability and life expectancies of magnetic tape for use with IBM 3590 and digital linear tape systems. In Proceedings of the 20th IEEE/11th NASA Goddard Conference on Mass Storage Systems and Technologies (MSST 2003), San Diego, CA, USA, 7–10 April 2003. [Google Scholar]
- Okazaki, Y.; Hara, K.; Kawashima, T.; Sato, A.; Hirano, T. Estimating the archival life of metal particulate tape. IEEE Trans. Magn. 1992, 28, 2365–2367. [Google Scholar] [CrossRef]
- Wheeler, J. Long-Term Storage of Videotape. SMPTE J. 1983, 92, 650–654. [Google Scholar] [CrossRef]
- Rusch, A.; Beekman, E.; Lascaro, C. newblock Reinforced Plastic Magnetic Tapes; Technical Report 2324; Technical Report; USAEL-RDL: Kanagawa, Japan, 1963. [Google Scholar]
- Goldade, A.; Bhushan, B. Measurement and Origin of Tape Edge Damage in a Linear Tape Drive. Tribol. Lett. 2003, 14, 167–180. [Google Scholar] [CrossRef]
- Brokerhof, A.W.; van Zanen, B.; den Teuling, A. Fluffy Stuff: Integrated Control of Mould in Archives; Netherlands Institute for Cultural Heritage (ICN): Amsterdam, The Netherlands, 2007. [Google Scholar]
- Florian, M.L.E. Heritage Eaters: Insects and Fungi in Heritage Collections; UBC Community and Partner Publications, James and James (Science Publishers) Ltd.: Northampton, UK, 1997. [Google Scholar]
- AES. AES Recommended Practice for Audio Preservation and Restoration—Storage of Polyester-Base Magnetic Tape; AES: New York, NY, USA, 1997. [Google Scholar]
- Bogart, J.W.V. Magnetic Tape Storage and Handling. A Guide for Libraries and Archives; Technical Report; National Media Laboratory: New York, NY, USA, 1995. [Google Scholar]
- Ma, Z.; Hong, Y.; Nelson, D.M.; Pichamuthu, J.E.; Leeson, C.E.; Wagner, W.R. Biodegradable Polyurethane Ureas with Variable Polyester or Polycarbonate Soft Segments: Effects of Crystallinity, Molecular Weight, and Composition on Mechanical Properties. Biomacromolecules 2011, 12, 3265–3274. [Google Scholar] [CrossRef]
- Costa, C.Z.; Albuquerque, M.d.C.C.d.; Brum, M.C.; Castro, A.M.d. Degradação microbiológica e enzimática de polímeros: uma revisão. Química Nova 2015, 38, 259–267. [Google Scholar]
- Lando, G.A.; Marconatto, L.; Kessler, F.; Lopes, W.; Schrank, A.; Vainstein, M.H.; Weibel, D.E. UV-Surface Treatment of Fungal Resistant Polyether Polyurethane Film-Induced Growth of Entomopathogenic Fungi. Int. J. Mol. Sci. 2017, 18, 1536. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of Polyester Polyurethane by Bacterial Polyester Hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef]
- Zafar, U.; Nzeram, P.; Langarica-Fuentes, A.; Houlden, A.; Heyworth, A.; Saiani, A.; Robson, G.D. Biodegradation of polyester polyurethane during commercial composting and analysis of associated fungal communities. Bioresour. Technol. 2014, 158, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Takács, E.; Krakovský, I.; Horváth, Z.; Rosta, L.; Almásy, L. Study on the microstructure of polyester polyurethane irradiated in air and water. Polymers 2015, 7, 1755–1766. [Google Scholar] [CrossRef]
- Labed, V.; Obeid, H.; Ressayre, K. Effect of relative humidity and temperature on PVC degradation under gamma irradiation: Evolution of HCl production Yields. Radiat. Phys. Chem. 2013, 84, 26–29. [Google Scholar] [CrossRef]
- Brown, D.W.; Lowry, R.E.; Smith, L.E. Kinetics of Hydrolytic Aging of Polyester Urethane Elastomers. Macromolecules 1980, 13, 248–252. [Google Scholar] [CrossRef]
- Wilhelm, C.; Gardette, J.L. Infrared analysis of the photochemical behaviour of segmented polyurethanes: 1 Aliphatic poly(ester-urethane). Polymer 1997, 38, 4019–4031. [Google Scholar] [CrossRef]
- Tosh, B. Thermal analysis of cellulose esters prepared from different molecular weight fractions of high a-cellulose pulp. Indian J. Chem. Technol. 2011, 18, 451–457. [Google Scholar]
- Edge, M.; Mohammadian, M.; Hayes, M.; Allen, N.S.; Brems, K.; Jones, K. Aspects of Polyester Degradation: Motion Picture Film and Videotape Materials. J. Imaging Sci. Technol. 1992, 36, 13–20. [Google Scholar]
- Pirzadeh, E.; Zadhoush, A.; Haghighat, M. Hydrolytic and thermal degradation of PET fibers and PET granule: The effects of crystallization, temperature, and humidity. J. Appl. Polym. Sci. 2007, 106, 1544–1549. [Google Scholar] [CrossRef]
- Vos, M.; Ashton, G.; Vanbogart, J.; Ensminger, R. Heat and moisture diffusion in magnetic tape packs. IEEE Trans. Magn. 1994, 30, 237–242. [Google Scholar] [CrossRef]
- Pellerin, C.; Pézolet, M.; Griffiths, P.R. Time-Resolved Infrared Spectroscopic Studies of Poly(ethylene terephthalate) Deformation. Macromolecules 2006, 39, 6546–6551. [Google Scholar] [CrossRef]
- Bertram, H.N.; Cuddihy, E.F. Kinetics of the Humid Aging of Magnetic Recording Tape. IEEE Trans. Magn. 1982, 27, 4388–4395. [Google Scholar] [CrossRef]
- Cuddihy, E. Hygroscopic Properties of Magnetic Recording Tape. IEEE Trans. Magn. 1976, MAG-12, 126–135. [Google Scholar] [CrossRef]
- Cuddihy, E. Aging of Magnetic Recording Tape. IEEE Trans. Magn. 1980, MAG-16, 558–568. [Google Scholar] [CrossRef]
- Norris, S. Effects of Desiccation on Degraded Binder Extraction in Magnetic Audio Tape. ARSC J. 2010, 41. [Google Scholar]
- Cuddihy, E. Storage, Preservation, and Recovery of Magnetic Recording Tape; Technical Report; NASA: Washington, DC, USA, 1994. [Google Scholar]
- Edge, M.; Allen, N.; Chen, W.; Horie, C. Degradation of magnetic tape: Binder oxidation studies. Eur. Polym. J. 1993, 29, 1031–1035. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.; Hayes, M.; Jewitt, T.; Brems, K.; Horie, V. Degradation of magnetic tape: Support and binder stability. Polym. Degrad. Stabil. 1993, 39, 207–214. [Google Scholar] [CrossRef]
- Nakamae, K.; Yamaguchi, K.; Asaoka, S.; Karube, Y.; Sudaryanto. Lifetime expectancy of polyurethane binder as magnetic recording media. Int. J. Adhes. Adhes. 1996, 16, 277–283. [Google Scholar] [CrossRef]
- Hahn, F. Head wear as a function of isolated asperities on the surface of magnetic tape. IEEE Trans. Magn. 1984, 20, 918–920. [Google Scholar] [CrossRef]
- Salazar, M.R.; Lightfoot, J.M.; Russell, B.G.; Rodin, W.A.; McCarty, M.; Wrobleski, D.A.; Orler, E.B.; Spieker, D.A.; Assink, R.A.; Pack, R.T. Degradation of a poly(ester urethane) elastomer. III. Estane 5703 hydrolysis: Experiments and modeling. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1136–1151. [Google Scholar] [CrossRef]
- Thompson, D.G.; Osborn, J.C.; Kober, E.M.; Schoonover, J.R. Effects of hydrolysis-induced molecular weight changes on the phase separation of a polyester polyurethane. Polym. Degrad. Stabil. 2006, 91, 3360–3370. [Google Scholar] [CrossRef]
- Pack, R.; Hanson, D.; Redondo, A. Chemical Kinetics of Estane Aging in PBX; Technical Report; UNT Libraries Government Documents Department: Denton, TX, USA, 1997. [Google Scholar]
- Bradshaw, R.; Falcone, S. Polymers in Information Storage Technology; Chapter Polyester-Polyurethane Interactions with Chromium Dioxide; Springer: New York, NY, USA, 1989; pp. 385–405. [Google Scholar]
- Hadad, A.S. An empirical approach to predicting long term behavior of metal particle based recording media. In Proceedings of the Conference on Mass Storage Systems and Technologies for Space and Earth Science Applications, Greenbelt, MD, USA, 23–25 July 1991. [Google Scholar]
- Djalali, A.; Seng, D.; Glatfelter, W.; Lambropoulos, H.; Judge, J.S.; Speliotis, D.E. Study of the Stability of Metal Particle Data Recording Tapes. J. Electrochem. Soc. 1991, 138, 2504–2509. [Google Scholar] [CrossRef]
- Swaddle, T.W.; Oltmann, P. Kinetics of the magnetite–maghemite–hematite transformation, with special reference to hydrothermal systems. Can. J. Chem. 1980, 58, 1763–1772. [Google Scholar] [CrossRef]
- Dee, R.H. Magnetic Tape for Data Storage: An Enduring Technology. Proc. IEEE 2008, 96, 1775–1785. [Google Scholar] [CrossRef]
- Mercer, T.; Bissell, P.; Tatarasanu, I. Effects of structure on noise in very thin particulate data storage media. J. Magn. Magn. Mater. 2007, 316, 199–202. [Google Scholar] [CrossRef]
- Nishio, H.; Yamamoto, H. Long-Term Magnetization Stability of Data Storage Tape Prepared from Ultrafine Metal Particles. IEEE Trans. Magn. 2010, 46, 3747–3751. [Google Scholar] [CrossRef]
- Nishio, H.; Yamamoto, H. Temperature dependence of long-term magnetization stability for data storage tapes prepared from ultrafine particles. Phys. Procedia 2011, 16, 58–62. [Google Scholar] [CrossRef][Green Version]
- Shimizu, O.; Murata, Y.; Kurihashi, Y.; Harasawa, T.; Asai, M.; Sueki, M.; Noguchi, H. Long-term Archival Stability of Barium Ferrite Magnetic Tape. J. Magn. Soc. Jpn. 2012, 36, 1–4. [Google Scholar] [CrossRef]
- Bowner, T.; Hull, G.; Plitz, I. Polymers in Information Storage Technology; Chapter Characterization and Hydrolysis of Magnetic Tapes; Springer: New York, NY, USA, 1989; pp. 331–344. [Google Scholar]
- Bottjer, W.; Ingersoll, H. Stabilized Ferromagnetic Chromium Dioxide. U.S. Patent US3512930, 19 May 1969. [Google Scholar]
- Grlesser, H.J. Polymers in Information Storage Technology; Chapter Plasma Polymer Films for Corrosion Protection of Cobalt-Nickel 80:20 Magnetic Thin Films; Springer: New York, NY, USA, 1989; pp. 351–372. [Google Scholar]
- Andanson, J.M.; Kazarian, S. In situ ATR-FTIR Spectroscopy of Poly(ethylene terephthalate) Subjected to High-Temperature Methanol. Macromol. Symp. 2008, 265, 195–204. [Google Scholar] [CrossRef]
- Gómez-Sánchez, E.; Simon, S.; Koch, L.C.; Wiedmann, A.; Weber, T.; Mengel, M. ATR/FT-IR spectroscopy for the characterisation of magnetic tape materials. ePRESERVATIONScience 2011, 8, 2–9. [Google Scholar]
- Hobaica, S. Analysis of audio magnetic tapes with sticky shed syndrome by ATR-FTIR. J. Appl. Polym. Sci. 2013, 128, 1962–1973. [Google Scholar] [CrossRef]
- Cassidy, B.M.; Lu, Z.; Fuenffinger, N.C.; Skelton, S.M.; Bringley, E.J.; Nguyen, L.; Myrick, M.L.; Breitung, E.M.; Morgan, S.L. Minimally Invasive Identification of Degraded Polyester-Urethane Magnetic Tape Using Attenuated Total Reflection Fourier Transform Infrared Spectroscopy and Multivariate Statistics. Anal. Chem. 2015, 87, 9265–9272. [Google Scholar] [CrossRef] [PubMed]
- de Faria, D.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Thiebaut, B.; Lattuati-Derieux, A.; Hocevar, M.; Vilmont, L.B. Application of headspace SPME-GC-MS in characterisation of odorous volatile organic compounds emitted from magnetic tape coatings based on poly(urethane-ester) after natural and artificial ageing. Polym. Test. 2007, 26, 243–256. [Google Scholar] [CrossRef]
- Thiébaut, B.; Vilmont, L.B.; Lavédrine, B. Characterization of U-matic videotape deterioration by size exclusion chromatography and pyrolysis gas chromatography/mass spectrometry and the role of adipic acid. J. Cult. Herit. 2009, 10, 183–197. [Google Scholar] [CrossRef]
- Bigourdan, J.L. Stability of Acetate Film Base: Accelerated-Aging Data Revisited. J. Imaging Sci. Technol. 2006, 50, 494–501. [Google Scholar] [CrossRef]
- Bressan, F.; Bertani, R. Reproducing a detection test for magnetic tapes degradation: Acetone extraction test. In Proceedings of the 5th International Multidisciplinary Scientific Conference on Social Sciences & Arts SGEM2018, Spa, Bulgaria, 24 August–2 September 2018; pp. 437–445. [Google Scholar]
- Bigourdan, J.; Reilly, J.M.; Santoro, K.; Salesin, G. The Preservation of Magnetic Tape Collections: A Perspective; Technical Report NEH GRANT PA-50123-03; Image Permanence Institute, Rochester Institute of Technology: Rochester, NY, USA, 2006. [Google Scholar]
- Bressan, F.; Canazza, S.; Bertani, R. Honey, I burnt the tapes! A study on thermal treatment for the recovery of magnetic tapes affected by Sticky Shed Syndrome. IASA J. 2015, 44, 53–64. [Google Scholar]
- Davis, A.R.; Monroe, E.; France, F.G. Understanding Magnetic Tape Degradation by Polymeric and Material Testing. In Proceedings of the Audio Engineering Society Conference: 2018 AES International Conference on Audio Archiving, Preservation and Restoration, New York, NY, USA, 28–30 June 2018. [Google Scholar]
- ISO/TC 42. Imaging Materials—Processed Films—Method for Determining Lubrication; Reference ISO 18904:2000; International Organization for Standardization (ISO): Geneva, Switzerland, 2000. [Google Scholar]
- Tseng, H.; Kolycheck, E. Polymers in Information Storage Technology; Chapter Dynamic Mechanical Behavior of Thermoplastic Polyurethane in Magnetic Coatings; Springer: New York, NY, USA, 1989; pp. 373–383. [Google Scholar]
- Weick, B.L. Correlations between creep, shrinkage, and dynamic mechanical characteristics of magnetic tape materials. J. Appl. Polym. Sci. 2011, 120, 226–241. [Google Scholar] [CrossRef]
- Mitin, D.; Grobis, M.; Albrecht, M. Scanning magnetoresistive microscopy: An advanced characterization tool for magnetic nanosystems. Rev. Sci. Instrum. 2016, 87. [Google Scholar] [CrossRef]
- Hempstock, M.; Sullivan, J. A study of the mechanical and magnetic performance of metal evaporated tape. J. Magn. Magn. Mater. 1996, 155, 323–328. [Google Scholar] [CrossRef]
- Medeiros, D.; Curtis, J.L.S.; Parry, R.; Underwood, J. Restored Magnetic Recording Media and Method of Producing Same. U.S. Patent 5,236,790, 17 August 1993. [Google Scholar]
- Richardson, C.A. The New “Non-Baking” Cure for Sticky Shed Tapes: How Forensic Chemistry Saved the Annapolis Sounds Masters. ARSC J. 2013, 44, 217–248. [Google Scholar]
- Hess, R.; Iraci, J.; Flak, K. The digitization of audio tapes. Techn. Bull. 2012, 30, 44. [Google Scholar]
- Dekant, W.; Klaunig, J.E. Toxicology of decamethylcyclopentasiloxane (D5). Regul. Toxicol. Pharmacol. 2016, 74, S67–S76, Toxicity and Human Health Risk Assessment of Decamethylcyclopentasiloxane (D5). [Google Scholar] [CrossRef] [PubMed]
- Ikonomov, N. Preservation and Digital Restoration of Audio Archives. In Review of the National Center for Digitization; Faculty of Mathematics: Belgrade, Serbia, 2003; pp. 40–45. [Google Scholar]
- IASA-TC 05. Handling and Storage of Audio and Video Carriers; IASA Technical Committee: Springfield, IL, USA, 2014. [Google Scholar]
- Bressan, F.; Rodà, A.; Bertani, R. The impact of thermal treatment on magnetic tapes: An exploratory study combining chemical analyses and audio features. In Proceedings of the 2018 AES International Conference on Audio Archiving, Preservation & Restoration, New York, NY, USA, 28–30 June 2018. [Google Scholar]
| 1. | [a] Engel, F.K. (ed.), “Oberlin Smith and the Invention of Magnetic Sound Recording [a] An Appreciation on the 150th Anniversary of the Inventor’s Birth”, 1990, revised version 2006. Full text available online: http://bit.ly/oberlin2006 (last visited 24 April 2019); [b] Engel F. and Hammar, P., “A Selected History of Magnetic Recording. by Friedrich Engel and Peter Hammar; additional editing by Hess R.L. in 2006. Full text available online: http://bit.ly/engels2006 (last visited 24 April 2019). |
| 2. | Hess, R.L. “-Degrading Tapes”, 2018. Link: http://bit.ly/DegradingTapes (last visited 24 April 2019). Other resources for basic restoration information include: [a] Copeland, A., “Manual of Analogue Sound Restoration Techniques”, The British Library, 2008. Full text available online: http://bit.ly/copland2008 (last visited 24 April 2019); [b] IASA 2014-2017, https://www.iasa-web.org/iasa-special-and-technical-publications (last visited 24 April 2019); [c] Marques 2014, https://www.loc.gov/folklife/sos/preserve1.html (last visited 24 April 2019). |
| 3. | For an overview of Agfa, BASF, and IG Farben’s tapes production, see: http://bit.ly/locHeritage (last visited 24 April 2019). Some of this history is discussed in: Engel F. and Hammar, P., “A Selected History of Magnetic Recording” additional editing by Hess R.L. in 2006. Full text available online: http://bit.ly/engels2006 (last visited 26 April 2019). |
| 4. | For an overview of 3M’s tape manufacturing history, see: http://www.aes.org/aeshc/docs/3mtape/aorprod-si.pdf (last visited 25 April 2019). |
| 5. | Personal communication with Bob Perry, former Director of Advanced Development in the Magnetic Tape Division of Ampex Corporation, 29 July 2006. |
| 6. | Further confirmation of running changes can be found in the online copies of the Scotch/3M Sound Talk publications, numbers 13-20-21-22. Available online: http://www.aes.org/aeshc/docs/3mtape/soundtalkindex.html (last visited 24 April 2019). |
| 7. | Personal communication with Dr. Richard Bradshaw, Tape Development, IBM Tucson, AZ, USA, 31 July 2006. Note that Bradshaw was the person who finally was able to safely unspool the Challenger tape after it was recovered from the ocean floor. He also led the team developing the IBM 3480 and 3490 data tapes, which have (so far) an enviable record of longevity and stability. |
| 8. | This is the actual 1965 NAB standard that includes the physical details of the NAB hub: http://bit.ly/nabHess (last visited on 27 April 2019). |
| 9. | See article published on Reuters: Fox, M., “Moon landing tapes got erased, NASA admits,” 16 July 2009. Full text online: http://bit.ly/eraseMoon (last visited 24 April 2019). |
| 10. | See Tim Stoffel’s webpage “Museum of Broadcast Technology” on Quadruplex Park Videotape Formats: http://www.lionlamb.us/quadpark.html (last visited on 25 April 2019). |
| 11. | Website of the Indiana University’s Media Digitization & Preservation Initiative: https://mdpi.iu.edu/ (last visited on 24 April 2019). |
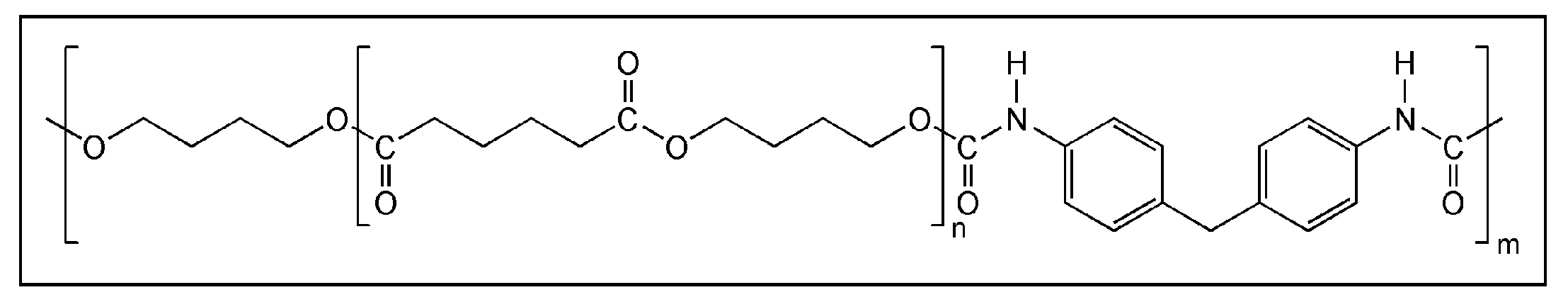
| 12. | The use of polyurethane appears to be the primary direction tape manufacturing went around the time this patent was issued, i.e., the mid-1970s. |
| 13. | Oe = Oersted; unit of magnetic field strength in CGS system. 1 Oe = (1000/)(A/m). In vacuum, if the magnetic field strength H is 1 Oe, the magnetic field density B is 1 Gauss. In a medium having permeability , B(Gauss) = −H(Oe). |
| 14. | Dew Point Calculator: http://www.dpcalc.org/ (last visited on 18 May 2019). |
| 15. | Online resource: “Field Strength for Partial Erasure of Magnetic Tape” derived from a report by Jay McKnight for Scully/Metrotech Div. of Dictaphone Corp. 29 October 1973. Link: http://www.mrltapes.com/field-strength-for-partial-erasure.pdf (last visited on 26 April 2019). |
| 16. | Online article: Morgan, S., Product life cycle of cassette tapes: http://www.avmediaplace.net/files/46347804.pdf (last visited on 26 April 2019). |
| 17. | Cinko, G.R., “TGA study of thermal degradation of plastic films”: http://bit.ly/giselaTapes (last visited on 26 April 2019). |
| 18. | See slides “Magnetic Forensics” presented by David Pappas of the National Institute of Standards & Technology at the THIC Meeting at the National Center for Atmospheric Research in 2006: http://www.thic.org/pdf/July06/nist.dpappas.060718.pdf (last visited on 25 April 2019). |
| 19. | Online resource: “Wet playing of reel tapes with Loss of Lubricant–A guest article by Marie O’Connell”, http://bit.ly/wetPlaying (last visited on 27 April 2019). |
| 20. | Personal communication to one of the authors on 3 February 2006. |
| 21. | See “Preservation Recording, Copying, and Storage Guidelines for Audio Tape Collections” published by Lyrasis in 2008: https://www.lyrasis.org/services/Documents/AudioTape-Guidelines.pdf (last visited on 25 April 2019). |
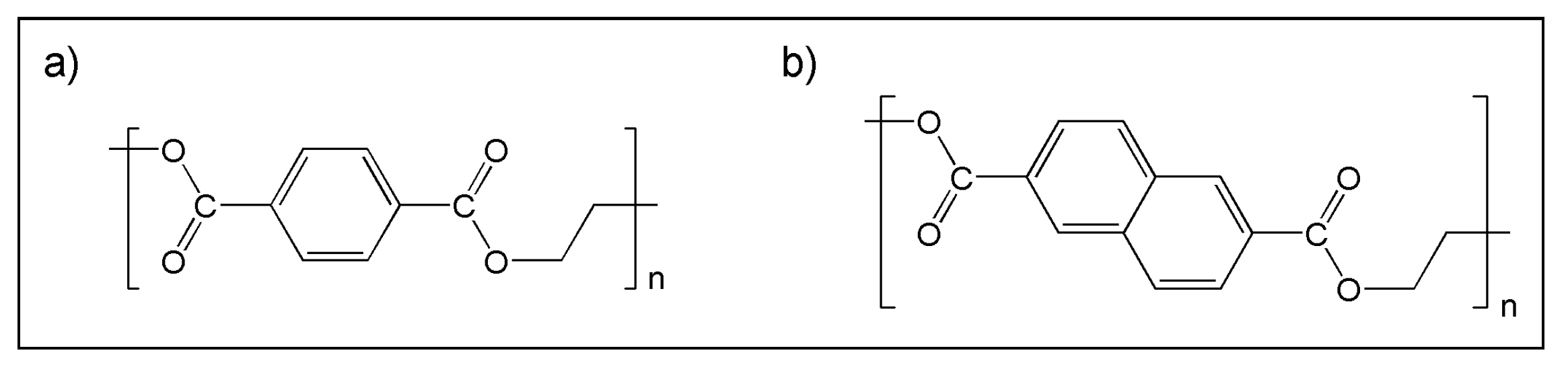
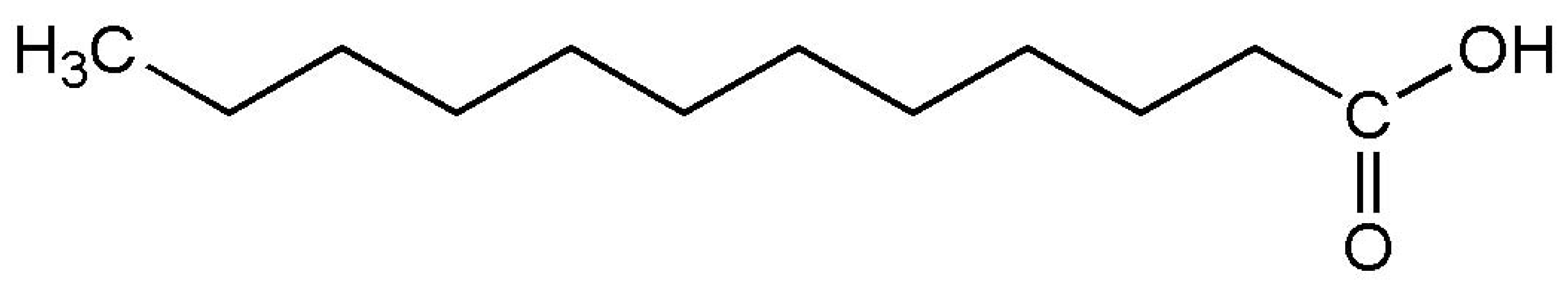 | Lauric acid (melting point ) |
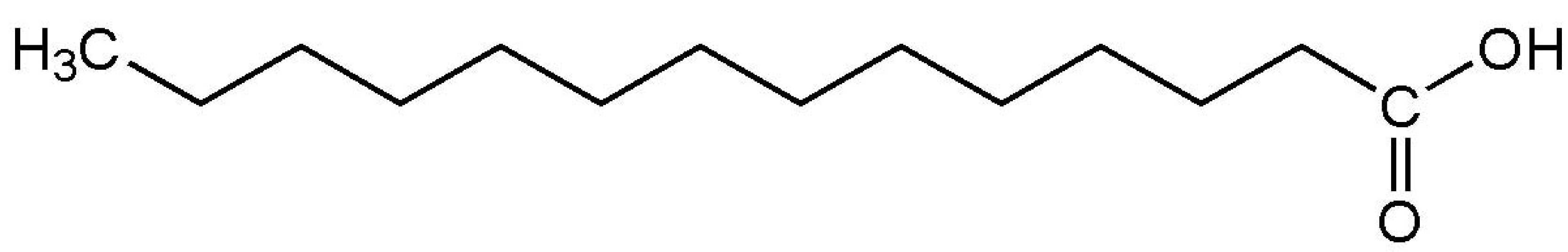 | Myristic acid (melting point ) |
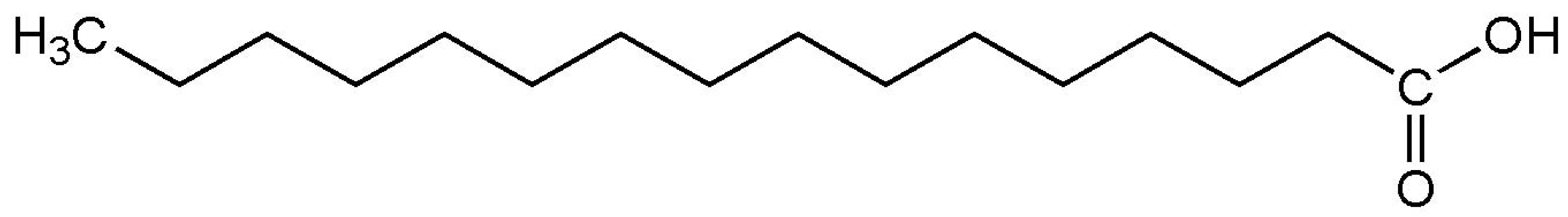 | Palmitic acid (melting point ) |
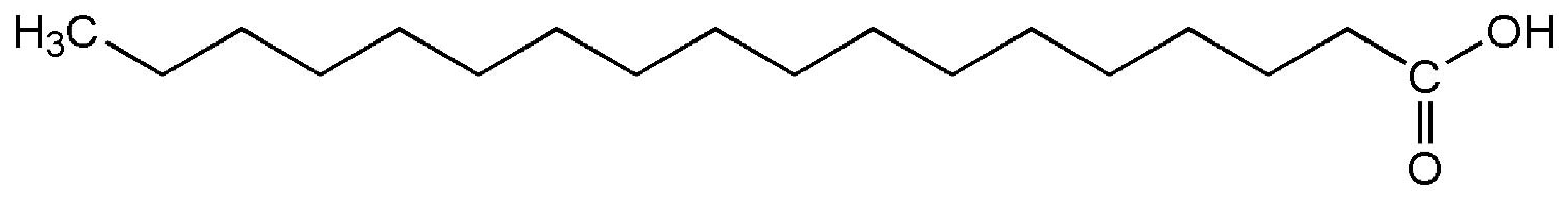 | Stearic acid (melting point ) |
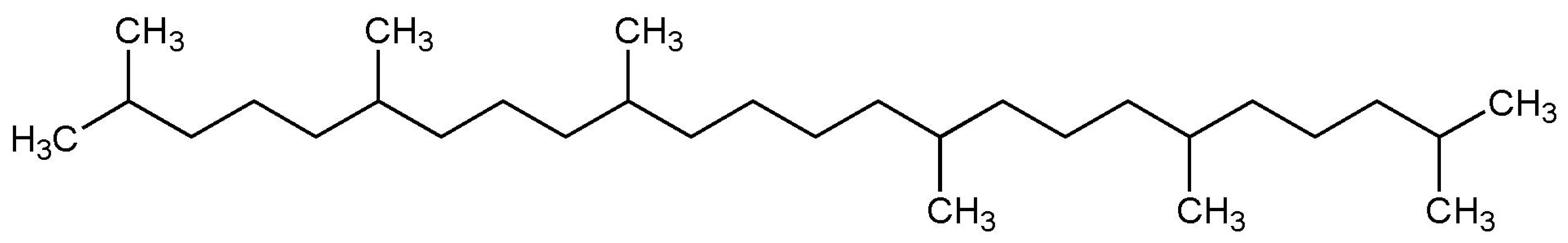 | Squalane (melting point ) |
(a) | (b) |
| Literature Assigned Segment Model | Literature Molecular Assignment (Peak Intensity) | Peak Wavenumber (cm) | Effect of Hydrolysis |
|---|---|---|---|
| Both | (C=O) free, (VS) | 1728–1721 | More intense in SSS tapes |
| Polyurethane | (C=O) hydrogen bonded, (VS) | 1701–1689 | Less intense in SSS tapes |
| Polyurethane | (C=C) aromatic ring, (S) | 1596–1591 | - |
| Polyurethane | (N–H) +(C-N), (S) | 1529–1522 | Amide peak: no change between SSS and non-SSS tapes |
| Polyester | (CH2), (W) | 1462–1453 | - |
| Polyurethane | (C–C) phenyl ring, (S) | 1413–1409 | - |
| Polyester | w(CH2), (W) | 1373–1356 | More intense in SSS tapes |
| Polyurethane | (N–H) +(C–N), (S) | 1311–1305 | Amide peak: no change between SSS and non-SSS tapes |
| Polyester | (C–O–C), w(CH2), (W-M) | 1257–1249 | Present in most SSS tapes (new C–O bonds) |
| Polyurethane | (N–H) +(C–N), (S) | 1220–1213 | Amide peak: no change between SSS and non-SSS tapes |
| Polyester | (C–O–C), (S) | 1173–1156 | - |
| Both | (C=O) +(O−CH2), (M) | 1141–1134 | Present in most SSS tapes (new O–CH2 moieties) |
| Both | (C–O–C), (S) | 1073–1060 | More intense in SSS tapes |
| Both | (Aryl–O), (M–S) | 1020–1015 | More intense in SSS tapes |
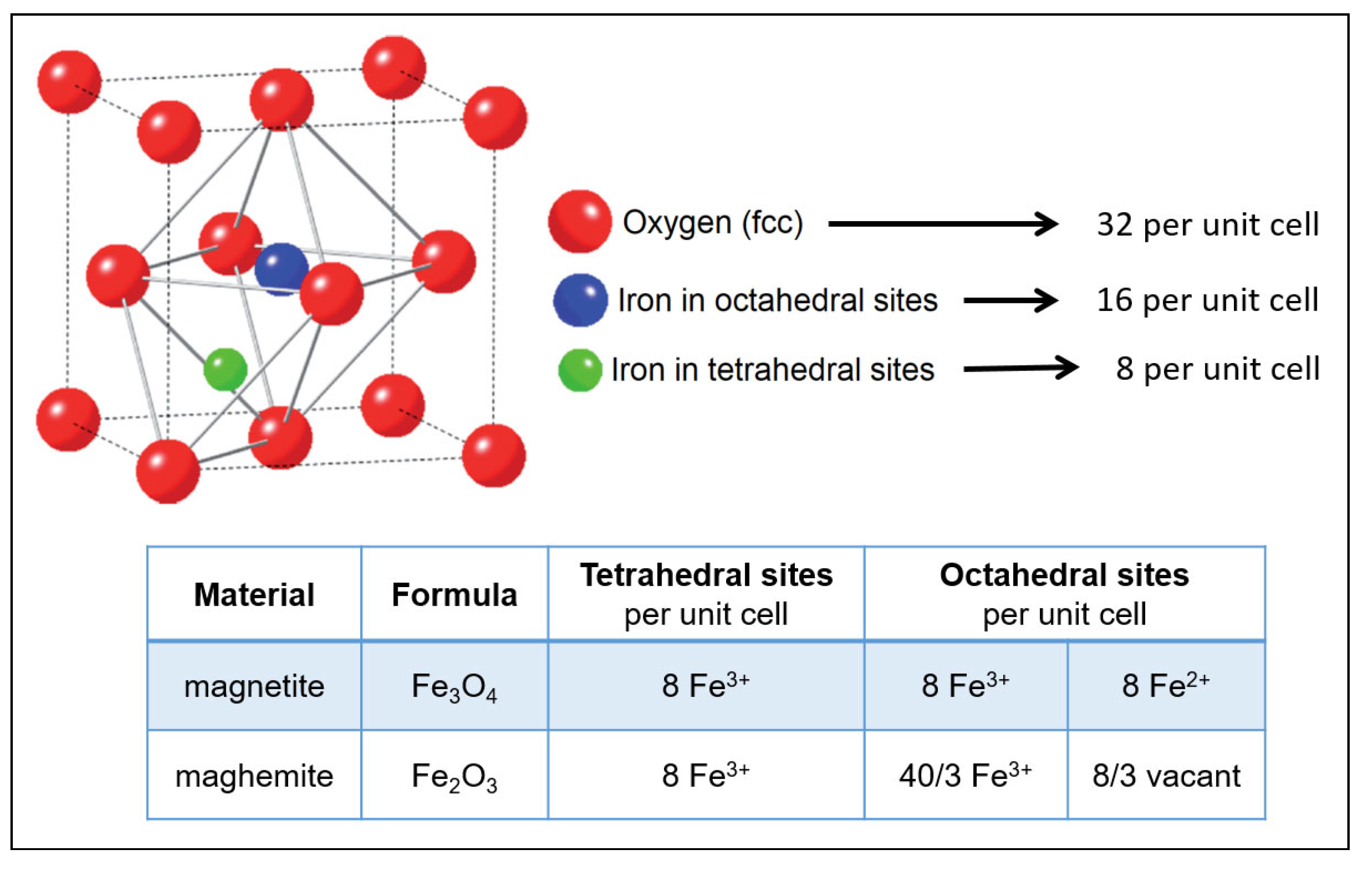
| Mineral Name | Goethite -FeOOH | Lepidocrocite -FeOOH | Hematite -Fe2O3 | Magnetite Fe3O4 | Maghemite -Fe2O3 |
|---|---|---|---|---|---|
| Infrared bands (cm) | 1667, 1399, 1260, 881, 793, 608 | 1625, 1152, 1017, 737 | 535, 464, 308 | 570, 390 | 730, 696, 636, 590, 570 |
| Raman lines (cm) | 243, 299, 385, 479, 550, 685, 993 | 220, 250, 309, 350, 377, 527, 648 | 225, 498, 247 | 300, 532, 661 | 350, 500, 700 |
| Mineral Name | Goethite -FeOOH | Lepidocrocite -FeOOH | Hematite -Fe2O3 | Magnetite Fe3O4 | Maghemite -Fe2O3 |
|---|---|---|---|---|---|
| a = 0.9956 b = 0.30215 c = 0.4608 | a = 0.307 b = 1.253 c = 0.388 | a = 0.50356(1) c = 1.37489(7) | a = 0.8396 | a = 0.83474l | |
| Cell dimension (nm) | orthorhombic | orthorhombic | rhombohedral hexagonal | cubic | cubic or tetragonal |
| Formula units, per cell | 4 | 4 | 6 | 8 | 8 |
| Density (gcm) | 4.26 | 4.09 | 5.26 | 5.18 | 4.87 |
| Color | yellow-brown | orange | red | black | reddish-brown |
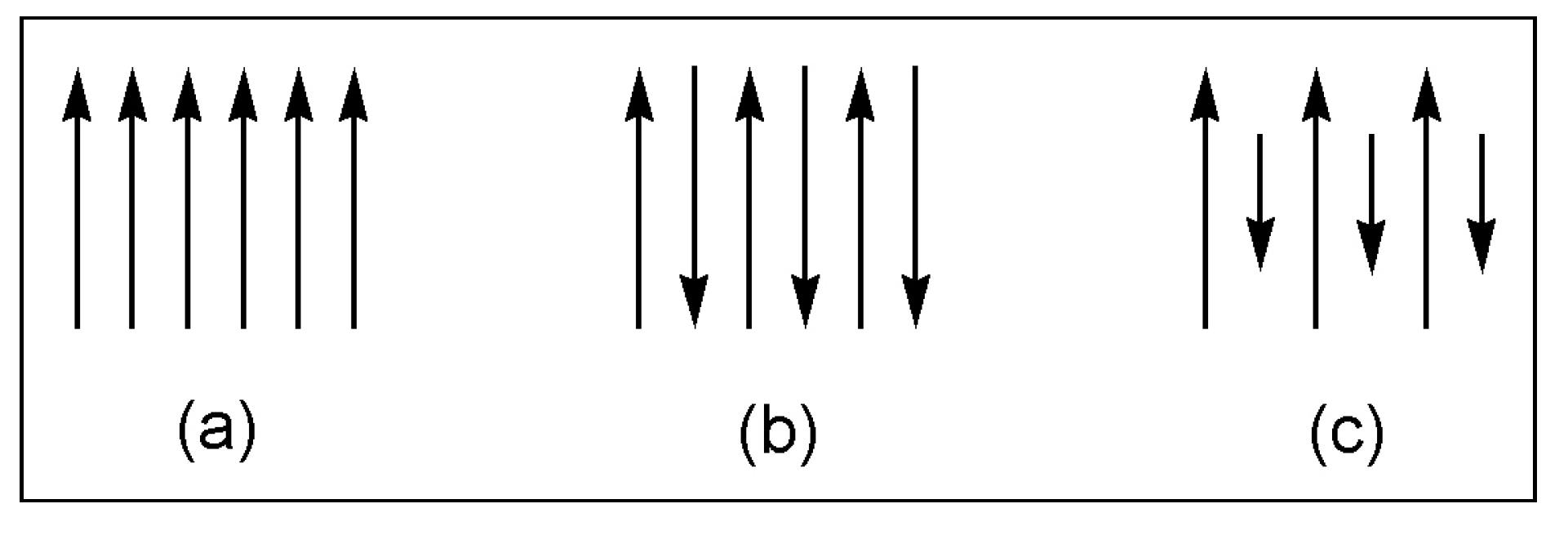
| Type of magnetism | antiferromag. | antiferromag. | weakly ferromag. or antiferromag. | ferrimag. | ferrimag. |
| Neel temperature () | 400 | 77 | (956)⋆ | (850)⋆ | (820-986)⋆ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bressan, F.; Hess, R.L.; Sgarbossa, P.; Bertani, R. Chemistry for Audio Heritage Preservation: A Review of Analytical Techniques for Audio Magnetic Tapes. Heritage 2019, 2, 1551-1587. https://doi.org/10.3390/heritage2020097
Bressan F, Hess RL, Sgarbossa P, Bertani R. Chemistry for Audio Heritage Preservation: A Review of Analytical Techniques for Audio Magnetic Tapes. Heritage. 2019; 2(2):1551-1587. https://doi.org/10.3390/heritage2020097
Chicago/Turabian StyleBressan, Federica, Richard L. Hess, Paolo Sgarbossa, and Roberta Bertani. 2019. "Chemistry for Audio Heritage Preservation: A Review of Analytical Techniques for Audio Magnetic Tapes" Heritage 2, no. 2: 1551-1587. https://doi.org/10.3390/heritage2020097
APA StyleBressan, F., Hess, R. L., Sgarbossa, P., & Bertani, R. (2019). Chemistry for Audio Heritage Preservation: A Review of Analytical Techniques for Audio Magnetic Tapes. Heritage, 2(2), 1551-1587. https://doi.org/10.3390/heritage2020097







