Medical Device Regulation Efforts for mHealth Apps during the COVID-19 Pandemic—An Experience Report of Corona Check and Corona Health
Abstract
1. Introduction
- Initially, little can be said about whether a selected app actually helps or is effective, with limited overall quality in most health promotion and healthcare domains [10,11,12,13,14,15]. Regulatory requirements cannot provide conclusive support in this regard, but they nevertheless give an indication of whether an app in question adheres to standards regarding validation, verification, and the fundamentals of data integrity.
- Given the vast number of medical apps offered, patients cannot know which app is actually the right one for their needs. Regulatory frameworks, including procedural quality assurance, can be a quality feature here and, at the same time, can serve as a clue to facilitate better navigation for those affected and to offer a more reliable assessment of an app.
- Establish a harmonized regulatory approach that draws the best from all rules and standards, thus avoiding over-regulation, but still showing conformity with the Medical Devices Regulation.
- Possibility of an iterative development approach while maintaining the necessary regulations.
- Creation of a simple and feasible development procedure, so that the high development speed is not slowed down, but at the same time, all necessary regulatory requirements are met for the app in question.
- Reliable project schedules can be created with reliable cost estimates.
- Reduction of error costs (i.e., a detailed requirements phase prevents errors in testing or after going live).
- We share take-home messages that we gained through the development of the two pandemic apps Corona Check and Corona Health in light of regulatory aspects.
- We share insights about the important aspects of the development process.
- We share insights of the costs incurred.
- We share insights about the perception of users based on a conducted study within the Corona Health app.
2. Background and Regulatory Basis
2.1. Collection of Common Standards in the Healthcare Sector
- IEC 62304—Medical Devices Software—Software Life Cycle Processes→ The IEC 62304 standard specifies the requirements for software life cycle processes for the development of medical software and software in medical devices. The processes, activities, and tasks prescribed in this standard form a common framework for action for all life cycle processes for software in the field of medical devices [22].
- GAMP5—Good Automated Manufacturing Practice Supplier Guide for Validation of Automated Systems in Pharmaceutical Manufacture→ This guide has become the standard set of rules for the validation of computerized systems in the pharmaceutical industry (manufacturers and suppliers). However, the GAMP5 set of rules is not legally binding. Therefore, different forms of validation of computerized systems are possible, which is useful for many systems [23].
- General Principles of Software Validation—Regulations of the U.S. Food and Drug Administration (FDA)—U.S. Agency for Food and Drugs.→ This guidance outlines general validation principles that the FDA considers applicable to the validation of medical device software or the validation of software used in the design, development, or manufacture of medical devices [24].
- PICS 11-3 PIC/S Guide to Good Manufacturing Practices (GMP)→ The PIC/S Guide to GMP is the basis for GMP inspections. In particular, its Annex 11, Computerized Systems, is used in the inspection of such systems.The purpose of this document is to provide recommendations and background information on computerized systems to assist inspectors in training and during the inspection of computerized systems. The document is intended to serve as good practice for inspectors that are responsible for inspecting applications in the regulated pharmaceutical sector [25].
- IEC 82304 Health software—Part 1: General requirements for product safety.→ This standard covers software as a medical device, including Mobile Medical Apps as well as other Health Software (outside the application of Regulation (EU) 2017/745 on medical devices). IEC 82304-1 has closed a gap in the normative coverage of validation and documentation of software placed on the market without specific hardware. The key points of this standard are requirements for product safety (SAFETY) as well as for information security (SECURITY) of health software products. This also includes requirements for usability (USABILITY) and instructions for use (INSTRUCTION FOR USE). The standard requires compliance with a software development process and refers to the IEC 62304/A1:2015 [26].
2.2. Overview of Relevant Regulatory Standards
- on the one hand, a positive vote by an ethics committee (here, from the University of Würzburg, which ultimately examines the content according to ethical, medical, and scientific aspects, among others), and,
- on the other hand, the support of a public body (Robert Koch Institute (RKI), Bavarian State Office for Health and Food Safety, …)
3. Resulting Concept of the Regulatory Compliant App Creation Process
3.1. Software Life Cycle Requirements
3.1.1. Roles and Assignment to Project Team Members
- Project Management:Responsible for the implementation and management of the entire product development.
- Quality Management:Responsible for ensuring software quality, regulatory requirements as well as the archiving of documents.
- Development:Responsible for development planning as well as related testing (except system testing).
- Test:Responsible for overall system testing (planning and execution) and risk management.Note: Risk management can be accomplished by a separate role.
- Main responsible person:Main responsible for the whole project. Most importantly, this person is responsible for the issuance of the final release.
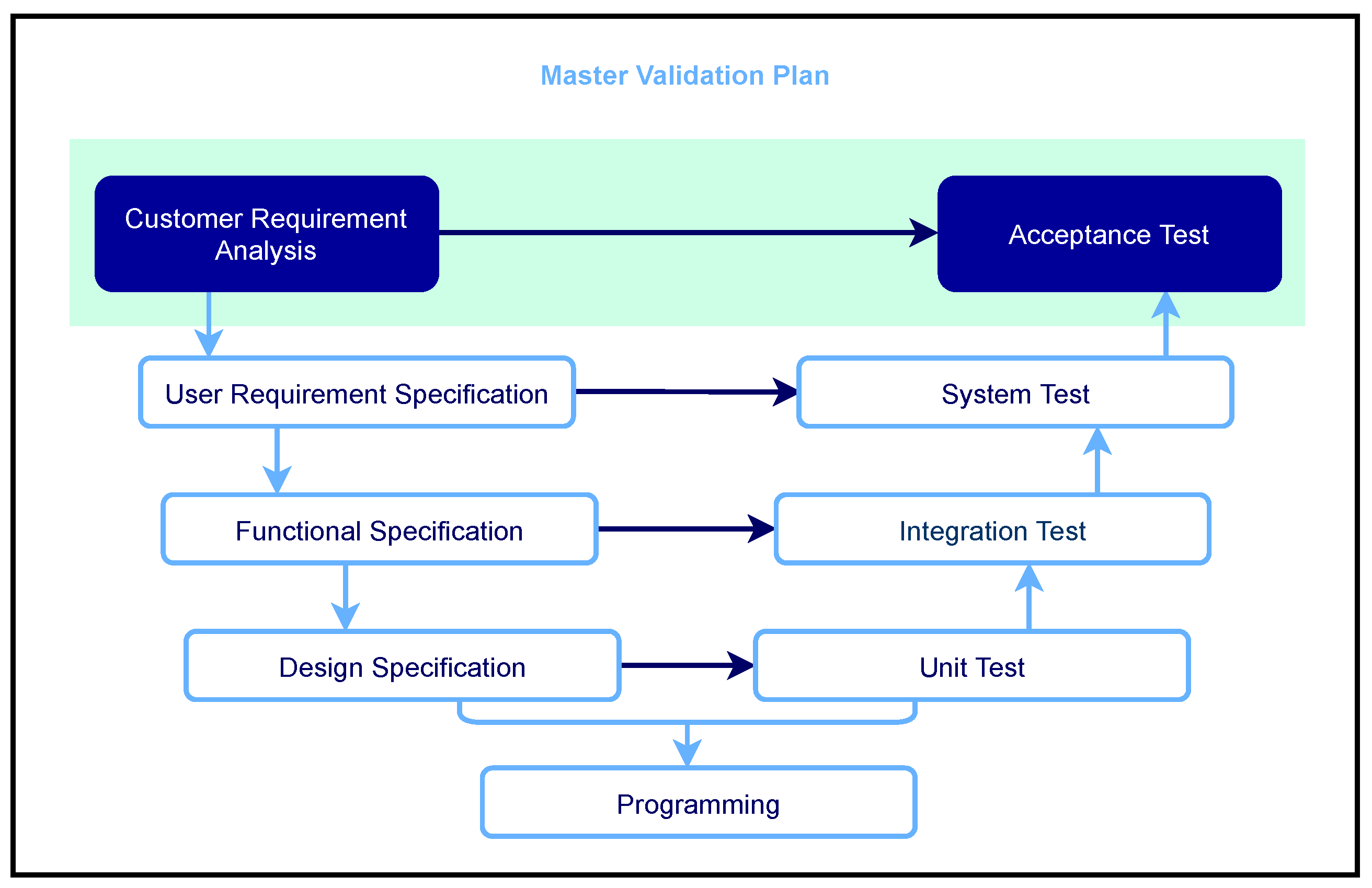
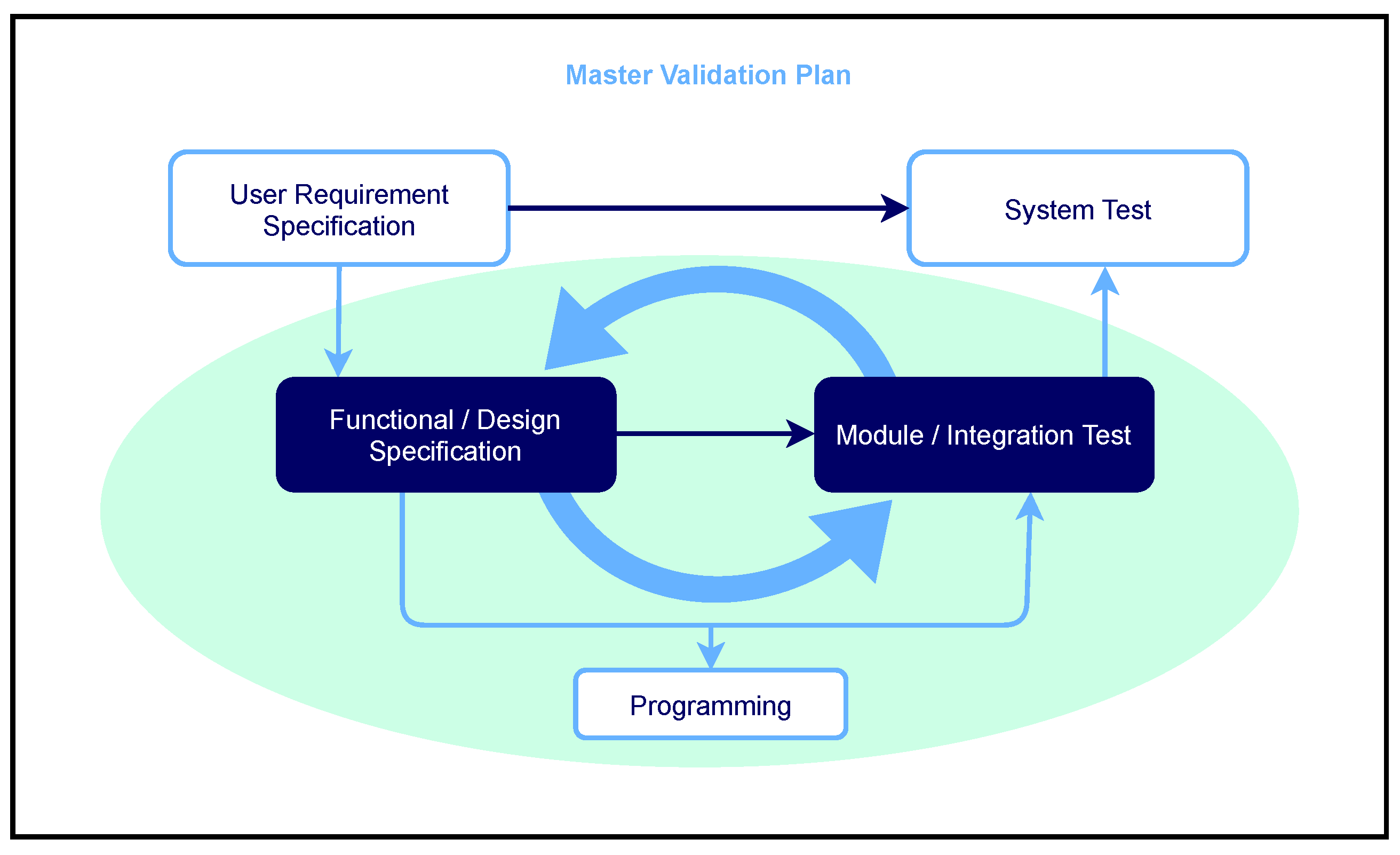
3.1.2. Development Phases/Documents, Responsibilities, and Reviews
- Planning phase (document title: Software Quality Management Plan or also Master Validation Plan)
- Requirements elicitation (document title: requirements specification or requirements specification)
- Summarized functional description + design (document title: combined functional specification/design specification)
- Programming (document title: no separate document is created here)
- Combined module/integration test (document title: Combined module/integration test with specification/protocol/evaluation)
- System test (document title: System test with specification/protocol/evaluation)
- Release (document title: release protocol, e.g., for ethics committee and store operator)
3.1.3. Involved Service Providers, Training, and Necessary SOPs
3.1.4. Tracing Processes
- Trace matrix Requirements specification—Requirements specification/design specification
- Trace Matrix Specifications—System Test Specification
- Trace matrix Requirements/design specification—Module/integration test specification
3.1.5. Test Concept
3.1.6. Interfaces
3.2. Release
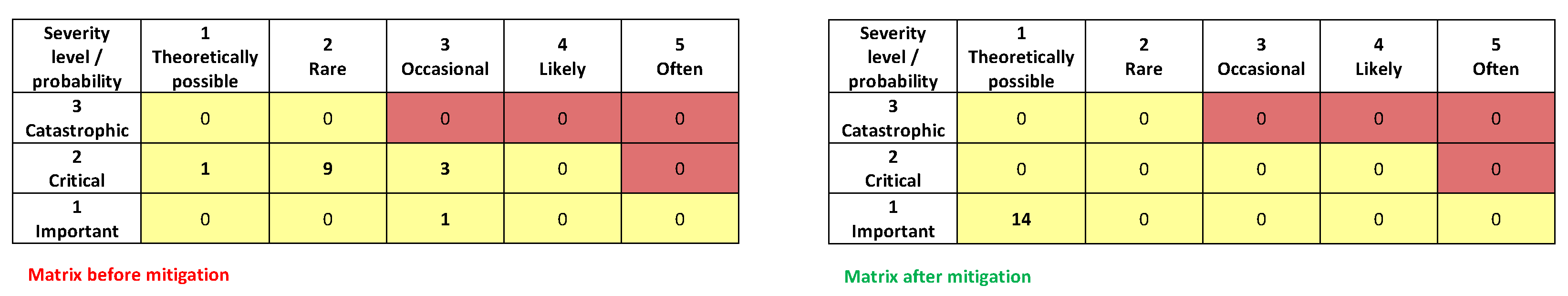
3.3. Risk Management Process
- collected,
- rated,
- mitigated, and
- monitored.
- Product Risks;
- Patient Risks; and
- Risks related to data integrity.
3.4. Acceptance and Evidence
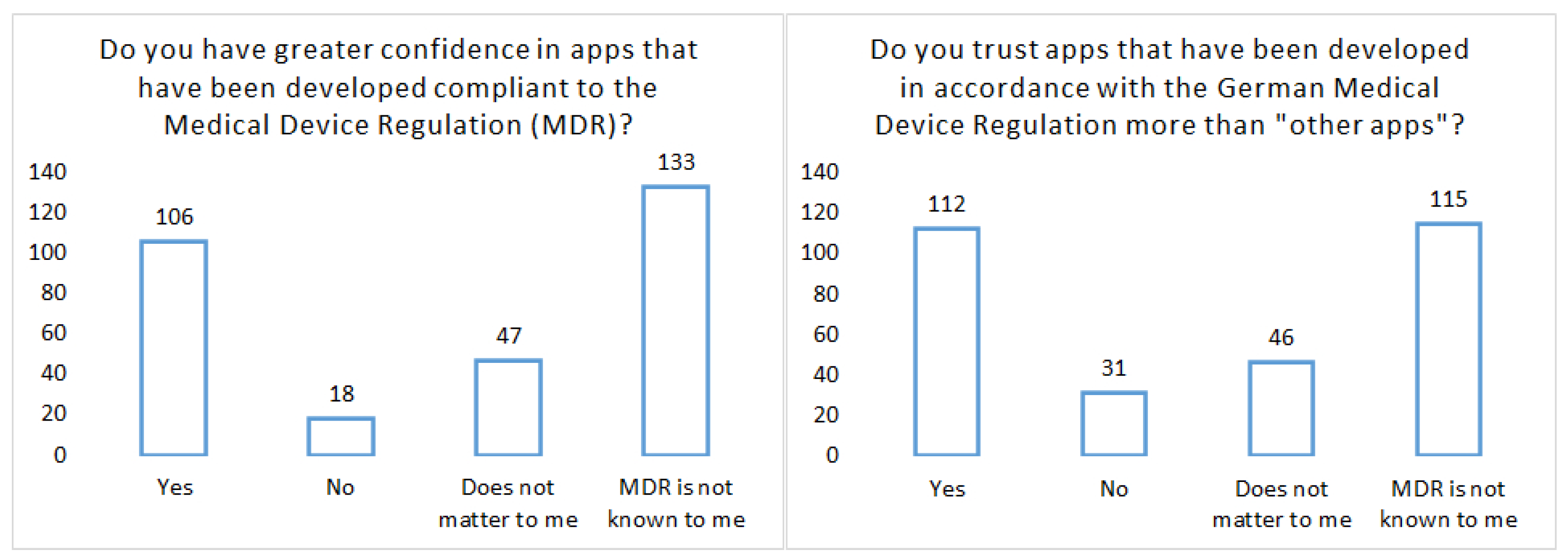
- Study on University Medicine Network: Compass project on the acceptance of pandemic apps (18 years and older): The variety of pandemic apps, apps that have been developed, for example, in hackathons to control COVID-19, shows the great potential that many experts see in them. However, for apps to be effective in the pandemic, they must be used by many people. This applies not only to the Corona warning app but also in particular to apps for assessing individual risk, for example in the case of certain pre-existing conditions. It is therefore necessary that such apps enjoy trust among the general population and that data can be analyzed together for medical research with the consent of the users. In COMPASS, scientists from a wide range of disciplines from university hospitals are joining forces with partners from science and industry in an interdisciplinary project to jointly develop a coordination and technology platform for pandemic apps. Help us with this survey so that pandemic apps can be developed even better and with broad acceptance and transparency in the future.
3.5. Costs
- Organizational matters (stakeholder definition, training, creation of SOPs, approval, and application to ethics committee). Involved persons: 2, work output needs per person: 1 week. Based on the following cost formula: 1.000 Euro/day full costs (=125, Euro/h mixed calculation rate), this phase can be summarized to 10.000 Euro.
- Planning/requirement phase. Involved persons: 3, work output needs per person: 3 weeks. Based on the following cost formula: 1.000 Euro/day full costs (=125, Euro/h mixed calculation rate), this phase can be summarized to 45.000 Euro.
- Development phase. Involved persons: 5, work output needs per person: 3 weeks. Based on the following cost formula: 1.000 Euro/day full costs (=125, Euro/h mixed calculation rate), this phase can be summarized to 75.000 Euro.
- Test phase iterative. Involved persons: 2, work output needs per person: 3 weeks. Based on the following cost formula: 1.000 Euro/day full costs (=125, Euro/h mixed calculation rate), this phase can be summarized to 30.000 Euro.
4. Discussion
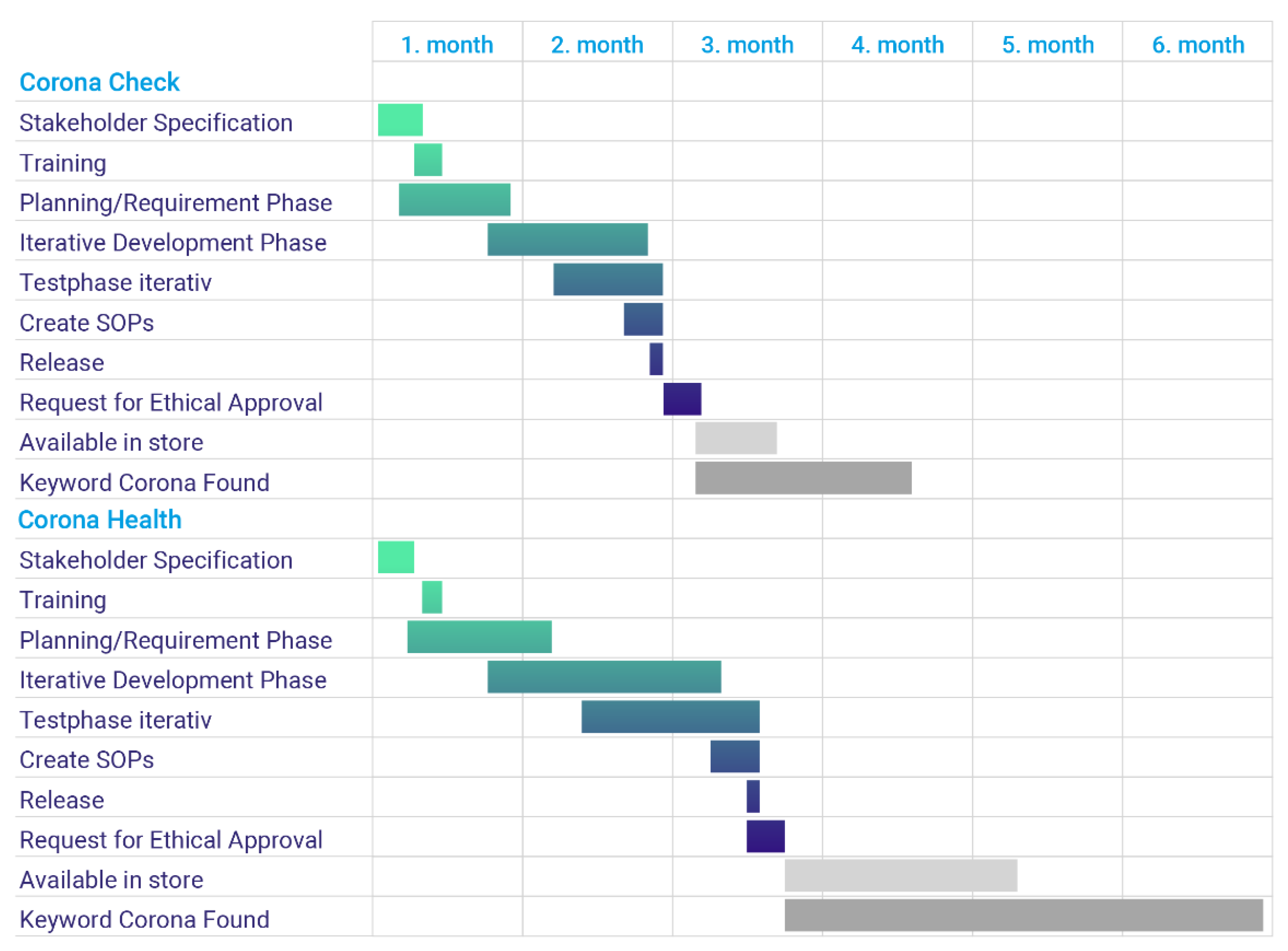
- Determination of the project participants (Day 1–Day 8)
- Training Phase (Day 9–Day 11)
- Planning Phase and Requirement Phase (Day 8–Day 35)
- Development phase (Day 30–Day 72)
- Test phase (Day 44–Day 79)
- Create and release SOPs (Day 70–Day 79)
- Release (Day 79)
- Request Ethics Committees (Day 79–Day 86)
- Release by the store operators (Day 86–Day?)
- Found on Google under the search term Corona (Day 86–Day?)
- Although mobile apps pose different characteristics to many other software projects (e.g., different mobile operating systems must be considered), a harmonized approach based on the existing standards is finally possible to accomplish, even in a short period of development time.
- Due to a clear distribution of roles and tasks as well as a detailed and extensive requirement engineering process (which is required for app development based on MDR anyway)—the complete development time of the app was hardly extended, since potential errors and the resulting unnecessary additional cycles were avoided. The product quality of medical apps required by the MDR was thus already given in a version 1.0.
- Especially for the risk analysis, a tight integration of the interdisciplinary team is required as mHealth apps require perspectives different from other medical software projects. For example, it includes app store involvement, particular requirements of an ethics committee, shorter development cycles, etc.
- Against the background that less mHealth apps that are compliant with the MDR exist, we assume that frequent change requirements (much more than in other software projects; e.g., changes in the underlying mobile operating system) are one major issue that prevent initiating endeavors aimed at the development of mHealth apps compliant to the MDR.
- The latter aspect emphasizes, among others, that experience reports such as that shown in this work could help and support others. In addition, more frameworks and templates are needed in the mHealth field that can be used in an off-the-shelf manner from a regulatory point of view.
- No strict, dogmatic approach, as specified in the extensive IEC 82304, was followed, but a pragmatic, harmonized approach based on IEC 82304 was chosen (in combination with other relevant standards), which was accepted by the ethics committee. Therefore, industrial quality can also be generated on this basis in the university environment.
- Costs play an important role and should be considered and minimized; we therefore recommend training an interdisciplinary team over time to manage costs that are caused by the MDR.
5. Conclusions
- Conformity to the Medical Devices Regulation and
- Consensus on iterative development approach, including high development speed, feasible project schedules, and low overall project costs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Statista. Anzahl der Verfügbaren Gesundheits-Apps Nach App Store im Jahr 2016. 2016. Available online: https://de.statista.com/statistik/daten/studie/688545/umfrage/anzahl-der-verfuegbaren-gesundheits-apps-nach-app-store/ (accessed on 30 March 2021).
- Statista. Number of mHealth Apps Available at Google Play from 1st Quarter 2015 to 4th Quarter 2020. 2021. Available online: https://www.statista.com/statistics/779919/health-apps-available-google-play-worldwide// (accessed on 1 April 2021).
- Statista. Number of mHealth Apps Available in the Apple App Store from 1st Quarter 2015 to 4th Quarter 2020. 2021. Available online: https://www.statista.com/statistics/779910/health-apps-available-ios-worldwide/ (accessed on 1 April 2021).
- OJ L 169. 12.7. 1993. Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices. 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31993L0042 (accessed on 26 March 2021).
- OJ L 117. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. 5 May 2017. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745 (accessed on 26 March 2021).
- Keutzer, L.; Simonsson, U.S. Medical Device Apps: An Introduction to Regulatory Affairs for Developers. JMIR Mhealth Uhealth 2020, 8, e17567. [Google Scholar] [CrossRef] [PubMed]
- Lang, M. Heart Rate Monitoring Apps: Information for Engineers and Researchers About the New European Medical Devices Regulation 2017/745. JMIR Biomed. Eng. 2017, 2. [Google Scholar] [CrossRef]
- Trektere, K.; McCaffery, F.; Lepmets, M.; Barry, G. Tailoring MDevSPICE® for Mobile Medical Apps. In Proceedings of the International Conference on Software and Systems Process, Austin, TX, USA, 14–15 May 2016. [Google Scholar] [CrossRef]
- Trektere, K.; Regan, G.; Caffery, F.M.; Flood, D.; Lepmets, M.; Barry, G. Mobile medical app development with a focus on traceability. J. Softw. Evol. Process 2017, 29, e1861. [Google Scholar] [CrossRef]
- Portenhauser, A.A.; Terhorst, Y.; Schultchen, D.; Sander, L.B.; Denkinger, M.D.; Stach, M.; Waldherr, N.; Dallmeier, D.; Baumeister, H.; Messner, E.M. Mobile Apps for Older Adults: Systematic Search and Evaluation Within Online Stores. JMIR Aging 2021, 4, e23313. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.B.; Schorndanner, J.; Terhorst, Y.; Spanhel, K.; Pryss, R.; Baumeister, H.; Messner, E.M. Help for trauma from the app stores’ A systematic review and standardised rating of apps for Post-Traumatic Stress Disorder (PTSD). Eur. J. Psychotraumatol. 2020, 11, 1701788. [Google Scholar] [CrossRef] [PubMed]
- Schultchen, D.; Terhorst, Y.; Holderied, T.; Stach, M.; Messner, E.M.; Baumeister, H.; Sander, L.B. Stay Present with Your Phone: A Systematic Review and Standardized Rating of Mindfulness Apps in European App Stores. Int. J. Behav. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stach, M.; Kraft, R.; Probst, T.; Messner, E.M.; Terhorst, Y.; Baumeister, H.; Schickler, M.; Reichert, M.; Sander, L.B.; Pryss, R. Mobile Health App Database—A Repository for Quality Ratings of mHealth Apps. In Proceedings of the 2020 IEEE 33rd International Symposium on Computer-Based Medical Systems (CBMS), Rochester, MN, USA, 28–30 July 2020; pp. 427–432. [Google Scholar] [CrossRef]
- Terhorst, Y.; Rathner, E.M.; Baumeister, H.; Sander, L.B. “Hilfe aus dem App-Store”: Eine systematische Übersichtsarbeit und Evaluation von Apps zur Anwendung bei Depressionen. Verhaltenstherapie 2018. [Google Scholar] [CrossRef]
- Terhorst, Y.; Messner, E.M.; Schultchen, D.; Paganini, S.; Portenhauser, A.; Eder, A.S.; Bauer, M.; Papenhoff, M.; Baumeister, H.; Sander, L.B. Systematic evaluation of content and quality of English and German pain apps in European app stores. Internet Interv. 2021, 24, 100376. [Google Scholar] [CrossRef] [PubMed]
- AppRadar. 2021. Available online: https://appradar.com/de (accessed on 1 April 2021).
- Corona Check. 2021. Available online: https://www.coronacheck.science/en/ (accessed on 8 April 2021).
- Corona Health. 2021. Available online: https://www.corona-health.net/en/ (accessed on 8 April 2021).
- Kraft, R.; Schlee, W.; Stach, M.; Reichert, M.; Langguth, B.; Baumeister, H.; Probst, T.; Hannemann, R.; Pryss, R. Combining mobile crowdsensing and ecological momentary assessments in the healthcare domain. Front. Neurosci. 2020, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Pryss, R. Mobile crowdsensing in healthcare scenarios: Taxonomy, conceptual pillars, smart mobile crowdsensing services. In Digital Phenotyping and Mobile Sensing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 221–234. [Google Scholar] [CrossRef]
- Anderson, R. New MRC guidance on evaluating complex interventions. BMJ 2008, 337. [Google Scholar] [CrossRef] [PubMed]
- IEC62304:2006/AMD1:2015 Amendment 1—Medical Device Software—Software Life Cycle Processes. 2015. Available online: https://webstore.iec.ch/publication/22790 (accessed on 30 March 2021).
- GAMP 5 Guide. 2008. Available online: https://www.ispe.org/publications/guidance-documents/gamp-5 (accessed on 30 March 2021).
- Food and Drug Administration. General Principles of Software Validation; Final Guidance for Industry and FDA Staff. 2011. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-principles-software-validation (accessed on 30 March 2021).
- Convention, P.I. Guide to Good Manufacturing Practice for Medicinal Products Annexes. 2018. Available online: https://picscheme.org/docview/2470 (accessed on 30 March 2021).
- IEC 82304-1:2016 Health Software—Part 1: General Requirements for Product Safety. 2016. Available online: https://www.iso.org/standard/59543.html (accessed on 30 March 2021).
- Agency, E.M. ICH Guideline Q9 on Quality Risk Management. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-3.pdf (accessed on 30 March 2021).
- NUM. Best Practices and Common Solutions for Mobile Pandemic Applications (COMPASS). 2021. Available online: https://num-compass.science/en/ (accessed on 24 May 2021).
- Vogel, C.; Pryss, R.; Schobel, J.; Schlee, W.; Beierle, F. Developing Apps for Researching the COVID-19 Pandemic with the TrackYourHealth Platform. In Proceedings of the 2021 IEEE/ACM 8th International Conference on Mobile Software Engineering and Systems (MOBILESoft); 2021. in print. Available online: https://doi.ieeecomputersociety.org/10.1109/MobileSoft52590.2021.00015 (accessed on 9 June 2021).
- Carroll, N.R. Mobile Medical App Regulation: Preventing a Pandemic of Mobilechondriacs. Louis U. J. Health L. Pol’y 2013, 7, 415. [Google Scholar]
- Psihogios, A.M.; Stiles-Shields, C.; Neary, M. The Needle in the Haystack: Identifying Credible Mobile Health Apps for Pediatric Populations during a Pandemic and beyond. J. Pediatr. Psychol. 2020, 45, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. You May Speed Now. The FDA’s Changing Digital Health Speed Limits During a Pandemic. Available online: https://nodehealth.org/2020/08/17/fdas-digital-health-speed-limits-during-a-pandemic-autobahn-or-the-interstate/ (accessed on 9 June 2021).
- Tarricone, R.; Petracca, F.; Ciani, O.; Cucciniello, M. Distinguishing features in the assessment of mHealth apps. Expert Rev. Pharmacoeconomics Outcomes Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kamenjasevic, E.; Biasin, E. A Commentary on Contact Proximity Tracing Apps in the Context of the EU Legal Framework for Medical Devices. EPLR 2020, 4, 110. [Google Scholar] [CrossRef]
- Sezgin, E.; Huang, Y.; Ramtekkar, U.; Lin, S. Readiness for voice assistants to support healthcare delivery during a health crisis and pandemic. NPJ Digit. Med. 2020, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gerke, S.; Shachar, C.; Chai, P.R.; Cohen, I.G. Regulatory, safety, and privacy concerns of home monitoring technologies during COVID-19. Nat. Med. 2020, 26, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly-Shah, V.N.; Gentry, K.R.; Van Cleve, W.; Kendale, S.M.; Jabaley, C.S.; Long, D.R. The COVID-19 pandemic highlights shortcomings in US health care informatics infrastructure: A call to action. Anesth. Analg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zamri, N.; Mohideen, F.B.S. The Practicality of Mobile Applications in Healthcare Administration and COVID-19 Pandemic. Ulum Islam. 2021, 117–130. [Google Scholar] [CrossRef]
| Subjects and Standard | General Principles of SW-Validation (2002) | IEC 62304-2015 | PIC/S 11-3-2007 | IEC 82304-2016 | AAMI TIR36:2007 | GAMP5:2008 |
|---|---|---|---|---|---|---|
| (1) Software Life Cycle—general, incl. validation plan | Sections 4.4 and 5.1 | Sections 5 and 5.1 | Section 9 | Section 6.1 | Chapter 4 | Sections 2, 3, 4, 3.3, 6.1.6 and M1 |
| (2) Software Life Cycle—V-model documentation (Planning-RE-Design-Test) | Sections 5.1–5.2.6 | Sections 5.2–5.8 | Sections 5–14 | Sections 5 and 6 | Sections 4.1–4.3 | Section 4 |
| (3a) Risk Management (classification) | Section 5.2 | Section 4.3 | Section 15 (reference to GAMP) | Section A3 (sub-item to 5.), as well as reference to IEC 62304 and thus to Section 7 | Section 7B | Sections 4.2.6 and M4 |
| (3b) Risk Management (continuous risk management process) | Section 5.2 | Section 7 | Sections 5–14 | Section A3 (sub-item to 5.), as well as reference to IEC 62304 and thus to Section 7 | Section 7B | Sections 5 and M3 |
| (4) Software Lifecycle—Software Maintenance and Support (CR Development) | Sections 4.7 and 5.2.7 | Sections 6.1–6.3 | Sections 17–18 | Sections 1.2 and 8.2 as well as reference to IEC 62304 and thus to Sections 6.1–6.3 | Section 4.4 | Sections 3.1, 4.2 and 4.3 |
| (5) Software Life Cycle—Decommissioning | Section 5.1 | - | Sections 14.2 and 17.2 | Sections 4.2, 7.2.2.9 and 8 | Section 4.5 | Sections 3.1, 4.2, 4.4 and M10 |
| (6) Involvement of software service providers | Section 6.3 | Referred in Section 4.1—by reference to QM system | Section 11 | Reference to IEC 62304 and thus indirectly to Section 4.1—reference to QM system | Indirectly in Section 2.2—reference to QM system | Sections 6.1.4 and 7 |
| (7) Training of employees | Section 2.4— Reference to QM system | Referred in Section 4.1—by reference to QM system | Section 22 | Chapters 6.1.e, as well as reference to IEC 62304 and thus indirectly to Section 4.1 | Indirectly in Section 2.2—reference to QM System | Section 6.1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holfelder, M.; Mulansky, L.; Schlee, W.; Baumeister, H.; Schobel, J.; Greger, H.; Hoff, A.; Pryss, R. Medical Device Regulation Efforts for mHealth Apps during the COVID-19 Pandemic—An Experience Report of Corona Check and Corona Health. J 2021, 4, 206-222. https://doi.org/10.3390/j4020017
Holfelder M, Mulansky L, Schlee W, Baumeister H, Schobel J, Greger H, Hoff A, Pryss R. Medical Device Regulation Efforts for mHealth Apps during the COVID-19 Pandemic—An Experience Report of Corona Check and Corona Health. J. 2021; 4(2):206-222. https://doi.org/10.3390/j4020017
Chicago/Turabian StyleHolfelder, Marc, Lena Mulansky, Winfried Schlee, Harald Baumeister, Johannes Schobel, Helmut Greger, Andreas Hoff, and Rüdiger Pryss. 2021. "Medical Device Regulation Efforts for mHealth Apps during the COVID-19 Pandemic—An Experience Report of Corona Check and Corona Health" J 4, no. 2: 206-222. https://doi.org/10.3390/j4020017
APA StyleHolfelder, M., Mulansky, L., Schlee, W., Baumeister, H., Schobel, J., Greger, H., Hoff, A., & Pryss, R. (2021). Medical Device Regulation Efforts for mHealth Apps during the COVID-19 Pandemic—An Experience Report of Corona Check and Corona Health. J, 4(2), 206-222. https://doi.org/10.3390/j4020017









