Evaluation of the Abbott Panbio™ COVID-19 Ag Rapid Antigen Test for Asymptomatic Patients during the Omicron Wave
Abstract
:1. Introduction
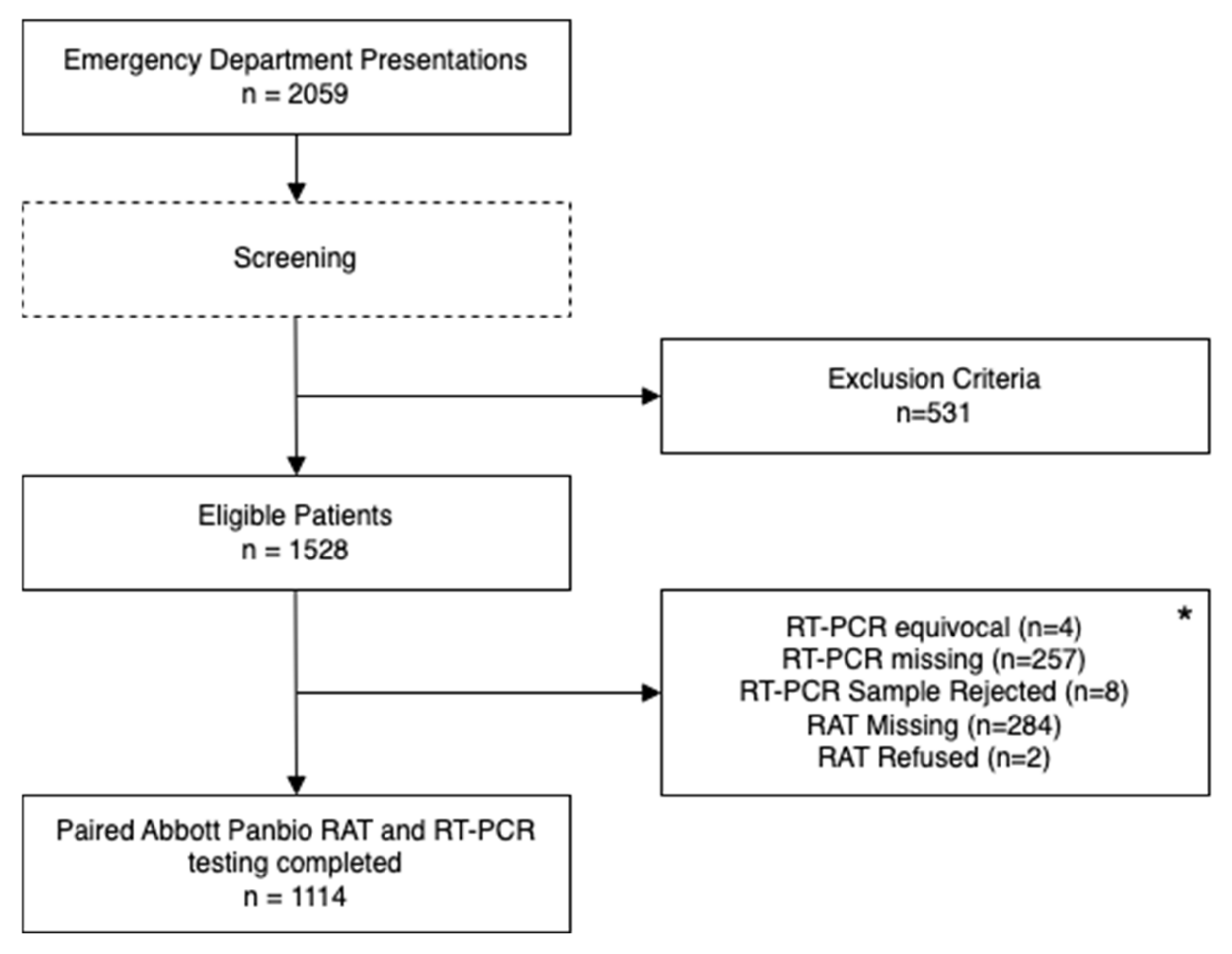
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Australian Government Department of Health. First Confirmed Case of Novel Coronavirus in Australia; Australian Government Department of Health: Canberra, Australia, 2020.
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Pieri, M.; Bernardini, S. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit. Rev. Clin. Lab. Sci. 2022, 59, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; VanInsberghe, D.; Koelle, K. Insights from SARS-CoV-2 sequences. Science 2021, 371, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control. COVID-19: SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 2 March 2023).
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance, 2 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Abbott. Ag Rapid Test Device|Abbott Point of Care Testing. Available online: https://dam.abbott.com/en-gb/panbio/120007883-v1-Panbio-COVID-19-Ag-Nasal-AsymptomaticSe.pdf (accessed on 20 December 2022).
- Winkel, B.; Schram, E.; Gremmels, H.; Debast, S.; Schuurman, R.; Wensing, A.; Bonten, M.; Goedhart, E.; Hofstra, M. Screening for SARS-CoV-2 infection in asymptomatic individuals using the Panbio COVID-19 antigen rapid test (Abbott) compared with RT-PCR: A prospective cohort study. BMJ Open 2021, 11, e048206. [Google Scholar] [CrossRef]
- Aranaz-Andrés, J.M.; Chávez, A.C.F.; Laso, A.M.; Abreu, M.; Núñez, P.M.; Galán, J.C.; Moreno, R.C. Analysis of the diagnostic accuracy of rapid antigenic tests for detection of SARS-CoV-2 in hospital outbreak situation. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 41, 305–312. [Google Scholar] [CrossRef]
- Barrera-Avalos, C.; Luraschi, R.; Vallejos-Vidal, E.; Mella-Torres, A.; Hernández, F.; Figueroa, M.; Rioseco, C.; Valdés, D.; Imarai, M.; Acuña-Castillo, C. The Rapid Antigen Detection Test for SARS-CoV-2 Underestimates the Identification of COVID-19 Positive Cases and Compromises the Diagnosis of the SARS-CoV-2 (K417N/T, E484K, and N501Y) Variants. Front. Public Health 2022, 9, 780801. [Google Scholar] [CrossRef]
- COVID-19 National Incident Room Surveillance Team. COVID-19 Australia: Epidemiology Report 58 Reporting Period Ending 13 February 2022. Commun. Dis. Intell. 2022, 46, 1–32. [Google Scholar] [CrossRef]
- Tsang, N.N.Y.; So, H.C.; Ng, K.Y.; Cowling, B.J.; Leung, G.M.; Ip, D.K.M. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1233–1245. [Google Scholar] [CrossRef]
- StataCorp, L. Stata Statistical Software: Release Vol. 16; StataCorp: College Station, TX, USA, 2019. [Google Scholar]
- Basseal, J.; Bennett, C.; Collignon, P.; Currie, B.; Durrheim, D.; Leask, J.; McBryde, E.; McIntyre, P.; Russell, F.; Smith, D. Key lessons from the COVID-19 public health response in Australia. Lancet Reg. Health West. Pac. 2023, 30, 100616. [Google Scholar] [CrossRef]
- Colavita, F.; Vairo, F.; Meschi, S.; Valli, M.B.; Lalle, E.; Castilletti, C.; Fusco, D.; Spiga, G.; Bartoletti, P.; Ursino, S. COVID-19 Rapid antigen test as screening strategy at points of entry: Experience in Lazio region, central Italy, August–October 2020. Biomolecules 2021, 11, 425. [Google Scholar] [CrossRef]
- O’Reilly, G.M.; Mitchell, R.D.; Mitra, B.; Akhlaghi, H.; Tran, V.; Furyk, J.S.; Buntine, P.; Bannon-Murphy, H.; Amos, T.; Udaya Kumar, M. Epidemiology and clinical features of emergency department patients with suspected and confirmed COVID-19: A multisite report from the COVID-19 Emergency Department Quality Improvement Project for July 2020 (COVED-3). Emerg. Med. Australas. 2021, 33, 114–124. [Google Scholar] [CrossRef]
- O’Reilly, G.M.; Mitchell, R.D.; Mitra, B.; Akhlaghi, H.; Tran, V.; Furyk, J.S.; Buntine, P.; Wong, A.; Gangathimmaiah, V.; Knott, J.; et al. Epidemiology and clinical features of emergency department patients with suspected COVID-19: Insights from Australia’s ‘second wave’ (COVED-4). Emerg. Med. Australas. 2021, 33, 331–342. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: What is the R number? BMJ 2020, 369, m1891. [Google Scholar] [CrossRef]
- Aronson, J.K.; Brassey, J.; Mahtani, K.R. When will it be over?”: An introduction to viral reproduction numbers, R0 and Re. Available online: https://www.cebm.net/covid-19/when-will-it-be-over-an-introduction-to-viral-reproduction-numbers-r0-and-re/ (accessed on 2 March 2023).
- O’Reilly, G.M.; Mitchell, R.D.; Mitra, B.; Akhlaghi, H.; Tran, V.; Furyk, J.S.; Buntine, P.; Wong, A.; Gangathimmaiah, V.; Knott, J.; et al. Outcomes for emergency department patients with suspected and confirmed COVID-19: An analysis of the Australian experience in 2020 (COVED-5). Emerg. Med. Australas. 2021, 33, 911–921. [Google Scholar] [CrossRef]
- COVID-19 National Incident Room Surveillance Team. COVID-19 Australia: Epidemiology Report 45 Reporting period ending 4 July 2021. Commun. Dis. Intell. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Brümmer, L.E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; Pollock, N.R. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 2021, 18, e1003735. [Google Scholar] [CrossRef]
- Schuit, E.; Venekamp, R.P.; Veldhuijzen, I.K.; van den Bijllaardt, W.; Pas, S.D.; Stohr, J.J.; Lodder, E.B.; Hellwich, M.; Molenkamp, R.; Igloi, Z. Accuracy and usability of saliva and nasal rapid antigen self-testing for detection of SARS-CoV-2 infection in the general population: A head-to-head comparison. medRxiv 2021. [Google Scholar] [CrossRef]
- Schrom, J.; Marquez, C.; Pilarowski, G.; Wang, C.-Y.; Mitchell, A.; Puccinelli, R.; Black, D.; Rojas, S.; Ribeiro, S.; Tulier-Laiwa, V. Comparison of SARS-CoV-2 Reverse Transcriptase Polymerase Chain Reaction and BinaxNOW Rapid Antigen Tests at a Community Site During an Omicron Surge: A Cross-Sectional Study. Ann. Intern. Med. 2022, 175, 682–690. [Google Scholar] [CrossRef]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Beaglehole, R.; Bonita, R.; Kjellström, T. Basic Epidemiology; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Gray, J.M. New concepts in screening. Br. J. Gen. Pract. 2004, 54, 292. [Google Scholar] [PubMed]
- Wilson, J.M.G.; Jungner, G.; World Health Organization. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- World Health Organisation. COVID-19 Target Product Profiles for Priority Diagnostics to Support Response to the COVID-19 Pandemic v.1.0. Available online: https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1 (accessed on 20 December 2022).
- Jung, J.; Kim, J.Y.; Park, H.; Park, S.; Lim, J.S.; Lim, S.Y.; Bae, S.; Lim, Y.-J.; Kim, E.O.; Kim, J.; et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Netw. Open 2022, 5, e2213606. [Google Scholar] [CrossRef] [PubMed]
- Poopalasingam, N.; Korenkov, M.; Ashurov, A.; Strobel, J.; Fish, I.; Hellmich, M.; Gruell, H.; Lehmann, C.; Heger, E.; Klein, F. Determining the reliability of rapid SARS-CoV-2 antigen detection in fully vaccinated individuals. J. Clin. Virol. 2022, 148, 105119. [Google Scholar] [CrossRef]
- Chu, V.T.; Schwartz, N.G.; Donnelly, M.A.P.; Chuey, M.R.; Soto, R.; Yousaf, A.R.; Schmitt-Matzen, E.N.; Sleweon, S.; Ruffin, J.; Thornburg, N.; et al. Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection. JAMA Intern. Med. 2022, 182, 701–709. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. Our Response to the Pandemic. Available online: https://www.health.gov.au/health-alerts/covid-19/government-response (accessed on 2 March 2023).
- Wu, S.; Neill, R.; De Foo, C.; Chua, A.Q.; Jung, A.-S.; Haldane, V.; Abdalla, S.M.; Guan, W.-J.; Singh, S.; Nordström, A. Aggressive containment, suppression, and mitigation of covid-19: Lessons learnt from eight countries. BMJ 2021, 375, e067508. [Google Scholar] [CrossRef]
| RAT Result | Total | ||||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| True | False | True | False | ||
| Demographics | 3 | 2 | 1089 | 20 | 1114 |
| Gender, n (%) | |||||
| Male | 1 (33) | 1 (50) | 556 (51) | 11 (55) | 569 (51) |
| Female | 2 (66) | 1 (50) | 533 (48.9) | 9 (45) | 545 (49) |
| Age | |||||
| Mean (years, ±SD) | 40.66 (33.5) | 51.0 (7.1) | 40.5 (23.5) | 35.75 (21.2) | 40.43 (23.4) |
| Number of patients < 14 years old, n (%) | 1 (33.3) | - | 126 (11.6) | 2 (10) | 129 (11.6) |
| Number of patients ≥ 65 years old, n (%) | 1 (33.3) | - | 202 (18.5) | 2 (10) | 205 (18.4) |
| Emergency Department | |||||
| Australian Triage Category | |||||
| 1, n (%) | - | 1 (0.1) | - | 1 (0.1) | |
| 2, n (%) | 1 (33.3) | 1 (50) | 128 (11.8) | 2 (10) | 132 (11.9) |
| 3, n (%) | 1 (33.3) | 1 (50) | 447 (41.1) | 9 (45) | 457 (41.0) |
| 4, n (%) | 1 (33.3) | - | 405 (37.2) | 9 (45) | 415 (37.3) |
| 5, n (%) | - | - | 108 (9.9) | - | 109 (9.8) |
| Mode of Arrival | |||||
| Private Vehicle, n (%) | - | 1 (50) | 105 (9.6) | 3 (15) | 109 (9.8) |
| Walking, n (%) | 2 (66.7) | - | 13 (65) | 643 (57.7) | |
| Ambulance, n (%) | 1 (33.3) | 1 (50) | 307 (28.2) | 2 (10) | 311 (27.9) |
| Police, n (%) | - | - | 12 (1.1) | 1 (5) | 13 (1.2) |
| Public Transport, n (%) | - | - | 1 (0.1) | - | 1 (0.1) |
| Other, n (%) | - | - | 36 (3.3) | 1 (5) | 37 (3.3) |
| ED Length of Stay (min), median (IQR) | 323 (732) | 319 (377) | 211 (209) | 274 (282) | 213 (210) |
| Disposition | |||||
| Discharged, n (%) | 2 (66.7) | 2 (100) | 694 (63.7) | 14 (70) | 712 (63.9) |
| Admitted, n (%) | 1 (33.3) | - | 395 (36.3) | 6 (30) | 402 (36.1) |
| RAT Tester | |||||
| Registered Nurse, n (%) | - | - | 89 (8.2) | 4 (20) | 93 (8.4) |
| Assistant in Nursing, n (%) | 2 (66.7) | - | 767 (70.4) | 15 (75) | 784 (70.4) |
| Ambulance Officer, n (%) | - | 2 (100) | 184 (16.9) | 186 (16.7) | |
| Other, n (%) | 1 (33.3) | - | 49 (4.5) | 1 (5) | 51 (4.6) |
| Variable | Agreement | κ | p |
|---|---|---|---|
| Screening | 100% | 1.0 | <0.0001 |
| PanBio RAT | 96.08% | 0.8571 | <0.0001 |
| RT-PCR | 96.08% | 0.8817 | <0.0001 |
| Tester | 94.12% | 0.8947 | <0.0001 |
| RT-PCR Positive | RT-PCR Negative | |
|---|---|---|
| Panbio positive | 3 | 2 |
| Panbio negative | 20 | 1089 |
| Binomial exact [95% conf] | ||
| Incidence (%) | 2.1% [1.3, 3.08] | |
| Sensitivity | 0.13 [0.027, 0.336] | |
| Specificity | 0.99 [0.993, 1.0] | |
| Positive predictive value | 0.6 [0.147, 0.947] | |
| Negative predictive value | 0.982 [0.972, 0.989] | |
| Study of Interest (x, n) | Comparative Study (est) | p-Value |
|---|---|---|
| Tran (3, 23) | Winkel (0.86) | <0.0001 |
| Tran (3, 23) | Aranaz-Andres (0.83) | <0.0001 |
| Aranaz-Andres (25, 30) | Winkel (0.86) | 0.6015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.; Barrington, G.; Aandahl, Z.; Lawrence, A.; Wijewardena, S.; Doyle, B.; Cooley, L. Evaluation of the Abbott Panbio™ COVID-19 Ag Rapid Antigen Test for Asymptomatic Patients during the Omicron Wave. J 2023, 6, 198-206. https://doi.org/10.3390/j6010015
Tran V, Barrington G, Aandahl Z, Lawrence A, Wijewardena S, Doyle B, Cooley L. Evaluation of the Abbott Panbio™ COVID-19 Ag Rapid Antigen Test for Asymptomatic Patients during the Omicron Wave. J. 2023; 6(1):198-206. https://doi.org/10.3390/j6010015
Chicago/Turabian StyleTran, Viet, Giles Barrington, Zach Aandahl, Amelia Lawrence, Senudi Wijewardena, Brian Doyle, and Louise Cooley. 2023. "Evaluation of the Abbott Panbio™ COVID-19 Ag Rapid Antigen Test for Asymptomatic Patients during the Omicron Wave" J 6, no. 1: 198-206. https://doi.org/10.3390/j6010015
APA StyleTran, V., Barrington, G., Aandahl, Z., Lawrence, A., Wijewardena, S., Doyle, B., & Cooley, L. (2023). Evaluation of the Abbott Panbio™ COVID-19 Ag Rapid Antigen Test for Asymptomatic Patients during the Omicron Wave. J, 6(1), 198-206. https://doi.org/10.3390/j6010015









