Design of Reliable Remobilisation Finger Implants with Geometry Elements of a Triple Periodic Minimal Surface Structure via Additive Manufacturing of Silicon Nitride
Abstract
1. Introduction
1.1. Biomechanical Simulation and Imaging
1.2. AI-Based Reconstruction of the Joint and Implant Generation
1.3. Materials and Processes for Implant Manufacturing
2. Materials and Methods
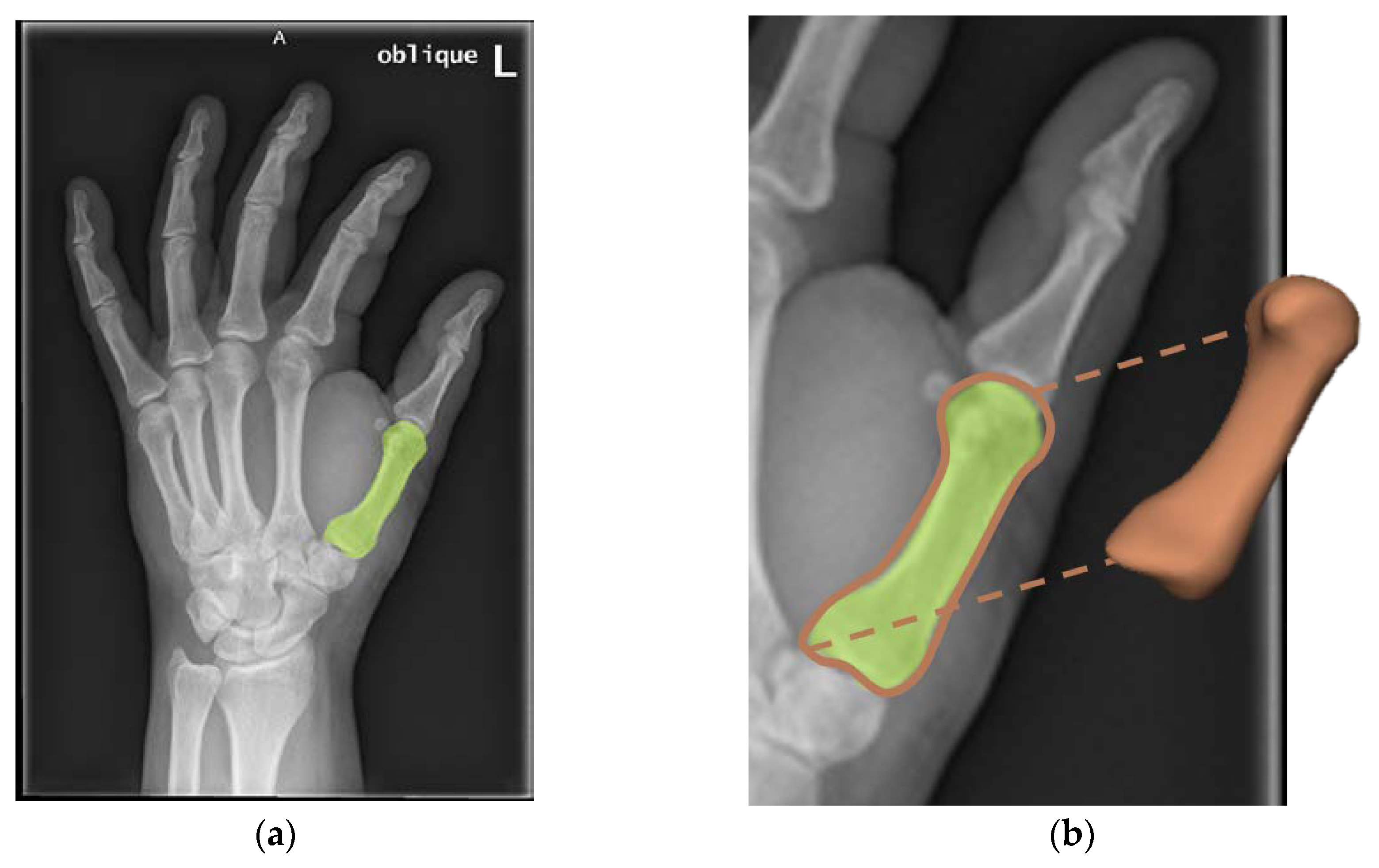
2.1. 3D Model Generation from 2D Medical Images
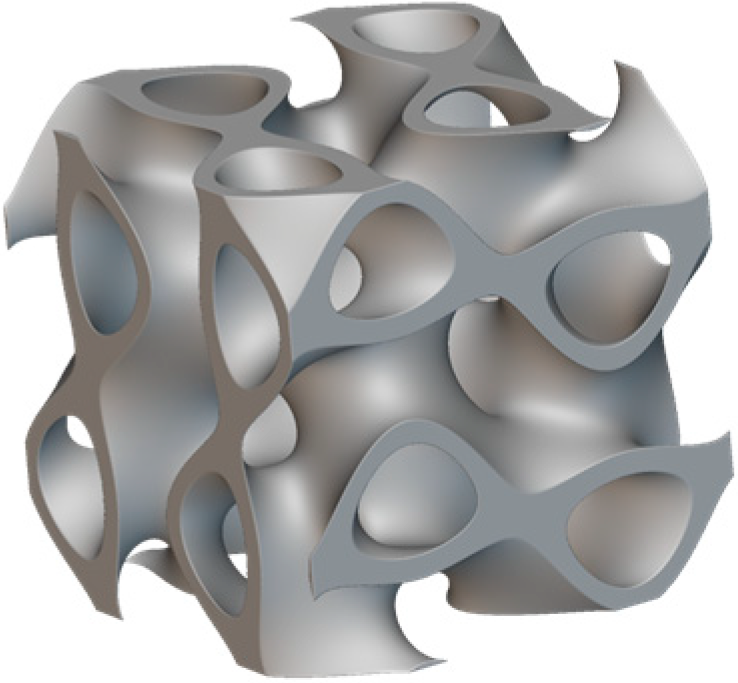
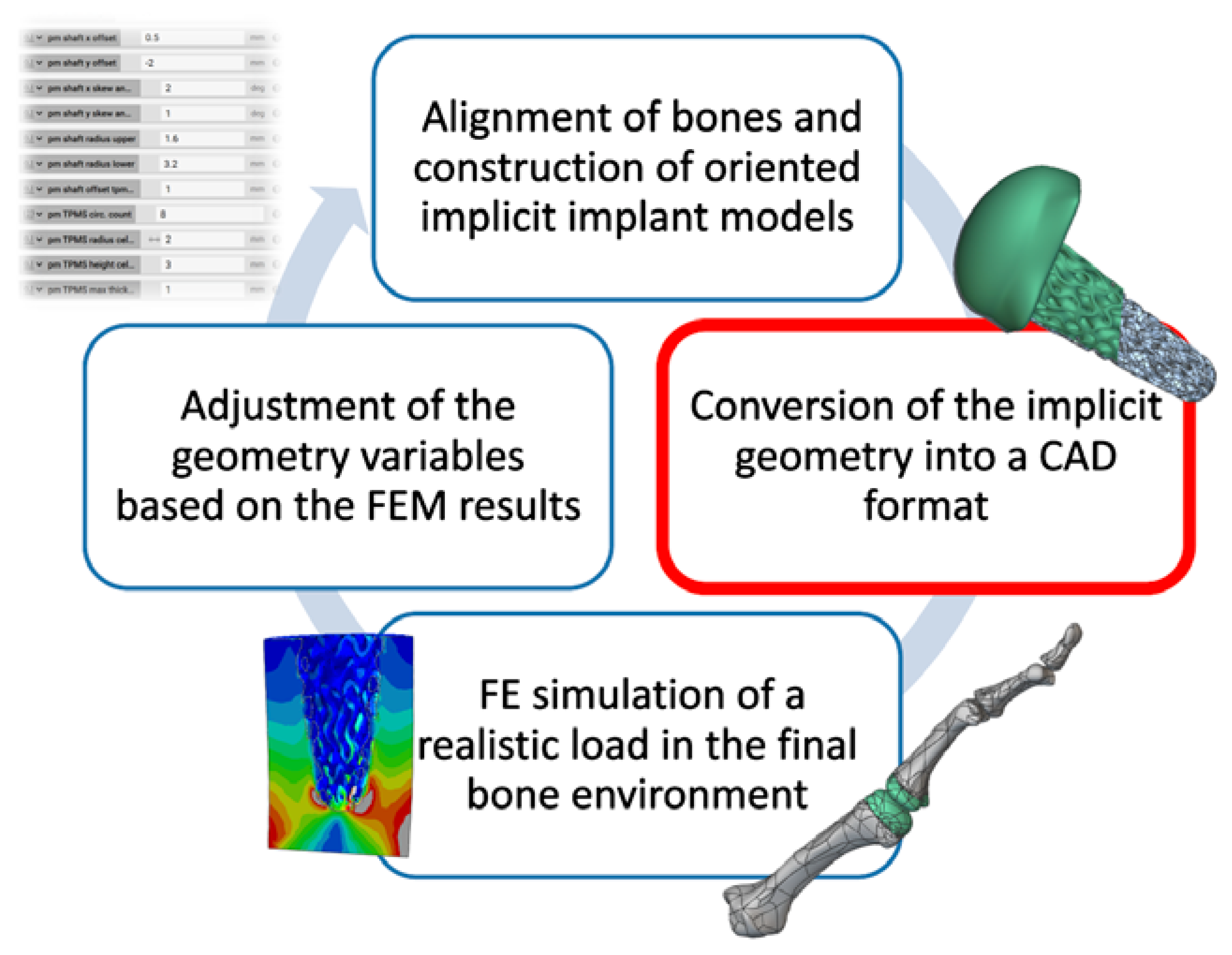
2.2. Design Process
2.3. CerAMfacturing of Test Components
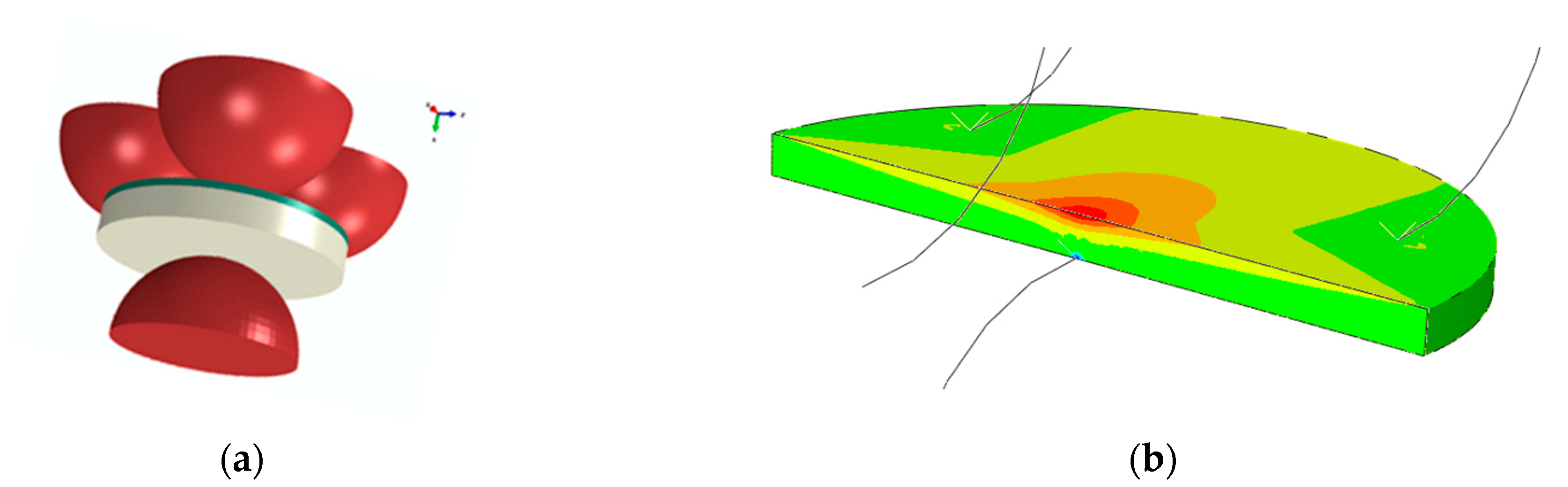
2.4. Mechanical Strength
3. Results and Discussion
3.1. 3D Model Generation from 2D Medical Images
3.2. Individual Implant Design
Creation of Implant Design
3.3. Additive Manufacturing of Silicon Nitride Specimen
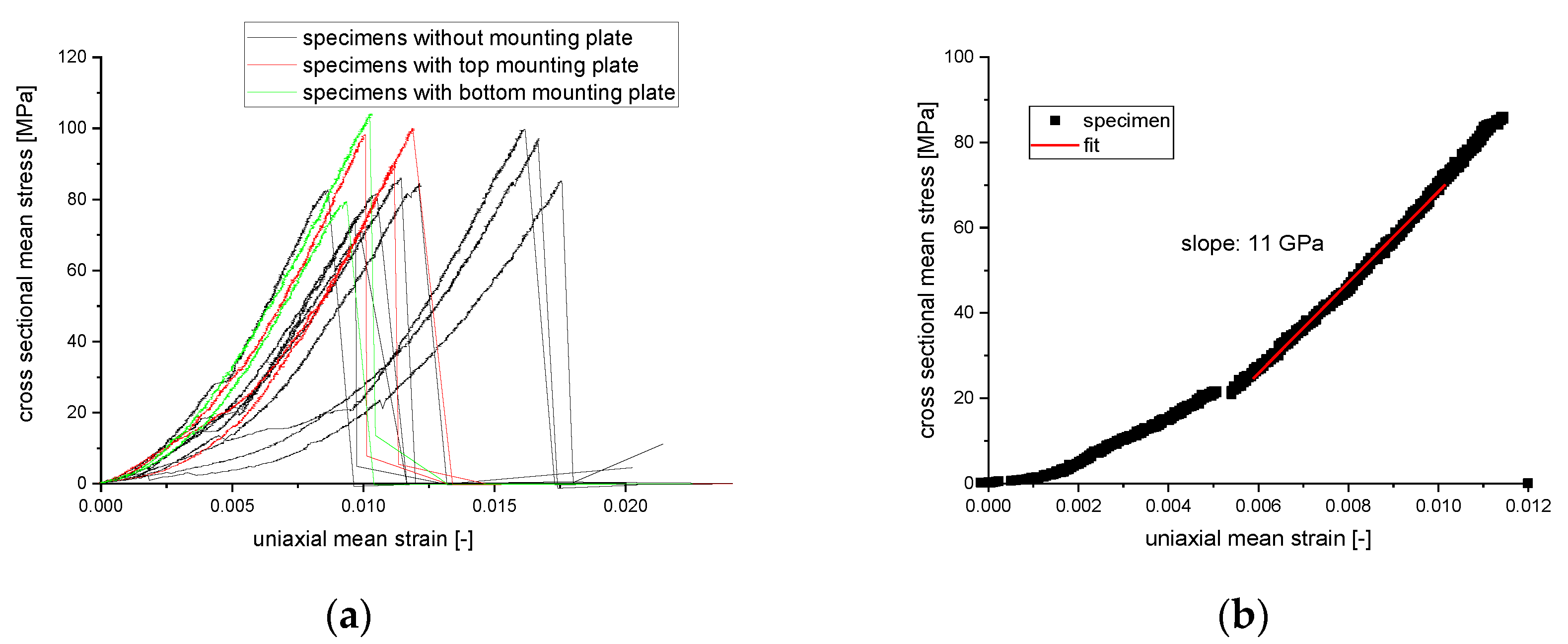
3.4. Fracture Test of Silicon Nitride Specimen
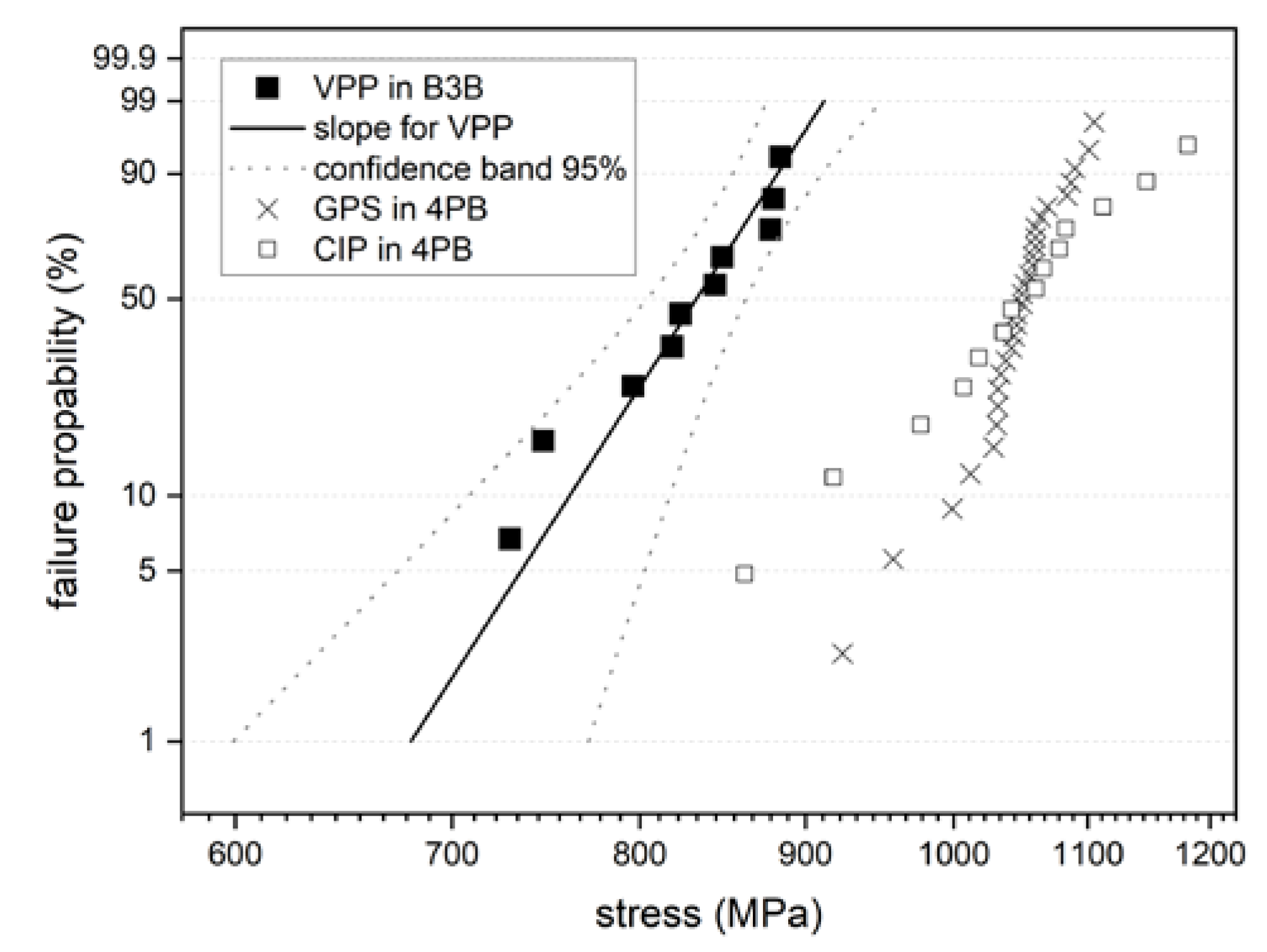
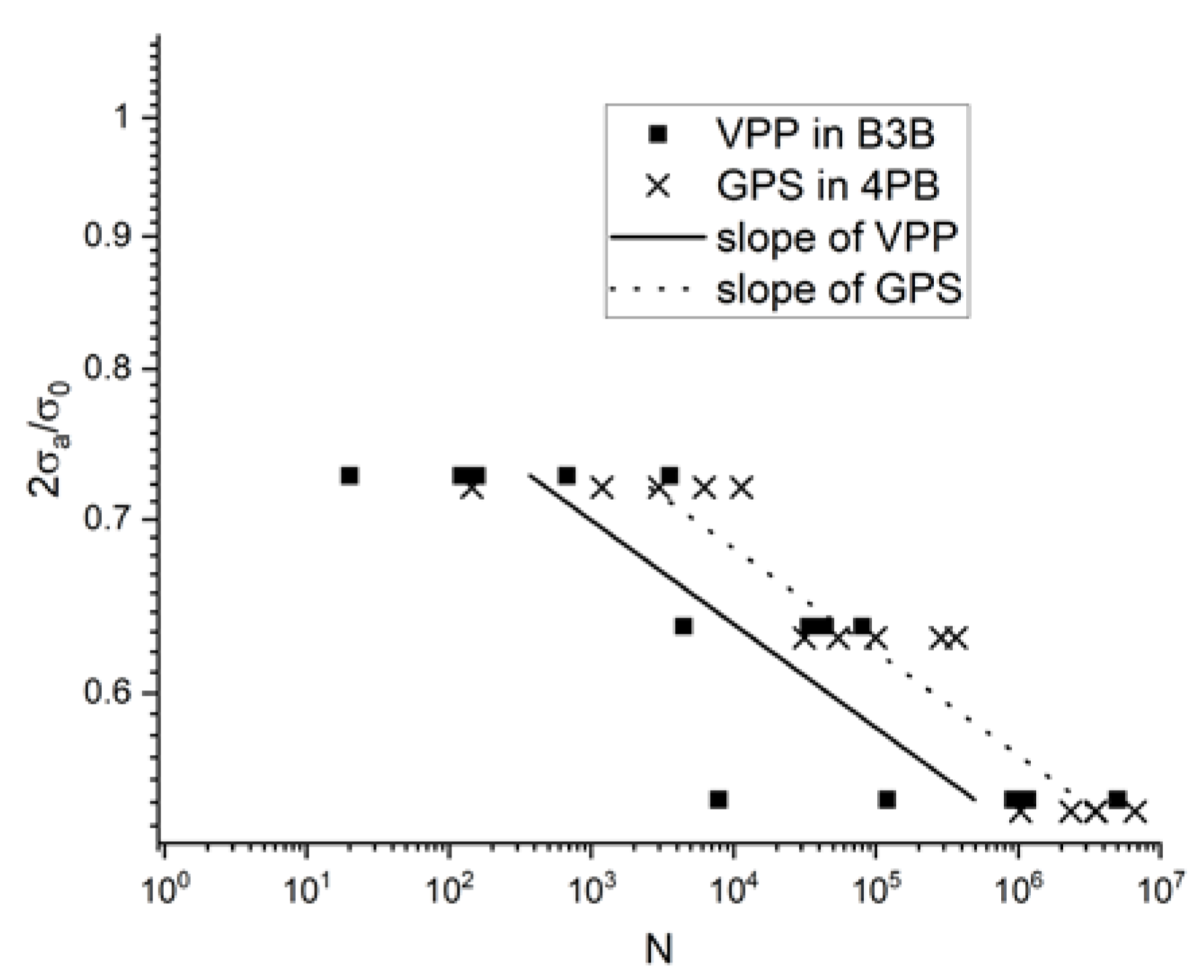
3.4.1. Discs for Ball-On-Three-Balls Test in Comparison to 4-Point Bending Results
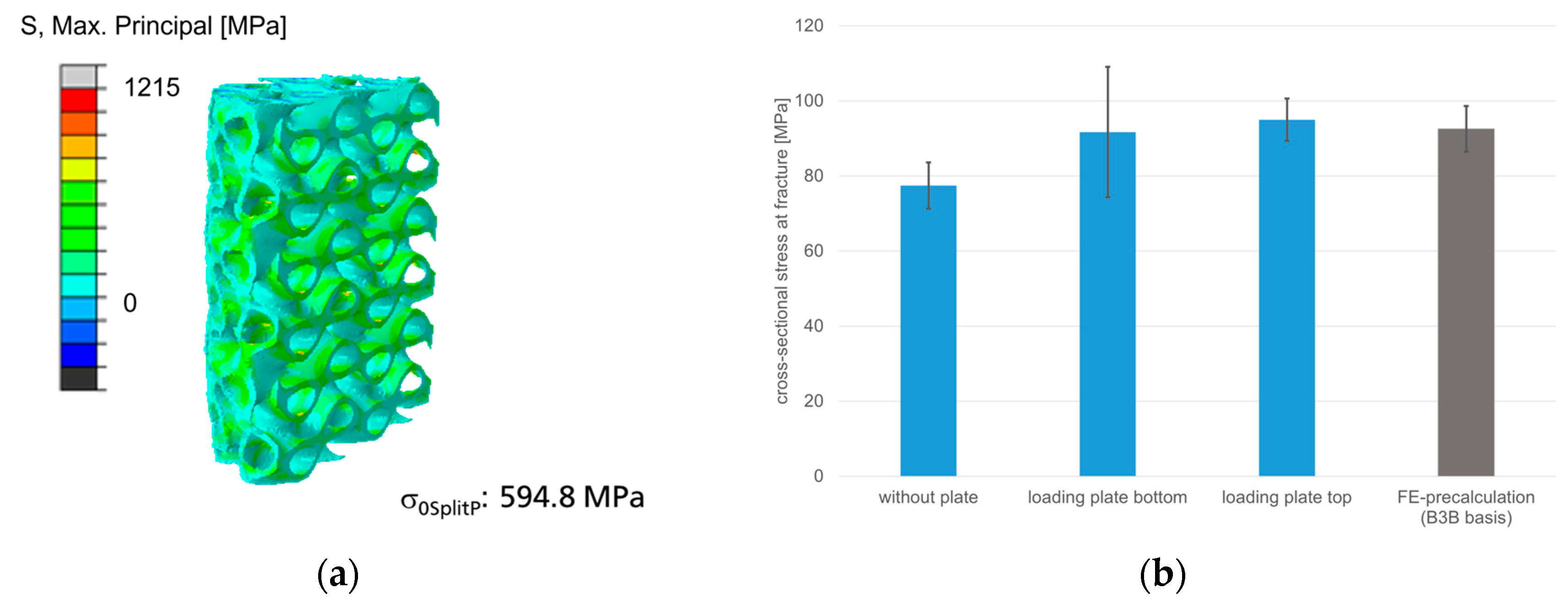
3.4.2. Mechanical Behavior of Cylinder with SplitP TPMS
3.5. Finite-Element-Modeling and Reliability Calculations
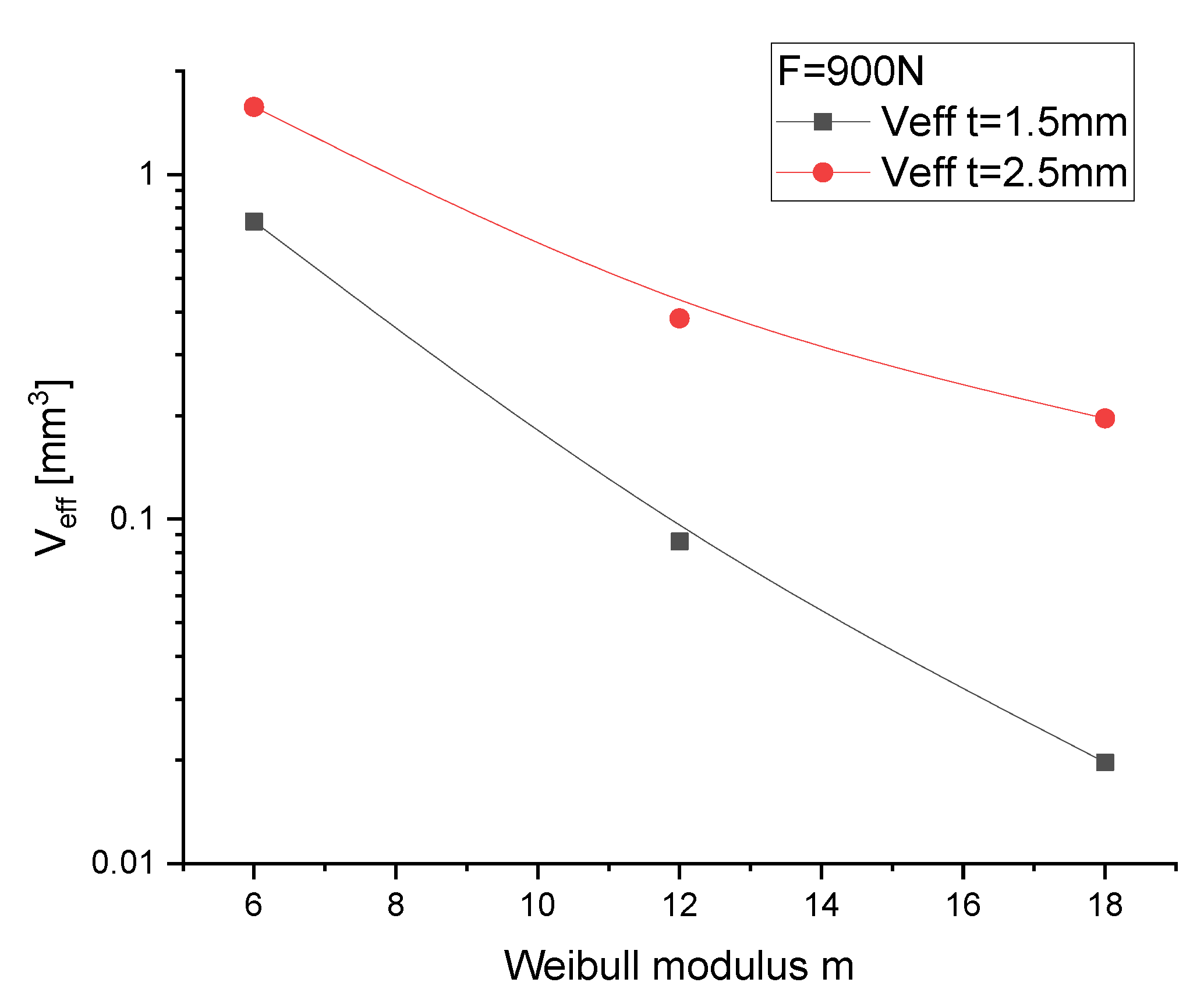
3.5.1. Discs for Ball-on-Three-Balls Test and Calculation of Weibull-Parameter
3.5.2. Cylinder with Cylindrical SplitP TPMS in Comparison to Experimental Results
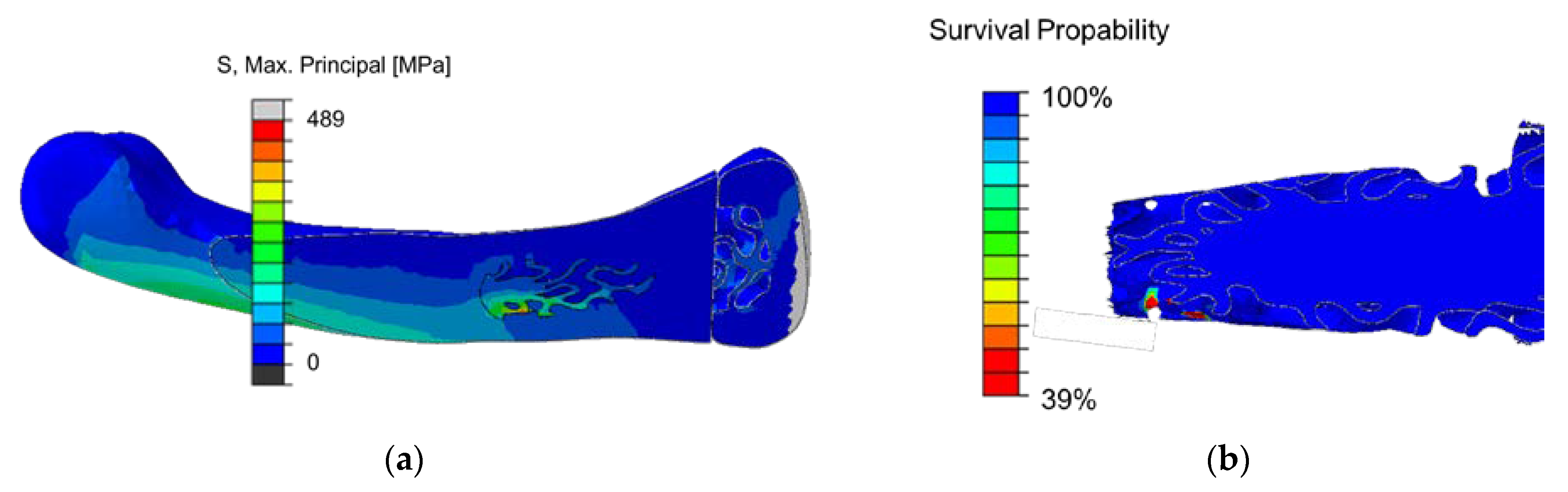
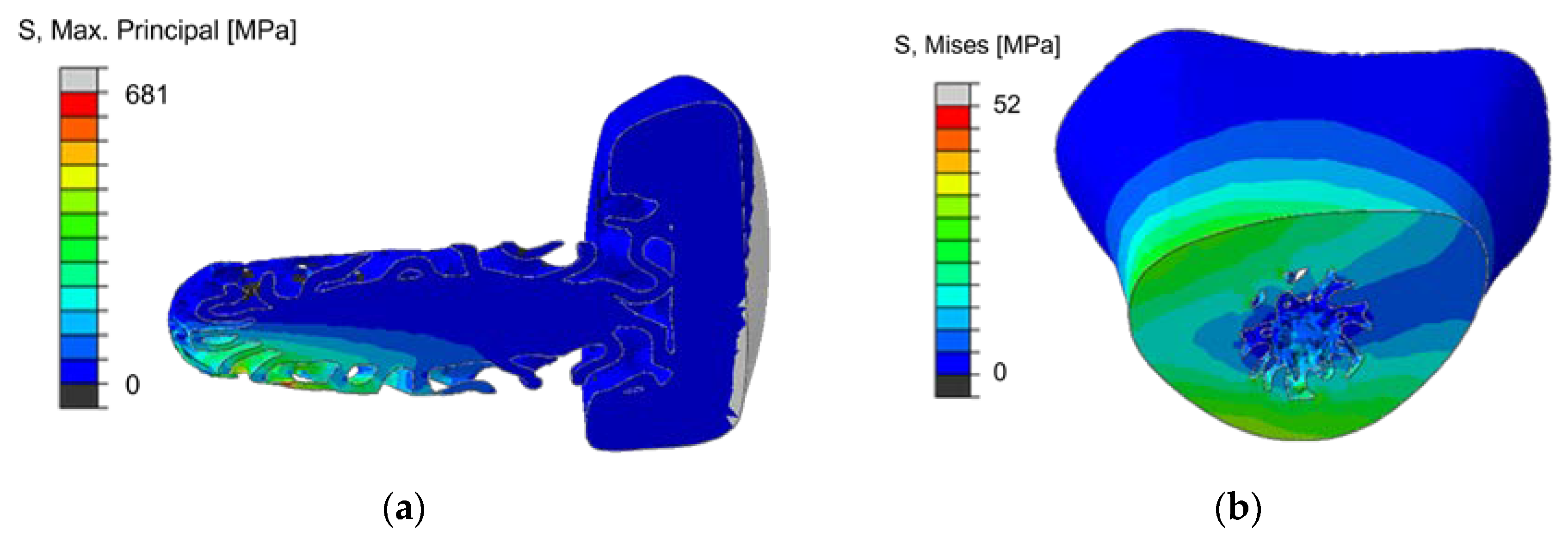
3.5.3. Calculation of Implant Load with Gradient SplitP TPMS for a Diagonal Joint Load
4. Conclusions
- The ceramics created by VPP can be reliably applied to filigree structures.
- The TPMS structures of the implant can be created in a graded form along the curvature of the complex implant. A full workflow for a specific gradient generation of a TPMS to solid structure was achieved in a CAD-nTopology loop for individual implants and bones.
- SplitP TPMS structures have been validated for brittle materials as excellent elasticity-mitigating structures (3.6%) with low stress factor (6.4).
- The ball-on-three-ball test is predestined for the brittle materials of submilimetre VPP ceramic structure.
- A full workflow converts joint bone models, matches and aligns them to implants.
- The submilimetre accuracy of the AI-based 3D shape construction of 2D real data is expected to be good, as it was validated on artificial reconstruction loop 3Dto2Dto3D.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Research and Markets. Foot and Ankle Devices Market by Product: Bracing & Support, Joint Implants, Soft Tissue Orthopedic Devices, Orthopedic Fixation, Prosthetics. Available online: https://www.grandviewresearch.com/industry-analysis/foot-and-ankle-devices-market (accessed on 22 October 2019).
- Hohendorff, B.; Spies, C.K.; Unglaub, F.; Müller, L.P.; Ries, C. Anatomie des Fingergrund- und -mittelgelenks unter Berücksichtigung der Endoprothetik. Orthopade 2019, 48, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Sauerbier, M.; Rauschmann, M.A. Geschichte der Endoprothetik der Fingergelenke. Orthopade 2003, 32, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Herren, D.B.; Simmen, B.R. Limited and complete fusion of the rheumatoid wrist. J. Am. Soc. Surg. Hand 2002, 2, 21–32. [Google Scholar] [CrossRef]
- Rizzo, M.; Cooney, W.P. Current concepts and treatment for the rheumatoid wrist. Hand Clin. 2011, 27, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D. Wrist arthroplasty: Partial and total. Hand Clin. 2013, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- van Harlingen, D.; Heesterbeek, P.J.C.; de Vos, M.J. High rate of complications and radiographic loosening of the biaxial total wrist arthroplasty in rheumatoid arthritis: 32 wrists followed for 6 (5–8) years. Acta Orthop. 2011, 82, 721–726. [Google Scholar] [CrossRef]
- Purves, W.K.; Berme, N. Resultant finger joint loads in selected activities. J. Biomed. Eng. 1980, 2, 285–289. [Google Scholar] [CrossRef]
- Marra, M.A.; Strzelczak, M.; van de Groes, S.; Heesterbeek, P.J.C.; Wymenga, A.B.; Koopman, H.F.J.M.; Janssen, D.; Verdonschot, N.J.J. Biomechanical effects of femoral component flexion in tka: A musculoskeletal modeling analysis. In Proceedings of the Orthopaedic Research Society Annual Meeting, Orlando, FL, USA, 5–8 March 2016. [Google Scholar]
- Lorenzetti, S. New Method to Determine the Young’s Modulus of Single Trabeculae; ETH Zurich: Zürich, Switzerland, 2006. [Google Scholar]
- Pickhardt, P.J.; Lee, L.J.; Del Rio, A.M.; Lauder, T.; Bruce, R.J.; Summers, R.M.; Pooler, B.D.; Binkley, N. Simultaneous screening for osteoporosis at CT colonography: Bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J. Bone Miner. Res. 2011, 26, 2194–2203. [Google Scholar] [CrossRef]
- Kaur, S.; Singla, J.; Nkenyereye, L.; Jha, S.; Prashar, D.; Joshi, G.P.; El-Sappagh, S.; Islam, M.S.; Islam, S.M.R. Medical Diagnostic Systems Using Artificial Intelligence (AI) Algorithms: Principles and Perspectives. IEEE Access 2020, 8, 228049–228069. [Google Scholar] [CrossRef]
- Ren, M.; Yi, P.H. Artificial intelligence in orthopedic implant model classification: A systematic review. Skelet. Radiol. 2022, 51, 407–416. [Google Scholar] [CrossRef]
- Maken, P.; Gupta, A. 2D-to-3D: A Review for Computational 3D Image Reconstruction from X-ray Images. Arch. Comput. Methods Eng. 2023, 30, 85–114. [Google Scholar] [CrossRef]
- Heimann, T.; Meinzer, H.-P. Statistical shape models for 3D medical image segmentation: A review. Med. Image Anal. 2009, 13, 543–563. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Reyes, M.; Bonaretti, S.; Reimers, N.; Lutz, C.; Ballester, M.Á.G. Evidence-based implant design using a statistical bone model and automated implant fitting. In Proceedings of the Computer Assisted Orthopaedic Surgery International—8th Annual Meeting, Hong Kong, China, 4–7 June 2008. [Google Scholar]
- Chanda, S.; Gupta, S.; Pratihar, D.K. A combined neural network and genetic algorithm based approach for optimally designed femoral implant having improved primary stability. Appl. Soft Comput. 2016, 38, 296–307. [Google Scholar] [CrossRef]
- Friederici, V.; Ellerhorst, M.; Imgrund, P.; Krämer, S.; Ludwig, N. Metal injection moulding of thin-walled titanium parts for medical applications. Powder Metall. 2014, 57, 5–8. [Google Scholar] [CrossRef]
- Lohmann, S. Properties of Biological Materials for Simulation of Human Movement. Basics of Functional Anatomy and Properties of Biomaterials to Create a 3d Modell of Human Body. Ph.D. Thesis, University of Konstanz, Konstanz, Germany, 2005. [Google Scholar]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Gorth, D.J.; Puckett, S.; Ercan, B.; Webster, T.J.; Rahaman, M.; Bal, B.S. Decreased bacteria activity on Si₃N₄ surfaces compared with PEEK or titanium. Int. J. Nanomed. 2012, 7, 4829–4840. [Google Scholar] [CrossRef]
- Heimann, R.B. Silicon Nitride, a Close to Ideal Ceramic Material for Medical Application. Ceramics 2021, 4, 208–223. [Google Scholar] [CrossRef]
- Schwarzer-Fischer, E.; Zschippang, E.; Kunz, W.; Koplin, C.; Löw, Y.M.; Scheithauer, U.; Michaelis, A. CerAMfacturing of silicon nitride by using lithography-based ceramic vat photopolymerization (CerAM VPP). J. Eur. Ceram. Soc. 2023, 43, 321–331. [Google Scholar] [CrossRef]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-12459-9. [Google Scholar]
- Jariwala, S.H.; Lewis, G.S.; Bushman, Z.J.; Adair, J.H.; Donahue, H.J. 3D Printing of Personalized Artificial Bone Scaffolds. 3d Print. Addit. Manuf. 2015, 2, 56–64. [Google Scholar] [CrossRef]
- Khaleghi, S.; Dehnavi, F.N.; Baghani, M.; Safdari, M.; Wang, K.; Baniassadi, M. On the directional elastic modulus of the TPMS structures and a novel hybridization method to control anisotropy. Mater. Des. 2021, 210, 110074. [Google Scholar] [CrossRef]
- Feng, J.; Fu, J.; Yao, X.; He, Y. Triply periodic minimal surface (TPMS) porous structures: From multi-scale design, precise additive manufacturing to multidisciplinary applications. Int. J. Extrem. Manuf. 2022, 4, 22001. [Google Scholar] [CrossRef]
- Shen, M.; Wang, C.; Zhao, Z. Mechanical Properties of ZrO 2 TPMS Structures Prepared by DLP 3D Printing. IOP Conf. Ser. Mater. Sci. Eng. 2019, 678, 12017. [Google Scholar] [CrossRef]
- Hua, S.-B.; Yuan, X.; Wu, J.-M.; Su, J.; Cheng, L.-J.; Zheng, W.; Pan, M.-Z.; Xiao, J.; Shi, Y.-S. Digital light processing porous TPMS structural HA & akermanite bioceramics with optimized performance for cancellous bone repair. Ceram. Int. 2022, 48, 3020–3029. [Google Scholar] [CrossRef]
- Wu, S.; Yang, L.; Wang, C.; Yan, C.; Shi, Y. Si/SiC ceramic lattices with a triply periodic minimal surface structure prepared by laser powder bed fusion. Addit. Manuf. 2022, 56, 102910. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Wenzel, M.; Romberg, D.; Köhn, A.; Kohlmann, P.; Link, F.; Hänsch, A.; Dicken, V.; Stein, R.; Haase, J.; et al. QuantMed: Component-based deep learning platform for translational research. In Medical Imaging 2020: Imaging Informatics for Healthcare, Research, and Applications, Proceedings of the Medical Imaging 2020: Imaging Informatics for Healthcare, Research, and Applications, Houston, TX, USA, 15–20 February 2020; Deserno, T.M., Chen, P.-H., Eds.; SPIE: Bellingham, DC, USA, 2020; Volume 3112020, p. 28. ISBN 9781510634039. [Google Scholar]
- CARS 2022—Computer Assisted Radiology and Surgery Proceedings of the 36th International Congress and Exhibition Tokyo, Japan, June 7–11, 2022. Int. J. CARS 2022, 17, 1–147. [CrossRef]
- MeVisLab 3.5; MeVis Medical Solutions AG: Bremen, Germany. Available online: https://www.mevislab.de/ (accessed on 23 February 2022).
- Matlab R2021b; The MathWorks Inc.: Natick, MA, USA. Available online: https://www.mathworks.com/ (accessed on 23 February 2022).
- D’Errico, J. Fminsearchbnd, Fminsearchcon; MATLAB Central File Exchange. Available online: https://www.mathworks.com/matlabcentral/fileexchange/8277-fminsearchbnd-fminsearchcon (accessed on 23 February 2022).
- nTopology; nTopology, Inc.: New York, NY, USA. Available online: https://ntopology.com/ (accessed on 23 February 2022).
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef]
- Pezzotti, G. Silicon Nitride: A Bioceramic with a Gift. ACS Appl. Mater. Interfaces 2019, 11, 26619–26636. [Google Scholar] [CrossRef]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Sonny Bal, B. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef]
- Schwentenwein, M.; Homa, J. Additive Manufacturing of Dense Alumina Ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Danzer, R.; Harrer, W.; Supancic, P.; Lube, T.; Wang, Z.; Börger, A. The ball on three balls test—Strength and failure analysis of different materials. J. Eur. Ceram. Soc. 2007, 27, 1481–1485. [Google Scholar] [CrossRef]
- Nohut, S. A general formulation for strength prediction of advanced ceramics by ball-on-three-balls (B3B)-test with different multiaxial failure criteria. Ceram. Int. 2012, 38, 2411–2420. [Google Scholar] [CrossRef]
- Börger, A.; Supancic, P.; Danzer, R. The ball on three balls test for strength testing of brittle discs: Stress distribution in the disc. J. Eur. Ceram. Soc. 2002, 22, 1425–1436. [Google Scholar] [CrossRef]
- Börger, A.; Supancic, P.; Danzer, R. The ball on three balls test for strength testing of brittle discs: Part II: Analysis of possible errors in the strength determination. J. Eur. Ceram. Soc. 2004, 24, 2917–2928. [Google Scholar] [CrossRef]
- Staudacher, M.; Lube, T.; Supancic, P. The Ball-on-Three-Balls strength test for discs and plates: Extending and simplifying stress evaluation. J. Eur. Ceram. Soc. 2023, 43, 648–660. [Google Scholar] [CrossRef]
- DIN EN 843-1; Advanced Technical Ceramics: Mechanical Properties of Monolithic Ceramics at Room Temperature. EN 843-1 (2006-12), IDT, Beuth Verlag GmbH. Available online: https://www.beuth.de/ (accessed on 24 March 2019).
- Khader, I.; Koplin, C.; Schröder, C.; Stockmann, J.; Beckert, W.; Kunz, W.; Kailer, A. Characterization of a silicon nitride ceramic material for ceramic springs. J. Eur. Ceram. Soc. 2020, 40, 3541–3554. [Google Scholar] [CrossRef]
- Abaqus R2018x; Dassault Systemes SIMULIA Corp.: Johnston, RI, USA. Available online: https://www.3ds.com/ (accessed on 1 January 2018).
- Fett, T.; Munz, D. Fracture Mechanics. In Handbook of Advanced Ceramics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 681–715. ISBN 9780123854698. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koplin, C.; Schwarzer-Fischer, E.; Zschippang, E.; Löw, Y.M.; Czekalla, M.; Seibel, A.; Rörich, A.; Georgii, J.; Güttler, F.; Yarar-Schlickewei, S.; et al. Design of Reliable Remobilisation Finger Implants with Geometry Elements of a Triple Periodic Minimal Surface Structure via Additive Manufacturing of Silicon Nitride. J 2023, 6, 180-197. https://doi.org/10.3390/j6010014
Koplin C, Schwarzer-Fischer E, Zschippang E, Löw YM, Czekalla M, Seibel A, Rörich A, Georgii J, Güttler F, Yarar-Schlickewei S, et al. Design of Reliable Remobilisation Finger Implants with Geometry Elements of a Triple Periodic Minimal Surface Structure via Additive Manufacturing of Silicon Nitride. J. 2023; 6(1):180-197. https://doi.org/10.3390/j6010014
Chicago/Turabian StyleKoplin, Christof, Eric Schwarzer-Fischer, Eveline Zschippang, Yannick Marian Löw, Martin Czekalla, Arthur Seibel, Anna Rörich, Joachim Georgii, Felix Güttler, Sinef Yarar-Schlickewei, and et al. 2023. "Design of Reliable Remobilisation Finger Implants with Geometry Elements of a Triple Periodic Minimal Surface Structure via Additive Manufacturing of Silicon Nitride" J 6, no. 1: 180-197. https://doi.org/10.3390/j6010014
APA StyleKoplin, C., Schwarzer-Fischer, E., Zschippang, E., Löw, Y. M., Czekalla, M., Seibel, A., Rörich, A., Georgii, J., Güttler, F., Yarar-Schlickewei, S., & Kailer, A. (2023). Design of Reliable Remobilisation Finger Implants with Geometry Elements of a Triple Periodic Minimal Surface Structure via Additive Manufacturing of Silicon Nitride. J, 6(1), 180-197. https://doi.org/10.3390/j6010014







