Emerging Roles of Cardiotrophin-1 in the Pathogenesis and Biomarker of Atherosclerosis
Abstract
1. Introduction
2. Structure, Expression, and Function of CT-1
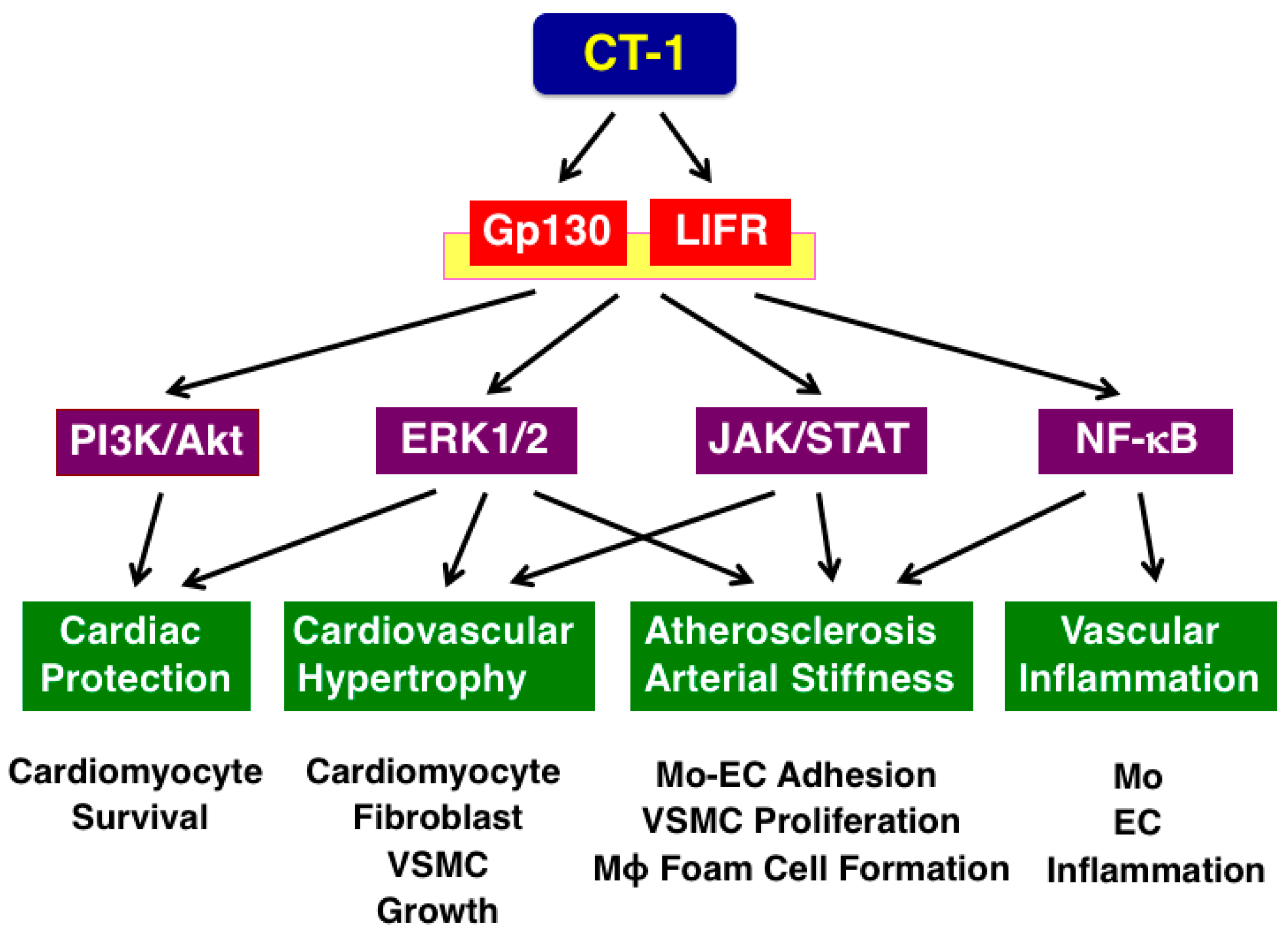
3. Receptor and Signaling of CT-1
4. Roles of CT-1 in Hypertension
5. Roles of CT-1 in Metabolic Syndrome
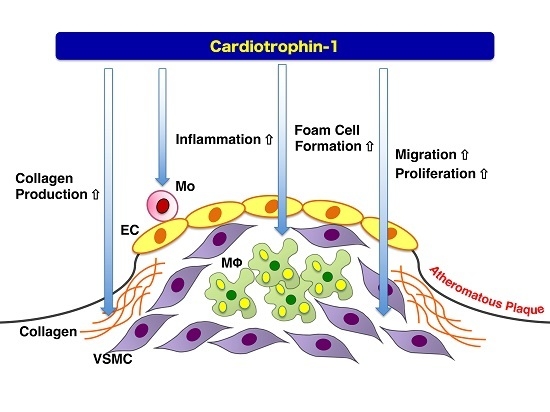
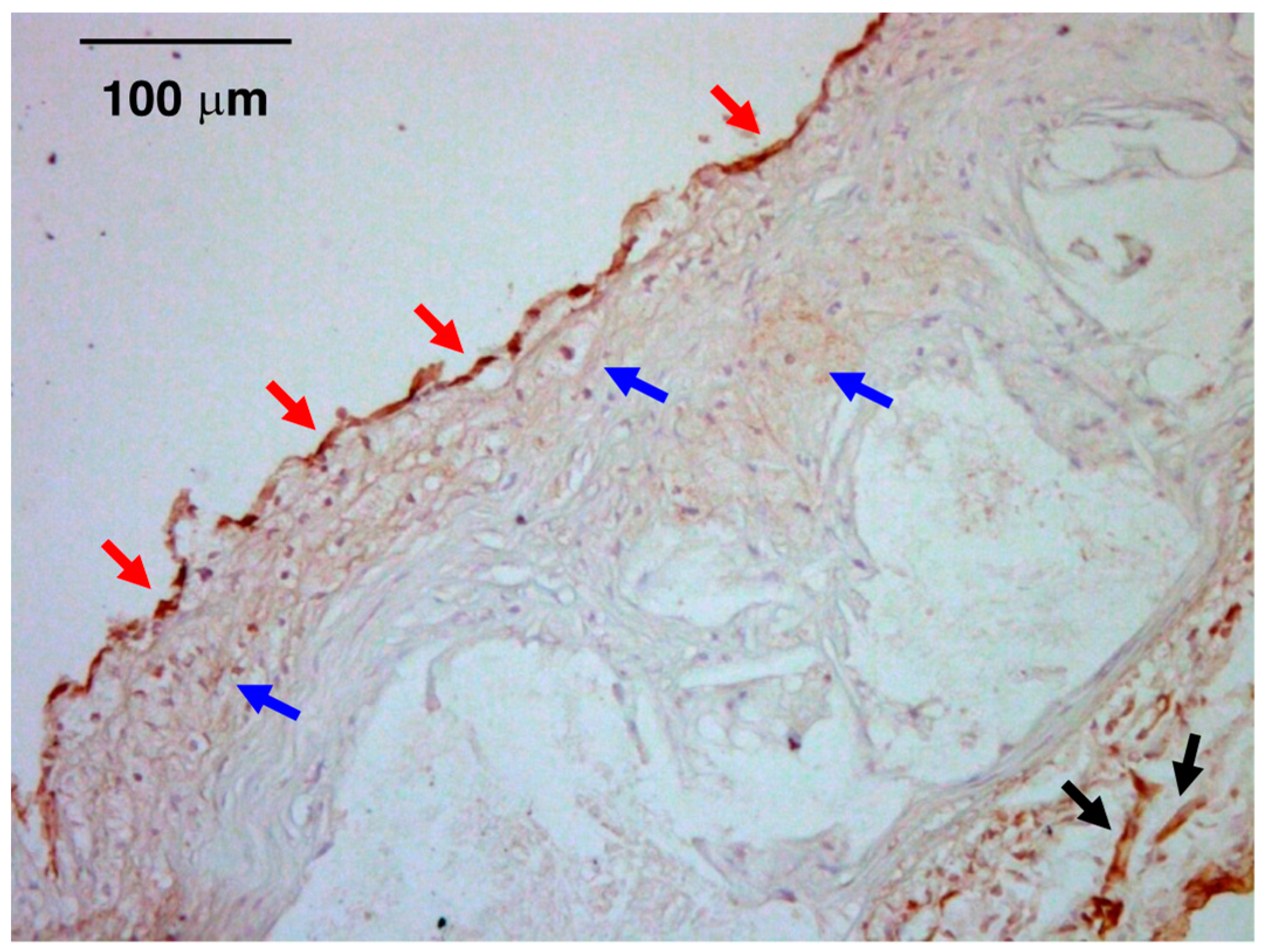
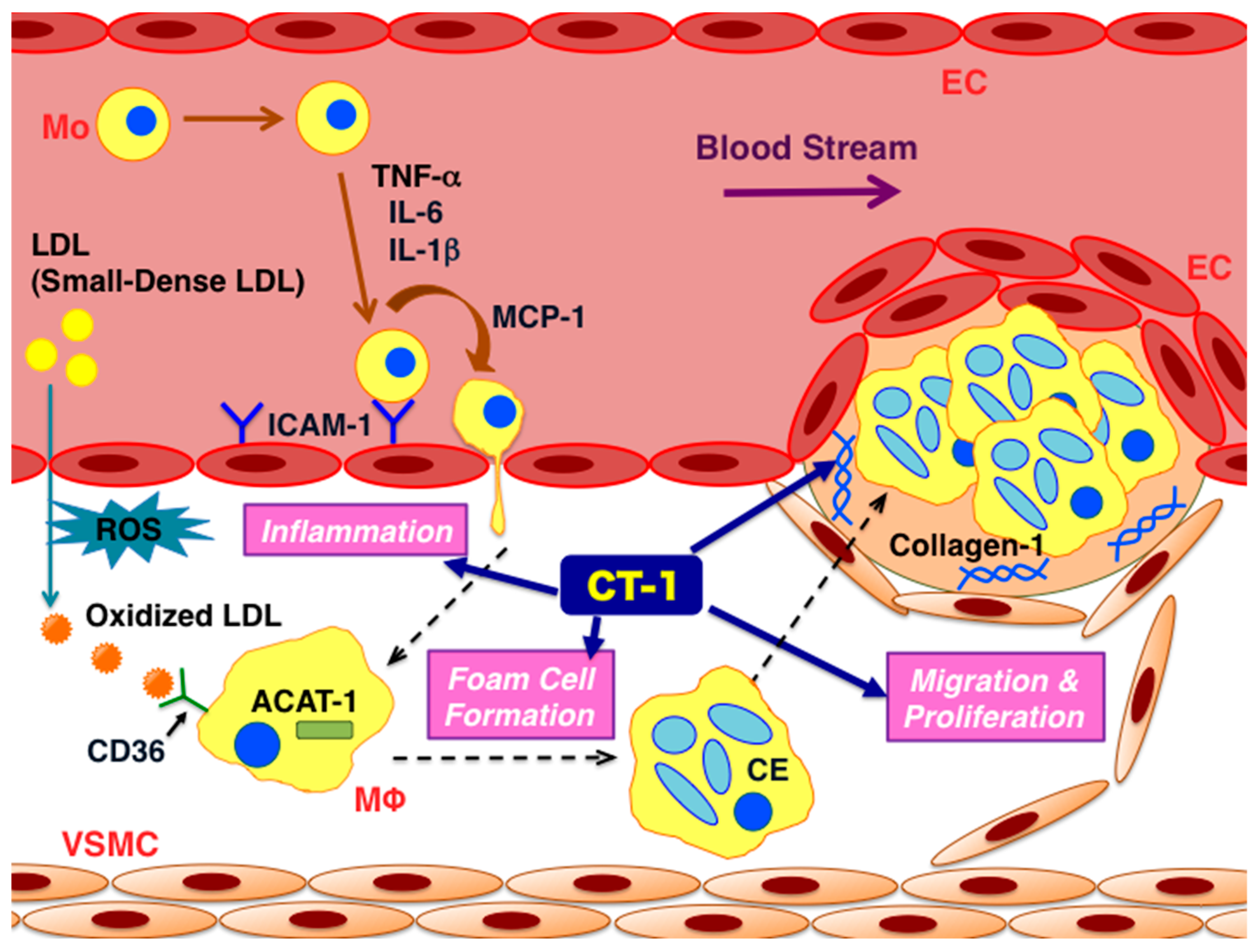
6. Atheroprone Effects of CT-1
7. Plasma/Tissue Levels of CT-1 in Atherosclerotic Cardiovascular Disease
8. Pharmacological Intervention Against CT-1
9. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Watanabe, T.; Kanome, T.; Miyazaki, A. Relationship between hypertension and atherosclerosis: From a viewpoint of the most potent vasoconstrictor human urotensin II. Curr. Hypertens. Rev. 2006, 2, 237–246. [Google Scholar] [CrossRef]
- Dzau, V.J. Atherosclerosis and hypertension: Mechanisms and interrelationships. J. Cardiovasc. Pharmacol. 1990, 15, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The role of oxidized low-density lipoproteins in atherosclerosis: The myths and the facts. Mediat. Inflamm 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sato, K.; Itoh, F.; Noguchi, Y.; Fujimoto, K.; Koyama, T.; Shichiri, M. Emerging roles for vasoactive peptides in diagnostic and therapeutic strategies against atherosclerotic cardiovascular diseases. Curr. Protein Pept. Sci. 2013, 14, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Pakala, R.; Katagiri, T.; Benedict, CR. Angiotensin II and serotonin potentiate endothelin-1-induced vascular smooth muscle cell proliferation. J. Hypertens. 2001, 19, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Arita, S.; Shiraishi, Y.; Suguro, T.; Sakai, T.; Hongo, S.; Miyazaki, A. Human urotensin II promotes hypertension and atherosclerotic cardiovascular diseases. Curr. Med. Chem. 2009, 16, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shirai, R.; Hontani, M.; Shinooka, R.; Hasegawa, A.; Kichise, T.; Yamashita, T.; Yoshizawa, H.; Watanabe, R.; Matsuyama, T.; et al. Potent vasoconstrictor kisspeptin-10 induces atherosclerotic plaque progression and instability: Reversal by its receptor GPR54 antagonist. J. Am. Heart Assoc. 2017, 6, e005790. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Koba, S. Roles of serotonin in atherothrombosis and related diseases. In Traditional and Novel Risk Factors in Atherothrombosis; Efrain Gaxiola, Ed.; INTECH: Rijeka, Croatia, 2012; pp. 57–70. [Google Scholar]
- Watanabe, T.; Sato, K.; Itoh, F.; Iso, Y.; Nagashima, M.; Hirano, T.; Shichiri, M. The roles of salusins in atherosclerosis and related cardiovascular diseases. J. Am. Soc. Hypertens. 2011, 5, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Watanabe, T.; Iso, Y.; Koba, S.; Sakai, T.; Nagashima, M.; Arita, S.; Hongo, S.; Ota, H.; Kobayashi, Y.; et al. Preventive effects of heregulin-β1 on macrophage foam cell formation and atherosclerosis. Circ. Res. 2009, 105, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Sato, K.; Shirai, R.; Watanabe, R.; Yamamoto, K.; Watanabe, K.; Nohtomi, K.; Hirano, T.; Watanabe, T. Vasoprotective effects of urocortin 1 against atherosclerosis in vitro and in vivo. PLoS ONE 2014, 9, e110866. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Ozawa, N.; Mori, Y.; Takahashi, Y.; Watanabe-Kominato, K.; Shirai, R.; Watanabe, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; et al. Catestatin prevents macrophage-driven atherosclerosis but not arterial injury-induced neointimal hyperplasia. Thromb. Haemost. 2018, 118, 182–194. [Google Scholar] [CrossRef] [PubMed]
- González, A.; López, B.; Ravassa, S.; Beaumont, J.; Zudaire, A.; Gallego, I.; Brugnolaro, C.; Díez, J. Cardiotrophin-1 in hypertensive heart disease. Endocrine 2012, 42, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Konii, H.; Sato, K.; Kikuchi, S.; Okiyama, H.; Watanabe, R.; Hasegawa, A.; Yamamoto, K.; Itoh, F.; Hirano, T.; Watanabe, T. Stimulatory effects of cardiotrophin 1 on atherosclerosis. Hypertension 2013, 62, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Squire, I.B.; Downie, P.F.; Davies, J.E.; Ng, L.L. Plasma N terminal pro-brain natriuretic peptide and cardiotrophin 1 are raised in unstable angina. Heart 2000, 84, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; King, K.L.; Shaw, K.J.; Luis, E.; Rullamas, J.; Luoh, S.M.; Darbonne, W.C.; Knutzon, D.S.; Yen, R.; Chien, K.R.; et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc. Natl. Acad. Sci. USA 1995, 92, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Swanson, T.A.; Shaw, K.J.; Kuang, W.J.; Gray, C.L.; Beatty, B.G.; Wood, W.I. Human cardiotrophin-1: Protein and gene structure, biological and binding activities, and chromosomal localization. Cytokine 1996, 8, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Saito, Y.; Miyamoto, Y.; Kuwahara, K.; Ogawa, E.; Nakagawa, O.; Harada, M.; Masuda, I.; Nakao, K. cDNA cloning of rat cardiotrophin-1 (CT-1): Augmented expression of ct-1 gene in ventricle of genetically hypertensive rats. Biochem. Biophys. Res. Commun. 1996, 219, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Latchman, D.S. Cardiotrophin-1: A novel cytokine and its effects in the heart and other tissues. Pharmacol. Ther. 2000, 85, 29–37. [Google Scholar] [CrossRef]
- Jougasaki, M. Cardiotrophin-1 in cardiovascular regulation. Adv. Clin. Chem. 2010, 52, 41–76. [Google Scholar] [PubMed]
- Stejskal, D.; Ruzicka, V. Cardiotrophin-1 review. Biomed. Pap Med. Fac. Univ. Palacky Olomuc Czechsolv. Repub. 2008, 152, 9–19. [Google Scholar] [CrossRef]
- Natal, C.; Fortuño, M.A.; Restituto, P.; Bazán, A.; Colina, I.; Díez, J.; Varo, N. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E52–E60. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Pennica, D.; Wood, W.I.; Chien, K.R. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development 1996, 122, 419–428. [Google Scholar] [PubMed]
- Tsuruda, T.; Jougasaki, M.; Boerrigter, G.; Huntley, B.K.; Chen, H.H.; D’Assoro, A.B.; Lee, S.C.; Larsen, A.M.; Cataliotti, A.; Burnett, J.C., Jr. Cardiotrophin-1 stimulation of cardiac fibroblast growth: Roles for glycoprotein 130/leukemia inhibitory factor receptor and the endothelin type A receptor. Circ. Res. 2002, 90, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Jougasaki, M.; Setoguchi, M.; Imamura, J.; Nakashima, H.; Matsuoka, T.; Sonoda, M.; Nakamura, K.; Minagoe, S.; Tei, C. Cardiotrophin-1 stimulates intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H750–H763. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, N.; Fortuño, M.A.; Diez, J.; Zannad, F.; Lacolley, P.; Rossignol, P. Vascular effects of cardiotrophin-1: A role in hypertension? J. Hypertens. 2010, 28, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, C.J.; Raudsepp, S.D.; Yandle, T.G.; Cameron, V.A.; Richards, A.M. Plasma cardiotrophin-1 is elevated in human hypertension and stimulated by ventricular stretch. Cardiovasc. Res. 2005, 68, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, S.; Funamoto, M.; Fujio, Y.; Kunisada, K.; Yamauchi-Takihara, K. Hypoxic stress induces cardiotrophin-1 expression in cardiac myocytes. Biochem. Biophys. Res. Commun. 1999, 264, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, J.; Booz, G.W.; Hunt, R.A.; Shimizu, N.; Karoor, V.; Baker, K.M.; Dostal, D.E. Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3: An autocrine loop for hypertrophy. Hypertension 2000, 35, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, N.; Iñigo, C.; Gallego, J.; Díez, J.; Fortuño, M.A. Aldosterone induces cardiotrophin-1 expression in HL-1 adult cardiomyocytes. Endocrinology 2008, 149, 4970–4978. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, M.; Hishinuma, S.; Fujio, Y.; Matsuda, Y.; Kunisada, K.; Oh, H.; Negoro, S.; Tone, E.; Kishimoto, T.; Yamauchi-Takihara, K. Isolation and characterization of the murine cardiotrophin-1 gene: Expression and norepinephrine-induced transcriptional activation. J. Mol. Cell. Cardiol. 2000, 32, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Janjua, S.; Lawrence, K.M.; Ng, L.L.; Latchman, D.S. The cardioprotective agent urocortin induces expression of CT-1. Cardiovasc. Toxicol. 2003, 3, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.S.; Jeyaraman, M.; Wen, G.B.; Fandrich, R.R.; Dixon, I.M.; Cattini, P.A.; Kardami, E. High-but not low-molecular weight FGF-2 causes cardiac hypertrophy in vivo; possible involvement of cardiotrophin-1. J. Mol. Cell. Cardiol. 2007, 42, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Park, J.H.; Lee, S.; Lim, H.J.; Choi, H.E.; Park, H.Y. HB-EGF induces delayed STAT3 activation via NF-kappaB mediated IL-6 secretion in vascular smooth muscle cell. Biochim. Biophys. Acta 2007, 1773, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Huang, F.; Xing, Z.; Wang, H.; Li, Z. Pioglitazone inhibits hypertrophy induced by high glucose and insulin in cultured neonatal rat cardiomyocytes. Pharmazie 2007, 62, 925–929. [Google Scholar] [PubMed]
- Ateghang, B.; Wartenberg, M.; Gassmann, M.; Sauer, H. Regulation of cardiotrophin-1 expression in mouse embryonic stem cells by HIF-1α and intracellular reactive oxygen species. J. Cell Sci. 2006, 119, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Saito, Y.; Kuwahara, K.; Mizuno, Y.; Yoshimura, M.; Higashikubo, C.; Tsuji, T.; Kishimoto, I.; Harada, M.; Hamanaka, I.; et al. The heart is a source of circulating cardiotrophin-1 in humans. Biochem. Biophys. Res. Commun. 2000, 279, 320–323. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Ravassa, S.; Loperena, I.; López, B.; Beaumont, J.; Querejeta, R.; Larman, M.; Díez, J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J. Hypertens. 2007, 25, 2148–2157. [Google Scholar]
- Limongelli, G.; Calabrò, P.; Maddaloni, V.; Russo, A.; Masarone, D.; D’Aponte, A.; Roselli, T.; Bonauro, R.; D’Alessandro, R.; D’Andrea, A.; et al. Cardiotrophin-1 and TNF-α circulating levels at rest and during cardiopulmonary exercise test in athletes and healthy individuals. Cytokine 2010, 50, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Chien, K.R. Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. J. Mol. Med. 1997, 75, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.; Limongelli, G.; Riegler, L.; Maddaloni, V.; Palmieri, R.; Golia, E.; Roselli, T.; Masarone, D.; Pacileo, G.; Golino, P.; et al. Novel insights into the role of cardiotrophin-1 in cardiovascular diseases. J. Mol. Cell. Cardiol. 2009, 46, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, I.; Saito, Y.; Nishikimi, T.; Magaribuchi, T.; Kamitani, S.; Kuwahara, K.; Ishikawa, M.; Miyamoto, Y.; Harada, M.; Ogawa, E.; et al. Effects of cardiotrophin-1 on hemodynamics and endocrine function of the heart. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H388–H396. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, R.; Keller, G.A.; Ryan, A.; Ko, A.; Finkle, D.; Swanson, T.A.; Li, W.; Pennica, D.; Wood, W.I.; et al. In vivo effects of cardiotrophin-1. Cytokine 1996, 8, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, D.; Sow, C.Y.; Bai, T.R. Cardiotrophin-1 alters airway smooth muscle structure and mechanical properties in airway explants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1165–L1171. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aliaga, M.J.; Pérez-Echarri, N.; Marcos-Gómez, B.; Larequi, E.; Gil-Bea, F.J.; Viollet, B.; Gimenez, I.; Martínez, J.A.; Prieto, J.; Bustos, M. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. 2011, 14, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Shaw, K.J.; Swanson, T.A.; Moore, M.W.; Shelton, D.L.; Zioncheck, K.A.; Rosenthal, A.; Taga, T.; Paoni, N.F.; Wood, W.I. Cardiotrophin-1: Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signalling complex. J. Biol. Chem. 1995, 270, 10915–10922. [Google Scholar] [CrossRef] [PubMed]
- Robledo, O.; Fourcin, M.; Chevalier, S.; Guillet, C.; Auguste, P.; Pouplard-Barthelaix, A.; Pennica, D.; Gascan, H. Signaling of the cardiotrophin-1 receptor. Evidence for a third receptor component. J. Biol. Chem. 1997, 272, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Escoté, X.; Gómez-Zorita, S.; López-Yoldi, M.; Milton-Laskibar, I.; Fernández-Quintela, A.; Martínez, L.A.; Moreno-Aliaga, M.J.; Portillo, M.P. Role of omentin, vaspin, cardiotrophin-1, TWEAK and NOV/CCN3 in obesity and diabetes development. Int. J. Mol. Sci. 2017, 18, 1770. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Gavriilaki, E.; Chatzopoulou, F.; Anyfanti, P.; Triantafyllou, A.; Petidis, K.; Zamboulis, C.; Douma, S. Association between cardiotrophin 1 levels and central blood pressure in untreated patients with essential hypertension. Am. J. Hypertens. 2014, 27, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wang, S.; Huang, B.; Luciano, A.; Srivastava, R.; Mani, A. Plasma cardiotrophin-1 levels are associated with hypertensive heart disease: A meta-analysis. J. Clin. Hypertens. 2014, 16, 686–692. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Castellano, J.M.; González, A.; Barba, J.; Díez, J. Association of increased plasma cardiotrophin-1 with inappropriate left ventricular mass in essential hypertension. Hypertension 2007, 50, 977–983. [Google Scholar] [CrossRef] [PubMed]
- López, B.; González, A.; Querejeta, R.; Barba, J.; Díez, J. Association of plasma cardiotrophin-1 with stage C heart failure in hypertensive patients: Potential diagnostic implications. J. Hypertens. 2009, 27, 418–424. [Google Scholar] [CrossRef] [PubMed]
- López, B.; González, A.; Querejeta, R.; Larman, M.; Rábago, G.; Díez, J. Association of cardiotrophin-1 with myocardial fibrosis in hypertensive patients with heart failure. Hypertension 2014, 63, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Zvonic, S.; Hogan, J.C.; Arbour-Reily, P.; Mynatt, R.L.; Stephens, J.M. Effects of cardiotrophin on adipocytes. J. Biol. Chem. 2004, 279, 47572–47579. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Mach, F.; Quercioli, A.; Dallegri, F.; Montecucco, F. Update on the pathophysiological activities of the cardiac molecule cardiotrophin-1 in obesity. Mediat. Inflamm. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- López-Yoldi, M.; Stanhope, K.L.; Garaulet, M.; Chen, X.G.; Marcos-Gómez, B.; Carrasco-Benso, M.P.; Santa Maria, E.M.; Escoté, X.; Lee, V.; Nunez, M.V.; et al. Role of cardiotrophin-1 in the regulation of metabolic circadian rhythms and adipose core clock genes in mice and characterization of 24-h circulating CT-1 profiles in normal-weight and overweight/obese subjects. FASEB J. 2017, 31, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Anik Ilhan, G.; Kanlioglu, C.; Arslan, G.; Yildizhan, B.; Pekin, T. Cardiotrophin-1 as a new metabolic biomarker in women with PCOS. Gynecol. Endocrinol. 2018, 30, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Lu, F.H.; Ou, H.Y.; Wu, H.T.; Wu, J.S.; Yang, Y.C.; Chang, C.J. Increased cardiotrophin-1 in subjects with impaired glucose tolerance and newly diagnosed diabetes. Int. J. Cardiol. 2013, 169, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, L.; Martínez-Salgado, C. Plasma cardiotrophin-1 as a marker of hypertension and diabetes-induced target organ damage and cardiovascular risk. Medicine 2015, 94, e1218. [Google Scholar]
- Lutz, S.Z.; Franck, O.; Böhm, A.; Machann, J.; Schick, F.; Machicao, F.; Fritsche, A.; Häring, H.; Staiger, H. Common genetic variation in the human CTF1 locus, encoding cardiotrophin-1, determines insulin sensitivity. PLoS ONE 2014, 9, e100391. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Downie, P.F.; Squire, I.B.; Davies, J.E.; Barnett, D.B.; Ng, L.L. Plasma N-terminal pro BNP and cardiotrophin-1 are elevated in aortic stenosis. Eur. J. Heart Fail. 2001, 3, 15–19. [Google Scholar] [CrossRef]
- Monserrat, L.; López, B.; González, A.; Hermida, M.; Fernández, X.; Ortiz, M.; Barriales-Villa, R.; Castro-Beiras, A.; Díez, J. Cardiotrophin-1 plasma levels are associated with the severity of hypertrophy in hypertrophic cardiomyopathy. Eur. Heart J. 2011, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Z.; Fu, X.T.; Liang, J.; Guo, Z.B. CT-1 induces angiogenesis by regulating the ADMA/DDAH pathway. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czechoslov. Repub. 2015, 159, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Fritzenwanger, M.; Meusel, K.; Foerster, M.; Kuethe, F.; Krack, A.; Figulla, H.R. Cardiotrophin-1 induces interleukin-6 synthesis in human umbilical vein endothelial cells. Cytokine 2006, 36, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Fritzenwanger, M.; Foerster, M.; Meusel, K.; Jung, C.; Figulla, H.R. Cardiotrophin-1 induces intercellular adhesion molecule-1 expression by nuclear factor κB activation in human umbilical vein endothelial cells. Chin. Med. J. 2008, 121, 2592–2598. [Google Scholar] [PubMed]
- Tokito, A.; Jougasaki, M.; Ichiki, T.; Hamasaki, S. Cardiotrophin-1 induces matrix metalloproteinase-1 in human aortic endothelial cells. PLoS ONE 2013, 8, e68801. [Google Scholar]
- Fritzenwanger, M.; Meusel, K.; Foerster, M.; Kuethe, F.; Krack, A.; Figulla, H.R. Cardiotrophin-1 induces interleukin-6 synthesis in human monocytes. Cytokine 2007, 38, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Fritzenwanger, M.; Jung, C.; Franz, M.; Foerster, M.; Figulla, H.R. Immunomodulatory effects of cardiotrophin-1 on in vitro cytokine production of monocytes & CD4 + T-lymphocytes. Indian J. Med. Res. 2012, 136, 471–476. [Google Scholar] [PubMed]
- Fritzenwanger, M.; Meusel, K.; Jung, C.; Franz, M.; Wang, Z.; Foerster, M.; Figulla, H.R. Cardiotrophin-1 induces tumor necrosis factor α synthesis in human peripheral blood mononuclear cells. Mediat. Inflamm. 2009, 2009, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.U.; San José, G.; Pejenaute, Á.; Landecho, M.F.; Díez, J.; Beloqui, Ó.; Fortuño, A.; Zalba, G. Association of phagocytic NADPH oxidase activity with hypertensive heart disease: A role for cardiotrophin-1? Hypertension 2014, 63, 468–474. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, N.; Calvier, L.; Labat, C.; Fay, R.; Díez, J.; Benetos, A.; Zannad, F.; Lacolley, P.; Rossignol, P. Absence of cardiotrophin 1 is associated with decreased age-dependent arterial stiffness and increased longevity in mice. Hypertension 2013, 61, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Demyanets, S.; Kaun, C.; Rychli, K.; Rega, G.; Pfaffenberger, S.; Afonyushkin, T.; Bochkov, V.N.; Maurer, G.; Huber, K.; Wojta, J. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI3-kinase and ERK1/2-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1962–H1968. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, N.; Rousseau, A.; Akhtar, R.; Calvier, L.; Iñigo, C.; Labat, C.; Zhao, X.; Cruickshank, K.; Díez, J.; Zannad, F.; et al. Cardiotrophin 1 is involved in cardiac, vascular, and renal fibrosis and dysfunction. Hypertension 2012, 60, 563–573. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, N.; Martin-Fernandez, B.; Rossignol, P.; Zannad, F.; Lahera, V.; Fortuño, M.A.; Cachofeiro, V.; Díez, J. A role for cardiotrophin-1 in myocardial remodeling induced by aldosterone. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2372–H2382. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Squire, I.B.; O’brien, R.J.; Downie, P.F.; Davies, J.E.; Ng, L.L. Plasma cardiotrophin-1 following acute myocardial infarction: Relationship with left ventricular systolic dysfunction. Clin. Sci. 2002, 102, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Squire, I.B.; Downie, P.F.; O’Brien, R.J.; Davies, J.E.; Ng, L.L. Elevated circulating cardiotrophin-1 in heart failure: Relationship with parameters of left ventricular systolic dysfunction. Clin. Sci. 2000, 99, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Sahin, S.; Koc, F.; Karayakali, M.; Sahin, M.; Benli, I.; Kadi, H.; Burucu, T.; Ceyhan, K.; Erkorkmaz, U. Cardiotrophin-1 plasma levels are increased in patients with diastolic heart failure. Med. Sci. Monit. 2012, 18, CR25–CR31. [Google Scholar] [CrossRef] [PubMed]
- Cottone, S.; Nardi, E.; Mulè, G.; Vadalà, A.; Lorito, M.C.; Riccobene, R.; Palermo, A.; Arsena, R.; Guarneri, M.; Cerasola, G. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin. Nephrol. 2007, 67, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Lu, F.H.; Ou, H.Y.; Wu, H.T.; Wu, J.S.; Yang, Y.C.; Chang, C.J. Cardiotrophin-1 is associated with increased risk of arterial stiffness. Biomark. Med. 2015, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bielecka-Dabrowa, A.; Gluba-Brzózka, A.; Michalska-Kasiczak, M.; Misztal, M.; Rysz, J.; Banach, M. The multi-biomarker approach for heart failure in patients with hypertension. Int. J. Mol. Sci. 2015, 16, 10715–10733. [Google Scholar] [CrossRef] [PubMed]
- Tsutamoto, T.; Asai, S.; Tanaka, T.; Sakai, H.; Nishiyama, K.; Fujii, M.; Yamamoto, T.; Ohnishi, M.; Wada, A.; Saito, Y.; et al. Plasma level of cardiotrophin-1 as a prognostic predictor in patients with chronic heart failure. Eur. J. Heart Fail. 2007, 9, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Heying, R.; Qing, M.; Schumacher, K.; Sokalska-Duhme, M.; Vazquez-Jimenez, J.F.; Seghaye, M.C. Myocardial cardiotrophin-1 is differentially induced in congenital cardiac defects depending on hypoxemia. Future Cardiol. 2014, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Freed, D.H.; Moon, M.C.; Borowiec, A.M.; Jones, S.C.; Zahradka, P.; Dixon, I.M.C. Cardiotrophin-1: Expression in experimental myocardial infarction and potential role in post-MI wound healing. Mol. Cell. Biochem. 2003, 254, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Freed, D.H.; Cunnington, R.H.; Dangerfield, A.L.; Sutton, J.S.; Dixon, I.M.C. Emerging evidence for the role of cardiotrophin-1 in cardiac repair in the infarcted heart. Cardiovasc. Res. 2005, 65, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Suen, C.; Jiang, B.; Deng, Y.; Weldrick, J.J.; Putinski, C.; Brunette, S.; Fernando, P.; Lee, T.T.; Flynn, P.; et al. Cardiotrophin 1 stimulates beneficial myogenic and vascular remodeling of the heart. Cell Res. 2017, 27, 1195–1215. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ruan, X.; Laurikka, J.; Laine, S.; Tarkka, M.; Wei, M. The human heart releases cardiotrophin-1 after coronary artery bypass grafting with cardiopulmonary bypass. Scand. Cardiovasc. J. 2011, 45, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Q.; Kelly, D.; Quinn, P.; Davies, J.E.; Ng, L.L. Cardiotrophin-1 predicts death or heart failure following acute myocardial infarction. J. Card. Fail. 2006, 12, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Morell-Azanza, L.; Rendo-Urteaga, T.; García-Calzón, S.; Ojeda-Rodríguez, A.; Martín-Calvo, N.; Moreno-Aliaga, M.J.; Martínez, J.A.; Azcona-San Julián, M.C. Serum and gene expression levels of CT-1, IL-6, and TNF-α after a lifestyle intervention in obese children. Pediatr. Diabetes 2018, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Qin, X.; Ren, T.; Cao, J. Baicalin reduces blood lipids and inflammation in patients with coronary artery disease and rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Peng, R.; Zhao, W.; Liu, Q.; Guo, Y.; Zhao, S.; Xu, D. Zhibital and low-dose atorvastatin reduce blood lipids and inflammation in patients with coronary artery disease. Medicine 2017, 96, 7. [Google Scholar]
- Wu, L.; Zhao, L.; Zheng, Q.; Shang, F.; Wang, X.; Wang, L.; Lang, B. Simvastatin attenuates hypertrophic responses induced by cardiotrophin-1 via JAK-STAT pathway in cultured cardiomyocytes. Mol. Cell. Biochem. 2006, 284, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, Q.; Wu, Y. Simvastatin prevents cardiac hypertrophy in vitro and in vivo via JAK-STAT pathway. Life Sci. 2008, 82, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Xu, X.; Wang, H.; Tran, H.; Taylor-Harding, B.; Li, A.; Karlan, B.Y. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol. Oncol. 2010, 116, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, Q.; Na, R.; Liu, B. Etanercept protects rat cardiomyocytes against hypertrophy by regulating inflammatory cytokines secretion and cell apoptosis. Braz. J. Med. Biol. Res. 2017, 50, e5868. [Google Scholar] [CrossRef] [PubMed]
| Disease | Control | p Value | Reference | |
|---|---|---|---|---|
| Hypertension (untreated) | 606 ± 18 ↑ | 546 ± 12 | <0.01 | [28] |
| Hypertension (treated) | 618 ± 10 ↑ | 546 ± 12 | <0.01 | [28] |
| Hypertension | 37.9 ± 2.0 ↑ | 17.9 ± 2.7 | >0.05 | [53] |
| Hypertension + LVH | 75.3 ± 7.3 ↑ | 17.9 ± 2.7 | <0.001 | [53] |
| Hypertension + heart failure | 116.6 ± 13.2 ↑ | 17.9 ± 2.7 | <0.001 | [53] |
| Metabolic syndrome | 5644 ± 356 ↑ | 4711 ± 178 | <0.05 | [23] |
| Obesity | 5467 ± 400 ↑ | 4889 ± 222 | <0.05 | [23] |
| Diabetes | 46.7 ± 7.7 ↑ | 21.1 ± 3.7 | <0.005 | [59] |
| Impaired glucose tolerance | 35.5 ± 5.4 ↑ | 21.1 ± 3.7 | <0.05 | [59] |
| Diabetes | 26.1 (10.9–86.0) ↑ | 14.2 (8.8–29.8) | <0.05 | [60] |
| Stable angina pectoris | 73.2 (41.5–102.1) ↑ | 27.0 (6.9–54.1) | <0.01 | [16] |
| Unstable angina pectoris | 142.5 (42.2–527.4) ↑ | 27.0 (6.9–54.1) | <0.01 | [16] |
| Aortic Stenosis | 57.3 (33.0–86.3) ↑ | 28.3 (6.9–48.3) | <0.0005 | [62] |
| Hypertrophic cardiomyopathy | 136.3 ± 72.0 ↑ | 17.9 ± 12.0 | <0.001 | [63] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, T.; Konii, H.; Sato, K. Emerging Roles of Cardiotrophin-1 in the Pathogenesis and Biomarker of Atherosclerosis. J 2018, 1, 94-105. https://doi.org/10.3390/j1010010
Watanabe T, Konii H, Sato K. Emerging Roles of Cardiotrophin-1 in the Pathogenesis and Biomarker of Atherosclerosis. J. 2018; 1(1):94-105. https://doi.org/10.3390/j1010010
Chicago/Turabian StyleWatanabe, Takuya, Hanae Konii, and Kengo Sato. 2018. "Emerging Roles of Cardiotrophin-1 in the Pathogenesis and Biomarker of Atherosclerosis" J 1, no. 1: 94-105. https://doi.org/10.3390/j1010010
APA StyleWatanabe, T., Konii, H., & Sato, K. (2018). Emerging Roles of Cardiotrophin-1 in the Pathogenesis and Biomarker of Atherosclerosis. J, 1(1), 94-105. https://doi.org/10.3390/j1010010