An Integrated Sulfide Removal Approach from a Substrate for Biogas Production and the Simultaneous Production of Electricity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fuel Cell Experiments
- i—electric current, A;
- m—mass of reacting substance, g;
- t—time, s;
- M—molar mass of reacting substance, g mol−1;
- n—number of exchanged electrons;
- F = 96,484 C mol−1, Faraday constant.
| No. | Reversible Anode Reaction | Number of Exchanged Electrons, n | Standard Electrode Potential, V, 25 °C |
|---|---|---|---|
| 1 | SO32− + 3H2O + 6e = S2− + 6OH− | 6 | −0.66 |
| 2 | SO42− + H2O + 2e = SO32− + 2OH− | 2 | −0.91 |
| 3 | S22− + 2e = 2S2− | 1 | −0.524 |
| 4 | S + 2e = S2− | 2 | −0.480 |
| 5 | S2O32− + 6H+ + 8e = 2S2− + 3H2O | 4 | −0.006 |
| 6 | SO42− + 4H2O + 8e = S2− + 8OH− | 8 | −0,693 |
2.2. Analyses
2.3. XRD Measurements
2.4. Biogas Production
3. Results and Discussion
3.1. The Sorbent Composition
3.2. Sulfide Sorption and Removal
3.3. Results for Biogas Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Qiu, Y.-Y.; Sharma, K.; Shi, T.; Song, Y.; Sun, J.; Liang, Z.; Yuan, Z.; Jiang, F. Hydrogen sulfide control in sewer systems: A critical review of recent progress. Water Res. 2023, 240, 120046. [Google Scholar] [CrossRef] [PubMed]
- Midilli, A.; Ay, M.; Kale, A.; Nejat Veziroglu, T. A parametric investigation of hydrogen energy potential based on H2S in Black Sea deep waters. Int. J. Hydrogen Energy 2007, 32, 117–124. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef] [PubMed]
- Mutegoa, E.; Sahini, M.G. Approaches to mitigation of hydrogen sulfide during anaerobic digestion process—A review. Helyon 2023, 9, e19768. [Google Scholar] [CrossRef] [PubMed]
- Jangam, K.; Chen, Y.-Y.; Qin, L.; Fan, L.-S. Perspectives on reactive separation and removal of hydrogen sulfide. Chem. Eng. Sci. 2021, 11, 100105. [Google Scholar] [CrossRef]
- Oil Field Team, The Oil & Gas Hub. 8 Innovations in Hydrogen Sulfide Removal. 2024. Available online: https://oilfieldteam.com/en/a/learning/Innovations-in-Hydrogen-Sulfide-Removal (accessed on 19 August 2025).
- Pudi, A.; Rezae, M.; Baschetti, M.G.; Signorini, V.; Mansouri, S.S. Hydrogen sulfide capture and removal technologies: A comprehensive review of recent developments and emerging trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- El Brahmi, A.; Abderafi, S. Hydrogen sulfide removal from wastewater using hydrogen peroxide in-situ treatment: Case study of Moroccan urban sewers. Mater. Today Proc. 2021, 45, 7424–7427. [Google Scholar] [CrossRef]
- Alayande, A.B.; Jee, H.; Kang, D.; Jang, J.K.; Chae, K.J.; Hwang, M.H.; Kim, C.; Chae, S.; Kim, I.S.; Chuah, C.Y.; et al. Membrane and adsorption technologies for efficient hydrogen sulfide removal from biogas: A review focused on the advancement of key components. Proc. Saf. Environ. Protec. 2024, 186, 448–473. [Google Scholar] [CrossRef]
- Jusoh, N.; Hassan, T.N.A.T.; Suhaimi, N.H.; Mubashir, M. Hydrogen sulfide removal from biogas: An overview of technologies emphasizing membrane separation. Sep. Purif. Technol. 2025, 373, 133466. [Google Scholar] [CrossRef]
- Vikrant, K.; Suresh Kumar Kailasa, S.K.; Tsang, D.C.W.; Sang Soo Lee, S.S.; Kumar, P.; Giri, B.S.; Ram Sharan Singh, R.S.; Kim, K.H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Gaj, K.; Cichuta, K. Combined biological method for simultaneous removal of hydrogen sulphide and volatile methylsiloxanes from biogas. Energies 2023, 16, 100. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ghanegaonkar, P.M. Hydrogen sulfide removal from biogas using chemical absorption technique in packed column reactors. Glob. J. Environ. Sci. Manag. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.; Choi, H.; Lee, C. A review of technologies for in-situ sulfide control in anaerobic digestion. Renew. Sustain. Energy Rev. 2022, 157, 112068. [Google Scholar] [CrossRef]
- Dutta, P.K.; Rabaey, K.; Yuan, Z.; Keller, J. Spontaneous electrochemical removal of aqueous sulfide. Water Res. 2008, 42, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Beschkov, V.; Razkazova-Velkova, E.; Martinov, M.; Stefanov, S. Electricity Production from Marine Water by Sulfide-Driven Fuel Cell. Appl. Sci. 2018, 8, 1926. [Google Scholar] [CrossRef]
- Stefanov, S.; Uzun, D.; Ljutzkanov, L.; Beschkov, V. Sulfide driven fuel cell performance enhanced by integrated chemosorption and electricity generation. Bul. Chem. Commun. 2024, 56, 379–387. [Google Scholar]
- Velichkova, P.; Bratkova, S.; Angelov, A.; Nikolova, K.; Genova, P.; Ivanov, R. Utilization of Distillery Wastewater in a Microbial Fuel Cell Based on Microbial Sulfate Reduction. J. Ecol. Nat. Resour. 2025, 9, 1–9. [Google Scholar] [CrossRef]
- Suhotin, A.M. Guidebook on Electrochemistry; Himia: Leningrad, Russia, 1981. (In Russian) [Google Scholar]
- Rees, T.D.; Gyllenpetz, A.B.; Docherty, A.C. The determination of trace amounts of sulphide in condensed steam with N-diethyl-P-phenylenediamine. Analyst 1971, 96, 201–208. [Google Scholar] [CrossRef]
- Available online: https://www.crystalimpact.com/match (accessed on 19 August 2025).
- Available online: https://www.icdd.com (accessed on 19 August 2025).
- Available online: https://www.crystallography.net/cod (accessed on 19 August 2025).
- Grazulis, S.; Chateigner, D.; Robert, T.; Downs, R.T.; Yokochi, A.F.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeckg, P.; et al. Crystallography Open Database–an open-access collection of crystal structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Gyawali, R.; Lens, P.N.L.; Lohani, S.P. Technologies for removal of hydrogen sulfide (H2S) from biogas, Chapter 11. In Emerging Technologies and Biological Systems for Biogas Upgrading; Aryal, N., Pant, D., Ottosen, L.D.M., Kofoed, M.V.W., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 295–320. [Google Scholar]
- Fonseca-Bermúdez, Ó.J.; Giraldo, L.; Sierra-Ramírez, R.; Giraldo, L.; Moreno-Piraján, J.C. Removal of hydrogen sulfide from biogas by adsorption and photocatalysis: A review. Environ. Chem. Lett. 2023, 21, 1059–1073. [Google Scholar] [CrossRef]






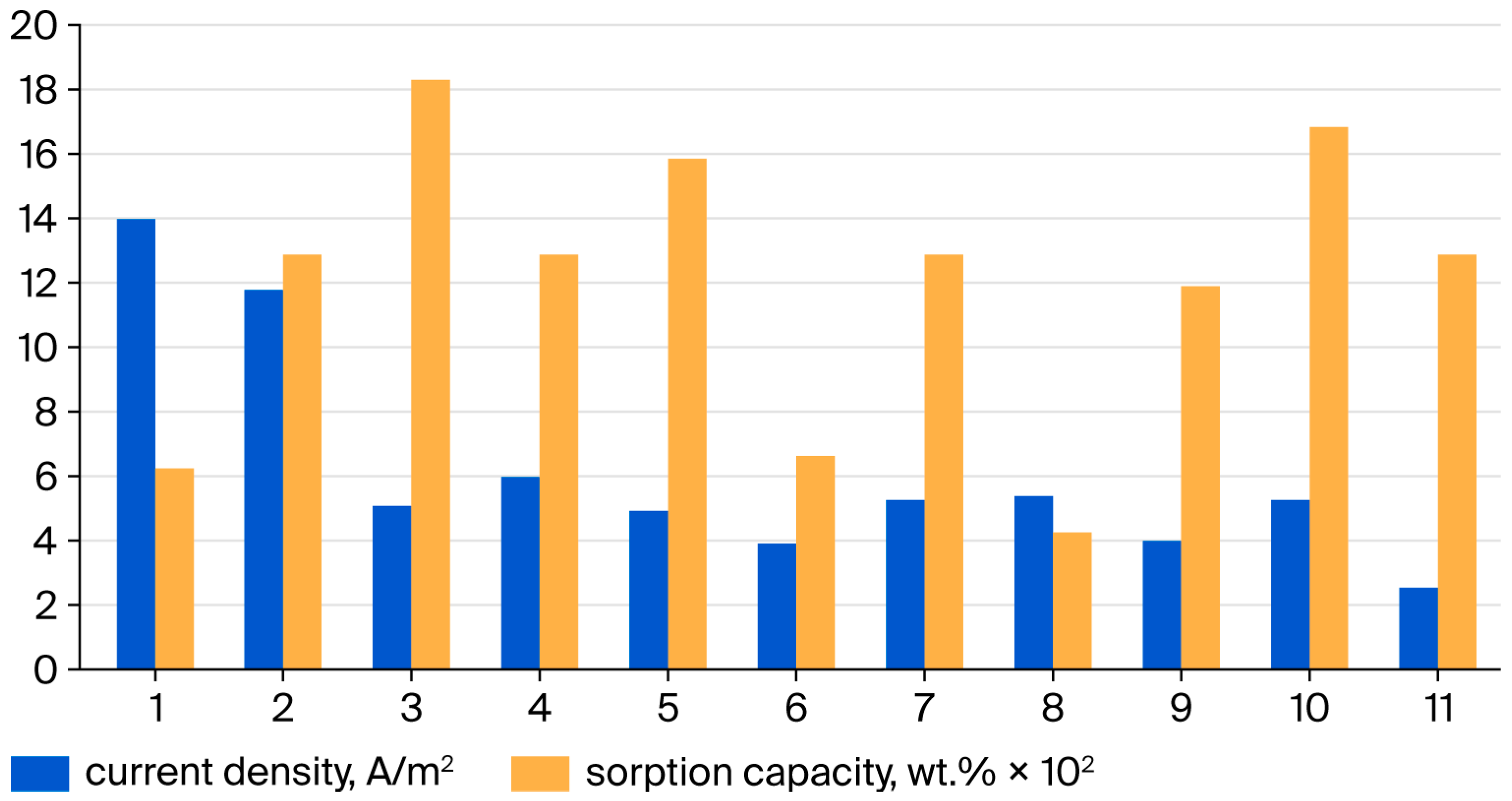


| Substrate | Initial Sulfide Concentration, mg dm−3 | Residual Sulfide Concentration, mg dm−3 | Initial Open Circuit Voltage, V | Maximum Power Density, W m−2 | Process |
|---|---|---|---|---|---|
| Vinasse | 54.3 | <1.0 | 0.330 | 0.132 | Continuous feed |
| Vinasse | 62.3 | <1.0 | 0.373 | 0.159 | Batch |
| Whey | 358 | 0.9 | 0.473 | 0.194 | Batch |
| Whey | 392 | <0.1 | 0.513 | 0.219 | Batch |
| Stillage | 18 | <0.1 | 0.300 | 0.169 | Batch |
| Stillage | 39.7 | <0.5 | 0.342 | 0.169 | Batch |
| Stillage | 79.1 | <0.5 | 0.454 | 0.384 | Batch |
| Average | 0.400 ± 0.080 | 0.204 ± 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beschkov, V.; Angelov, I.; Stefanov, S.; Ljutzkanov, L. An Integrated Sulfide Removal Approach from a Substrate for Biogas Production and the Simultaneous Production of Electricity. Clean Technol. 2025, 7, 77. https://doi.org/10.3390/cleantechnol7030077
Beschkov V, Angelov I, Stefanov S, Ljutzkanov L. An Integrated Sulfide Removal Approach from a Substrate for Biogas Production and the Simultaneous Production of Electricity. Clean Technologies. 2025; 7(3):77. https://doi.org/10.3390/cleantechnol7030077
Chicago/Turabian StyleBeschkov, Venko, Ivan Angelov, Stefan Stefanov, and Ljutzkan Ljutzkanov. 2025. "An Integrated Sulfide Removal Approach from a Substrate for Biogas Production and the Simultaneous Production of Electricity" Clean Technologies 7, no. 3: 77. https://doi.org/10.3390/cleantechnol7030077
APA StyleBeschkov, V., Angelov, I., Stefanov, S., & Ljutzkanov, L. (2025). An Integrated Sulfide Removal Approach from a Substrate for Biogas Production and the Simultaneous Production of Electricity. Clean Technologies, 7(3), 77. https://doi.org/10.3390/cleantechnol7030077






