From Waste to Resource: Algal–Bacterial Systems and Immobilization Techniques in Aquaculture Effluent Treatment
Abstract
1. Introduction and Rationale for ABSS in Aquaculture Effluent Treatment
1.1. Global Challenge of Aquaculture Effluents
1.2. Pollutant Composition and Ecological Risks
1.2.1. Nutrient Overloading and Its Ecological Consequences
1.2.2. Organic Matter Accumulation and Oxygen Depletion
1.2.3. Suspended Solids and Particle-Induced Turbidity
1.2.4. Antibiotics and Antibiotic Resistance Genes (ARGs)
1.2.5. Heavy Metals and Co-Selection of Antibiotic Resistance
1.3. Conventional Treatment Technologies: Performance and Limitations
1.3.1. Mechanical and Physical Filtration Techniques for Aquaculture Effluents
1.3.2. Chemical Coagulation in Aquaculture
1.3.3. Traditional Biological Treatments
1.4. Rationale for ABSS and Immobilization
2. Algae–Bacteria Consortia: A Synergistic Platform for Integrated Remediation and Resource Recovery
2.1. Microalgae-Based Aquaculture Wastewater Treatment and Species Performance
2.2. Bacterial Taxa in Algae–Bacteria Symbiotic System
2.3. Environmental Drivers Shaping Microbial Interactions and Treatment Efficiency
2.3.1. Algae to Bacterial Ratio
2.3.2. Light Regulation
2.3.3. Temperature and pH
2.3.4. External Carbon Source and Carbon-to-Nitrogen Ratio
2.3.5. Aeration Rate and DO
2.4. Microbial Synergy and Pollutant Removal Mechanisms in ABSS
2.4.1. Microbial Interactions Underpinning Metabolic Complementarity and Ecological Stability in ABSS
2.4.2. Synergistic Degradation Mechanism for Integrated Multi-Pollutant Purification
2.4.3. Molecular Mechanisms Underlying Efficient Nitrogen and Phosphorus Removal
3. Application of Algal–Bacterial Immobilization Technology
3.1. Principles of Immobilization Technology
3.2. Carrier Types and Approaches for Algal-Bacterial Immobilization Technologies
3.2.1. Inorganic Carrier
3.2.2. Organic Carriers
3.3. Immobilized ABSS: Advances and Justifications
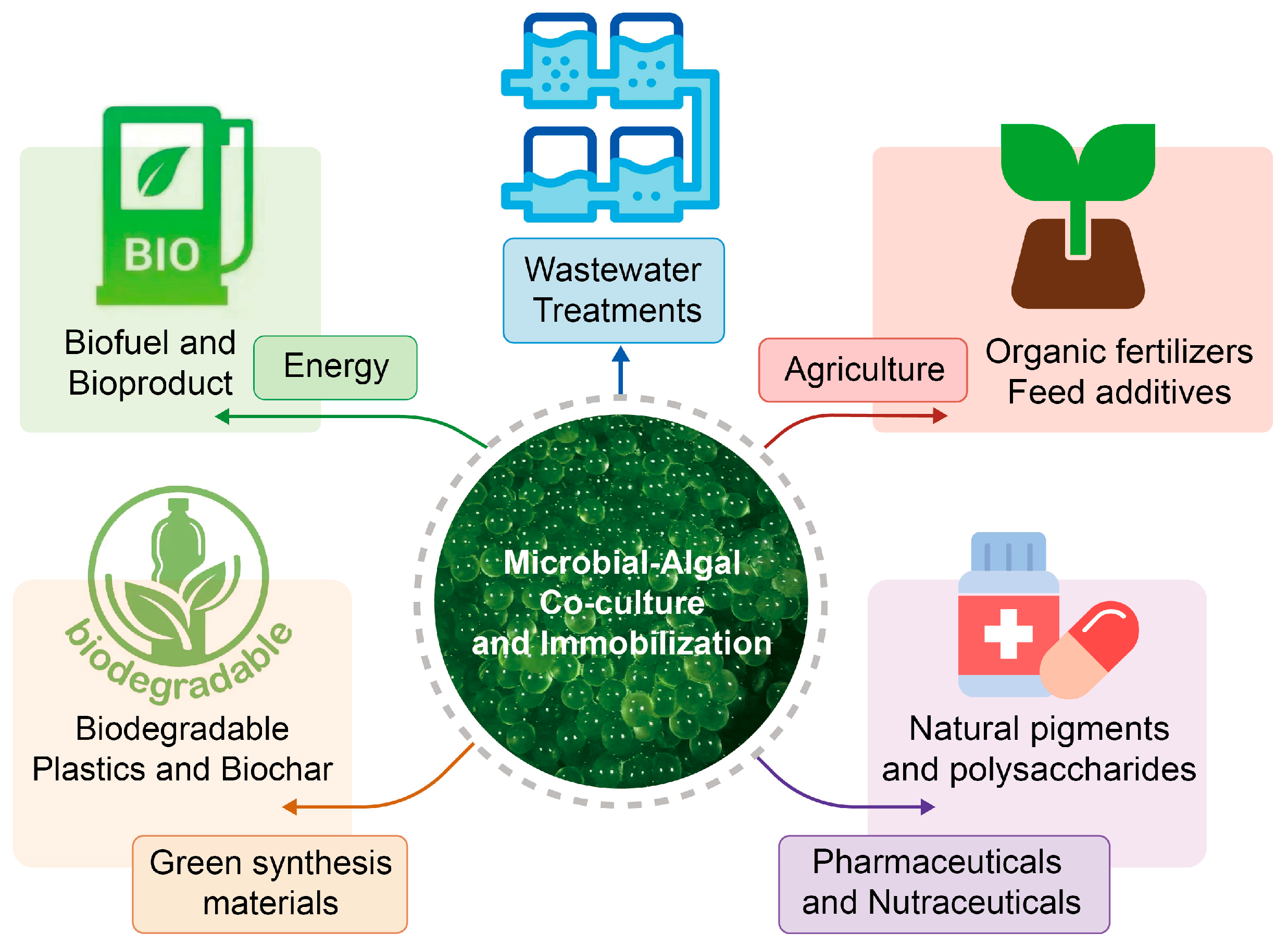
3.4. ABSS Enhanced by Immobilization: A Promising Strategy for Biomass Resource Valorization
3.5. Biomass Valorization and Circular Applications
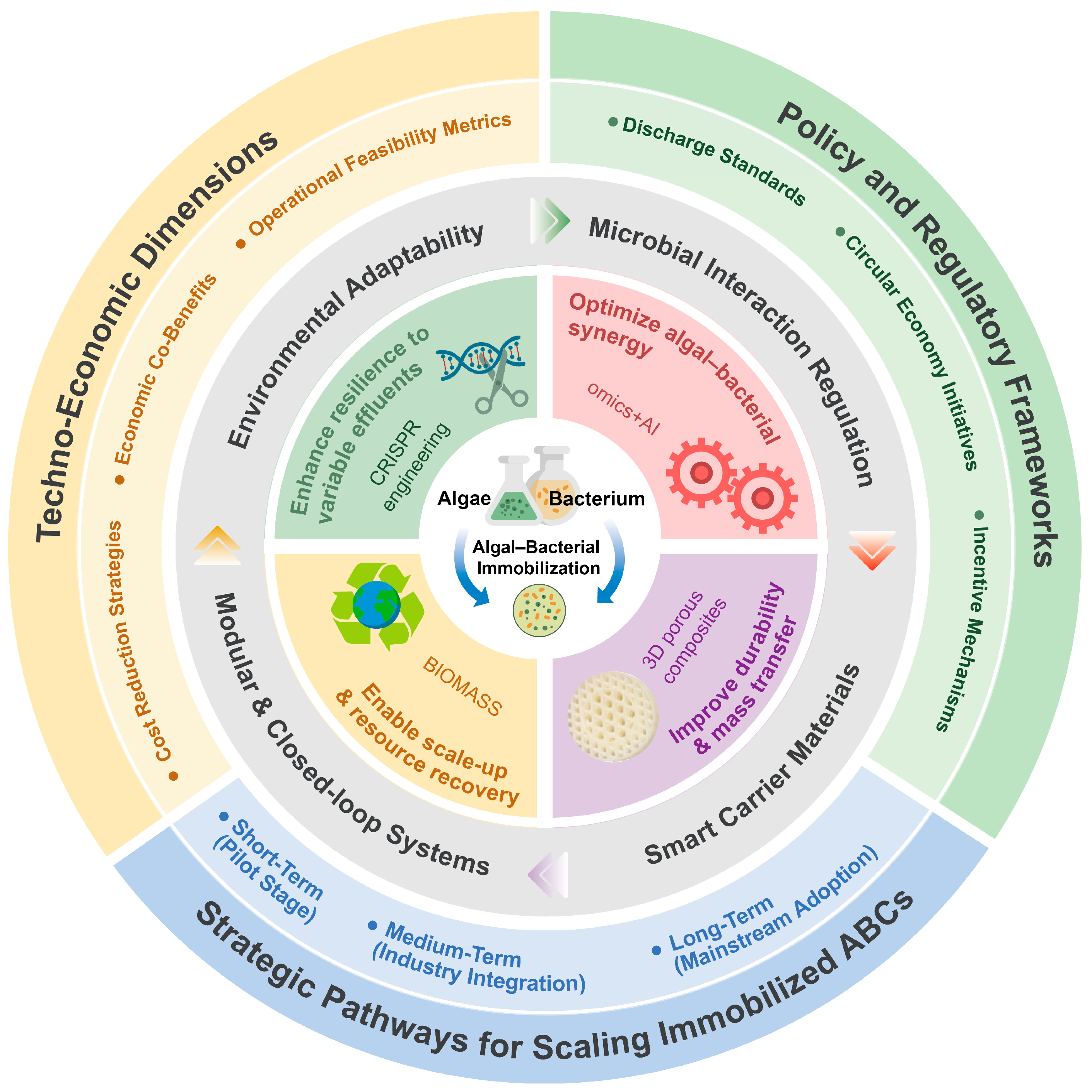
4. Key Findings and Critical Discussion
4.1. Aquaculture Effluents: Distinct Composition and Ecological Implications
4.2. Principles for Designing Effective ABSS
4.3. Why Immobilization? Mechanistic and Kinetic Advantages
4.4. Techno-Economic and Regulatory Dimensions
4.5. Critical Insights
5. Overcoming Limitations and Future Directions
5.1. Scale-Up and Reactor Design
5.2. Environmental Fluctuations
5.3. Carrier Durability and Fouling
5.4. Mass Transfer Limitations and Metabolic Imbalance
5.5. Biomass Recovery and Risk of Secondary Pollution
5.6. Economic and Policy Considerations
5.7. Integrated Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABSS | Algal–bacterial symbiotic systems |
| N | Nitrogen |
| P | Phosphorus |
| TN | Total Nitrogen |
| TP | Total Phosphorus |
| NH4+-N | ammonium |
| NO2−-N | Nitrite nitrogen |
| NO3−-N | Nitrate nitrogen |
| PO43− | Orthophosphate |
| COD | Chemical oxygen demand |
| DOC | Dissolved organic carbon |
| DO | Dissolved oxygen |
| ARGs | Antibiotic resistance genes |
| RAS | Recirculating aquaculture systems |
| AOB | ammonia -oxidizing bacteria |
| NOB | Nitrite-oxidizing bacteria |
| ADPA | Aerobic denitrifying and phosphorus-accumulating bacteria |
| HNADPR | Heterotrophic nitrification, aerobic denitrification, and aerobic phosphate removal |
| C/N | Carbon-to-nitrogen |
| EPS | Extracellular polymeric substances |
| SA | Sodium alginate |
| PAM | Polyacrylamide |
References
- Albrektsen, S.; Kortet, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.; Hansen, J.Ø.; Lock, E.; Mørkøre, T.; et al. Future Feed Resources in Sustainable Salmonid Production: A Review. Rev. Aquac. 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-138763-4. [Google Scholar]
- Tacon, A.G.J.; Shumway, S.E. Critical Need to Increase Aquatic Food Production and Food Supply from Aquaculture and Capture Fisheries: Trends and Outlook. Rev. Fish. Sci. Aquac. 2024, 32, 389–395. [Google Scholar] [CrossRef]
- Bjørndal, T.; Dey, M.; Tusvik, A. Economic Analysis of the Contributions of Aquaculture to Future Food Security. Aquaculture 2024, 578, 740071. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Sutcliffe, M.; Suleria, H.A.R.; Giri, S.S. Global Issues in Aquaculture. Anim. Front. 2024, 14, 3–5. [Google Scholar] [CrossRef]
- Verdegem, M.; Buschmann, A.H.; Latt, U.W.; Dalsgaard, A.J.T.; Lovatelli, A. The Contribution of Aquaculture Systems to Global Aquaculture Production. J. World Aquac. Soc. 2023, 54, 206–250. [Google Scholar] [CrossRef]
- Mavraganis, T.; Constantina, C.; Kolygas, M.; Vidalis, K.; Nathanailides, C. Environmental Issues of Aquaculture Development. Egypt. J. Aquat. Biol. Fish. 2020, 24, 441–450. [Google Scholar] [CrossRef]
- Zhang, B.; Bian, Y.; Chen, J.; Zhang, Z.; Sun, S.; Yang, F.; Lei, Z.; Huang, W. Nitrogen Reclamation from Aquaculture Wastewater as Potential Fish Feed Additives via Bacterial and Algal-Bacterial Granular Sludge Systems. Bioresour. Technol. Rep. 2023, 24, 101609. [Google Scholar] [CrossRef]
- Luo, G. Review of Waste Phosphorus from Aquaculture: Source, Removal and Recovery. Rev. Aquac. 2023, 15, 1058–1082. [Google Scholar] [CrossRef]
- Schumann, M.; Brinker, A. Understanding and Managing Suspended Solids in Intensive Salmonid Aquaculture: A Review. Rev. Aquac. 2020, 12, 2109–2139. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Kawahata, T.; Fujibayashi, M.; Nishimura, O.; Sakamaki, T. Sources and Oxygen Consumption of Particulate Organic Matter Settling in Oyster Aquaculture Farms: Insights from Analysis of Fatty Acid Composition. Estuar. Coast. Shelf Sci. 2021, 254, 107328. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, J.; Jin, R.; Li, T.; Zhao, Y.; Shen, M. Microplastics in Aquaculture Systems: Occurrence, Ecological Threats and Control Strategies. Chemosphere 2023, 340, 139924. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics 2025, 14, 598. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Umi, W.A.D.; Ramli, N.M.; Harun, R. Water Quality Management in Aquaculture. Camb. Prism. Water 2024, 2, e8. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Chin, J.Y.; Mohd Harun, M.H.Z.; Low, S.C. Environmental Impacts and Imperative Technologies towards Sustainable Treatment of Aquaculture Wastewater: A Review. J. Water Process Eng. 2022, 46, 102553. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, H.; Zhang, Y.; Zhou, Q.; Wen, X.; Guo, W.; Zhang, Z. A Systematic Review on Aquaculture Wastewater: Pollutants, Impacts, and Treatment Technology. Environ. Res. 2024, 262, 119793. [Google Scholar] [CrossRef]
- Ahmad, A.; Sheikh Abdullah, S.R.; Hasan, H.A.; Othman, A.R.; Ismail, N. Aquaculture Industry: Supply and Demand, Best Practices, Effluent and Its Current Issues and Treatment Technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.; Li, Y.; Liu, Y.; Wang, X. Environmental Effects of Mariculture in China: An Overall Study of Nitrogen and Phosphorus Loads. Acta Oceanol. Sin. 2022, 41, 4–11. [Google Scholar] [CrossRef]
- Kolek, L.; Inglot, M.; Jarosiewicz, P. Nutrient Cycling Enhancement in Intensive-Extensive Aquaculture through C/N Ratio Manipulation and Periphyton Support. Bioresour. Technol. 2023, 368, 128309. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, J.; Du, C.; Yang, Q.; Huang, J.; Wang, Z.; Xu, J.; Zhang, M. Relationship between Nitrogen Dynamics and Key Microbial Nitrogen-Cycling Genes in an Intensive Freshwater Aquaculture Pond. Microorganisms 2024, 12, 266. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Huang, S.; Wu, X.; Zhou, P.; Geng, Y.; Zhu, Y.; Wang, Y.; Wu, Y.; Chen, Q.; et al. Promoting Effect and Mechanism of Residual Feed Organic Matter on the Formation of Cyanobacterial Blooms in Aquaculture Waters. J. Clean. Prod. 2023, 417, 138068. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]
- Hu, F.; Ye, J.; Wang, B.; Zhang, W.; Chen, P.; Yuan, Z.; Xu, Z. Transformation of Dissolved Organic Matter during Aquaculture Wastewater Treatment: Insights into the Biological Toxicity, Spectral Indices and Molecular Signatures. Water Res. 2025, 283, 123834. [Google Scholar] [CrossRef]
- Dindar, E. Assessment of Pollution Status Using Water Quality Index (WQI) and Hydrochemical Indicators in the Gemlik Gulf, Marmara Sea, Türkiye: A Spatial and Temporal Perspective. Environ. Sci. Pollut. Res. 2025, 32, 14860–14890. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, Z.; Qi, M.; Cai, W.; Saraiva, J.L.; Wen, Y.; Liu, G.; Zhu, Z.; Zhu, S.; Zhao, J. Water Quality Impact on Fish Behavior: A Review From an Aquaculture Perspective. Rev. Aquac. 2025, 17, e12985. [Google Scholar] [CrossRef]
- Kumari, S.; Das, S. Bacterial Enzymatic Degradation of Recalcitrant Organic Pollutants: Catabolic Pathways and Genetic Regulations. Environ. Sci. Pollut. Res. 2023, 30, 79676–79705. [Google Scholar] [CrossRef]
- Dong, S.-L.; Li, L. Sediment and Remediation of Aquaculture Ponds. In Aquaculture Ecology; Dong, S.-L., Tian, X.-L., Gao, Q.-F., Dong, Y.-W., Eds.; Springer Nature: Singapore, 2023; pp. 309–334. ISBN 978-981-19-5486-3. [Google Scholar]
- Kong, W.; Xu, Q.; Lyu, H.; Kong, J.; Wang, X.; Shen, B.; Bi, Y. Sediment and Residual Feed from Aquaculture Water Bodies Threaten Aquatic Environmental Ecosystem: Interactions among Algae, Heavy Metals, and Nutrients. J. Environ. Manag. 2023, 326, 116735. [Google Scholar] [CrossRef]
- Boyd, C.E. Dissolved and Suspended Solids in Aquaculture. CABI Rev. 2019, 2018, 1–13. [Google Scholar] [CrossRef]
- Kim, D.; Chung, S. Enhancing Harmful Algal Bloom Predictions through Integrated Modeling of Turbidity and Nutrient Dynamics in Monsoon Climate Reservoirs. J. Environ. Manag. 2025, 381, 125291. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Smith, D.G. Turbidity Suspeni)Ed Sediment, and Water Clarity: A Review. JAWRA J. Am. Water Resour. Assoc. 2001, 37, 1085–1101. [Google Scholar] [CrossRef]
- Rossi, L.; Chèvre, N.; Fankhauser, R.; Margot, J.; Curdy, R.; Babut, M.; Barry, D.A. Sediment Contamination Assessment in Urban Areas Based on Total Suspended Solids. Water Res. 2013, 47, 339–350. [Google Scholar] [CrossRef]
- Bilotta, G.; Brazier, R.E. Understanding the Influence of Suspended Solids on Water Quality and Aquatic Biota. Water Res. 2008, 42, 2849–2861. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of Alternatives to Antibiotic Use in Aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Limbu, S.M.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. A Global Analysis on the Systemic Effects of Antibiotics in Cultured Fish and Their Potential Human Health Risk: A Review. Rev. Aquac. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, Antibiotic-Resistant Bacteria, and Resistance Genes in Aquaculture: Risks, Current Concern, and Future Thinking. Environ. Sci. Pollut. Res. Int. 2022, 29, 11054–11075. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy Use of Prophylactic Antibiotics in Aquaculture: A Growing Problem for Human and Animal Health and for the Environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Singh, A.K.; Kaur, R.; Verma, S.; Singh, S. Antimicrobials and Antibiotic Resistance Genes in Water Bodies: Pollution, Risk, and Control. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar] [CrossRef]
- Emenike, E.C.; Iwuozor, K.O.; Anidiobi, S.U. Heavy Metal Pollution in Aquaculture: Sources, Impacts and Mitigation Techniques. Biol. Trace Elem. Res. 2022, 200, 4476–4492. [Google Scholar] [CrossRef]
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental Persistence, Bioaccumulation, and Ecotoxicology of Heavy Metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Huang, L.; Qin, D.; Tang, S.; Wang, P.; Gao, L. Trace Element Content and Health Risk Assessment of Main Aquaculture Products in Northeast China. Qual. Assur. Saf. Crops Foods 2025, 17, 86–105. [Google Scholar] [CrossRef]
- Singh, C.K.; Sodhi, K.K.; Shree, P.; Nitin, V. Heavy Metals as Catalysts in the Evolution of Antimicrobial Resistance and the Mechanisms Underpinning Co-Selection. Curr. Microbiol. 2024, 81, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, J.; Han, Y.; Chen, J.; Liu, G.; Lu, H.; Yan, B.; Chen, S. Nutrients, Heavy Metals and Microbial Communities Co-Driven Distribution of Antibiotic Resistance Genes in Adjacent Environment of Mariculture. Environ. Pollut. 2017, 220, 909–918. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A Review on Algal-Bacterial Symbiotic System for Effective Treatment of Wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Padeniya, U.; Davis, D.A.; Wells, D.E.; Bruce, T.J. Microbial Interactions, Growth, and Health of Aquatic Species in Biofloc Systems. Water 2022, 14, 4019. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, X.; He, M.; Jin, W.; Chen, Y.; Sun, J. Pollutant Removal and Resource Recovery of Co-Cultivated Microalgae Chlorella Sp. and Phaeodactylum Tricornutum for Marine Aquaculture Wastewater. J. Water Process Eng. 2024, 67, 106182. [Google Scholar] [CrossRef]
- Hasnain, M.; Zainab, R.; Ali, F.; Abideen, Z.; Yong, J.W.H.; El-Keblawy, A.; Hashmi, S.; Radicetti, E. Utilization of Microalgal-Bacterial Energy Nexus Improves CO2 Sequestration and Remediation of Wastewater Pollutants for Beneficial Environmental Services. Ecotoxicol. Environ. Saf. 2023, 267, 115646. [Google Scholar] [CrossRef]
- Ahmad Ali, S. Design and Evaluate a Drum Screen Filter Driven by Undershot Waterwheel for Aquaculture Recirculating Systems. Aquac. Eng. 2013, 54, 38–44. [Google Scholar] [CrossRef]
- de Jesus Gregersen, K.J.; Olsson, P.; Pellicer-Nàcher, C.; Pedersen, P.B. Effect of Drum Filter Mesh Size on RAS Water Quality. Aquac. Eng. 2025, 109, 102508. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen Removal Techniques in Aquaculture for a Sustainable Production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) Analysis: Main Issues on Management and Future Challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Muñoz Alegría, J.A.; Muñoz España, E.; Flórez Marulanda, J.F. Dissolved Air Flotation: A Review from the Perspective of System Parameters and Uses in Wastewater Treatment. TecnoLógicas 2021, 24, e2111. [Google Scholar] [CrossRef]
- Tango, M.S.; Gagnon, G.A. Impact of Ozonation on Water Quality in Marine Recirculation Systems. Aquac. Eng. 2003, 29, 125–137. [Google Scholar] [CrossRef]
- Jokela, P.; Lepistö, R. Lamella Dissolved Air Flotation Treatment of Fish Farming Effluents as a Part of an Integrated Farming and Effluent Treatment Concept. Environ. Technol. 2014, 35, 2727–2733. [Google Scholar] [CrossRef]
- Mota, V.C.; Brenne, H.; Kojen, M.; Marhaug, K.R.; Jakobsen, M.E. Evaluation of an Ultrafiltration Membrane for the Removal of Fish Viruses and Bacteria in Aquaculture Water. Front. Mar. Sci. 2022, 9, 1037017. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Mahdi Zerafat, M.; Fauzi Ismail, A.; Sean Goh, P. Emerging Membrane Technologies for Sustainable Water Treatment: A Review on Recent Advances. Environ. Sci. Adv. 2025, 4, 530–570. [Google Scholar] [CrossRef]
- Acarer, S. A Review of Microplastic Removal from Water and Wastewater by Membrane Technologies. Water Sci. Technol. 2023, 88, 199–219. [Google Scholar] [CrossRef]
- Fatima, F.; Du, H.; Kommalapati, R.R. A Sequential Membrane Process of Ultrafiltration Forward Osmosis and Reverse Osmosis for Poultry Slaughterhouse Wastewater Treatment and Reuse. Membranes 2023, 13, 296. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Sibrell, P.L.; Ogden, S.R.; Summerfelt, S.T. Evaluation of Chemical Coagulation-Flocculation Aids for the Removal of Suspended Solids and Phosphorus from Intensive Recirculating Aquaculture Effluent Discharge. Aquac. Eng. 2003, 29, 23–42. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Coagulation–Flocculation of Aquaculture Effluent Using Biobased Flocculant: From Artificial to Real Wastewater Optimization by Response Surface Methodology. J. Water Process Eng. 2023, 53, 103869. [Google Scholar] [CrossRef]
- Jensen, H.S.; Reitzel, K.; Egemose, S. Evaluation of Aluminum Treatment Efficiency on Water Quality and Internal Phosphorus Cycling in Six Danish Lakes. Hydrobiologia 2015, 751, 189–199. [Google Scholar] [CrossRef]
- Botté, A.; Zaidi, M.; Guery, J.; Fichet, D.; Leignel, V. Aluminium in Aquatic Environments: Abundance and Ecotoxicological Impacts. Aquat. Ecol. 2022, 56, 751–773. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Alzghoul, T.M.; Nassani, D.E.; Bashir, M.J.K. Natural Coagulants for Sustainable Wastewater Treatment: Current Global Research Trends. Processes 2025, 13, 1754. [Google Scholar] [CrossRef]
- Tran, T.; Phuong, T.T.B.; Giang, L.V. Study on Application of Cationic Modified Starch in Combination with Poly Aluminium Chloride for Treatment of Flocculation of Aquatic Wastewater. In Proceedings of the 2nd Energy Security and Chemical Engineering Congress (ESChE 2021), Gambang, Malaysia, 3–5 November 2021; AIP: Melville, NY, USA, 2022; Volume 2610, p. 040010. [Google Scholar]
- Schroeder, J.P.; Croot, P.L.; Von Dewitz, B.; Waller, U.; Hanel, R. Potential and Limitations of Ozone for the Removal of Ammonia, Nitrite, and Yellow Substances in Marine Recirculating Aquaculture Systems. Aquac. Eng. 2011, 45, 35–41. [Google Scholar] [CrossRef]
- Powell, A.; Scolding, J.W.S. Direct Application of Ozone in Aquaculture Systems. Rev. Aquac. 2018, 10, 424–438. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Zhou, Y.-T.; Li, W.; Zhang, X.; Du, Y.; Hu, H.-Y. Underestimated Risk from Ozonation of Wastewater Containing Bromide: Both Organic Byproducts and Bromate Contributed to the Toxicity Increase. Water Res. 2019, 162, 43–52. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Gagnon, G.A. Ozone Application in Recirculating Aquaculture System: An Overview. Ozone Sci. Eng. 2011, 33, 345–367. [Google Scholar] [CrossRef]
- Li, B.; Jia, R.; Hou, Y.; Zhang, C.; Zhu, J.; Ge, X. The Sustainable Treatment Effect of Constructed Wetland for the Aquaculture Effluents from Blunt Snout Bream (Megalobrama Amblycephala) Farm. Water 2021, 13, 3418. [Google Scholar] [CrossRef]
- Chen, C.; Yang, G.; Chen, X.; Li, P.; Chen, J.; Yan, M.; Guo, C. Treatment Effect of Long-Term Subsurface-Flow Constructed Wetland on Mariculture Water and Analysis of Wetland Bacterial Community. Water 2024, 16, 1054. [Google Scholar] [CrossRef]
- Chao, C.; Gong, S.; Xie, Y. The Performance of a Multi-Stage Surface Flow Constructed Wetland for the Treatment of Aquaculture Wastewater and Changes in Epiphytic Biofilm Formation. Microorganisms 2025, 13, 494. [Google Scholar] [CrossRef]
- Meng, T.; Cheng, W.; Li, D. Effects of Ammonium/Nitrate Ratios on Plant Growth and Nitrogen and Phosphorus Removal in an Ecological Floating Bed System. Chem. Ecol. 2025, 41, 408–422. [Google Scholar] [CrossRef]
- Betanzo-Torres, E.A.; Ballut-Dajud, G.; Aguilar-Cortés, G.; Delfín-Portela, E.; Sandoval Herazo, L.C. Plants Used in Constructed Wetlands for Aquaculture: A Systematic Review. Sustainability 2025, 17, 6298. [Google Scholar] [CrossRef]
- Letelier-Gordo, C.O.; Huang, X.; Aalto, S.L.; Pedersen, P.B. Activated Sludge Denitrification in Marine Recirculating Aquaculture System Effluent Using External and Internal Carbon Sources. Aquac. Eng. 2020, 90, 102096. [Google Scholar] [CrossRef]
- Davidson, J.; Summerfelt, S.; Schrader, K.K.; Good, C. Integrating Activated Sludge Membrane Biological Reactors with Freshwater RAS: Preliminary Evaluation of Water Use, Water Quality, and Rainbow Trout Oncorhynchus Mykiss Performance. Aquac. Eng. 2019, 87, 102022. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Process Performance of Anaerobic Co-Digestion of Waste Activated Sludge and Aquaculture Sludge. Aquac. Eng. 2020, 90, 102090. [Google Scholar] [CrossRef]
- Sam, T.; Le Roes-Hill, M.; Hoosain, N.; Welz, P.J. Strategies for Controlling Filamentous Bulking in Activated Sludge Wastewater Treatment Plants: The Old and the New. Water 2022, 14, 3223. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Jiang, X. Application of Biofilm Water Conservation and Emission Reduction Technology in the Pond Culture of Largemouth Bass and Japanese Eel. Sustainability 2023, 15, 6663. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Zhao, Y.; Liu, Y.; Li, F. Bacterial Biofilm Probed by Scanning Electrochemical Microscopy: A Review. ChemElectroChem 2022, 9, e202200470. [Google Scholar] [CrossRef]
- Shao, S.; Wang, M.; Zhong, J.; Wu, X. Characteristics of Tetracycline Degradation Coupled Simultaneous Nitrification-Denitrification and Phosphorus Removal in Aquaculture Wastewater. Geomicrobiol. J. 2023, 40, 399–412. [Google Scholar] [CrossRef]
- Hoang, V.; Delatolla, R.; Abujamel, T.; Mottawea, W.; Gadbois, A.; Laflamme, E.; Stintzi, A. Nitrifying Moving Bed Biofilm Reactor (MBBR) Biofilm and Biomass Response to Long Term Exposure to 1 °C. Water Res. 2014, 49, 215–224. [Google Scholar] [CrossRef]
- Aqeel, H.; Liss, S.N. Fate of Sloughed Biomass in Integrated Fixed-Film Systems. PLoS ONE 2022, 17, e0262603. [Google Scholar] [CrossRef]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture Wastewater Treatment Technologies and Their Sustainability: A Review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Chanakya Varma, V.; Shyamala, G.; Sri Bala, G.; Nagaraju, T.V. Aquaculture Effluent Treatment and Waste-to-Energy: Driving Inland Aquaculture Sustainability. In Inland Aquaculture Sustainability and Effective Water Management Strategies: Optimizing Resources for Environmental Harmony; Nagaraju, T.V., Das, B., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 49–67. ISBN 978-3-031-88384-2. [Google Scholar]
- Xu, J.; Du, Y.; Xu, Y.; Chen, F.; Zhou, L.; Sun, J.; Qiu, T. Review of the Application of Bacterial–Algae Symbiotic Systems in Nitrogen Removal from Aquaculture Effluent: Principles, Performance Enhancement, Resource Utilization, and Challenges. Water Reuse 2025, 15, 230–254. [Google Scholar] [CrossRef]
- Lin, C.; Cao, P.; Xu, X.; Ye, B. Algal-Bacterial Symbiosis System Treating High-Load Printing and Dyeing Wastewater in Continuous-Flow Reactors under Natural Light. Water 2019, 11, 469. [Google Scholar] [CrossRef]
- Neissi, A.; Rafiee, G.; Rahimi, S.; Farahmand, H.; Pandit, S.; Mijakovic, I. Enriched Microbial Communities for Ammonium and Nitrite Removal from Recirculating Aquaculture Systems. Chemosphere 2022, 295, 133811. [Google Scholar] [CrossRef]
- Li, Q.; Hasezawa, R.; Saito, R.; Okano, K.; Shimizu, K.; Utsumi, M. Abundance and Diversity of Nitrifying Microorganisms in Marine Recirculating Aquaculture Systems. Water 2022, 14, 2744. [Google Scholar] [CrossRef]
- Gao, J.; Mang, Q.; Li, Q.; Sun, Y.; Xu, G. Microbial-Algal Symbiotic System Drives Reconstruction of Nitrogen, Phosphorus, and Methane Cycles for Purification of Pollutants in Aquaculture Water. Bioresour. Technol. 2025, 430, 132531. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Z.; Liu, M.; Liu, C. Enhancing Simultaneous Nitrification and Denitrification in a Plant-Scale Integrated Fixed-Film Activated Sludge System: Focusing on the Cooperation between Activated Sludge and Biofilm. Chem. Eng. J. 2024, 496, 154322. [Google Scholar] [CrossRef]
- Ding, Q.; Zhou, Z.; Cui, L.; Liu, J.; You, G.; Chen, Q.; Hou, J.; Fan, X.; Yang, Y. Study on the Treatment of Livestock and Poultry Wastewater Using Algae-Bacteria Symbiotic System: Effect of Inoculation Proportion and Performance. Environ. Technol. 2025, 46, 2597–2614. [Google Scholar] [CrossRef]
- Tang, C.-C.; Zuo, W.; Tian, Y.; Sun, N.; Wang, Z.-W.; Zhang, J. Effect of Aeration Rate on Performance and Stability of Algal-Bacterial Symbiosis System to Treat Domestic Wastewater in Sequencing Batch Reactors. Bioresour. Technol. 2016, 222, 156–164. [Google Scholar] [CrossRef]
- Markou, G.; Economou, C.N.; Petrou, C.; Tzovenis, I.; Doulgeraki, A.; Zioga, M.; Saganas, N.; Kougia, E.; Arapoglou, D. Biofloc Technology Combined with Microalgae for Improved Nitrogen Removal at Lower C/N Ratios Using Artificial Aquaculture Wastewater. Aquac. Int. 2024, 32, 1537–1557. [Google Scholar] [CrossRef]
- Li, S.-N.; Zhang, C.; Li, F.; Ren, N.-Q.; Ho, S.-H. Recent Advances of Algae-Bacteria Consortia in Aquatic Remediation. Crit. Rev. Environ. Sci. Technol. 2023, 53, 315–339. [Google Scholar] [CrossRef]
- Satiro, J.; dos Santos Neto, A.G.; Marinho, T.; Sales, M.; Marinho, I.; Kato, M.T.; Simões, R.; Albuquerque, A.; Florencio, L. The Role of the Microalgae–Bacteria Consortium in Biomass Formation and Its Application in Wastewater Treatment Systems: A Comprehensive Review. Appl. Sci. 2024, 14, 6083. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, Y.; Ji, H.; Li, Z. Algae-Bacteria Symbiotic Biofilm System for Low Carbon Nitrogen Removal from Municipal Wastewater: A Review. World J. Microbiol. Biotechnol. 2025, 41, 218. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y. Microalgae Biofilm and Bacteria Symbiosis in Nutrient Removal and Carbon Fixation from Wastewater: A Review. Curr. Pollut. Rep. 2022, 8, 128–146. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Liu, Y.; Taidi, B. Immobilized Microalgae: Principles, Processes and Its Applications in Wastewater Treatment. World J. Microbiol. Biotechnol. 2024, 40, 150. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Gao, F.; Yang, H.-L.; Wu, H.-W.-J.; Li, C.; Lu, M.-M.; Yang, Z.-Y. Simultaneous Removal of Nutrient and Sulfonamides from Marine Aquaculture Wastewater by Concentrated and Attached Cultivation of Chlorella Vulgaris in an Algal Biofilm Membrane Photobioreactor (BF-MPBR). Sci. Total Environ. 2020, 725, 138524. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Böjti, T.; Shetty, P.; Lakatos, G.; Bagi, Z.; Kovács, K.L.; Maróti, G. Chlorella Vulgaris and Its Phycosphere in Wastewater: Microalgae-Bacteria Interactions During Nutrient Removal. Front. Bioeng. Biotechnol. 2020, 8, 557572. [Google Scholar] [CrossRef]
- Mubashar, M.; Zhang, J.; Liu, Q.; Chen, L.; Li, J.; Naveed, M.; Zhang, X. In-Situ Removal of Aquaculture Waste Nutrient Using Floating Permeable Nutrient Uptake System (FPNUS) under Mixotrophic Microalgal Scheme. Bioresour. Technol. 2022, 363, 128022. [Google Scholar] [CrossRef]
- Xu, T.; Liu, W.; Liu, X.; Li, X.; Xian, Q.; Huo, S.; Zhao, C.; Guo, B.; Li, Q. The Symbiotic System between Tribonema Sp. and Aerobic Denitrifying Phosphorus Accumulating Bacteria: A Promising Method for Wastewater Treatment. Algal Res. 2025, 85, 103824. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Kumar, V.; Vlaskin, M.S.; Grigorenko, A.V. Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment. Water 2022, 14, 3784. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Cui, Y.-W.; Huang, J.-L.; Sun, F.-L.; Chen, S. A Novel Pseudomonas Aeruginosa Strain Performs Simultaneous Heterotrophic Nitrification-Aerobic Denitrification and Aerobic Phosphate Removal. Water Res. 2022, 221, 118823. [Google Scholar] [CrossRef]
- Makarani, N.; Kaushal, R.S. Advances in Actinobacteria-Based Bioremediation: Mechanistic Insights, Genetic Regulation, and Emerging Technologies. Biodegradation 2025, 36, 24. [Google Scholar] [CrossRef]
- James, G.; Prasannan Geetha, P.; Thavarool Puthiyedathu, S.; Vattringal Jayadradhan, R.K. Applications of Actinobacteria in Aquaculture: Prospects and Challenges. 3 Biotech 2023, 13, 42. [Google Scholar] [CrossRef]
- Qi, L.; Liu, X.; Gao, Y.; Yang, Q.; Wang, Z.; Zhang, N.; Su, X. Interactions between Bacteria and Microalgae in Microalgal-Bacterial Symbiotic Wastewater Treatment Systems: Mechanisms and Influencing Factors. Aquat. Microb. Ecol. 2024, 90, 41–60. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, M.; Zhang, J.; Jiang, X.; Zheng, Z. The Interactions of Algae-Bacteria Symbiotic System and Its Effects on Nutrients Removal from Synthetic Wastewater. Bioresour. Technol. 2018, 247, 44–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Zhu, Y.; Yuan, C.; Zhao, T. Effect of Bacteria-to-Algae Volume Ratio on Treatment Performance and Microbial Community of a Novel Heterotrophic Nitrification-Aerobic Denitrification Bacteria-Chlorella Symbiotic System. Bioresour. Technol. 2021, 342, 126025. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, L.; Liu, C. Effect of Bacteria-Algae Ratio on Treatment of Anaerobic Digested Wastewater by Symbiotic Coupling of Bacteria and Algae under the Background of Carbon Neutralization. Environ. Res. 2024, 251, 118771. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, S.-A.; Ko, S.-R.; Oh, H.-M.; Ahn, C.-Y. Effects of Photoperiod on Nutrient Removal, Biomass Production, and Algal-Bacterial Population Dynamics in Lab-Scale Photobioreactors Treating Municipal Wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef]
- Wang, S.; Jin, Z.; Chen, Z.; Zheng, Z.; Li, L.; Ding, X.; Zhang, C.; Lv, G. Effect of Light Wavelengths on Algal-Bacterial Symbiotic Particles (ABSP): Nitrogen Removal, Physicochemical Properties, Community Structure. J. Clean. Prod. 2023, 429, 139465. [Google Scholar] [CrossRef]
- Glemser, M.; Heining, M.; Schmidt, J.; Becker, A.; Garbe, D.; Buchholz, R.; Brück, T. Application of Light-Emitting Diodes (LEDs) in Cultivation of Phototrophic Microalgae: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1077–1089. [Google Scholar]
- Chu, G.; Gao, C.; Wang, Q.; Zhang, W.; Tian, T.; Chen, W.; Gao, M. Effect of Light Intensity on Nitrogen Removal, Enzymatic Activity and Metabolic Pathway of Algal-Bacterial Symbiosis in Rotating Biological Contactor Treating Mariculture Wastewater. Bioresour. Technol. 2025, 417, 131872. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, G.; Gao, C.; Tian, T.; Zhang, W.; Chen, W.; Gao, M. Effect of Light Intensity on Performance, Microbial Community and Metabolic Pathway of Algal-Bacterial Symbiosis in Sequencing Batch Biofilm Reactor Treating Mariculture Wastewater. Bioresour. Technol. 2025, 433, 132726. [Google Scholar] [CrossRef]
- Li, S.; Xing, D.; Sun, C.; Jin, C.; Zhao, Y.; Gao, M.; Guo, L. Effect of Light Intensity and Photoperiod on High-Value Production and Nutrient Removal Performance with Bacterial-Algal Coupling System. J. Environ. Manag. 2024, 356, 120595. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Xue, Y.; Qu, Y.; Li, Y. The Impact of Seasonal Variations about Temperature and Photoperiod on the Treatment of Municipal Wastewater by Algae-Bacteria System in Lab-Scale. Algal Res. 2021, 54, 102175. [Google Scholar] [CrossRef]
- Ji, B.; Zhu, L.; Wang, S.; Liu, Y. Temperature-Effect on the Performance of Non-Aerated Microalgal-Bacterial Granular Sludge Process in Municipal Wastewater Treatment. J. Environ. Manag. 2021, 282, 111955. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Barat, R.; Ferrer, J. Effect of Ambient Temperature Variations on an Indigenous Microalgae-Nitrifying Bacteria Culture Dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef]
- González-Camejo, J.; Barat, R.; Pachés, M.; Murgui, M.; Seco, A.; Ferrer, J. Wastewater Nutrient Removal in a Mixed Microalgae-Bacteria Culture: Effect of Light and Temperature on the Microalgae-Bacteria Competition. Environ. Technol. 2018, 39, 503–515. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, Y.; Ge, F.; Liu, N.; Wong, M. A pH-Dependent Enhancement Effect of Co-Cultured Bacillus Licheniformis on Nutrient Removal by Chlorella Vulgaris. Ecol. Eng. 2015, 75, 258–263. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, Y.; Ge, F.; Xu, Y.; Tao, N.; Peng, F.; Wong, M. Efficiency Assessment and pH Effect in Removing Nitrogen and Phosphorus by Algae-Bacteria Combined System of Chlorella Vulgaris and Bacillus Licheniformis. Chemosphere 2013, 92, 1383–1389. [Google Scholar] [CrossRef]
- Salisu, A.; Umar, B.I.; Zhong, Z.; Yu, C.; Wang, Y. Charcoal Stabilizes pH and Improves Nutrients Removal of Bacteria-Microalgae Interaction: A Potential for Improving Water Quality in Aquaculture. Environ. Eng. Res. 2023, 28, 220620. [Google Scholar] [CrossRef]
- Silaban, A.; Bai, R.; Gutierrez-Wing, M.T.; Negulescu, I.I.; Rusch, K.A. Effect of Organic Carbon, C:N Ratio and Light on the Growth and Lipid Productivity of Microalgae/Cyanobacteria Coculture. Eng. Life Sci. 2014, 14, 47–56. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, H.; Li, H.; Lu, Q.; Sun, Y. Does Exogenous Carbon Source Always Promote Algal Biomass and Nutrients Removal in Algal-Bacterial Wastewater Remediation? J. Clean. Prod. 2021, 281, 125371. [Google Scholar] [CrossRef]
- Casagli, F.; Rossi, S.; Steyer, J.P.; Bernard, O.; Ficara, E. Balancing Microalgae and Nitrifiers for Wastewater Treatment: Can Inorganic Carbon Limitation Cause an Environmental Threat. Environ. Sci. Technol. 2021, 55, 3940–3955. [Google Scholar] [CrossRef]
- Ji, M.; Gao, H.; Zhang, J.; Hu, Z.; Liang, S. Environmental Impacts on Algal–Bacterial-Based Aquaponics System by Different Types of Carbon Source Addition: Water Quality and Greenhouse Gas Emission. Environ. Sci. Pollut. Res. 2024, 31, 26665–26674. [Google Scholar] [CrossRef]
- Pereira, A.S.A.d.P.; Magalhães, I.B.; Silva, T.A.; dos Reis, A.J.D.; Couto, E.d.A.d.; Calijuri, M.L. Municipal and Industrial Wastewater Blending: Effect of the Carbon/Nitrogen Ratio on Microalgae Productivity and Biocompound Accumulation. J. Environ. Manag. 2024, 370, 122760. [Google Scholar] [CrossRef]
- Shen, X.-F.; Xu, Y.-P.; Tong, X.-Q.; Huang, Q.; Zhang, S.; Gong, J.; Chu, F.-F.; Zeng, R.J. The Mechanism of Carbon Source Utilization by Microalgae When Co-Cultivated with Photosynthetic Bacteria. Bioresour. Technol. 2022, 365, 128152. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, S.; Zhang, Y.; Teng, J.; Zhang, M.; Lin, H. Optimizing Aeration Intensity to Enhance Self-Flocculation in Algal-Bacterial Symbiosis Systems. Chemosphere 2023, 341, 140064. [Google Scholar] [CrossRef]
- Nguyen, N.-K.-Q.; Bui, X.-T.; Dao, T.-S.; Pham, M.-D.-T.; Ngo, H.H.; Lin, C.; Lin, K.-Y.A.; Nguyen, P.-D.; Huynh, K.-P.-H.; Vo, T.-K.-Q.; et al. Influence of Hydrodynamic Shear Stress on Activated Algae Granulation Process for Wastewater Treatment. Environ. Technol. Innov. 2024, 33, 103494. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Khorshidi Nazloo, E.; Hajinajaf, N.; Higgins, B. Interactions of Microalgae-Bacteria Consortia for Nutrient Removal from Wastewater: A Review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Feng, S.; Yang, T.; Xu, L.; Shen, B.; Bi, Y.; Lyu, H. Cultivation of Microalgae–Bacteria Consortium by Waste Gas–Waste Water to Achieve CO2 Fixation, Wastewater Purification and Bioproducts Production. Biotechnol. Biofuels Bioprod. 2024, 17, 26. [Google Scholar] [CrossRef]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability 2023, 15, 5521. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, Minerals and Microbes: Geomicrobiology and Bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- da Silva Rodrigues, D.A.; da Cunha, C.C.R.F.; do Espirito Santo, D.R.; de Barros, A.L.C.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Removal of Cephalexin and Erythromycin Antibiotics, and Their Resistance Genes, by Microalgae-Bacteria Consortium from Wastewater Treatment Plant Secondary Effluents. Environ. Sci. Pollut. Res. 2021, 28, 67822–67832. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water. Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Show, P.L. Microalgal-Bacterial Consortia as Future Prospect in Wastewater Bioremediation, Environmental Management and Bioenergy Production. Indian J. Microbiol. 2021, 61, 262–269. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete Nitrification by Nitrospira Bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Muro-Pastor, M.I.; Reyes, J.C.; Florencio, F.J. Ammonium Assimilation in Cyanobacteria. Photosynth. Res. 2005, 83, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, X.; Lu, H. Overlooked Nitrogen-Cycling Microorganisms in Biological Wastewater Treatment. Front. Environ. Sci. Eng. 2021, 15, 133. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, Z.; Zhao, C.; He, L.; Lin, Z.; Liu, M. Homogeneous Environmental Selection Mainly Determines the Denitrifying Bacterial Community in Intensive Aquaculture Water. Front. Microbiol. 2023, 14, 1280450. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Molecular Mechanisms of Phosphate Sensing, Transport and Signalling in Streptomyces and Related Actinobacteria. Int. J. Mol. Sci. 2021, 22, 1129. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Ukaigwe, S.; Dang, H.; Liu, Y. Phosphorus Removal from Aerobic Granular Sludge: Proliferation of Polyphosphate-Accumulating Organisms (PAOs) under Different Feeding Strategies. Processes 2022, 10, 1399. [Google Scholar] [CrossRef]
- Felz, S.; Kleikamp, H.; Zlopasa, J.; van Loosdrecht, M.C.; Lin, Y. Impact of Metal Ions on Structural EPS Hydrogels from Aerobic Granular Sludge. Biofilm 2019, 2, 100011. [Google Scholar] [CrossRef]
- Muthu, M.; Wu, H.-F.; Gopal, J.; Sivanesan, I.; Chun, S. Exploiting Microbial Polysaccharides for Biosorption of Trace Elements in Aqueous Environments-Scope for Expansion via Nanomaterial Intervention. Polymers 2017, 9, 721. [Google Scholar] [CrossRef]
- Law, Y.; Kirkegaard, R.H.; Cokro, A.A.; Liu, X.; Arumugam, K.; Xie, C.; Stokholm-Bjerregaard, M.; Drautz-Moses, D.I.; Nielsen, P.H.; Wuertz, S.; et al. Integrative Microbial Community Analysis Reveals Full-Scale Enhanced Biological Phosphorus Removal under Tropical Conditions. Sci. Rep. 2016, 6, 25719. [Google Scholar] [CrossRef]
- Zhang, L.; Ali, A.; Su, J.; Huang, T.; Wang, Z. Ammonium Nitrogen and Phosphorus Removal by Bacterial-Algal Symbiotic Dynamic Sponge Bioremediation System in Micropolluted Water: Operational Mechanism and Transformation Pathways. Sci. Total Environ. 2024, 947, 174636. [Google Scholar] [CrossRef]
- Schaedig, E.; Cantrell, M.; Urban, C.; Zhao, X.; Greene, D.; Dancer, J.; Gross, M.; Sebesta, J.; Chou, K.J.; Grabowy, J.; et al. Isolation of Phosphorus-Hyperaccumulating Microalgae from Revolving Algal Biofilm (RAB) Wastewater Treatment Systems. Front. Microbiol. 2023, 14, 1219318. [Google Scholar] [CrossRef]
- Mauret, M.; Ferrand, F.; Boisdon, V.; Spérandio, M.; Paul, E. Process Using DO and ORP Signals for Biological Nitrification and Denitrification: Validation of a Food-Processing Industry Wastewater Treatment Plant on Boosting with Pure Oxygen. Water Sci. Technol. 2001, 44, 163–170. [Google Scholar] [CrossRef]
- Jin, Y.; Zhan, W.; Wu, R.; Han, Y.; Yang, S.; Ding, J.; Ren, N. Insight into the Roles of Microalgae on Simultaneous Nitrification and Denitrification in Microalgal-Bacterial Sequencing Batch Reactors: Nitrogen Removal, Extracellular Polymeric Substances, and Microbial Communities. Bioresour. Technol. 2023, 379, 129038. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of Microbial Cells for the Biotreatment of Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Jhariya, U.; Chien, M.-F.; Umetsu, M.; Kamitakahara, M. New Insights into Immobilized Bacterial Systems for Removal of Heavy Metals from Wastewater. Int. J. Environ. Sci. Technol. 2025, 22, 8319–8334. [Google Scholar] [CrossRef]
- Bustos-Terrones, Y.A. A Review of the Strategic Use of Sodium Alginate Polymer in the Immobilization of Microorganisms for Water Recycling. Polymers 2024, 16, 788. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, X.; Li, H.; Hao, R.; Liu, F. New Insights into Microalgal-Bacterial Immobilization Systems for Wastewater Treatment: Mechanisms, Enhancement Strategies, and Application Prospects. Bioresour. Technol. 2025, 431, 132609. [Google Scholar] [CrossRef]
- Sun, N.; Xing, D.; Liu, F.; Liang, F.; Liu, L. Purification Effect of Algae and Immobilized Bacteria Combination System in Aquaculture Wastewater Treatment with Targeted Removal of Pathogenic Vibrio and Antibiotic Resistance Genes. Aquaculture 2025, 607, 742673. [Google Scholar] [CrossRef]
- Emami Moghaddam, S.A.; Harun, R.; Mokhtar, M.N.; Zakaria, R. Potential of Zeolite and Algae in Biomass Immobilization. BioMed Res. Int. 2018, 2018, 6563196. [Google Scholar] [CrossRef]
- Gneedy, A.H.; Dryaz, A.R.; Said, M.S.; AlMohamadi, H.A.; Ahmed, S.A.; Elsayed, R.; Soliman, N.K. Application of Marine Algae Separate and in Combination with Natural Zeolite in Dye Adsorption from Wastewater; A Review. Egypt. J. Chem. 2022, 65, 589–616. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Liu, X.; Xu, H.; Yang, D.; Hua, Y.; Dai, X. Diatomite Powder Carrier Improved Nitrogen and Phosphorus Removal from Real Municipal Wastewater: Insights into Micro-Granule Formation and Enhancement Mechanism. Chem. Eng. J. 2024, 484, 149482. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Grillini, V.; Mutavdžić Pavlović, D.; Verlicchi, P. Activated Carbon Coupled with Advanced Biological Wastewater Treatment: A Review of the Enhancement in Micropollutant Removal. Sci. Total Environ. 2021, 790, 148050. [Google Scholar] [CrossRef]
- Berillo, D.; Malika, T.; Baimakhanova, B.B.; Sadanov, A.K.; Berezin, V.E.; Trenozhnikova, L.P.; Baimakhanova, G.B.; Amangeldi, A.A.; Kerimzhanova, B. An Overview of Microorganisms Immobilized in a Gel Structure for the Production of Precursors, Antibiotics, and Valuable Products. Gels 2024, 10, 646. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, K.; Bai, B. A Critical Review of Sodium Alginate-Based Composites in Water Treatment. Carbohydr. Polym. 2024, 331, 121850. [Google Scholar] [CrossRef] [PubMed]
- Kube, M.; Fan, L.; Roddick, F. Alginate-Immobilised Algal Wastewater Treatment Enhanced by Species Selection. Algal Res. 2021, 54, 102219. [Google Scholar] [CrossRef]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Ablouh, E.; Hanani, Z.; Eladlani, N.; Rhazi, M.; Taourirte, M. Chitosan Microspheres/Sodium Alginate Hybrid Beads: An Efficient Green Adsorbent for Heavy Metals Removal from Aqueous Solutions. Sustain. Environ. Res. 2019, 29, 5. [Google Scholar] [CrossRef]
- Ye, T.; Li, M.; Lin, Y.; Su, Z. An Effective Biological Treatment Method for Marine Aquaculture Wastewater: Combined Treatment of Immobilized Degradation Bacteria Modified by Chitosan-Based Aerogel and Macroalgae (Caulerpa Lentillifera). Aquaculture 2023, 570, 739392. [Google Scholar] [CrossRef]
- Satpati, G.G.; Dikshit, P.K.; Mal, N.; Pal, R.; Sherpa, K.C.; Rajak, R.C.; Rather, S.-U.; Raghunathan, S.; Davoodbasha, M. A State of the Art Review on the Co-Cultivation of Microalgae-Fungi in Wastewater for Biofuel Production. Sci. Total Environ. 2023, 870, 161828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cheng, K.; Mei, H. Stability Enhancement of Microalgae–Fungal Pellets. Water 2025, 17, 1766. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Su, D.; Zhao, L.; Leng, K.; Miao, J.; Yu, Y. Enhancing Astaxanthin Accumulation in Immobilized Haematococcus Pluvialis via Alginate Hydrogel Membrane. Int. J. Biol. Macromol. 2025, 292, 139145. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [PubMed]
- Zorn, S.M.F.E.; Reis, C.E.R.; Silva, M.B.; Hu, B.; De Castro, H.F. Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation. Energies 2020, 13, 3648. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Youssef, I.M.I.; Saleh, E.S.E.; Tawfeek, S.S.; Abdel-Fadeel, A.A.A.; Abdel-Razik, A.-R.H.; Abdel-Daim, A.S.A. Effect of Spirulina Platensis on Growth, Hematological, Biochemical, and Immunological Parameters of Nile Tilapia (Oreochromis Niloticus). Trop. Anim. Health Prod. 2023, 55, 275. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Erasmus, M. Green Synthesis of Bioplastics from Microalgae: A State-of-the-Art Review. Polymers 2024, 16, 1322. [Google Scholar] [CrossRef] [PubMed]
- Roy Chong, J.W.; Tan, X.; Khoo, K.S.; Ng, H.S.; Jonglertjunya, W.; Yew, G.Y.; Show, P.L. Microalgae-Based Bioplastics: Future Solution towards Mitigation of Plastic Wastes. Environ. Res. 2022, 206, 112620. [Google Scholar] [CrossRef]
- Diaz, C.J.; Douglas, K.J.; Kang, K.; Kolarik, A.L.; Malinovski, R.; Torres-Tiji, Y.; Molino, J.V.; Badary, A.; Mayfield, S.P. Developing Algae as a Sustainable Food Source. Front. Nutr. 2023, 9, 1029841. [Google Scholar] [CrossRef]
- Villaseñor Camacho, J.; Fernández Marchante, C.M.; Rodríguez Romero, L. Analysis of a Photobioreactor Scaling up for Tertiary Wastewater Treatment: Denitrification, Phosphorus Removal, and Microalgae Production. Environ. Sci. Pollut. Res. 2018, 25, 29279–29286. [Google Scholar] [CrossRef]
- Velásquez-Orta, S.B.; Yáñez-Noguez, I.; Ramírez, I.M.; Ledesma, M.T.O. Pilot-Scale Microalgae Cultivation and Wastewater Treatment Using High-Rate Ponds: A Meta-Analysis. Environ. Sci. Pollut. Res. Int. 2024, 31, 46994. [Google Scholar] [CrossRef] [PubMed]
- Kant Bhatia, S.; Ahuja, V.; Chandel, N.; Mehariya, S.; Kumar, P.; Vinayak, V.; Saratale, G.D.; Raj, T.; Kim, S.-H.; Yang, Y.-H. An Overview on Microalgal-Bacterial Granular Consortia for Resource Recovery and Wastewater Treatment. Bioresour. Technol. 2022, 351, 127028. [Google Scholar] [CrossRef]
- Head, M.A.; Oleszkiewicz, J.A. Bioaugmentation for Nitrification at Cold Temperatures. Water Res. 2004, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Béchet, Q.; Laviale, M.; Arsapin, N.; Bonnefond, H.; Bernard, O. Modeling the Impact of High Temperatures on Microalgal Viability and Photosynthetic Activity. Biotechnol. Biofuels 2017, 10, 136. [Google Scholar] [CrossRef]
- Caldwell, G.S.; In-na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J.G.M. Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Ho, S.-H. Immobilized Microalgal System: An Achievable Idea for Upgrading Current Microalgal Wastewater Treatment. Environ. Sci. Ecotechnol. 2022, 14, 100227. [Google Scholar] [CrossRef]
- Weber, S.; Schaepe, S.; Freyer, S.; Kopf, M.; Dietzsch, C. Monitoring Gradient Formation in a Jet Aerated Bioreactor. Eng. Life Sci. 2018, 19, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]


| Water Body/System | Culture Type | Major Pollutants | Typical Concentration Range | Risk Level/Impact | References |
|---|---|---|---|---|---|
| Freshwater ponds | Carp, tilapia | TN, TP, COD | TN 20–40 mg/L; TP 2–6 mg/L; COD 40–100 mg/L | Local eutrophication; algal blooms | [8,9,18,44] |
| Intensive shrimp ponds (marine/brackish) | Shrimp, other freshwater species | NH4+-N, PO43−-P, antibiotics | NH4+-N 5–15 mg/L; PO43−-P 5–12 mg/L; antibiotics 10–200 µg/L | Benthic hypoxia, ARG proliferation | [8,9,18] |
| Net-pen/cage aquaculture | Salmon, seabass | Dissolved nutrients, organic matter | TN < 20 mg/L; TP < 3 mg/L; COD < 50 mg/L | Diffuse eutrophication; localized sediment impact | [23,24,25] |
| Recirculating aquaculture systems (RAS, high-density) | Tilapia, other freshwater species | TAN, nitrate, TDS | TAN 10–30 mg/L; NO3−-N 50–150 mg/L; TDS 300–1200 mg/L | Water reuse challenge, biofilter overload | [45,46] |
| Brackish aquaculture effluents | Shrimp/fish mix | conductivity, heavy metals | Conductivity 2–40 mS/cm; Na+, Cl− 50–300 mg/L; Cu/Zn up to 0.1–0.3 mg/L | Salinity stress to freshwater biota, sediment contamination | [46,47,48] |
| Treatment Type | Pollutant Removal/Efficiency | Advantages | Limitations | References |
|---|---|---|---|---|
| Sedimentation/Oxidation ponds | SS: 40–70%; TN: 20–40%; TP: 15–35% | Low cost; simple operation; natural processes | Large land footprint; poor control; limited N and P removal | [47,86,87] |
| Biofilters (trickling, moving bed) | NH4+-N: 40–80%; TN: 30–60% | Effective for ammonium oxidation; suitable for RAS | Requires aeration and biofilm maintenance; limited P removal | [88,89,90] |
| Constructed wetlands | TN: 40–70%; TP: 30–60%; COD: 40–80% | Eco-friendly; habitat provision | Large area; seasonal performance variation; pathogen risk | [44,46,86] |
| Activated sludge systems | TN: 60–80%; TP: 40–70%; COD: 70–90% | High efficiency; widely used; adaptable | High energy demand; sludge production; less stable under salinity | [86,91,92] |
| Sequencing batch reactors (SBR) | TN: 70–90%; TP: 50–80%; COD: 70–90% | Flexible operation; enhanced N removal | Skilled operation required; sensitive to fluctuations | [47,93] |
| Membrane bioreactors (MBR) | TN: 80–95%; TP: 70–90%; COD: 85–95% | High effluent quality; small footprint | High cost; fouling issues; energy-intensive | [47,76,91] |
| Chemical precipitation (e.g., lime, alum) | TP: >90%; COD: 40–60% | Rapid P removal; simple | High chemical cost; sludge disposal problem; not sustainable | [60,61] |
| Biofloc technology | TN: 40–70%; TP: 30–50% | Converts waste into microbial protein; feed supplement | Requires high aeration; may not meet discharge standards | [45,94] |
| Feature | Algae-Only | Bacteria-Only | Free-Living ABSS | Immobilized ABSS | Advantages/Disadvantages | References |
|---|---|---|---|---|---|---|
| Nutrient Removal Efficiency | NH4+-N: 30–70%; TP: 20–50% | NH4+-N: 60–85%; COD: 50–90% | NH4+-N: 70–90%; TP: 60–80% | NH4+-N: 85–95%; TP: 75–90% | Immobilized systems achieve higher and more stable removal; algae-only limited by light; bacteria-only poor in P removal | [44,100,101,102,104,109,111,155,158,159] |
| Kinetic Parameters | Vmax: low (0.2–0.5 mg N/L·h); t1/2: >48 h | Vmax: 0.6–1.0 mg N/L·h; t1/2: 24–36 h | Vmax: 0.8–1.2 mg N/L·h; t1/2: 20–30 h | Vmax: 1.2–1.8 mg N/L·h; t1/2: 12–24 h | Immobilization improves volumetric rates and shortens half-life | [101,158,159] |
| Biomass Retention | Low (washout prone) | Low–moderate | Moderate, but unstable | High; cells firmly attached | Immobilization ensures stable biomass over long operation | [155,156,157,158,160,168] |
| Reusability/Operational Lifetime | Limited, mostly batch | Limited | Moderate, prone to collapse | High; reusable carriers, >10 cycles reported | Improves process economics | [158,159,160,162] |
| Environmental Stability | Sensitive to pH/light/temp | Sensitive to organic shock loads | Moderate | High tolerance to fluctuations | Key advantage for aquaculture effluents | [155,156,157,158,160] |
| Operational Complexity & Cost | Low-cost, simple | Low-cost | Moderate | Higher setup cost, carrier preparation required | Trade-off: stability vs. cost | [155,156,157,158,160] |
| Limitations | Light dependency, limited P uptake | Insufficient P removal, poor resilience | Biomass washout, unstable long-term | Mass transfer limits, carrier fouling, higher capital cost | Choice depends on effluent type & scale | [155,156,157,158,160] |
| Algal Partner | Bacterial Partner(s) | Carrier/System Type | Scale | Pollutant Removal Efficiency | Additional Outcomes | References |
|---|---|---|---|---|---|---|
| C. vulgaris | Indigenous bacteria | BF-MPBR | Semi-pilot (outdoor) | TN > 90%; sulfonamides 70–85% | Simultaneous removal of nutrients and antibiotics | [100] |
| Microalgae (mixed) | Indigenous bacterial community | FPNUS | Pilot (aquaculture pond) | TN 75–85%; TP 60–70% | Operated under outdoor mixotrophic conditions | [102] |
| C. vulgaris | Mixed bacterial community | Alginate–chitosan beads | Lab/pilot | NH4+-N > 90%; TP 75–85% | Reduction in Vibrio spp. and ARGs | [160] |
| C. vulgaris | Indigenous bacteria | Alginate beads | Lab (batch) | NH4+-N 85–90%; TP 70–80% | Reusability across ≥ 4 cycles | [156,159] |
| Scenedesmus sp. | Indigenous bacteria | Alginate beads | Lab (continuous) | TP > 80% | Stable operation for 42 days without carrier degradation | [167] |
| Mixed algal culture (Chlorella sp., Scenedesmus sp.) | Nitrifiers, denitrifiers | Zeolite granules | Lab | NH4+-N > 90%; TP ~80% | Improved pH buffering; salinity tolerance | [161] |
| C. vulgaris | Indigenous bacteria | BF-MPBR | Semi-pilot (outdoor) | TN > 90%; sulfonamides 70–85% | Simultaneous removal of nutrients and antibiotics | [100] |
| Valorization Pathway | Key Biomass Composition/Yield | Application/Benefit | References |
|---|---|---|---|
| Aquafeed | Protein 45–55% DW; essential amino acids; feed substitution up to 30% | Improves fish growth, immunity, and feed conversion ratios | [176] |
| Biofertilizer | N, P-rich biomass; residual organic matter | Enhances soil fertility, nutrient recycling, reduces chemical fertilizer demand | [170] |
| Biofuels | Lipid 20–40% DW (biodiesel); residual biomass for methane | Renewable energy generation, integration with circular aquaculture systems | [170,178] |
| Bioplastics | Polyhydroxyalkanoates (PHAs) and carbohydrate-rich fractions | Pilot-scale biopolymer production; biodegradable alternatives to plastics | [179] |
| Pigments & High-Value Products | Astaxanthin (>20% higher under immobilization), phycocyanin | High-value nutraceuticals, natural pigments, antioxidant supplements | [172,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Ren, R.; Wu, Z.; Huang, J.; Zhang, Q. From Waste to Resource: Algal–Bacterial Systems and Immobilization Techniques in Aquaculture Effluent Treatment. Clean Technol. 2025, 7, 97. https://doi.org/10.3390/cleantechnol7040097
Qu J, Ren R, Wu Z, Huang J, Zhang Q. From Waste to Resource: Algal–Bacterial Systems and Immobilization Techniques in Aquaculture Effluent Treatment. Clean Technologies. 2025; 7(4):97. https://doi.org/10.3390/cleantechnol7040097
Chicago/Turabian StyleQu, Jiangqi, Ruijun Ren, Zhanhui Wu, Jie Huang, and Qingjing Zhang. 2025. "From Waste to Resource: Algal–Bacterial Systems and Immobilization Techniques in Aquaculture Effluent Treatment" Clean Technologies 7, no. 4: 97. https://doi.org/10.3390/cleantechnol7040097
APA StyleQu, J., Ren, R., Wu, Z., Huang, J., & Zhang, Q. (2025). From Waste to Resource: Algal–Bacterial Systems and Immobilization Techniques in Aquaculture Effluent Treatment. Clean Technologies, 7(4), 97. https://doi.org/10.3390/cleantechnol7040097






