1. Introduction
Over the past five decades, olive oil production has increased by 200% worldwide, with the main producers located in the Mediterranean region. Despite its economic importance, olive mill wastewaters (OMWW) are the primary environmental challenge associated with this growth. Countries like Spain, Italy, Turkey, Greece and Portugal, responsible for over 55% of global olive oil production, are particularly affected by this issue, generating considerable volumes of OMWW annually [
1]. These effluents are acidic, dark in colour, and rich in organic compounds, which, when inadequately managed, pose significant risks to ecosystems [
2,
3]. OMWW are characterised by their high phenolic content, strong phytotoxic and bactericidal effects, and resistance to biodegradation, making them a persistent environmental threat to soil, aquatic systems, and living organisms [
4,
5].
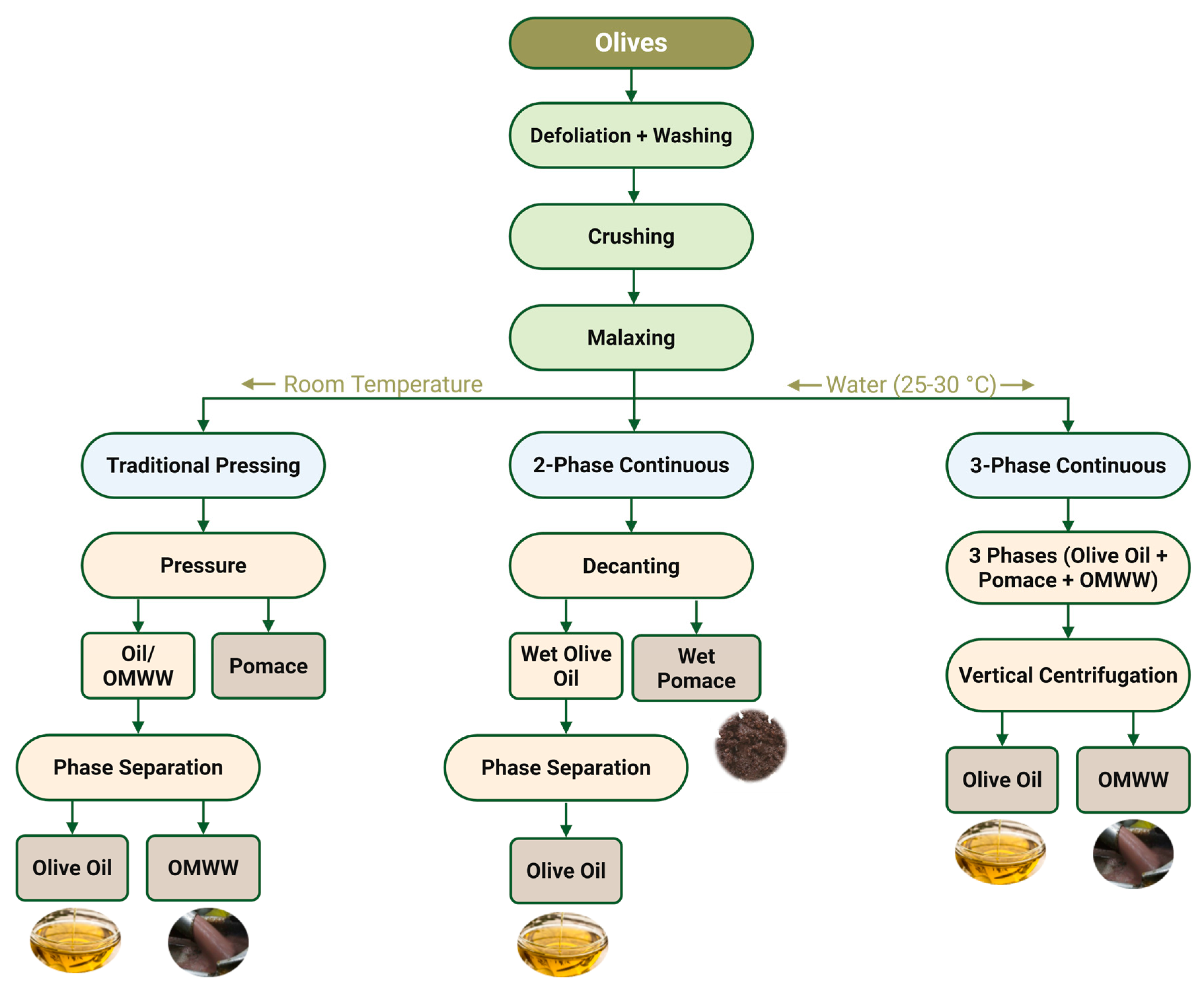
OMWW are a by-product of the olive oil extraction process, wherein only 20% of the olive fruit is converted into oil, leaving the remaining 80% as waste [
5]. This residue, composed of stones, pulp, vegetation water, and processing effluents, is voluminous and chemically complex. Its characteristics are significantly influenced by the extraction method used, with traditional systems generating lower volumes of OMWW but higher concentrations of bioactive compounds. In contrast, continuous systems, which involve additional water input, produce larger volumes of OMWW with diluted properties [
6,
7,
8]. These differences are illustrated in
Figure 1, which compares the traditional discontinuous, the two-phase continuous and the three-phase continuous centrifugal extraction processes.
The choice of extraction method not only influences the physicochemical properties of OMWW but also determines their environmental impact and potential for valorisation. While all major extraction systems (traditional, two-phase, and three-phase) are present in the majority of olive oil-producing countries, the prevalence of each varies regionally. For instance, in Spain, over 95% of olive mills operate with two-phase continuous systems, generating semi-solid residues with high moisture and phenolic contents [
9,
10]. In contrast, Portugal and Italy maintain a significant number of traditional discontinuous systems, especially among small and medium-scale producers [
11,
12]. Morocco and Tunisia also rely heavily on conventional extraction techniques in rural regions [
13,
14]. These regional differences impact not only the volume and composition of OMWW but also the feasibility of treatment and valorisation options. Producers operating on small landholdings, often below 10 hectares, typically lack access to centralised or high-cost treatment infrastructures, reinforcing the need for simple, adaptable pre-treatment strategies aligned with the extraction method in use [
9,
10].
Previous studies have reported substantial variability in the physicochemical properties of OMWW, such as pH, total solids, and phenolic content, depending on the extraction method used in different countries [
15,
16,
17,
18,
19].
Table 1 summarises these variations, highlighting the broader range of values typically observed in OMWW obtained from traditional and three-phase continuous extraction processes.
OMWW contain bioactive compounds that have been used in applications such as biofertilizers, biopolymers, microbial bioproducts, or biogas recovery [
23,
24,
25]. However, their intense pollutant load (high levels of organic matter, acidity, and phenolics) prevents their direct use in agriculture or fermentation without prior treatment [
26]. For this reason, several treatment techniques have been developed to detoxify and enable the valorisation of OMWW, including phenolic adsorption, coagulation/flocculation, or hybrid chemical-biological systems [
27,
28,
29]. Across these approaches, only a few incorporate simple physical pre-treatments like filtration. A recent study combining olive stone filtration with coagulation–flocculation reported <82% TSS removal and moderate COD and phenolic reduction (23–31%) after filtration alone [
30]. These findings suggest that initial low-cost steps can meaningfully reduce pollutant load, yet their systematic influence on OMWW composition remains largely unexamined.
Together, the current evidence shows that while treatment methods are effective, they frequently require high-cost infrastructure or sequential treatments not readily available to small or even medium-sized mills [
4]. Therefore, there is a need to evaluate whether simple filtration alone can modify key physicochemical and biological properties of OMWW to obtain a more accessible valorisation, especially for small-scale production settings in the Mediterranean.
This study offers a novel perspective for OMWW management by focusing on the interplay between extraction methods and low-cost filtration levels, a research topic that remains underexplored. Specifically, the objective is to compare physicochemical properties, biological characteristics, and the preservation or removal of relevant bioactive compounds. By doing so, we aim to support more accessible valorisation approaches for small and medium-scale producers, contributing to more environmentally responsible and economically viable OMWW management in the Mediterranean regions.
2. Materials and Methods
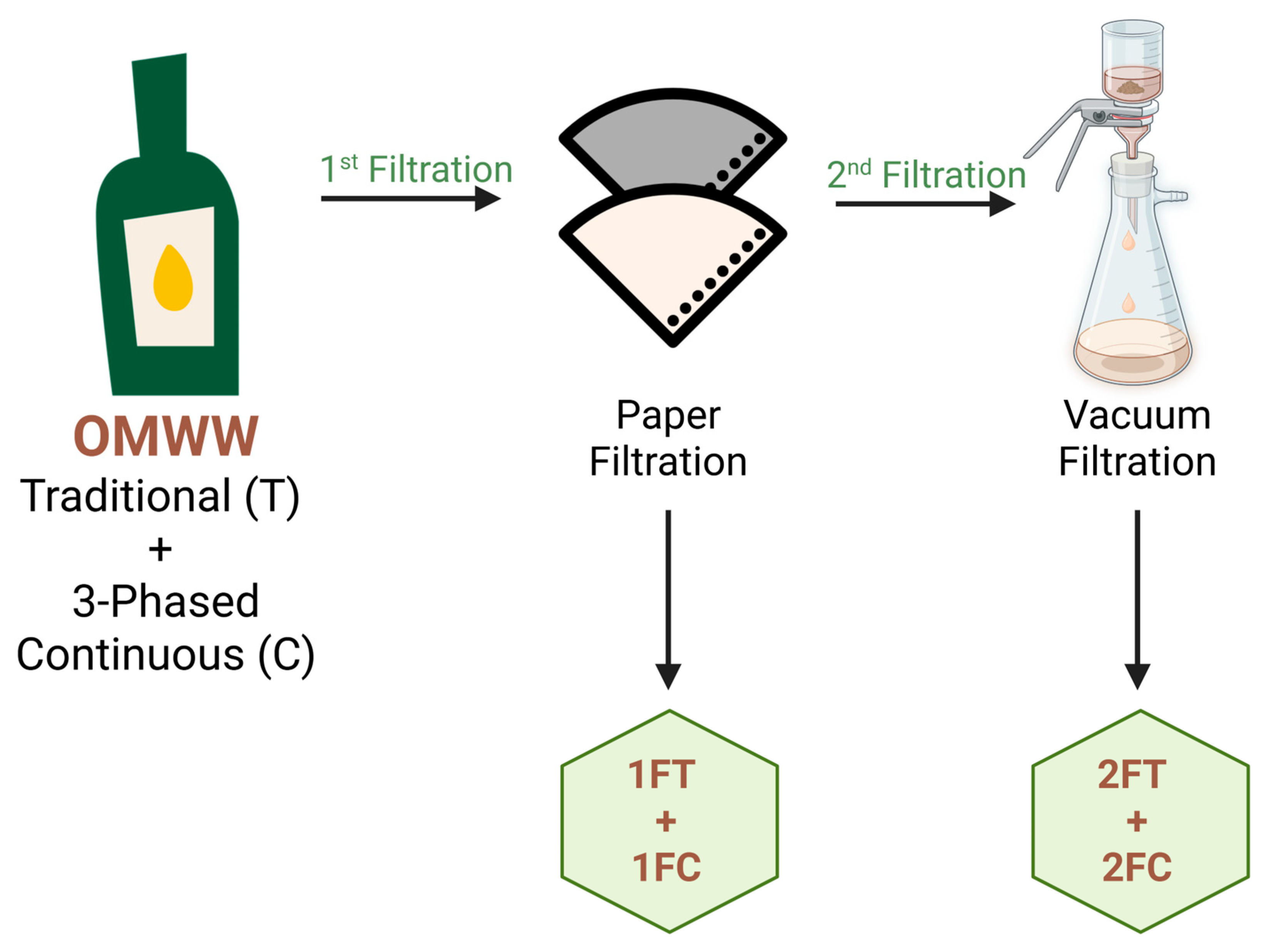
Fresh OMWW samples were obtained from mills located in Macedo de Cavaleiros, northern Portugal, representing two different extraction systems: the traditional discontinuous press (processed at room temperature) and three-phase continuous centrifugal systems (processed at 27 °C). The olive cultivars used were Cobrançosa, Madural, and Verdeal Transmontana, which are predominant in the region of Trás-os-Montes. The samples were collected in sterile polyethylene containers and stored at 4 °C until the analysis. Given their high density, some of the OMWW were submitted to a first filtration using a rough coffee filter No. 4, before vacuum filtration using Whatman No. 32 filter paper with a pore size of 0.45 µm. The samples submitted only to the first filtration were denoted as 1 (1FT for single-filtered traditional OMWW and 1FC for the single-filtered continuous samples) and the samples submitted to both filtrations were referred to as 2 (2FT for the traditional system and 2FC for the continuous system), as illustrated in
Figure 2.
The samples (1FT, 1FC, 2FT, 2FC) were evaluated for their water content (WC), density, pH value, electric conductivity (EC), composition in fatty acids (presented as fatty acid methyl esters (FAME)), acidity, total solids (TS), total suspended solids (TSS), chemical oxygen demand (COD), biological oxygen demand (BOD), biodegradability, total phenolic compounds (TPC), and reducing power (RC). At least three replicates were performed for each parameter.
2.1. Water Content
The WC was determined by automatic titration using a Metrohm 831 Karl Fischer coulometer with Hydranal Coulomat AG (Riedel-de Haen) with an uncertainty of ±0.001 g/g. The analysis was performed after the dilution of samples using methanol as a solvent and adding 250 µL of the sample to 25 mL volumetric flasks. After the calibration of the equipment, four drops of each diluted sample were introduced to the septum of the coulometer.
2.2. Density
The density measurements were carried out using a vibrating tube densimeter (DMA 5000 M, Anton Paar, Ashland, VA, USA) coupled with a U-shaped tube (uncertainty of ±5 × 10−5 g/cm−3). The densities were measured at 0.1 MPa. All the measurements were preceded by a calibration step with ultra-pure water and air, and the solvent used to prepare the solutions was methanol.
2.3. pH Value
The pH measurement was carried out using a multiparameter meter (HANNA edge, 230V, Woonsocket, RI, USA), with an uncertainty of ±0.01. Values were measured between 23.7 °C and 24.4 °C. All the measurements were preceded by a calibration step with buffer solutions with pH values of 4.0, 7.0, and 10.0. After each measurement, the electrode was washed in deionized water.
2.4. Electric Conductivity
The electric conductivity (EC) measurements were carried out using a similar procedure and the same equipment used for the pH value measurements. The measurements were performed from 14.2 °C to 15.9 °C, with uncertainty within ±1 mS/cm2.
2.5. Fatty Acids Analysis
The organic phase of the OMWW samples (150 mL) was extracted using hexane, and the solvent was subsequently removed using a rotary evaporator (Buchi R-114, Buchi, Spain). The dried extract was then derivatised with boron trifluoride (BF
3) in methanol to convert fatty acids into their methyl esters (FAMEs). The analysis was performed using a gas chromatography (GC) system (Nexis GC-2030, Shimadzu, Tokyo, Japan), equipped with a flame ionisation detector, an autoinjector AOC-20i, and an OPTIMA Biodiesel (30 m × 0.25 mm × 0.23 μm) capillary column, following the EN 14103/2003 Standard [
31]. All the individual FAME compounds were identified by comparing the chromatograms of the obtained samples with the obtained chromatogram for the Standard 37 Component FAME mix (Supelco, CRM47885, Bellefonte, PA, USA), using the same operation conditions and the same equipment. For comparison purposes, in addition to the 4 OMWW extracts (1FT, 1FC, 2FT, 2FC), the corresponding olive oils were also analysed.
After the identification of the peaks in the sample, the percentage of FAME was determined using Equation (1).
where
is the total peak area of all methyl esters from C4:0 to C24:1n9,
is the peak area corresponding to methyl heptadecanoate (IS),
is the concentration (mg/mL) of the IS solution,
is the volume of the IS solution, and
is the mass, in milligrams, of the olive oil sample or OMWW extract.
2.6. Acidity
The acidity of the samples was measured by titration, based on the
EN 14,104 [
32] Standard, using an alcoholic potassium hydroxide solution. The acid value (AV) was determined using Equation (2).
where
is the volume of the titrant (mL),
is the concentration of the titrant (mol/L),
is the molar weight of the titrant (56.1 g/mol), and
is the mass of olive oil samples (g).
2.7. Solid Content
The solids content was determined for the TS and TSS contents. TS include any dissolved salts and solid particles, and a high level indicates a large amount of solid material is present in the liquid sample, while
TSS represent all mineral and organic particles in the olive oil mill wastewaters, and a large concentration can be regarded as a form of pollution. TS and TSS measurements were carried out according to the 2540/-B procedure of the Standard Methods for the Examination of Water and Wastewater [
32], where 20 mL of OMWW samples were taken and transferred to a previously weighted crucible. The content of the
TS, in g/L, is obtained from Equation (3).
where
m2 represents the weight of the crucible with the OMWW after drying at 105 °C (g),
m1 is the weight of the empty crucible (g), and
V is the volume of the sample (mL). For TSS,
m2 corresponds to the mass of the filter with the dried residue (g) and
m1 the mass of the empty filter (g).
2.8. Chemical Oxygen Demand
The COD of OMWW samples was determined according to the 5220/-C procedure of the Standard Methods for the Examination of Water and Wastewater [
32], using a closed reflux method. Before the analysis, OMWW samples were diluted to ensure absorbance values were within the calibration range, where the traditional mill OMWW were diluted using a factor of 1:10 and 1:50 and the samples from the 3-phase mill were diluted 1:100 and 1:250 times, with distilled water. A digestion solution containing potassium dichromate and sulfuric acid with silver sulphate was added to the samples, which were then heated to 150 °C for 2 h under reflux. After cooling, COD values were measured spectrophotometrically (Jasco V530, Tokyo, Japan) and analysed using the WinASPECT software, where the results provided were then adjusted with a linear calibration curve:
(
2.9. Biological Oxygen Demand
The BOD over five days (BOD
5) was determined following the 5210/-B. procedure of the Standard Methods for the Examination of Water and Wastewater [
32]. OMWW samples were first neutralised and diluted using an oxygen-saturated dilution water containing an inoculum of aerobic microorganisms. For each sample, 22.7 mL of diluted OMWW was transferred to OxiTop bottles, along with sodium hydroxide tablets to absorb CO
2. The bottles were incubated at 20 ± 1 °C for five days under continuous agitation. The decrease in dissolved oxygen was monitored using an OxiTop respirometric system (OxiTop OC 100, WTW, Sao Paulo, Brazil), in which the evolution of the total pressure of a closed vessel is monitored over the days of the test. Equation (4) demonstrates the progress of oxygen being consumed by converting the pressure provided by the sensors, in mg/L:
where
is the dissolved oxygen of the diluted sample immediately after preparation [mg/L],
is the dissolved oxygen of the diluted sample after 5 days incubation at 20 °C [mg/L], and
is the decimal volumetric fraction of the used sample.
2.10. Biodegradability
Biodegradability corresponds to the BOD/COD ratio and measures the capacity for the biological degradation of organic materials by living organisms down to base substances such as water, carbon dioxide, methane, basic elements, and biomass. The biodegradability measurements considered that the BOD
5 values were smaller than the COD ones. The biodegradability ratio was determined according to Equation (5).
2.11. Total Phenolic Content
The TPC of OMWW samples was determined using the Folin–Ciocalteu method [
33]. The lyophilised aqueous phase was reconstituted in water, mixed with Folin–Ciocalteu reagent and sodium carbonate, and incubated at 40 °C for colour development. After centrifugation, absorbance was measured at 765 nm using a microplate reader (Epoch 2, Biotek). TPC values were quantified using a gallic acid calibration curve and expressed as mg gallic acid equivalents per gram of lyophilised extract (mg GAE/g extract). Some of the TPC and antioxidant activity data were previously published in a study focused on the antimicrobial evaluation of OMWW by our research team [
8]. They are reanalysed here in a broader physicochemical context, with added statistical analysis.
2.12. Reducing Power
The reducing power of OMWW extracts was determined using the potassium ferricyanide method [
34]. Serial dilutions of each sample were prepared (from 1.00 to 0.0156 mg/mL). Then, 500 µL of the extract was mixed with 500 µL of sodium phosphate buffer (0.2 mol/L, pH 6.6) and 500 µL of potassium ferricyanide solution (1%
w/
v). The mixture was homogenised and incubated at 50 °C for 20 min. After cooling, 500 µL of trichloroacetic acid (10%
w/
v) was added, and the mixture was centrifuged at 12,000 rpm for 2 min.
Subsequently, 1 mL of the supernatant was transferred to a test tube and mixed with 1 mL of deionised water and 200 µL of ferric chloride solution (0.1% w/v). Absorbance was measured at 690 nm using a microplate reader (Epoch 2, BioTek Instruments, Winooski, VT, USA). A blank was prepared using distilled water instead of the sample extract. The reducing power was expressed as IC50 (the extract concentration corresponding to 0.5 absorbance), calculated from the linear regression curve of absorbance versus concentration. Trolox was used as a positive control.
2.13. Statistical Analysis
Results were expressed as means ± standard deviation (S.D.) (n = 3). A two-way analysis of variance (ANOVA) was performed using Minitab® 17 (Minitab LLC, State College, PA, USA) to evaluate the effect of the extraction system (traditional or continuous) and the number of filtration steps (one or two), as well as their interaction. When significant differences were detected (p < 0.05), Tukey’s post hoc test was applied to identify statistically different groups. For parameters where only the extraction system was tested, one-way ANOVA, followed by Tukey’s test, was used.
3. Results and Discussion
3.1. Analytical Parameters
Table 2 reports the analytical data for the filtered OMWW, highlighting the statistical significance of the effects of the extraction system, filtration level, and their interaction.
Table 3 presents the main physicochemical parameters of the unfiltered samples, enabling a baseline comparison between traditional and three-phase continuous extraction methods.
3.2. Water Content
WC differed significantly between extraction systems and filtration levels (
p < 0.05), as shown in
Table 2. The continuous system consistently exhibited higher WC, with the highest value observed in the double-filtered sample (2FC, 98.37%), and the lowest in the single-filtered traditional sample (1FT, 91.63%). This pattern reflects the greater water addition inherent to the three-phase extraction process. Within each system, increased filtration was associated with a slightly higher water content, likely due to the removal of TSS.
Although the measured values exceed the typical ranges reported in the literature for raw OMWW (65–89%) [
35,
36], this variation may result from regional cultivars, olive maturity or milling conditions. From a practical standpoint, a higher WC can ease downstream separation processes, though it may dilute compounds of interest.
3.3. Density
As illustrated in
Table 2, OMWW density was significantly influenced by the extraction method (
p < 0.05) but not by the filtration level. The traditional system produced denser samples, with values ranging from 1.030 to 1.3032 g/cm
3, while continuous OMWW presented lower densities between 1.019 and 1.021 ± 0.00126 g/cm
3. Once again, these results reflect the higher content of suspended and dissolved solids typically found in traditional OMWW, as less water was added during processing.
Bouknana et al. [
37] conducted a study on the density of OMWW samples obtained from 21 different mills in eastern Morocco, focusing on the type of mill used. Their findings align with the results of the present work, with densities ranging from 1.001 to 1.050 g/cm
3. The highest density value, 1.05 g/cm
3, was observed in the samples obtained through the three-phase extraction process. Interestingly, this process also exhibited a wide range of density values, including some similar to those obtained through the traditional extraction process, which were close to 1.00 g/cm
3.
Understanding density is important for practical applications, as it influences the sedimentation rates and separation efficiency during OMWW treatment. The slight variation between extraction systems reinforces the importance of tailoring downstream processes to the specific physicochemical profile of each effluent type.
3.4. pH Value
The pH values of the OMWW samples had a narrow range, between 4.71 and 4.74, with no statistically significant differences observed between extraction systems, filtration levels, or their interaction (
Table 2). This consistency indicates a stable acidic profile across all treatments. These findings are consistent with previous studies that also reported more acidic effluents from traditional mills. Specifically, the pH range for the traditional OMWW falls between 4.7 and 5.7, as documented by Di Giovacchino [
38]. For the continuous OMWW, the pH range is slightly broader, from 4.6 to 5.9. These values are in agreement with those reported by Fiestas and Borja [
39], with a pH range of 4.3 to 4.8 for the traditional and 4.6 to 5.3 for the continuous OMWW.
The relatively acidic nature of OMWW, with pH values typically below 6, indicates the presence of organic acids and other acidic compounds. This acidity can pose challenges for direct disposal and requires neutralisation or other treatment processes to mitigate potential environmental impacts [
40]. However, the low pH could be advantageous in scenarios where microbial growth needs to be suppressed, such as in antimicrobial applications.
3.5. Electric Conductivity
EC varied significantly with both the extraction system and the filtration level (
p < 0.05), as shown in
Table 2. Traditional OMWW samples presented higher EC values (up to 20.31 mS/cm in 1FT), while the lowest was recorded in the double-filtered continuous sample (2FC, 13.87 mS/cm). These differences reflect the lower water input and higher concentration of dissolved ions typically found in traditional systems. The filtration levels did not significantly influence the EC values, suggesting that most ionic species remain in solution regardless of the filtration method. These findings highlight the role of extraction processes in determining ionic concentration in OMWW, consistent with previous studies. Elabdouni et al. [
18] found 18–25 mS/cm for the traditional OMWW and 16–22 mS/cm for the continuous OMWW. Aires [
17] reported 65–128 mS/cm for the traditional OMWW and 13–118 mS/cm for the continuous OMWW, and Bouknana et al. [
37] found 13–55 mS/cm for the traditional OMWW and 23–41 mS/cm for the continuous OMWW.
EC serves as a valuable indicator of the level of mineralisation in OMWW. High EC values indicate a high concentration of dissolved salts and other inorganic substances, which can impact both the environmental discharge quality and the treatment processes [
37]. However, these effluents could also serve as a source of recoverable salts and minerals if appropriate treatment and recovery methods are applied.
3.6. Fatty Acids Methyl Esters Content
FAMEs are crucial components in the analysis of OMWW, as they provide insights into the lipid composition and potential oxidative stability of these effluents. The GC-FID analysis revealed distinct FAME profiles across OMWW samples, influenced by both the extraction method and filtration level (
Figure 3). In traditional OMWW, the most abundant methyl esters were oleic acid (C18:1n9), palmitic acid (C16:0), and linoleic acid (C18:2n6). In contrast, continuous samples contained stearic acid (C18:0), oleic acid, and palmitic acid as major components.
Comparing filtered samples to their corresponding olive oils, traditional OMWW showed greater similarity in FAME composition than continuous samples. This suggests that traditional extraction retains more lipid-rich fractions, likely due to lower mechanical stress and water dilution. Filtration level also influenced FAME profiles, since double-filtered samples exhibited lower overall FAME content, particularly in traditional OMWW, likely due to the removal of lipid-bound solids.
This variation aligns with previous studies reporting that mechanical intensity and water addition during extraction can alter the partitioning of lipophilic compounds [
41]. However, Bouknana et al. [
37] reported that filtration reduces suspended solids but can lead to the loss of some bioactive compounds. Similarly, Amaral et al. [
15] highlighted that different filtration levels influenced the COD and BOD values, indicating that filtration can change the biodegradability and potential environmental impact of OMWW. The presence of significant FAME levels underscores the potential of OMWW for valorisation pathways, including biodiesel production, as oleic acid is highly stable and suitable for biofuel applications [
42]. However, the variation in FAME profiles between traditional and continuous systems highlights the need for different approaches that maximise resource recovery from these effluents.
3.7. Acidity
Acidity levels varied significantly between extraction systems and filtration levels (
p < 0.05), with the highest value recorded in the single-filtered traditional sample (1FT, 10.57 mg KOH/mg OMWW) and the lowest in the double-filtered continuous sample (2FC, 3.12 mg KOH/mg OMWW) (
Table 2). Although the interaction effect was not statistically significant, the influence of the extraction method was particularly prominent.
The higher acidity in traditional OMWW is a result of hydrolytic reactions that occur during the pressing of olives, where enzymes such as lipases break down triglycerides into free fatty acids (FFA). In contrast, the continuous system has higher water volumes and mechanical forces that may dilute these fatty acids or alter the reaction kinetics, resulting in lower acidity levels.
These findings deviate significantly from those reported in previous studies (1.190–1.755) [
37,
43]. The higher acidity levels observed in our study can be attributed to the presence of solid parts in the single-filtered samples, which retain more acidic components, such as phenolic compounds. This is supported by studies that identified these compounds as major contributors to the acidity of OMWW. For example, Benitez et al. [
44] and Bouknana et al. [
37] have highlighted that the solid fraction of OMWW, which includes suspended solids and other particulates, often contains concentrated amounts of phenolic compounds. These compounds are known for their acidic nature and contribute significantly to the overall acidity of the OMWW. Furthermore, self-oxidation reactions and phenolic compound polymerisation during storage may further increase acidity, as evidenced by the noticeable change in colour from an initial dark black hue, likely due to self-oxidation and phenolic compound polymerisation during storage. From an application perspective, high acidity may limit the use of OMWW in agronomic contexts without pH adjustment but may enhance its antimicrobial potential due to the combined presence of FFAs and phenolics.
3.8. Solid Content
TS and TSS were measured in unfiltered OMWW samples to assess the baseline load before filtration (
Table 3). TS values were higher in the traditional system (74.5 mg/L) compared to the continuous system (43.8 mg/L), with a statistically significant difference (
p < 0.05). In contrast, TSS values were similar between systems, with 33.3 mg/L for traditional and 29.3 mg/L for continuous OMWW, showing no significant difference.
The elevated TS content in traditional OMWW reflects a greater presence of both dissolved and particulate material, which is consistent with the lower water dilution and less efficient solid–liquid separation. Although TSS values did not differ significantly, their relatively high levels in both systems highlight the importance of filtration in reducing solid loads.
These findings agree with previous reports showing that traditional extraction tends to yield OMWW with higher solid concentrations [
15,
17,
37]. From a treatment standpoint, a high solids content can hinder sedimentation and clog filtration units, but also indicates a higher potential for resource recovery, particularly through composting or anaerobic digestion of the organic-rich fraction [
45].
3.9. Chemical Oxygen Demand
COD indicates the amount of dissolved oxygen needed to oxidise organic substances in wastewater. High COD levels in OMWW suggest a significant presence of oxidizable organic matter, which can pose environmental pollution risks if discharged untreated. Dark coloured OMWW samples were diluted for spectrophotometer measurements. As a result, a 10-fold dilution sufficed for the conventional samples to be read in the spectrophotometer, whereas a minimum 100-fold dilution was required for the continuous OMWW samples. The COD values for the traditional OMWW were higher, with 93.361 g/L for the 10-fold diluted sample and 90.627 g/L for the 50-fold diluted sample. In contrast, the 100-fold diluted continuous OMWW yielded COD results of 63.500 g/L and 75.562 g/L (
Table 3). This trend is consistent with the lower water addition in traditional systems, resulting in more concentrated organic matter in the effluent.
Despite the lack of statistical significance, when compared with the values reported in the literature, it is evident that the COD values do not follow a consistent pattern, suggesting that the extraction method may not significantly influence COD levels. For instance, the studies by Amaral et al. [
15] and Bouknana et al. [
37] reported varying COD values, highlighting the influence of regional and processing differences on COD levels.
3.10. Biological Oxygen Demand
The BOD
5 results revealed a substantial organic matter content in the OMWW samples, with values of 23 g O2/L for the traditional OMWW and 20.5 g O2/L for the continuous OMWW, as shown in
Table 3, indicating no statistically significant difference. These results align with the COD findings, which also indicated higher organic matter levels in the traditional samples, though the discrepancy in BOD was not as pronounced as that in COD. Traditional OMWW tend to retain more fermentable material due the to lower water dilution and the inclusion of suspended solids, which may explain the slightly higher BOD. Nevertheless, the relatively small difference suggests that both systems generate effluents with similarly high biological loads.
From an environmental perspective, high BOD values pose a risk of oxygen depletion in receiving water bodies, reinforcing the need for treatment before discharge. On the other hand, these results demonstrate the potential of OMWW for anaerobic digestion or other biological valorisation routes, where the organic content can be converted into biogas or biomass [
25,
46].
3.11. Biodegradability
The BOD/COD ratio for the continuous OMWW was around 25% and 29.5% for the traditional samples (
Table 3). These findings indicate that, although OMWW contain organic matter that can undergo biological degradation, a significant portion remains resistant to microbial breakdown.
The limited biodegradability may be attributed to the presence of recalcitrant compounds, such as certain phenolics, that inhibit microbial activity [
47]. Also, the presence of complex organic molecules that are not easily broken down by microorganisms further contributes to the lower biodegradability [
2]. Traditional extraction methods may also lead to higher concentrations of these recalcitrant compounds when compared to continuous methods [
6]. The results we obtained are comparable but generally higher than those reported in some studies in the literature. For instance, Amaral et al. [
15] found similar biodegradability levels, while Bouknana et al. [
37] reported lower ratios, suggesting variability based on regional and methodological differences.
3.12. Total Phenolic Content
The gallic acid calibration curve was calculated from the obtained values for the absorbance at 765 nm, using a standard gallic acid solution with a defined concentration and respective dilutions (, ).
The TPC varied significantly with the extraction system and the interaction between system and filtration level (
p < 0.05), as shown in
Table 2. Traditional OMWW exhibited higher TPC values than continuous samples, with the maximum observed in the single-filtered traditional sample (1FT, 29.81 mg GAE/g OMWW), where GAE represents Gallic Acid Equivalents. In both systems, double filtration slightly reduced the TPC, likely due to the removal of phenol-rich particulates.
These results confirm that traditional extraction retains more phenolic compounds, consistent with the lower dilution and less intensive mechanical processing. The reduction in TPC with increased filtration is in line with reports suggesting that phenolics may bind to suspended solids and be partially removed during fine filtration.
A study by Dali et al. [
48] discovered that the ranges of TPC from OMWW in different regions of Tunisia varied between 79.10 and 216.10 mg GAE/g of dry matter. Another study by Gueboudji et al. [
49] found those values to be around 950 µg GAE/mg of extract. Previous studies [
15,
16,
17,
18,
37,
43] have reported TPC ranging from 0.40 to 14.30 g/L, varying with the type of extraction. These values were not directly compared with the ones found in this work. However, when considering an average density of approximately 1 g/cm
3 for OMWW, their findings align closely with our results, highlighting similar ranges of phenolic content.
Phenolic compounds are known for their antioxidant and antimicrobial properties. The higher TPC in traditional OMWW indicates a greater potential for applications in pharmaceuticals, food preservation, or agricultural biostimulants. However, the presence of these bioactive compounds also poses challenges for wastewater treatment due to their recalcitrant nature. It is also known that the phenolic composition of OMWW depends not only on the variety, fruit maturity, and climatic conditions but also on the technological processes used to separate the aqueous phase from the oil phase. Consequently, the discrepancies between the results obtained in this study and those reported in the literature may be attributed to these variations.
3.13. Reducing Power
The RP of the OMWW extracts was evaluated by measuring the absorbance of the reaction mixture at 690 nm, with IC
50 values corresponding to the extract concentration required to reach an absorbance of 0.5 (
Table 2). All samples demonstrated reducing activity, with differences observed between extraction systems and filtration levels. Among the traditional extracts, 1FT (
,
) presented the highest reducing capacity (IC
50 = 0.337 mg/mL), followed by 2FT (
,
). In the continuous system, 2FC (
,
) showed slightly better performance (IC
50 = 0.359 mg/mL) than 1FC (
,
).
These results partially reflect those observed in the DPPH assay from our previous studies, where 2FC also exhibited strong antioxidant activity [
8]. However, in the RP assay, 1FT performed better, suggesting that different antioxidant compounds or mechanisms may be favoured in each method. The observed variability may be attributed to the differences in extraction method and filtration, which can affect the retention or removal of bioactive phenolic fractions. The overall pattern suggests that both the extraction method and filtration level influence antioxidant potential, likely through the retention or removal of phenol-bound solids.
Figure 4 illustrates the concentration–response profiles of all samples, including Trolox as a reference standard.
3.14. Integrated Discussion
This study aimed to determine whether simple filtration steps, applied to OMWW using both traditional and three-phase continuous extraction systems, could significantly influence their physicochemical and biological properties. While most parameters were primarily affected by the extraction method, filtration also had measurable effects, particularly on solid content, acidity, EC, and antioxidant potential.
The unfiltered OMWW samples showed substantial solid content, particularly in the traditional system. While the effect of filtration on solids was not measured directly, it is reasonable to assume that the removal of suspended material through sequential filtering contributed to the clearer appearance of the extracts used in subsequent analyses. This is relevant from a practical perspective, as high particulate loads are known to increase treatment complexity and cost. Slight reductions in TPC and RP were also observed with double filtration, suggesting that some bioactive compounds may be partially retained in the removed solid fraction. However, the magnitude of these reductions was moderate and may be acceptable depending on the intended application.
From an operational perspective, the two-step filtration used in this study relies on widely available materials: commercial coffee filters (first step) and Whatman No. 32 filter paper (second step). While laboratory-grade filters can be costly in bulk, their use here remains affordable at a small scale. Filtering 1 L of OMWW typically required the use of 2–3 coffee filters and 4–6 Whatman discs, depending on turbidity and viscosity. Based on current retail prices, this corresponds to an estimated consumable cost between EUR 0.15 and EUR 0.30 per litre, excluding labour. If scaled up using fabric-based filtration or gravity-fed setups, the cost may drop below EUR 0.10/L, making this method highly feasible for small-scale producers without access to industrial wastewater systems.
These findings complement our previous work, in which the same OMWW extracts demonstrated significant antimicrobial activity against multiple pathogens [
8]. That study used the same samples and filtration approach but focused exclusively on biological activity (DPPH and MIC assays). The current results allow us to better interpret that bioactivity in light of the physicochemical profile, confirming the strong link between phenolic content and antioxidant behaviour. Together, both studies provide a more complete picture of OMWW’s potential across valorisation paths.
4. Conclusions
This study demonstrated that both the olive oil extraction method and the number of filtration steps influence the physicochemical and biological properties of OMWW. Traditional systems consistently produced effluents with higher organic and phenolic loads, while continuous systems yielded more diluted samples. Filtration, although simple, had measurable effects on parameters such as acidity, EC, and antioxidant potential, particularly in traditional OMWW.
The use of low-cost filtration materials proved to be a practical and scalable pre-treatment strategy for small producers. While some bioactive compounds were partially lost during filtration, the trade-off may be justified when aiming for easier handling, reduced solid content, and improved compatibility with downstream applications.
This approach offers a viable first step toward OMWW valorisation, especially in contexts lacking access to advanced treatment infrastructure. However, this study was conducted at laboratory scale, and the long-term behaviour of filtered OMWW remains to be assessed under real-world storage and usage conditions. Future studies should explore the integration of this filtration strategy with biological or oxidative treatments and assess the economic feasibility and stability of filtered OMWW in practical settings.