Inflammation and Peripheral Arterial Disease
Abstract
:1. Introduction
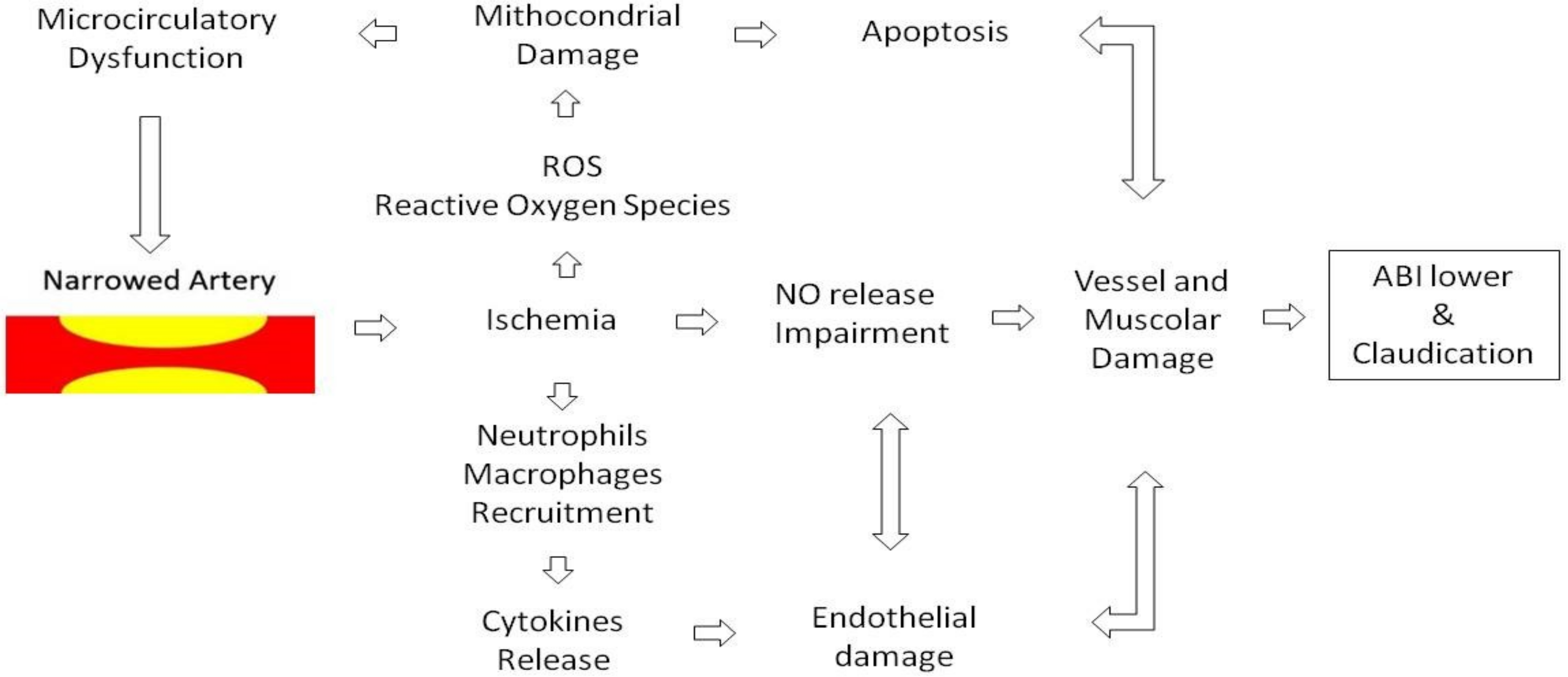
2. Endothelial Dysfunction
3. Inflammation and PAD
4. C-Reactive Protein (CRP)
5. Interleukin-6 (IL-6)
- Expression of Vascular endothelial growth factor (VEGF) [60]
- Production of neutrophils in the bone marrow
- Secretion of MCP-1 and IL-8 by endothelial cells and macrophages.
- Changing leukocytes into atherosclerotic plaque by producing ICAM-1 by smooth muscle cells (SMCs)
- Transformation of SMCs into foam cells
6. Interleukins in PAD
7. Selectins
8. Matrix Metalloproteinases (MMPs)
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Savji, N.; Rockman, C.; Skolnick, A. Association Between Advanced Age and Vascular Disease in Different Arterial Territories: A Population Database of Over 3.6 Million Subjects. J. Vasc. Surg. 2013, 58, 1719–1720. [Google Scholar] [CrossRef] [Green Version]
- Shu, J.; Ssntilli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; Evans, J.C.; Nieto, K.; Larson, M.G.; Levy, D.; Wilson, P.W. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am. Heart J. 2002, 143, 961–965. [Google Scholar] [CrossRef]
- Selvin, E.; Erlinger, T.P. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999 ± 2000. Circulation 2004, 110, 738–743. [Google Scholar] [CrossRef]
- Sigvant, B.; Wiberg-Hedman, K.; Bergqvist, D.; Rolandsson, O.; Andersson, B.; Persson, E.; Wahlberg, E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J. Vasc. Surg. 2007, 45, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Alzamora, M.T.; Forés, R.; Baena-Díez, J.M.; Pera, G.; Toran, P.; Sorribes, M.; Vicheto, M.; Reina, M.D.; Sancho, A.; Albaladejo, C.; et al. The peripheral arterial disease study (PERART/ARTPER): Prevalence and risk factors in the general population. BMC Public Health 2010, 10, 38. [Google Scholar] [CrossRef]
- Santo Signorelli, S.; Anzaldi, M.; Fiore, V.; Simili, M.; Puccia, G.; Libra, M.; Malaponte, G.; Neri, S. Patients with unrecognized peripheral arterial disease (PAD) assessed by ankle-brachial index (ABI) present a defined profile of proinflammatory markers compared to healthy subjects. Cytokine 2012, 59, 294–298. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Housley, E.; Cawood, E.H.; Macintyre, C.C.; Ruckley, C.V.; Prescott, R.J. Edinburgh Artery Study: Prevalence of Asymptomatic and Symptomatic Peripheral Arterial Disease in the General Population. Int. J. Epidemiol. 1991, 20, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update. A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar]
- Ramos, R.; Quesada, M.; Solanas, P.; Subirana, I.; Sala, J.; Vilá, J.; Masia, R.; Cerezo, C.; Elosua, R.; Grau, M.; et al. Prevalence of Symptomatic and Asymptomatic Peripheral Arterial Disease and the Value of the Ankle-brachial Index to Stratify Cardiovascular Risk. J. Vasc. Surg. 2009, 50, 703–704. [Google Scholar] [CrossRef] [Green Version]
- Norgern, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. TASC II Working Group: Inter-society consensus for management of peripheral arterial diseases (TASCII). J. Vasc. Surg. 2007, 45 (Suppl, S), S5–S67. [Google Scholar] [CrossRef]
- Santo Signorelli, S.; Anzaldi, M.; Fiore, V.; Catanzaro, S.; Simili, M.; Torrisi, B.; Neri, S. Study on unrecognized peripheral arterial disease (PAD) by ankle/brachial index and arterial co-morbidity in Catania (Sicily, Italy). Angiology 2010, 61, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douek, P.C.; Revel, D.; Chazel, S.; Falise, B.; Villard, J.; Amiel, M. Fast MR angiography of the aortoiliac arteries and arteries of the lower extremity: Whole of bolus enhanced, whole-volume subtraction technique. AJR Am. J. Roentgenol. 1995, 165, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Cranny, G.; Burch, J.; Aguiar-Ibáñez, R.; Craig, D.; Wright, K.; Berry, E.; Gough, M.; Kleijnen, J.; Westwood, M. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol. Assess. 2007, 11, 1–184. [Google Scholar] [CrossRef]
- Criqui, M.; Fronek, A.; Criqui, D.M.H.; Langer, R.D.; Feigelson, H.S. Coronary Disease and Stroke in Patients with Large-Vessel Peripheral Arterial Disease. Drugs 1991, 42, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis as an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, E.M.; Kapadia, S.R.; Tutar, E.; Ziada, K.M.; Hobbs, R.E.; McCarthy, P.M.; Young, J.B.; Nissen, S.E. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: Evidence from intravascular ultrasound. Circulation 2001, 103, 2705. [Google Scholar] [CrossRef]
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Peripheral artery disease in patients with type 2 diabetes. J. Its Complicat. 2014, 28, 912. [Google Scholar] [CrossRef]
- Lu, L.; Mackay, D.F.; Pell, J.P. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart 2014, 100, 414–423. [Google Scholar] [CrossRef]
- Brevetti, G.; Schiano, V.; Chiariello, M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 2008, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.; Stampfer, M.; Rifai, N. Novel risk factors for systemic atherosclerosis. A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein (a), and standard cholesterol screening as predictors of peripheral arterial disease. ACC Curr. J. Rev. 2001, 10, 25–26. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Goldstone, J.; Smith, S.C., Jr.; McDermott, M.; Moneta, G.; Oka, R.; Newman, A.B.; Pearce, W.H.; Writing Group 1. Atherosclerotic peripheral vascular disease symposium II. Circulation 2008, 118, 2826–2829. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Chen, J.; Sutton-Tyrrell, K.; He, J. Relation of Inflammation to Peripheral Arterial Disease in the National Health and Nutrition Examination Survey, 1999–2002. Am. J. Cardiol. 2005, 96, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Plasma Concentration of C-Reactive Protein and Risk of Developing Peripheral Vascular Disease. Circulation 1998, 97, 425–428. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y.; Ali, F. Atherosclerotic cardiovascular disease: A review of initiators and protective factors. Inflammopharmacol 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Husain, K.; Hernandez, W.; Ansari, R.A.; Ferder, L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. J. Boil. Chem. 2015, 6, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amroc, S.M.; Weitzman, M. Multiple biomarkers for mortality prediction in peripheral arterial disease. Vasc. Med. 2016, 21, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Casini, A.; Ignarro, L.J.; Cirino, G.; Napoli, C. Nitric Oxide as a Signaling Molecule in the Vascular System: An Overview. J. Cardiovasc. Pharmacol. 1999, 34, 879–886. [Google Scholar]
- Sandoo, A.; Van Zanten, J.J.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Dejam, A.; Kleinbongard, P.; Rassaf, T.; Hamada, S.; Gharini, P.; Rodriguez, J.; Feelisch, M.; Kelm, M. Thiols enhance NO formation from nitrate photolysis. Free Radic. Biol. Med. 2003, 35, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.L.; Skyrme-Jones, R.A.P.; Meredith, I.T. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation. Clin. Sci. 2000, 99, 261. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Rac-albu, M.; Iliuta, L.; Guberna, S.M.; Sinescu, C. The role of ankle-brachial index for predicting peripheral arterial disease. Maedica 2014, 9, 295–302. [Google Scholar]
- Cohen, R.A.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation 1995, 92, 3337–3349. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ. Res. 2015, 116, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Kitta, Y.; Obata, J.-E.; Nakamura, T.; Hirano, M.; Kodama, Y.; Fujioka, D.; Saito, Y.; Kawabata, K.-I.; Sano, K.; Kobayashi, T.; et al. Persistent Impairment of Endothelial Vasomotor Function Has a Negative Impact on Outcome in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2009, 53, 323–330. [Google Scholar] [CrossRef]
- Fort-Gallifa, I.; García-Heredia, A.; Hernández-Aguilera, A.; Simó, J.M.; Sepúlveda, J.; Martín-Paredero, V.; Camps, J.; Joven, J. Biochemical indices of oxidative stress and inflammation in the evaluation of peripheral artery disease. Free Radic. Biol. Med. 2016, 97, 568–576. [Google Scholar] [CrossRef]
- Rossi, E.; Biasucci, L.; Citterio, F. Risk of myocardial infarction and angina in patients with severe peripheral vascular disease. Predictive role of C-reactive protein. ACC Curr. J. Rev. 2002, 11, 34. [Google Scholar] [CrossRef]
- Violi, F.; Criqui, M.; Longoni, A.; Castiglioni, C. Relation between risk factors and cardiovascular complications in patients with peripheral vascular disease. Results from the A.D.E.P. study. Atherosclerosis 1996, 120, 25–35. [Google Scholar] [CrossRef]
- Li, J.J.; Fang, C.H. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med. Hypotheses 2004, 62, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Mach, F.; Steffens, S. The specific role of chemokines in atherosclerosis. Thromb. Haemost. 2007, 97, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Roldán, V.; Marín, F.; Lip, G.Y.H.; Blann, A.D. Soluble E-selectin in cardiovascular disease and its risk factors. Thromb. Haemost. 2003, 90, 1007–1020. [Google Scholar] [CrossRef]
- Nielsen, L.B. Atherogenecity of lipoprotein(a) and oxidized low density lipoprotein: Insight from in vivo studies of arterial wall influx, degradation and efflux. Atherosclerosis 1999, 143, 229–243. [Google Scholar] [CrossRef]
- Carmelli, D.; Fabsitz, R.R.; Swan, G.E.; Reed, T.; Miller, B.; Wolf, P.A. Contribution of Genetic and Environmental Influences to Ankle-Brachial Blood Pressure Index in the NHLBI Twin Study. Am. J. Epidemiol. 2000, 151, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.C.; Vohra, R.S.; Beer, S.; Bhatti, K.; Ponnambalam, S.; Homer-Vanniasinkam, S. Biomarkers in Peripheral Arterial Disease. Cardiovasc. Med. 2009, 19, 147–151. [Google Scholar] [CrossRef]
- Chambers, R.E.; Hutton, C.W.; Dieppe, P.A.; Whicher, J.T. Comparative study of C reactive protein and serum amyloid A protein in experimental inflammation. Ann. Rheum. Dis. 1991, 50, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W. Function of C-reactive protein. Ann. Med. 2000, 32, 274–278. [Google Scholar] [CrossRef]
- Ridker, P.M.; Tracy, R.P.; Hennekens, C.H.; Cushman, M.; Stampfer, M.J. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Pankow, J.S.; Tracy, R.P.; Arnett, D.K.; Peacock, J.M.; Hong, Y.; Djoussé, L.; Eckfeldt, J.H. Investigators of the NHBLI Family Heart Study: Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am. J. Cardiol. 2001, 88, 112–117. [Google Scholar] [CrossRef]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The structure and biological features of fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 11–30. [Google Scholar] [CrossRef]
- McDermott, M.M.; Guralnik, J.M.; Corsi, A.; Albay, M.; Macchi, C.; Bandinelli, S.; Ferrucci, L. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am. Heart J. 2005, 150, 276–281. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Dalmon, J.; Laurent, M.; Courtois, G. The human b fibrinogen promoter contains a hepatocyte nuclear factor 1-dependent interleukin-6-responsive element. Mol. Cell Biol. 1993, 13, 1183–1193. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Greininger, G.; Tosato, G. Acute phase and immune responses: Interleukin-6. Ann. N. Y. Acad. Sci. 1989, 557, 1–583. [Google Scholar]
- Wei, L.-H.; Kuo, M.-L.; Chen, C.-A.; Chou, C.-H.; Lai, K.-B.; Lee, C.-N.; Hsieh, C.-Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003, 22, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- McDermott, M.M.; Liu, K.; Ferrucci, L.; Tian, L.; Guralnik, J.M.; Tao, H.; Ridker, P.M.; Criqui, M.H. Relation of Interleukin-6 and Vascular Cellular Adhesion Molecule-1 Levels to Functional Decline in Patients with Lower Extremity Peripheral Arterial Disease. Am. J. Cardiol. 2011, 107, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martone, V.; De Cristofaro, T.; Corrado, S.; Silvestro, A.; Di Donato, A.M.; Bucur, R.; Scopacasa, F.; Brevetti, G. High Levels of Adhesion Molecules Are Associated with Impaired Endothelium-dependent Vasodilation in Patients with Peripheral Arterial Disease. Thromb. Haemost. 2001, 85, 63–66. [Google Scholar] [CrossRef]
- Nylænde, M.; Stranden, E.; Morken, B.; Sandbæk, G.; Lindahl, A.; Seljeflot, I.; Kroese, A.; Arnesen, H. Markers of vascular inflammation are associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vasc. Med. 2006, 11, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Murabito, J.M.; Keyes, M.J.; Guo, C.Y.; Keaney, J.F., Jr.; Vasan, R.S.; D’Agostino Sr, R.B.; Benjamin, E.J. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis 2009, 203, 509–514. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Murray, G.; Lee, A.J.; Rumley, A.; Lowe, G.D.; Fowkes, F.G.R. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur. Heart J. 2007, 28, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Potaczek, D.P.; Undas, A.; Nowakowski, T.; Szczeklik, A. Association between inflammatory markers and the interleukin-6 -174 G/C polymorphism is abolished in peripheral arterial occlusive disease. Int. Angiol. 2007, 26, 318–323. [Google Scholar] [PubMed]
- Libra, M.; Signorelli, S.S.; Bevelacqua, Y.; Navolanic, P.M.; Bevelacqua, V.; Polesel, J.; Talamini, R.; Stivala, F.; Mazzarino, M.C.; Malaponte, G. Analysis of G(-174)C IL-6 polymorphism and plasma concentrations of inflammatory markers in patients with type 2 diabetes and peripheral arterial disease. J. Clin. Pathol. 2006, 59, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Murray, G.; Lee, A.; Rumley, A.; Lowe, G.; Fowkes, G. C-Reactive Protein, Interleukin-6, and Soluble Adhesion Molecules as Predictors of Progressive Peripheral Atherosclerosis in the General Population. Edinburgh Artery Study. ACC Curr. J. Rev. 2005, 14, 16. [Google Scholar] [CrossRef]
- Marino, F.; Guasti, L.; Tozzi, M.; Maio, R.C.; Castiglioni, L.; Rasini, E.; Schembri, L.; Maroni, L.; Legnaro, M.; De Leo, A.; et al. Angiotensin Type 1 Receptor Expression and Interleukin-8 Production in Polymorphonuclear Leukocytes of Patients with Peripheral Arterial Disease. J. Cardiovasc. Pharmacol. 2009, 54, 520–525. [Google Scholar] [CrossRef]
- Malek, T.R.; Castro, I. Interleukin-2 receptor signaling: At the interface between tolerance and immunity. Immunity 2010, 33, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Shi, G.P. Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze, V.; Emmanuel, F.; Bureau, M.F.; Soubrier, F.; Esposito, B.; Duez, H.; Fievet, C.; et al. Protective Role of Interleukin-10 in Atherosclerosis. Circ. Res. 1999, 85, 17–24. [Google Scholar] [CrossRef]
- Mallat, Z.; Heymes, C.; Ohan, J.; Faggin, E.; Lesèche, G.; Tedgui, A. Expression of interleukin-10 in advanced human atherosclerotic plaques: Relation to inducible nitric oxide synthase expression and cell death. Arter. Thromb. Vasc. Boil. 1999, 19, 611–616. [Google Scholar] [CrossRef]
- Anguera, I.; Miranda-Guardiola, F.; Bosch, X.; Filella, X.; Sitges, M.; Marin, J.L.; Betriu, A.; Sanz, G. Elevation of serum levels of the anti-inflammatory cytokine interleukin-10 and decreased risk of coronary events in patients with unstable angina. Am. Heart J. 2002, 144, 811–817. [Google Scholar] [CrossRef]
- Smith, D.A.; Irving, S.D.; Sheldon, J.; Cole, D.; Kaski, J.C. Serum Levels of the Antiinflammatory Cytokine Interleukin-10 Are Decreased in Patients with Unstable Angina. Circulation 2001, 104, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Merten, M.; Thiagarajan, P. P-selectin in arterial thrombosis. Z. Kardiol. 2004, 93, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. The role of selectins in inflammation and disease. Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Boulbou, M.S.; Koukoulis, G.N.; Vasiou, K.G.; Petinaki, E.A. Increased soluble E-selectin levels in type 2 diabetic patients with peripheral arterial disease. Int. Angiol. 2004, 23, 18–24. [Google Scholar] [PubMed]
- Signorelli, S.S.; Mazzarino, M.C.; Di Pino, L.; Malaponte, G.; Porto, C.; Pennisi, G.; Marchese, G.; Costa, M.P.; DiGrandi, D.; Celotta, G.; et al. High circulating levels of cytokines (IL-6 and TNFalpha), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc. Med. 2003, 8, 15–19. [Google Scholar] [CrossRef]
- Saetre, T.; Enoksen, E.; Lyberg, T.; Stranden, E.; Jørgensen, J.J.; Sundhagen, J.O.; Hisdal, J. Supervised exercise training reduces plasma levels of the endothelial inflammatory markers E-selectin and ICAM-I in patients with peripheral arterial disease. Angiology 2011, 62, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Dollery, C.M.; McEwan, J.R.; Henney, A.M. Matrix metalloproteinasesand cardiovascular disease. Circ. Res. 1995, 77, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Malaponte, G.; Libra, M.; Di Pino, L.; Celotta, G.; Bevelacqua, V.; Petrina, M.; Nicotra, G.S.; Indelicato, M.; Navolanic, P.M.; et al. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc. Med. 2005, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Busti, C.; Falcinelli, E.; Momi, S.; Gresele, P. Matrix metalloproteinases and peripheral arterial disease. Intern. Emerg. Med. 2010, 5, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aguilar, E.; Gomez-Rodriguez, V.; Orbe, J.; Rodríguez, J.A.; Fernández-Alonso, L.; Roncal, C.; Paramo, J.A. Matrix metalloproteinase 10 is associated with disease severity and mortality in patients with peripheral arterial disease. J. Vasc. Surg. 2015, 61, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giagtzidis, I.; Karkos, C.; Pitoulias, G.; Papazoglou, K. Matrix metalloproteinases and peripheral arterial disease. Int. Angiol. 2015, 34, 195–201. [Google Scholar] [PubMed]
| Author | Prevalence (%) | Number |
|---|---|---|
| Murabito, J.M. et al., 2002 [4] | 3.9 | 5124 |
| Selvin, E. et al., 2004 [5] | 4.3 | 2174 |
| Sigvant, B. et al., 2007 [6] | 18 | 5080 |
| Alzamora, M.T. et al., 2010 [7] | 7.6 | 3786 |
| Signorelli, S. et al., 2010 [8] | 2.3 | 9100 |
| Fowkes, F.G.R. et al., 2013 [9] | PAD prevalence increases by 28.7% in low-income or middle-income countries (LMIC) and 13.1% in high income countries. | Review of 34 published studies (2000–2010). |
| American Heart Association (AHA), 2018 [10] | individuals ≥ 80 years 22.7% individuals 40–49 years 1.6% | |
| Ramos, R. et al., 2009 [11] | 4.5 | 6262 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorelli, S.S.; Marino, E.; Scuto, S. Inflammation and Peripheral Arterial Disease. J 2019, 2, 142-151. https://doi.org/10.3390/j2020012
Signorelli SS, Marino E, Scuto S. Inflammation and Peripheral Arterial Disease. J. 2019; 2(2):142-151. https://doi.org/10.3390/j2020012
Chicago/Turabian StyleSignorelli, Salvatore Santo, Elisa Marino, and Salvatore Scuto. 2019. "Inflammation and Peripheral Arterial Disease" J 2, no. 2: 142-151. https://doi.org/10.3390/j2020012
APA StyleSignorelli, S. S., Marino, E., & Scuto, S. (2019). Inflammation and Peripheral Arterial Disease. J, 2(2), 142-151. https://doi.org/10.3390/j2020012





