Abstract
Kupffer cells (KCs) are resident macrophages in the liver. Recent studies have revealed that KCs are closely related to inflammatory liver diseases, including nonalcoholic liver diseases (NAFLD). From this point of view, KC transplantation can be a candidate for immunotherapy against inflammatory diseases. Similar to general macrophages, KCs show several different phenotypes according to their environment. Activated KCs are involved in either proinflammatory responses or anti-inflammatory responses. Thus, to manipulate KCs for immunotherapy, it is crucial to control the direction of KC activation. Here, we summarize the outlook and the issues hindering immunotherapy using KC transplantation.
1. Introduction
Immunotherapy using macrophage-based approaches has received increasing attention in recent years. Macrophages have high plasticity that serves multiple functions, including phagocytosis against cellular debris (e.g., microbial products, apoptotic or damaged cells), the classical M1 proinflammatory phenotype (induced by TLR ligands and IFN-γ) or the alternative M2 anti-inflammatory phenotype (induced by IL-4/IL-13) [1]. According to the multiple phenotypes, macrophages are sometimes associated with the development of inflammatory diseases, including cancer. Several strategies have been developed to target tumor-associated macrophages (TAMs) with the tumor-promoting roles [2].
Kupffer cells (KCs) are resident macrophages in the liver. They have a high phagocytic competency, allowing them to remove the foreign medium, for example, viruses and bacteria, apoptotic cells, and cellular debris. Accordingly, KCs in the liver sinusoids serve a crucial role as gatekeepers in the hepatic immune system [3]. KCs play a central role in immunity, tissue injury, and repair in the liver [4,5]. KCs generate various inflammatory mediators containing cytokines, prostaglandins, and reactive oxygen species mainly through NADPH-oxidase or inducible NO-synthase (iNOS) activities [4,5]. There is no doubt that KC dysfunction contributes to the pathogenesis of nonalcoholic fatty liver diseases (NAFLD) [6,7]. Nevertheless, the role of KCs in regulating liver metabolism and the incidence of metabolic disease remains unknown [8]. Thus, if KCs were transplanted efficaciously in the liver with engraftment and long-term survival, the main issues regarding their characteristics in microbial clearance, antigen presentation, and tissue inflammation or repair could be addressed [9,10,11]. Some studies have shown that KC transplantation is helpful [12,13]. On the other hand, the kinetics of how the administered KCs settle in the liver remain unclear.
2. The Importance of KCs as a Therapeutic Target for Inflammatory Diseases
Nutrients absorbed from the small intestine flow through the portal vein or lymph vessels to the liver, where they are metabolized as an energy source [14,15,16]. An excessive influx of nutrients can impose excessive stress on the liver by promoting the production of reactive oxygen species and waste products during metabolism. In addition, various infectious microorganisms and wastes, including hepatitis viruses, enter the liver through the circulatory system, causing damage to the liver. The liver is equipped with a hepatic immune system to counter these factors. KCs reside in sinusoids and monitor blood influx to the liver. KCs have also been reported to be involved in lipid metabolism and iron metabolism derived from aged red blood cells [17]. KCs are supposed to be directly involved in various liver diseases, including infectious diseases, lifestyle-induced alcoholic and nonalcoholic liver diseases, autoimmune diseases, and drug-induced diseases. In each case, the inflammatory response progresses as the disease progresses, and in some cases, the disease becomes more severe, leading to cirrhosis and hepatocellular carcinoma (HCC). Some studies have reported the involvement of KCs in these diseases [18,19]. Based on these studies, there is no doubt about the importance of KCs as a therapeutic target for the inflammatory diseases.
3. KC Transplantation as an Immunotherapeutic Method
Several reports have demonstrated the usefulness of KC transplantation in several animals. Cheng et al. reported that the transplantation of KCs into the liver by administration via the portal vein in rats reduced liver injury at the time of liver transplantation and improved the liver transplantation rate [20]. Merlin et al. also reported a liver transplantation method using transvenous administration of KCs in a mouse system [13]. We recently established a method for intraperitoneal administration of KCs [21].
4. Migration Pathway of Intraperitoneally Administered KCs to the Liver
The route of translocation of intraperitoneally administered KCs to the liver is not clear. In the intraperitoneal administration of drugs, there are four possible routes by which administered drugs reach the liver: (1) diffusion and absorption from the wall-side intraperitoneal capillary bed via the portal vein, (2) absorption from the visceral side intraperitoneal capillary bed via the body’s circulatory system, (3) via the lymphatic vascular system, and (4) direct absorption from the liver surface. According to Nishida et al., the critical size of molecules that can be absorbed from the liver surface covered by the capsule is estimated to be about 70,000 [22]. This is slightly larger than the 6.6 K molecular weight of albumin. This suggests that it would be very difficult for KCs to pass directly through the liver surface. Hoppo et al. reported that hepatocytes enter the lymphatic system via lymph vessels [23]. Considering the possibility of lymphatic mediation, we investigated the spleen and lymph nodes to confirm the presence of transplanted KCs but we were unable to detect them [21]. Given the affinity of KCs for sinusoidal cavities, they most likely migrated via the portal vein from the wall-side intraperitoneal capillary bed. Further clarification of the kinetics of KCs administered in vivo is essential for establishing a highly efficient KC transplantation method.
5. Nucling/NF-κB Signal and KC Activation
Nucling/UACA (uveal autogatigen with coiled coil domains and ankyrin repeats), a novel NF-κB regulatory molecule, is crucial for maintaining KCs [24]. Nucling/UACA is a novel apoptosis-associated molecule regulating the apoptosome pathway, galectin-3 and NF-κB [25]. The NF-κB signal is spontaneously activated in cells or tissues prepared from Nucling-knockout (KO) mice compared with those prepared from wild-type mice. Especially in the liver, KCs prepared from Nucling-KO mice expressed inflammatory cytokines (TNFα, IL-1β, IL-6) with NF-κB activation. Based on the above findings, we hypothesize that KCs normally act protectively as scavengers against the initiation of inflammation (Figure 1). When a robust inflammatory response begins, they rapidly remove the diseased cells by apoptosis through a proinflammatory reaction as one of their diverse functions as macrophages. At this point, KCs are shifted to the M1 dominant phenotype. Severely damaged hepatocytes are removed by apoptosis through a proinflammatory reaction. The liver is a unique organ with a strong regenerative capacity. When pathological hepatocytes are removed by apoptosis, they are quickly regenerated. However, if they are continuously exposed to an inflammatory environment, healthy hepatocytes cannot be regenerated in time, leading to fibrosis and cancer in the liver [26,27]. In other words, proinflammatory KCs may act as an aggravating factor in a highly inflammatory environment.
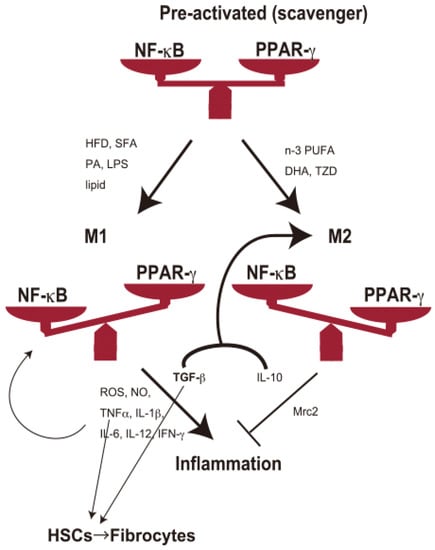
Figure 1.
KCs maintain a heterogeneous population of macrophages. KCs act as scavengers (preactivated (unpolarized) phase) for debris from dead cells or damaged cells to maintain normal conditions. Spontaneous KC damage or depletion may lead to the accumulation of damaged cells in the liver, followed by the prevalence of NAFLD. TGF-β and IL-10 may skew KCs to the M2 phenotype. Pro-fibrotic cytokines (TNF-α and TGF-β) also promote HSCs to fibrogenesis, followed by cirrhosis. Hepatic stressors include overnutrition (HFD, SFA, lipids) and endotoxins (e.g., LPS). HFD, high-fat diet; SFA, saturated fatty acid; PA, palmitic acid; LPS, lipopolysaccharide; PUFA, polyunsaturated fatty acid; DHA, docosahexaenoic acid; TZD, thiazolidinedione.
6. KCs-HSCs Axis
As a type of macrophage, KCs undergo transdifferentiation into several cell types. Thus, it is crucial to keep KCs in a healthy, stable state for use in immunotherapy. When inoculated KCs enter the injured hepatic parenchyma, they can be differentiated into specific phenotypes by activated hepatic stellate cells (HSCs). Transdifferentiation of KCs has been well studied in NAFLD. KCs and HSCs play vital roles in the development of NAFLD. It is well documented that HSCs directly participate in the development of fibrosis through their transdifferentiation into myofibroblasts with excessive fibrotic matrix deposition [28]. A recent study revealed that KCs also participate in liver fibrosis through transdifferentiation into fibrocytes [29]. In the context, Li et al. mention two distinct parallel processes of HSCs and KCs. KCs make cytokines that cause HSC transdifferentiation, but that KCs also undergo transdifferentiation in an autocrine manner.
7. Discussion
It is crucial to maintain proper characteristics to manipulate KCs as a material for immunotherapy because KCs exhibit multiple properties according to their conditions. KCs present heterogeneity in terms of their volume, phagocytotic activity, and enzymatic activities, even in typical physiological situations. The smaller inactive cells predominantly localize in the central zone of the liver lobule [30,31]. The larger active cells are localized much more in the periphery of the liver lobule. The physiological conditions can change the heterogeneity because KCs can transdifferentiate into several cell fates. There are differences in the direction of KC involvement. In the pathogenesis of NAFLD, accumulated lipids and their metabolites activate KCs [32]. Activated KCs release proinflammatory cytokines that promote NAFLD development. Progressive NAFLD further stimulates KC activation, aiding the progression to hepatitis, cirrhosis, and HCC.
On the other hand, there are also reports of cases in which KCs act protectively against liver injury [33]. Many of these differences are supposed to arise from differences in the activation direction of KCs (so-called M1/M2 polarization). In other words, KCs secrete pro-(M1 phenotype) or anti-inflammatory cytokines (M2 phenotype) depending on the direction of their activation, which may exacerbate inflammation in some cases and protect against inflammation in others. The critical mediators for M1/M2 polarization are the nuclear factor-kappa B (NF-κB) signaling pathway and peroxisome proliferator-activated receptor γ (PPAR-γ) [34].
Administration of HFD, SFA, PA, and lipids induces NF-κB activation in KCs. LPS also induces NF-κB activation of KCs to enter the M1 phase [35]. NF-κB activation enters KCs into the M1 phenotype, which produces tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-12, transforming growth factor (TGF)-β, IFN-γ, etc. These proinflammatory cytokines induce further activation of KCs into the M1 phase and act as pathogenic or exacerbating factors for NAFLD, NASH, and HCC [36]. TNF-α and IL-6 play crucial roles in the process of steatosis. Meanwhile, TGF-β promotes the activation of HSCs, which play a central role in the development of fibrosis, leading to the progression of cirrhosis [28]. On the other hand, n-3 PUFA, DHA, and TZD induce PPAR-γ activation and induce KCs to enter the M2 phenotype. The resulting IL-10, Mrc2, etc., may suppress inflammation and prevent NAFLD (Figure 1). TGF-β, together with IL-10, skews macrophages to the M2 subset in cancer [37]. These studies suggest that synergistic immunomodulation among M1/M2 KCs and HSCs plays a crucial role in NAFLD development.
In addition, KCs have the potential to transdifferentiate into alternative phenotypes. It has been reported that KCs participate in liver fibrosis through transdifferentiation into fibrocytes [29]. KCs originate from two cell lineages, circulating monocytes and yolk sac erythro-myeloid progenitors. The significant population in the adult murine liver comes from embryonic precursors. Embryo-derived KCs maintain self-renewal and are better resistant to cellular stresses than their monocyte-derived counterparts [38]. This heterogeneity contributes to the expression profile of cytokines. The expression level of cytokines in KCs is directly linked to the effect of immunotherapy using KC transplantation. These facts suggest the importance of KC maintenance for effective transplantation therapy.
8. Conclusions
Abnormalities in the number and activation state of KCs may be risk factors for the development of inflammatory liver diseases (such as NAFLD, NASH, or HCC) and their related disorders (e.g., metabolic syndrome). As a regulatory factor of NF-κB signaling in KCs, Nucling/UACA is supposed to be one of these markers. However, KCs are undoubtedly a powerful tool for treating inflammatory liver diseases. In vivo or ex vivo manipulation of KC status may be a therapeutic strategy for these disorders. In recent years, cancer therapy targeting tumor-associated macrophages (TAMs) has attracted much attention [2], and related studies have shown that the activation status of TAMs is critical. Based on these findings, the regulation of KC activation is crucial in progressive phases of diseases such as NASH and HCC. Novel therapeutic strategies for the in vivo modulation of KCs by chemicals, diet or exercise or ex vivo methods using KC transplantation are expected to be established.
The usefulness of T cells as tools for cancer immunotherapy has been attracting attention, as well as macrophages. However, as with the KCs transplantation described here, several problems must be overcome in the T cell transplantation method. One of the main problems is T cell dysfunction at the time of transplantation [39], and macrophage-derived cytokines are deeply involved in the state of T cells. Further research on KC and T cell transplantation are expected to establish a powerful immuno-cell transplantation method with synergistic effects.
Author Contributions
T.S. and W.-L.L. conceived and wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MEXT KAKENHI, Japan [Grant Number JP16K15333].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Decker, K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur. J. Biochem. FEBS 1990, 192, 245–261. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Gomez, C.; Delzenne, N.M. Precision-cut liver slices in culture as a tool to assess the physiological involvement of Kupffer cells in hepatic metabolism. Comp. Hepatol. 2004, 3 (Suppl. S1), S45. [Google Scholar] [CrossRef][Green Version]
- Diehl, A.M. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am. J. Phys. Gastrointest. Liver Physiol. 2002, 282, G1–G5. [Google Scholar] [CrossRef]
- Yang, S.Q.; Lin, H.Z.; Lane, M.D.; Clemens, M.; Diehl, A.M. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. USA 1997, 94, 2557–2562. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Cani, P.D.; Dewulf, E.M.; De Backer, F.; Bindels, L.B.; Delzenne, N.M. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem. Biophys. Res. Commun. 2009, 385, 351–356. [Google Scholar] [CrossRef]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef]
- Ikarashi, M.; Nakashima, H.; Kinoshita, M.; Sato, A.; Nakashima, M.; Miyazaki, H.; Nishiyama, K.; Yamamoto, J.; Seki, S. Distinct development and functions of resident and recruited liver Kupffer cells/macrophages. J. Leukoc. Biol. 2013, 94, 1325–1336. [Google Scholar] [CrossRef]
- Klein, I.; Cornejo, J.C.; Polakos, N.K.; John, B.; Wuensch, S.A.; Topham, D.J.; Pierce, R.H.; Crispe, I.N. Kupffer cell heterogeneity: Functional properties of bone marrow derived and sessile hepatic macrophages. Blood 2007, 110, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, F. Infusion of Kupffer Cells Expanded In Vitro Ameliorated Liver Fibrosis in a Murine Model of Liver Injury. Cell Transpl. 2021, 30, 9636897211004090. [Google Scholar] [CrossRef] [PubMed]
- Merlin, S.; Bhargava, K.K.; Ranaldo, G.; Zanolini, D.; Palestro, C.J.; Santambrogio, L.; Prat, M.; Follenzi, A.; Gupta, S. Kupffer Cell Transplantation in Mice for Elucidating Monocyte/Macrophage Biology and for Potential in Cell or Gene Therapy. Am. J. Pathol. 2016, 186, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Greiner, T.U.; Torz, L.; Bookout, A.; Gerstenberg, M.K.; Castorena, C.M.; Kuhre, R.E. Targeting the Gut in Obesity: Signals from the Inner Surface. Metabolites 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’s Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef]
- Scott, C.L.; Guilliams, M. The role of Kupffer cells in hepatic iron and lipid metabolism. J. Hepatol. 2018, 69, 1197–1199. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Z. Roles of Hepatic Innate and Innate-Like Lymphocytes in Nonalcoholic Steatohepatitis. Front. Immunol. 2020, 11, 1500. [Google Scholar] [CrossRef]
- Guillot, A.; Tacke, F. Liver Macrophages: Old Dogmas and New Insights. Hepatol. Commun. 2019, 3, 730–743. [Google Scholar] [CrossRef]
- Chen, G.S.; Qi, H.Z. Effect of Kupffer cells on immune tolerance in liver transplantation. Asian Pac. J. Trop. Med. 2012, 5, 970–972. [Google Scholar] [CrossRef]
- Lin, W.L.; Mizobuchi, M.; Kawahigashi, M.; Nakahashi, O.; Maekawa, Y.; Sakai, T. Functional kupffer cells migrate to the liver from the intraperitoneal cavity. Biochem. Biophys. Rep. 2021, 27, 101103. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sato, N.; Sasaki, H.; Nakamura, J. Mechanism for drug absorption from rat-liver surface membrane: Effect of dose and transport inhibitors on the pharmacokinetics of phenol red. J. Pharm. Pharmacol. 1995, 47, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Hoppo, T.; Komori, J.; Manohar, R.; Stolz, D.B.; Lagasse, E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology 2011, 140, 656–666.e2. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Liu, L.; Teng, X.; Ishimaru, N.; Mukai-Sakai, R.; Tran, N.H.; Kim, S.M.; Sano, N.; Hayashi, Y.; Kaji, R.; et al. Inflammatory disease and cancer with a decrease in Kupffer cell numbers in Nucling-knockout mice. Int. J. Cancer 2010, 126, 1079–1094. [Google Scholar] [CrossRef]
- Sakai, T.; Liu, L.; Teng, X.; Mukai-Sakai, R.; Shimada, H.; Kaji, R.; Mitani, T.; Matsumoto, M.; Toida, K.; Ishimura, K.; et al. Nucling recruits Apaf-1/pro-caspase-9 complex for the induction of stress-induced apoptosis. J. Biol. Chem. 2004, 279, 41131–41140. [Google Scholar] [CrossRef]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef]
- Parlati, L.; Regnier, M.; Guillou, H.; Postic, C. New targets for NAFLD. JHEP Rep. 2021, 3, 100346. [Google Scholar] [CrossRef]
- Araujo Junior, R.F.; Garcia, V.B.; Leitao, R.F.; Brito, G.A.; Miguel Ede, C.; Guedes, P.M.; de Araujo, A.A. Carvedilol Improves Inflammatory Response, Oxidative Stress and Fibrosis in the Alcohol-Induced Liver Injury in Rats by Regulating Kuppfer Cells and Hepatic Stellate Cells. PLoS ONE 2016, 11, e0148868. [Google Scholar] [CrossRef]
- Li, X.; Hollingshead, N.; Lampert, S.; Truong, C.D.; Li, W.; Niu, J.; Crispe, I.N.; Soysa, R. A conserved pathway of transdifferentiation in murine Kupffer cells. Eur. J. Immunol. 2021, 51, 2452–2463. [Google Scholar] [CrossRef]
- Bilzer, M.; Roggel, F.; Gerbes, A.L. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006, 26, 1175–1186. [Google Scholar] [CrossRef]
- Sleyster, E.C.; Knook, D.L. Relation between localization and function of rat liver Kupffer cells. Lab. Invest. 1982, 47, 484–490. [Google Scholar] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chai, Y.; Dong, D.; Zhang, N.; Liu, W.; Ma, T.; Wu, R.; Lv, Y.; Hu, L. AICAR-Induced AMPK Activation Inhibits the Noncanonical NF-kappaB Pathway to Attenuate Liver Injury and Fibrosis in BDL Rats. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6181432. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xu, Q.; Wang, Q.; Wu, H.; Hua, J. Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 2017, 7, 44612. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ogasawara, K.; Takeda, K.; Hashimoto, W.; Sakihara, H.; Kumagai, K.; Anzai, R.; Satoh, M.; Seki, S. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 1996, 156, 2436–2442. [Google Scholar]
- Chen, J.; Deng, X.; Liu, Y.; Tan, Q.; Huang, G.; Che, Q.; Guo, J.; Su, Z. Kupffer Cells in Non-Alcoholic Fatty Liver Disease: Friend or Foe? Int. J. Biol. Sci. 2020, 16, 2367–2378. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Govaere, O.; Petersen, S.K.; Martinez-Lopez, N.; Wouters, J.; Van Haele, M.; Mancina, R.M.; Jamialahmadi, O.; Bilkei-Gorzo, O.; Lassen, P.B.; Darlay, R.; et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 1001–1012. [Google Scholar] [CrossRef]
- Titov, A.; Kaminskiy, Y.; Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Rakhmatullina, A.; Petukhov, A.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Knowns and Unknowns about CAR-T Cell Dysfunction. Cancers 2022, 14, 1078. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).