Abstract
Municipal sewage sludge represents a significant environmental challenge due to its large-scale production and limited disposal options. Pyrolysis, a thermal decomposition process, offers a promising approach for converting sewage sludge into biochar, a carbon-rich material with diverse environmental applications. Sewage sludge-derived biochars were prepared at pyrolysis temperatures of 300 °C, 500 °C, 700 °C, and 900 °C (denoted as B300 to B900) and evaluated for their structural, adsorption, and catalytic performance. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET), and energy dispersive X-ray spectrometry (EDS) analyses revealed a distinct temperature-dependent morphological evolution and mineral exposure. The B900 biochar exhibited a BET surface area of 83.8 m2/g and the highest pore volume of 0.101 cm3/g, indicating a well-developed mesoporous structure. In catalytic degradation tests using 20 mg/L persulfate and 500 mg/L B900, rapid oxidation was observed, achieving 91% methylene blue (MB) degradation in 30 min, highlighting its role in activating persulfate via surface-bound Fe and Al species. Optimization studies confirmed that MB removal efficiency was highest at 500 mg/L biochar and 40 mg/L persulfate, and the system was not significantly affected by the tap and synthetic wastewater matrices. This work demonstrates that biochar obtained from sewage sludge can serve as an eco-friendly and multifunctional material for resource recovery and environmental cleanup.
1. Introduction
Due to the increasing population of Kazakhstan and the rapid pace of urbanization, wastewater generation grows steadily every year in all major cities of the country. Wastewater treatment plants are based on biological processes in which the decomposition of organic pollutants occurs due to activated sludge, which is mainly composed of bacteria and protozoa [1,2]. For efficient wastewater treatment, it is crucial to maintain a certain ratio of activated sludge to the incoming concentration of organic pollutants. As a result, excessive activated sludge is constantly removed from the system, generating large amounts of sewage sludge. In Astana and Almaty, sewage sludge is just dehydrated and disposed of in a solid waste landfill [3,4]. Every day, about 240–250 tons of dehydrated sewage sludge with a moisture content of about 70–75% is disposed of near the capital of Kazakhstan. The storage of biological waste in landfills is not an environmentally friendly solution, as sewage sludge may contain pathogenic microorganisms and organic pollutants absorbed into the cells of the microorganisms [5].
In world practice, thermal utilization is one of the promising methods for biological waste processing as it falls into waste-to-energy solutions. Among them, pyrolysis gained much attention due to the possibility of biochar production [6]. Pyrolysis refers to the breakdown of organic matter by heat under limited or no oxygen conditions, typically occurring at 400 and 900 °C. Pyrolysis is the most suitable method for producing biochar from organic material and has been used to obtain biochar from various biological waste, such as corn cob, pine wood, rice straw, corn straw, and sea mango [7]. Despite its potential, limited studies have focused on producing biochar from sewage sludge, and the efficiency of pyrolysis is strongly influenced by sludge composition, which changes across regions and seasons.
Biological processes used in conventional wastewater treatment may be unable to fully degrade certain persistent pollutants, notably pharmaceuticals, pesticides, surfactants, endocrine disruptors, personal care substances, and multiple additive compounds [8,9]. Despite the low ambient concentration of these organic pollutants, they can adversely affect the environment and aquatic life. In addition, due to their complex structure, they can accumulate in water, soil, and biomass. To remove these substances from the water, an additional wastewater treatment method is required.
Among the emerging strategies for treating refractory wastewater contaminants, sulfate radical-based advanced oxidation processes (SR-AOPs) are considered especially effective. In this approach, active radicals such as sulfate (SO4•−) and hydroxyl (•OH) are employed to degrade organic molecules [10,11]. The sulfate radical is particularly potent, with a redox potential of 2.5–3.1 V and reacts with organic pollutants at remarkably high rate constants between 105 and 109 M−1s−1 [12]. Persulfate salts (S2O82−) are commonly used as radical precursors and are advantageous compared with hydrogen peroxide because of their chemical stability, lower transportation and storage costs, higher radical yield, reduced sensitivity to operational parameters, and flexible activation methods [13,14,15]. A key advantage of SR-AOPs is their environmental safety, as sulfate radicals are eventually reduced to sulfate ions, which are non-toxic and do not require further treatment [16,17].
Carbon materials such as carbon nanotubes, graphene oxide, and biochar are considered as promising alternatives for existing heterogeneous catalysts due to the abundance, high surface area, and low cost of these materials [18]. Therefore, sewage sludge biochar could be utilized as an activator for persulfate (PS) [17]. Biochar is a pyrogenic material and contains persistent free radicals along with hydroxyl, carboxyl, carbonyl, and amino groups, which participate in electron transfer reactions for the generation of sulfate radicals [19]. Previous studies have demonstrated that biochar, when combined with persulfate as a heterogeneous catalyst, can effectively degrade pollutants such as bisphenol A [20], phenolic compounds [19], sulfamethoxazole [21,22], and orange G [23].
Miserli et al. used commercially available biochar with a specific surface area of 459 m2/g and a pore size of 3.78 nm for the removal of the phenolic compounds from wastewater [19]. The authors reported that there was no difference in the removal efficiencies between the biochar/PS and biochar/PS/Fe2+ system, indicating that the biochar was the main activator of the PS. In another research, Lykoudi et al. collected spent coffee grounds and pyrolyzed them at 850 °C to make biochar [21]. The obtained biochar had a surface area of 492 m2/g and was used without any post-treatment. The authors reported that almost complete removal of sulfamethoxazole (2 mg/L) has been achieved using 0.2 g/L biochar and 1 g/L sodium persulfate after 75 min.
Recent studies have demonstrated that the catalytic performance of biochar can be significantly enhanced through various modification strategies. For instance, adjusting pyrolysis conditions, particularly temperature, influences biochar’s porosity, graphitization, and surface chemistry, thereby affecting its reactivity [24]. Chemical activation using acids or bases (e.g., HCl, KOH, NaOH) is another common approach to increase surface area and expose active sites [7,18]. In addition, heteroatom doping or metal incorporation has been widely employed to improve electron transfer and radical generation [25]. Biochar composites with metal oxides or minerals have also been reported as efficient persulfate activators [18]. However, many of these modifications involve additional processing steps and chemical inputs, which may limit large-scale applications. In contrast, sewage sludge offers a sustainable feedstock, as it is naturally enriched with minerals that can act as redox-active sites during persulfate activation. Therefore, sewage sludge-derived biochar provides an opportunity to obtain catalytically active materials without the need for extensive modification, while simultaneously addressing the environmental challenge of sludge disposal.
This paper aims to characterize biochar derived from municipal sewage sludge of the Astana municipal wastewater treatment plant and to use it as a catalyst for persulfate-based degradation of methylene blue (MB). MB was selected as the target contaminant as it is a widely used cationic dye in the textile, printing, and pharmaceutical industries, and is frequently discharged into wastewater. It is highly visible even at low concentrations and is commonly employed as a model organic pollutant in adsorption and advanced oxidation studies [26]. This work aligns with the United Nations Sustainable Development Goals (SDGs), particularly SDG 6 (Clean Water and Sanitation), SDG 11 (Sustainable Cities and Communities), and SDG 12 (Responsible Consumption and Production), by proposing an efficient and circular approach to wastewater treatment and sludge management.
2. Materials and Methods
Sewage sludge was collected from the municipal wastewater treatment plant “Astana su arnasy” in Astana, Kazakhstan. The material was oven-dried at 105 °C overnight and then transferred into sealed ceramic vessels designed to prevent oxygen penetration during heating. These vessels were subsequently placed in a gradient furnace (LH 60/12, Nabertherm GmbH, Lilienthal, Germany) and subjected to thermal treatment at temperatures ranging from 300 to 900 °C.
Thermogravimetric behavior of the biochar was examined using a Simultaneous Thermal Analyzer STA 6000 (Perkin Elmer, Waltham, MA, USA). Morphological features were investigated with an Auriga Crossbeam 540 SEM (Carl Zeiss, Oberkochen, Germany), while microstructural details were further studied by TEM (JEM2010F, JEOL Ltd., Tokyo, Japan). Elemental composition was obtained through EDX using a JEOL JSM-IT200 SEM (JEOL, Tokyo, Japan). Crystalline phases and structural purity were analyzed with a SmartLab X-ray Diffractometer (Rigaku Corp., Tokyo, Japan) employing Cu Kα radiation. Textural properties, including surface area and pore size distribution, were determined by nitrogen adsorption with an Autosorb iQ porosimeter (Quantachrome Instruments, Boynton Beach, FL, USA). Fourier Transform Infrared (FTIR) spectra were collected on a Nicolet iS10 spectrometer (Thermo Fisher Scientific, Madison, WI, USA).
MB solutions (5–50 mg/L) were used as model pollutants. Potassium persulfate (≥98%) acted as the persulfate source. All reagents were analytical grade, and ultrapure water was used in all procedures. In 100 mL beakers, 50 mL of MB solution was mixed with biochar, and persulfate was then introduced to initiate reactions. The mixtures were stirred at ambient temperature, and samples were collected at selected intervals. After filtration through 0.22 µm syringe filters (Thermo Fisher Scientific, Waltham, MA, USA), MB concentrations were analyzed by Thermo Scientific Genesys 150 UV–Vis spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 664 nm. Experiments were run in duplicate, and average results were reported.
3. Results and Discussion
3.1. Characterization of the Sewage Sludge-Derived Biochar
Characterization of the biochars’ physicochemical properties was carried out using several analytical techniques. Figure 1 presents the outcome of the thermogravimetric analysis (TGA) measurements.
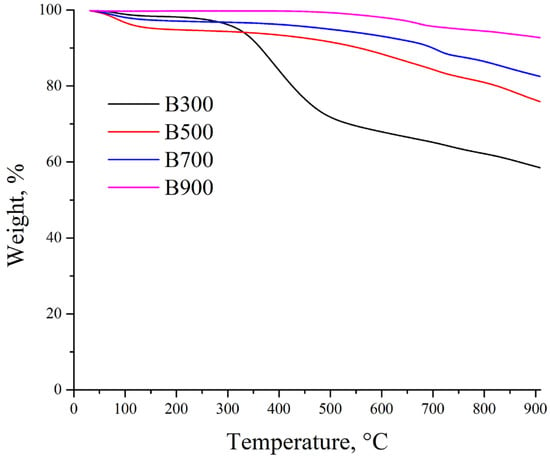
Figure 1.
Thermogravimetric analysis of the biochars.
The presented TGA graph shows the dependence of biochar mass change on temperature, produced from wastewater sludge pyrolysis at various temperatures. As the thermal treatment temperature increases, the biochars exhibit different degrees of thermal resistance, which is associated with the removal of volatile substances and the decomposition/mineralization of organic compounds [27]. The most pronounced weight reduction occurs below 500 °C, primarily attributed to volatile components that remain after low-temperature pyrolysis [28]. For biochar produced at 500 °C, the mass loss is less pronounced compared to B300, as the main volatile components have already been removed by this temperature [28]. The mass loss stabilizes above 600 °C, indicating a higher degree of carbonization and structural stability [29]. Biochar produced at higher temperatures (e.g., 700 °C and 900 °C) shows minimal mass loss throughout the heating interval. The high pyrolysis temperature results in maximum material stabilization due to the complete decomposition of organic impurities [29,30]. With increasing pyrolysis temperature (from B300 to B900), the thermal stability of the biochar significantly improves. Biochar produced at 900 °C is characterized by minimal mass loss, indicating its high degree of carbonization and low volatile content. In contrast, materials produced at lower temperatures (B300 and B500) contain more organic and volatile components, which reduce their thermal resistance.
Surface functional groups of biochars obtained at various pyrolysis temperatures were characterized by FTIR spectroscopy, with the results presented in Figure 2.
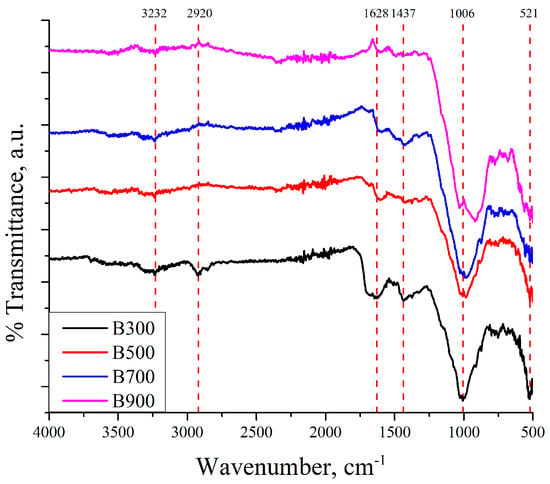
Figure 2.
FTIR analysis of the biochars.
All spectra exhibit characteristic features that reflect the chemical evolution of the biochar structure with increasing temperature. An absorption band centered around 3232 cm−1 is characteristic of hydroxyl functional groups and is assigned to their O-H stretching, likely originating from adsorbed moisture or residual phenolic structures [31]. The peak at 2920 cm−1 corresponds to the asymmetric stretching of aliphatic C-H bonds (-CH2 and -CH3), indicating the presence of residual organic matter, which gradually diminishes with increasing pyrolysis temperature [31,32]. The absorption band at 1628 cm−1 can be attributed either to C=C stretching in aromatic structures or to C=O stretching of conjugated carbonyl groups [33]. The absorption observed at 1437 cm−1 is attributed either to aliphatic C-H bending vibrations or to the asymmetric stretching of carboxylate (COO−) groups [31,34]. With rising temperature, these bands diminish or shift, indicating the breakdown of oxygen-containing groups and the development of condensed, aromatic carbon structures. The intense band near 1006 cm−1 corresponds to Si-O stretching, indicating the retention of silicate minerals from the original sewage sludge [32,35]. The sharp band at 521 cm−1 is typically assigned to metal-O bonds such as Fe-O, Si-O, or Al-O, indicating the retention of inorganic mineral phases in the char matrix [32,35]. In summary, FTIR analysis demonstrates that higher pyrolysis temperatures lead to a gradual disappearance of organic functional groups, while mineral phases remain stable. These findings are consistent with the X-ray Diffraction (XRD) and EDS results.
The XRD patterns of biochar samples (B300, B500, B700, and B900) are shown in Figure 3.
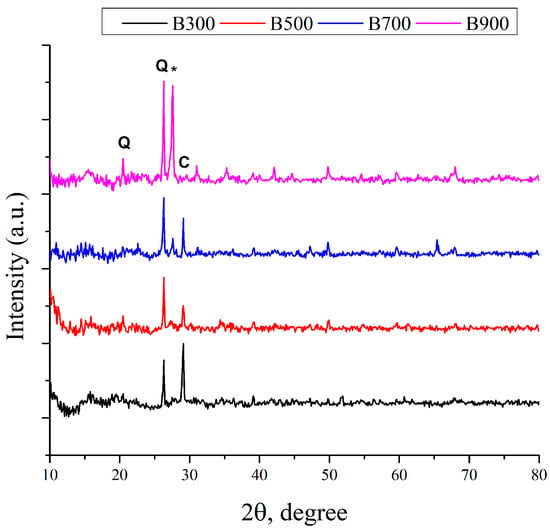
Figure 3.
XRD analysis of the biochar samples: a) B300, b) B500, c) B700, and d) B900 (Q—quartz, *—feldspar-type minerals, and C—calcite).
For comparative purposes, all patterns were normalized to the most intense peak in each sample. As a result, broad and low-intensity features such as the typical amorphous carbon peak around 2θ ≈ 20–25° may not be visible, particularly in the B300 and B500 samples [35]. As the pyrolysis temperature increased, the XRD patterns showed progressively sharper and more intense peaks, indicating the formation of well-defined crystalline mineral phases. In the B900 sample, several strong diffraction peaks were observed. A prominent reflection at 2θ ≈ 26.3° corresponds to the (101) plane of quartz (SiO2), confirming the presence of silica-rich components [25]. The diffraction peak at ~27.5° could be attributed to feldspar-type minerals, which had been previously detected in sewage sludge [36]. Another intense peak at 2θ ≈ 29.1° could be attributed to the (104) plane of calcite (CaCO3) [25,32]. Additional strong peaks at 2θ ≈ 31.0° and 32.7° could be assigned to iron oxide (Fe2O3) and calcium silicate (CaSiO3), respectively [32].
A distinct diffraction peak at 2θ ≈ 35.2° could be attributed to the (311) plane of either magnetite (Fe3O4) [37] or magnesium ferrite (MgFe2O4) [38], suggesting the thermal transformation of iron-containing phases. Minor but well-defined peaks observed at 2θ ≈ 39.1°, 49.9°, and 54.7° could correspond to aluminum oxide (Al2O3), secondary quartz reflections, and mixed Fe/Al oxides [32,39]. These findings were in good agreement with EDS results, which confirmed the presence of major inorganic elements, including Si, Ca, Fe, and Al. Overall, the increase in temperature led to a transition from an amorphous to a crystalline structure, reflecting both mineral reorganization and the partial graphitization of the carbon matrix.
The surface morphology of the biochar samples was investigated using SEM, as shown in Figure 4.

Figure 4.
SEM images of biochar samples: (a) B300, (b) B500, (c) B700, and (d) B900.
The B300 sample exhibited a relatively dense and compact surface with limited porosity, reflecting the incomplete decomposition of organic matter at low temperature [40]. As the temperature rose to 500 °C, the biochar structure became more fragmented and layered, with the appearance of micro-cracks and loosely bound flakes, suggesting partial volatilization and restructuring of organic components [41].
At 700 °C, the biochar developed a noticeably more porous and irregular structure with visible lamellar features and particle agglomerates. In the B900 sample, the morphology became highly porous and rough, with numerous cavities and granular deposits distributed across the surface. These structural changes reflect the progressive carbonization and mineral exposure that occur with increasing temperature, ultimately leading to the formation of a well-developed porous network favorable for surface reactions [26,42]. The observed morphological evolution is consistent with trends in surface area and porosity confirmed by BET analysis. The evaluation of the elemental composition of the biochar samples was conducted using EDS (Table 1).

Table 1.
EDS analysis results.
Carbon and oxygen were the predominant elements in all samples, indicative of a carbonaceous matrix with surface-bound oxygen-containing functional groups. As the pyrolysis temperature rose from 300 °C to 900 °C, the carbon content decreased significantly from 52.74% to 28.28%, while oxygen content showed a slight increase from 33.74% to 40.09%. This trend suggests the progressive loss of volatile organic matter and the relative enrichment of thermally stable inorganic constituents at higher temperatures as temperature controls the elemental composition, organic carbon content, presence of functional groups, and other properties of the biochar [40].
Among the inorganic elements, notable increases were observed for silicon, calcium, aluminum, and iron. Calcium content increased markedly from 1.96% in B300 to 9.28% in B900, likely due to the concentration of calcium phosphates or carbonates. Similarly, silicon rose from 4.25% to 9.62%, and iron from 1.51% to 4.46%, consistent with the accumulation of mineral phases derived from sewage sludge. Aluminum content also increased steadily, reaching 4.13% in B900. Phosphorus exhibited a peak concentration at B500 (3.46%) and then declined, while potassium remained relatively stable. Minor but consistent levels of magnesium and sulfur were also observed. Titanium, though absent at lower temperatures, was detected at 0.36% in both B700 and B900, likely originating from TiO2-containing consumer or industrial waste. Its persistence at high pyrolysis temperatures suggests potential catalytic relevance or involvement in radical generation during persulfate activation. Trace amounts of sodium and chlorine appeared sporadically. The findings suggest that rising pyrolysis temperatures lead to higher mineral content, which increases the physicochemical diversity of the biochars and may improve their catalytic and adsorptive properties. A similar composition of pyrolyzed sewage sludge was reported previously [43].
Figure 5a–h presents TEM images of biochars B300, B500, B700, and B900. These images provide insight into the structural and morphological changes in the biochar samples.
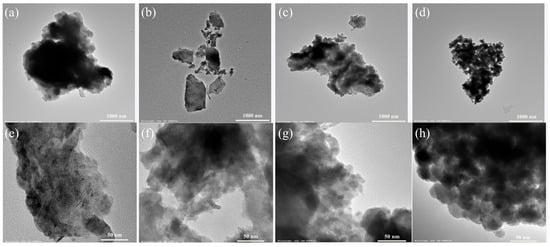
Figure 5.
TEM images of biochar samples: (a,e) B300, (b,f) B500, (c,g) B700, and (d,h) B900.
The B300 biochar displays an aggregated structure with undefined and irregular particle shapes. The particles are primarily compact and lack well-developed porosity. The internal structure appears dense with limited visible pore formation, indicating minimal carbonization due to the low pyrolysis temperature. The B500 biochar reveals a more fragmented structure compared to B300. The particles form irregularly shaped aggregates, and the structure shows a transition toward a more heterogeneous carbon framework.
The B700 biochar exhibits a noticeable increase in structural uniformity and porosity. The particles are more defined and well-separated, indicating significant material degradation and carbonization. The internal structure shows an interconnected network of pores, suggesting enhanced porosity due to further decomposition of organic matter. The B900 biochar shows highly carbonized structures. The particles are compact and exhibit a more defined morphology compared to the other samples. A well-developed pore network is visible, indicating advanced carbonization. The structure is indicative of high thermal stability.
TEM observations reveal that higher pyrolysis temperatures induce gradual structural modifications in the biochars, including greater porosity, clearer particle boundaries, and a higher degree of carbonization. B300 demonstrates an amorphous structure with minimal porosity, while B900 exhibits a highly porous and crystalline structure, consistent with trends reported in previous studies on thermally treated biochars derived from sewage sludge and lignocellulosic materials [26,40,44].
Figure 6 displays the nitrogen adsorption–desorption isotherms of the biochars (B300, B500, B700, B900), with the associated BET surface area and pore volume values provided in Table 2.
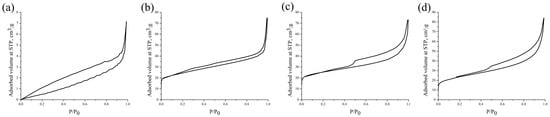
Figure 6.
N2 adsorption–desorption isotherms of the biochar samples: (a) B300, (b) B500, (c) B700, and (d) B900.

Table 2.
BET surface area and pore volume of the obtained biochar samples.
The biochar produced at 300 °C exhibits negligible nitrogen adsorption, corresponding to a low SBET of 3.649 m2/g and the absence of measurable pore characteristics, indicating a lack of developed porosity. Biochar obtained at 500 °C demonstrates a significant increase in nitrogen adsorption, reflected in a SBET of 87.453 m2/g. This increase is accompanied by the development of microporosity, as demonstrated by the pore volume of 0.074 cm3/g. At 700 °C, the nitrogen adsorption is further enhanced, resulting in the highest SBET of 92.33 m2/g. The pore characteristics show a shift toward mesoporosity, with a larger pore volume of 0.079 cm3/g. For biochar produced at 900 °C, nitrogen adsorption remains high, but the BET surface area slightly decreases to 83.801 m2/g. This reduction may be attributed to structural densification or partial pore collapse at elevated temperatures. Nonetheless, B900 exhibits the highest pore volume (0.101 cm3/g), indicating well-developed mesoporosity and enhanced structural stability. The results show that pyrolysis temperatures rising to 700 °C markedly improve the surface area and porosity of the biochars [28]. At 900 °C, the biochar transitions to a more mesoporous structure with lower surface area but higher pore volume, making it potentially suitable for applications requiring large mesoporous volumes and high adsorption capacities.
A similar trend has been reported for biosolid-derived biochars, where the specific surface area increased from 48.3 m2/g to 65.7 m2/g and the micropore volume from 0.031 cm3/g to 0.037 cm3/g as the pyrolysis temperature was elevated in the range of 700–900 °C, confirming that higher temperatures promote improved pore development and surface characteristics [45]. Likewise, Zhao et al. (2019) reported a BET surface area of 31.4 m2/g for sewage sludge biochar produced via slow pyrolysis at 500–550 °C [46]. Fachini et al. (2022) prepared biochar from sewage sludge collected at the Melchior wastewater treatment plant (Brazil) via pyrolysis at 300 °C, achieving a BET surface area of 27.8 m2/g, which is relatively high considering the low pyrolysis temperature [43].
3.2. Adsorption and Catalytic Results
The adsorption capacity of the biochars for methylene blue was assessed during a 120-min period, with the outcomes presented in Figure 7. Experiments were carried out using 500 mg/L of biochar at an initial MB concentration of 10 mg/L in the absence of persulfate. The normalized concentration values (C/C0) indicate a clear dependence on the pyrolysis temperature of the biochar.
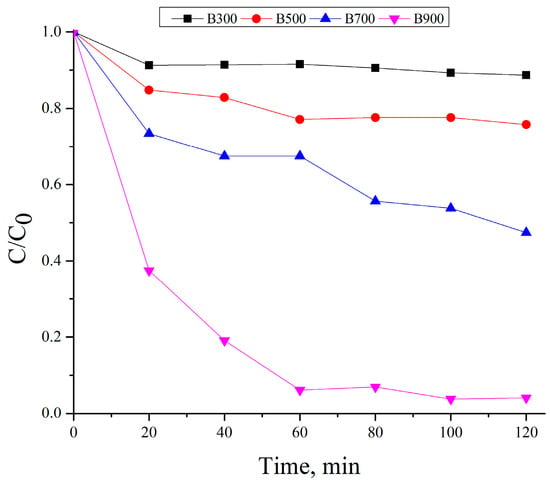
Figure 7.
Time-dependent adsorption of methylene blue (10 mg/L) onto biochar samples B300, B500, B700, and B900 at a fixed dosage of 500 mg/L. C0 is the initial MB concentration, C is the concentration at time t, and C/C0 represents the relative concentration.
B300 exhibited negligible adsorption capacity, with C/C0 remaining above 0.88 throughout the experiment, likely due to its dense, non-porous structure and limited surface functionalization. B500 showed slightly better performance, reaching a final C/C0 of approximately 0.76. A more pronounced adsorption effect was observed for B700, which reached 0.47 after 120 min, indicating enhanced porosity and surface area development. The highest adsorption efficiency was achieved by B900, with C/C0 dropping to 0.04, demonstrating its well-developed porous network and favorable surface characteristics for dye uptake. These results are consistent with BET and SEM analyses and confirm that increasing pyrolysis temperature enhances the adsorption capacity of biochar toward cationic dyes [22,47,48].
Figure 8 displays the results of tests evaluating the catalytic activity of the biochars in persulfate activation for methylene blue removal. The experimental setup included 500 mg/L of biochar, 20 mg/L of PS, and an initial MB concentration of 10 mg/L. A clear enhancement in MB removal was observed across all samples compared to adsorption alone, indicating that the biochars acted as effective catalysts for persulfate activation.
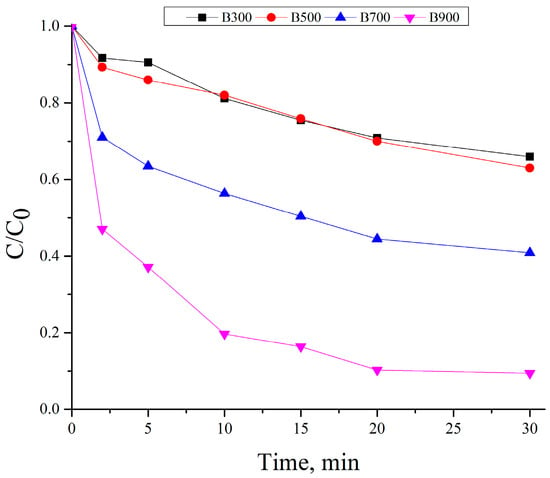
Figure 8.
Time-dependent degradation of methylene blue (10 mg/L) using biochar samples (500 mg/L) and persulfate (20 mg/L) at different pyrolysis temperatures (B300–B900).
In the case of B300 and B500, only modest improvements were observed. B300 showed a gradual decrease in C/C0 from 1.00 to 0.66 over 30 min, while B500 reached 0.63, reflecting limited surface activity and weak PS activation. In contrast, B700 and especially B900 demonstrated significantly accelerated degradation kinetics. B700 achieved a C/C0 of 0.41 after 30 min, while B900 reduced the MB concentration to just 0.09 in the same time frame. This high efficiency is attributed to the well-developed porous structure, increased surface area, and greater exposure of mineral components such as Fe and Al in B900, which may promote electron transfer and radical generation during persulfate activation [25,49]. The clear difference from the adsorption-only tests indicates that persulfate-driven catalytic oxidation is the dominant pathway for MB degradation, especially in biochars produced at elevated pyrolysis temperatures.
The influence of key operational parameters on methylene blue removal using the biochar/persulfate system was evaluated through a series of single-factor experiments, as presented in Figure 9. All tests were conducted for 30 min under standard conditions (10 mg/L MB, 500 mg/L biochar, and 20 mg/L PS), unless otherwise specified.
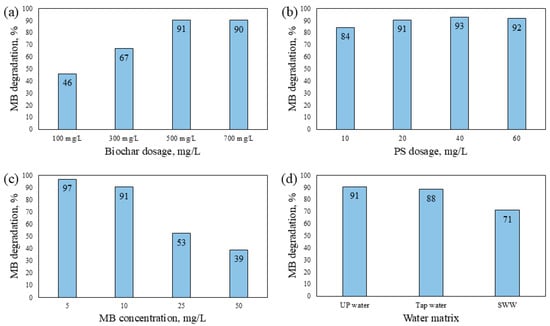
Figure 9.
Effects of operational parameters on methylene blue degradation in the biochar/persulfate system after 30 min: (a) effect of biochar dosage (100–700 mg/L); (b) effect of persulfate dosage (10–60 mg/L); (c) effect of initial MB concentration (5–50 mg/L); (d) effect of water matrix (ultrapure water, tap water, and synthetic wastewater (SWW) containing glucose 150 mg/L, Cl− 50 mg/L, HCO3− 200 mg/L, and NO3− 150 mg/L).
Increasing the biochar dosage from 100 to 500 mg/L significantly enhanced the degradation efficiency from approximately 46% to over 90% (Figure 9a). A further increase to 700 mg/L yielded no substantial improvement, suggesting that sufficient reactive sites and surface area were already available at 500 mg/L. As shown in Figure 9b, MB degradation improved with increasing PS concentration, reaching a maximum of ~93% at 40 mg/L. A slight decrease at 60 mg/L (91.9%) may be attributed to radical self-quenching at excess oxidant concentrations [50,51]. As shown in Figure 9c, degradation efficiency decreased with increasing initial MB concentration: 96.8% removal occurred at 5 mg/L, whereas only 39% was achieved at 50 mg/L, attributed to adsorption site saturation and insufficient radical availability. Lastly, Figure 9d compares different water matrices. High removal efficiencies were maintained in ultrapure (90.6%) and tap water (88.4%), whereas the efficiency decreased to 71.3% in synthetic wastewater (SWW). The reduced performance in SWW can be attributed to the presence of radical scavengers such as glucose (150 mg/L), bicarbonate (200 mg/L), and chloride (50 mg/L), which compete with MB for sulfate and hydroxyl radicals, thereby suppressing degradation [52]. These findings emphasize the importance of optimizing system conditions and accounting for water matrix effects in practical applications.
Table 3 compares the biochar developed in this work with previously reported biochar-based systems, highlighting differences in precursor type, activation methods, and degradation efficiency.

Table 3.
Comparison table.
As shown in Table 3, the performance of the present sewage sludge-derived biochar (91% degradation of MB in 30 min with 20 mg/L PS) is comparable to previously reported biochar samples. This demonstrates that sewage sludge can potentially serve as a precursor for biochar production with a strong catalytic activity.
4. Conclusions
This study investigated sewage sludge-derived biochar samples prepared at different pyrolysis temperatures (300–900 °C) for their adsorption and catalytic activity toward methylene blue degradation via persulfate activation. Increasing the pyrolysis temperature enhanced porosity, mineral crystallinity, and surface activity, with the B900 biochar showing the most favorable characteristics, including a BET surface area of 83.8 m2/g and a pore volume of 0.101 cm3/g. In the absence of persulfate, B900 achieved approximately 96% MB removal within 120 min at a dosage of 500 mg/L (initial MB concentration = 10 mg/L), demonstrating strong adsorption capacity compared to lower-temperature biochars. When combined with persulfate, B900 exhibited excellent catalytic activity, achieving 91% MB degradation within 30 min, which was attributed to the presence of surface-bound Fe, Al, and other mineral phases that facilitated persulfate activation. Optimization experiments revealed that the best MB removal occurred at 500 mg/L biochar and 40 mg/L persulfate, whereas a further increase to 60 mg/L resulted in a slight decline, probably due to radical self-quenching. Importantly, the system maintained high efficiency in both ultrapure water (91%) and tap water (88%), with somewhat reduced performance in synthetic wastewater (71%) as a result of competing scavenging species. Overall, these findings demonstrated that sewage sludge-derived biochar, particularly B900, was a sustainable and effective persulfate activator for rapid dye removal. The results demonstrate that sewage sludge-derived biochar can serve as a sustainable catalyst in advanced oxidation processes, contributing to improved wastewater treatment systems and supporting global efforts toward SDGs 6, 11, and 12.
Author Contributions
Conceptualization, Y.N.K. and S.G.P.; methodology, Y.N.K. and S.G.P.; software, Y.N.K.; validation, Y.N.K., R.T. and M.B.; formal analysis, Y.N.K.; investigation, Y.N.K. and R.T.; resources, Y.N.K. and S.G.P.; data curation, Y.N.K., R.T. and M.B.; writing—original draft preparation, Y.N.K.; writing—review and editing, Y.N.K. and S.G.P.; visualization, Y.N.K.; supervision, Y.N.K. and S.G.P.; project administration, Y.N.K. and S.G.P.; funding acquisition, Y.N.K. and S.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP22684942). S.G. Poulopoulos acknowledges funding from the Nazarbayev University project “Valorization of local kaolin as adsorbent and catalyst for water/wastewater treatment,” Faculty Development Competitive Research Grant Program (General) 2024–2026, Grant Number 201223FD8809.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors gratefully acknowledge the assistance of the engineering staff of the Astana Wastewater Treatment Plant, Kazakhstan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Öfverström, S.; Davidsson, Å.; Haghighatafshar, S.; Kjerstadius, H.; Jansen, J.L.C. Waste Ochre for Control of Phosphates and Sulfides in Digesters at Wastewater Treatment Plants with Enhanced Biological Phosphorus Removal. Clean Technol. 2020, 2, 116–126. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Kakimov, Y.; Adamov, A.; Makhatova, A.; Yeshmuratov, A.; Poulopoulos, S.G.; Inglezakis, V.J.; Arkhangelsky, E. The Effect of Caffeine, Metronidazole and Ibuprofen on Continuous Flow Activated Sludge Process. J. Chem. Technol. Biotechnol. 2021, 96, 1370–1380. [Google Scholar] [CrossRef]
- Ospanov, K.; Myrzahmetov, M.; Zapparov, M. Study of the Products of Pyrolysis Recycling. Sewage Sludge in the Aeration Station Almaty, Kazakhstan. Procedia Eng. 2015, 117, 288–295. [Google Scholar] [CrossRef]
- Andraka, D.; Ospanov, K.; Myrzakhmetov, M. Current State of Communal Sewage Treatment in the Republic of Kazakhstan. J. Ecol. Eng. 2015, 16, 101–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faria, W.M.; Figueiredo, C.C.d.; Coser, T.R.; Vale, A.T.; Schneider, B.G. Is Sewage Sludge Biochar Capable of Replacing Inorganic Fertilizers for Corn Production? Evidence from a Two-Year Field Experiment. Arch. Agron. Soil Sci. 2018, 64, 505–519. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Ahrenfeldt, J.; Henriksen, U.B.; Frandsen, F.J.; Müller-Stöver, D.S. Changes Imposed by Pyrolysis, Thermal Gasification and Incineration on Composition and Phosphorus Fertilizer Quality of Municipal Sewage Sludge. J. Environ. Manag. 2017, 198, 308–318. [Google Scholar] [CrossRef]
- Beckinghausen, A.; Reynders, J.; Merckel, R.; Wu, Y.W.; Marais, H.; Schwede, S. Post-Pyrolysis Treatments of Biochars from Sewage Sludge and A. Mearnsii for Ammonia (NH4-n) Recovery. Appl. Energy 2020, 271, 115212. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Abduvalov, A.; Kaikanov, M.; Poulopoulos, S.G.; Atabaev, T.S. A Review on WO3 Photocatalysis Used for Wastewater Treatment and Pesticide Degradation. Heliyon 2025, 11, e40788. [Google Scholar] [CrossRef]
- Kamal, A.; Kanafin, Y.N.; Satayeva, A.; Kim, J.; Poulopoulos, S.G.; Arkhangelsky, E. Removal of Carbamazepine, Sulfamethoxazole and Aspirin at Municipal Wastewater Treatment Plant of Astana, Kazakhstan: Paths to Increase the Efficiency of the Treatment Process. J. Chem. Technol. Biotechnol. 2024, 99, 2248–2258. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Makhatova, A.; Meiramkulova, K.; Poulopoulos, S.G. Treatment of a Poultry Slaughterhouse Wastewater Using Advanced Oxidation Processes. J. Water Process Eng. 2022, 47, 102694. [Google Scholar] [CrossRef]
- Stathoulopoulos, A.; Mantzavinos, D.; Frontistis, Z. Coupling Persulfate-Based AOPs: A Novel Approach for Piroxicam Degradation in Aqueous Matrices. Water 2020, 12, 1530. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, M.S. Degradation of Ciprofloxacin by UV/S2O82-Process in a Large Photoreactor. J. Photochem. Photobiol. A Chem. 2014, 285, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Hong, J. Sulfate Radical Degradation of Acetaminophen by Novel Iron–Copper Bimetallic Oxidation Catalyzed by Persulfate: Mechanism and Degradation Pathways. Appl. Surf. Sci. 2017, 422, 443–451. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and Treatment of Landfill Leachate: A Review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, N.; Deng, Y.; Yang, Y.; Ma, Y. Ultraviolet (UV) Light-Activated Persulfate Oxidation of Sulfamethazine in Water. Chem. Eng. J. 2012, 195–196, 248–253. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.F.; An, L.; Wu, D.D. The Use of Carbon Materials in Persulfate-Based Advanced Oxidation Processes: A Review. New Carbon Mater. 2020, 35, 667–683. [Google Scholar] [CrossRef]
- Miserli, K.; Kogola, D.; Paraschoudi, I.; Konstantinou, I. Activation of Persulfate by Biochar for the Degradation of Phenolic Compounds in Aqueous Systems. Chem. Eng. J. Adv. 2022, 9, 100201. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of Persulfate with Biochar for Degradation of Bisphenol A in Soil. Chem. Eng. J. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of Sulfamethoxazole with Persulfate Using Spent Coffee Grounds Biochar as Activator. J. Environ. Manag. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of Persulfate by Biochars from Valorized Olive Stones for the Degradation of Sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Li, M.; Sang, W.; Qiu, Y.; Xie, C. Activation of Persulfate by Manganese Oxide-Modified Sludge-Derived Biochar to Degrade Orange G in Aqueous Solution. Environ. Pollut. Bioavailab. 2019, 31, 70–79. [Google Scholar] [CrossRef]
- Simetić, T.; Marjanović Srebro, T.; Apostolović, T.; Anojčić, J.; Đukanović, N.; Mutić, S.; Molnar Jazić, J.; Beljin, J. Biochar as a Catalyst in Persulfate Activation: A Sustainable Approach to Remove Pesticides from Water. Processes 2025, 13, 1856. [Google Scholar] [CrossRef]
- Zang, T.; Wang, H.; Liu, Y.; Dai, L.; Zhou, S.; Ai, S. Fe-Doped Biochar Derived from Waste Sludge for Degradation of Rhodamine B via Enhancing Activation of Peroxymonosulfate. Chemosphere 2020, 261, 127616. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef] [PubMed]
- Călin, C.; Sîrbu, E.E.; Tănase, M.; Győrgy, R.; Popovici, D.R.; Banu, I. A Thermogravimetric Analysis of Biomass Conversion to Biochar: Experimental and Kinetic Modeling. Appl. Sci. 2024, 14, 9856. [Google Scholar] [CrossRef]
- Kujawska, J.; Wojtaś, E.; Charmas, B. Biochar Derived from Sewage Sludge: The Impact of Pyrolysis Temperature on Chemical Properties and Agronomic Potential. Sustainability 2024, 16, 8225. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Thermogravimetric Analysis and Carbon Stability of Chars Produced from Slow Pyrolysis and Hydrothermal Carbonization of Manure Waste. J. Anal. Appl. Pyrolysis 2019, 140, 434–443. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Chen, J.; Li, S. Characterization of Biofuel Production from Hydrothermal Treatment of Hyperaccumulator Waste (Pteris Vittata L.) in Sub- and Supercritical Water. RSC Adv. 2020, 10, 2160–2169. [Google Scholar] [CrossRef]
- Kritikaki, A.; Karmali, V.; Vathi, D.; Bartzas, G.; Komnitsas, K. Advanced Characterization of Biochars Produced from Three Different Organic-Based Feedstocks and Their Potential Applications. Circ. Econ. Sustain. 2025, 1–24. Available online: https://link.springer.com/article/10.1007/s43615-025-00580-w (accessed on 20 April 2025). [CrossRef]
- Zafeiriou, I.; Karadendrou, K.; Ioannou, D.; Karadendrou, M.A.; Detsi, A.; Kalderis, D.; Massas, I.; Gasparatos, D. Effects of Biochars Derived from Sewage Sludge and Olive Tree Prunings on Cu Fractionation and Mobility in Vineyard Soils over Time. Land 2023, 12, 416. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Xiao, Z.; Li, Z.; Zhang, D. Characterization of Biochars Derived from Different Materials and Their Effects on Microbial Dechlorination of Pentachlorophenol in a Consortium. RSC Adv. 2019, 9, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Ariyanti, D.; Widiasa, I.N.; Widiyanti, M.; Lesdantina, D.; Gao, W. Agricultural Waste-Based Magnetic Biochar Produced via Hydrothermal Route for Petroleum Spills Adsorption. Int. J. Renew. Energy Dev. 2023, 12, 499–507. [Google Scholar] [CrossRef]
- Czechowska-Kosacka, A. Application of Sewage Sludge for the Production of Construction. MATEC Web Conf. 2019, 252, 05025. [Google Scholar] [CrossRef]
- Ouyang, D.; Yan, J.; Qian, L.; Chen, Y.; Han, L.; Su, A.; Zhang, W.; Ni, H.; Chen, M. Degradation of 1,4-Dioxane by Biochar Supported Nano Magnetite Particles Activating Persulfate. Chemosphere 2017, 184, 609–617. [Google Scholar] [CrossRef]
- Kadam, R.H.; Alone, S.T.; Bichile, G.K.; Jadhav, K.M. Measurement of Atomic Number and Mass Attenuation Coefficient in Magnesium Ferrite. Pramana-J. Phys. 2007, 68, 869–874. [Google Scholar] [CrossRef]
- Nardis, B.O.; Santana Da Silva Carneiro, J.; Souza, I.M.G.D.; Barros, R.G.D.; Azevedo Melo, L.C. Phosphorus Recovery Using Magnesium-Enriched Biochar and Its Potential Use as Fertilizer. Arch. Agron. Soil Sci. 2021, 67, 1017–1033. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of Pyrolysis Temperature and Heating Time on Biochar Obtained from the Pyrolysis of Straw and Lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The Impact of Pyrolysis Temperature on Biochar Properties and Its Effects on Soil Hydrological Properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Fachini, J.; de Figueiredo, C.C. Pyrolysis of Sewage Sludge: Physical, Chemical, Morphological and Mineralogical Transformations. Braz. Arch. Biol. Technol. 2022, 65, e22210592. Available online: https://www.scielo.br/j/babt/a/TZnv3QqjqDm8WdKN4FF47ZM/?format=html&lang=en (accessed on 20 April 2025). [CrossRef]
- Li, S.; Zhou, Y.; Wang, J.; Dou, M.; Zhang, Q.; Huo, K.; Han, C.; Shi, J. Sewage Sludge Pyrolysis ‘Kills Two Birds with One Stone’: Biochar Synergies with Persulfate for Pollutants Removal and Energy Recovery. Chemosphere 2024, 363, 142824. [Google Scholar] [CrossRef]
- Kim, D.; Hadigheh, S.A.; Wei, Y. Unlocking Biosolid Pyrolysis: Towards Tailored Biochar with Different Surface Properties. Mater. Today Sustain. 2024, 27, 100868. [Google Scholar] [CrossRef]
- Zhao, J.J.; Shen, X.J.; Domene, X.; Alcañiz, J.M.; Liao, X.; Palet, C. Comparison of Biochars Derived from Different Types of Feedstock and Their Potential for Heavy Metal Removal in Multiple-Metal Solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Frigana, A.; Vakros, J.; Frontistis, Z.; Mantzavinos, D. Persulfate Activation Using Biochar from Pomegranate Peel for the Degradation of Antihypertensive Losartan in Water: The Effects of Pyrolysis Temperature, Operational Parameters, and a Continuous Flow Reactor. Catalysts 2024, 14, 127. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, L.; Tang, Y.; Zhou, J.; Shi, B. An Iron–Based Biochar for Persulfate Activation with Highly Efficient and Durable Removal of Refractory Dyes. J. Environ. Chem. Eng. 2022, 10, 106979. [Google Scholar] [CrossRef]
- Guo, L.; Liu, D.; Han, R.; Yin, A.; Gong, G.; Li, S.; Chen, R.; Yang, J.; Liu, Z.; Zhi, K. Advances in Activation of Persulfate by Novel Carbon-Based Materials: Degradation of Emerging Contaminants, Mechanisms, and Perspectives. Crystals 2025, 15, 432. [Google Scholar] [CrossRef]
- Hadi, S.; Taheri, E.; Amin, M.M.; Fatehizadeh, A.; Aminabhavi, T.M. Synergistic Degradation of 4-Chlorophenol by Persulfate and Oxalic Acid Mixture with Heterogeneous Fenton like System for Wastewater Treatment: Adaptive Neuro-Fuzzy Inference Systems Modeling. J. Environ. Manag. 2020, 268, 110678. [Google Scholar] [CrossRef] [PubMed]
- Kanafin, Y.N.; Abdirova, P.; Arkhangelsky, E.; Dionysiou, D.D.; Poulopoulos, S.G. UVA and Goethite Activated Persulfate Oxidation of Landfill Leachate. Chem. Eng. J. Adv. 2023, 14, 100452. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Makhatova, A.; Zarikas, V.; Arkhangelsky, E.; Poulopoulos, S.G. Photo-Fenton-like Treatment of Municipal Wastewater. Catalysts 2021, 11, 1206. [Google Scholar] [CrossRef]
- Giannakopoulos, S.; Vakros, J.; Frontistis, Z.; Manariotis, I.D.; Venieri, D.; Poulopoulos, S.G.; Mantzavinos, D. Biochar from Lemon Stalks: A Highly Active and Selective Carbocatalyst for the Oxidation of Sulfamethoxazole with Persulfate. Catalysts 2023, 13, 233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).