Abstract
The maritime industry, while indispensable to global trade, is a significant contributor to greenhouse gas (GHG) emissions, accounting for approximately 3% of global emissions. As international regulatory bodies, particularly the International Maritime Organization (IMO), push for ambitious decarbonization targets, hydrogen-based technologies have emerged as promising alternatives to conventional fossil fuels. This review critically examines the potential of hydrogen fuels—including hydrogen fuel cells (HFCs) and hydrogen internal combustion engines (H2ICEs)—for maritime applications. It provides a comprehensive analysis of hydrogen production methods, storage technologies, onboard propulsion systems, and the associated techno-economic and regulatory challenges. A detailed life cycle assessment (LCA) compares the environmental impacts of hydrogen-powered vessels with conventional diesel engines, revealing significant benefits particularly when green or blue hydrogen sources are utilized. Despite notable hurdles—such as high production and retrofitting costs, storage limitations, and infrastructure gaps—hydrogen holds considerable promise in aligning maritime operations with global sustainability goals. The study underscores the importance of coordinated government policies, technological innovation, and international collaboration to realize hydrogen’s potential in decarbonizing the marine sector.
1. Introduction
When contrasted with air or road-based logistics, maritime shipping stands out as an exceptionally effective approach for transporting substantial cargo volumes, accounting for approximately three-quarters of global merchandise trade by volume and nearly two-thirds by monetary worth [1,2]. However, maritime transportation remains largely dependent on fossil fuels, positioning it as a significant source of greenhouse gas emissions, which pose significant risks to both public health and the stability of the Earth’s climate. [3,4]. In 2018, the global maritime industry released more than one million tonnes of greenhouse gases (GHG) and carbon dioxide (CO2), marking a notable rise of 9.6% in overall GHG emissions and 9.3% in CO2 emissions relative to the levels reported in 2012. [5]. Additionally, the emission of pollutants such as sulfur oxides (SOX), nitrogen oxides (NOX), and various particulate matter significantly affects both human health and the environment, particularly within a 400 km radius from coastlines [6,7,8]. While the maritime industry plays an indispensable role in facilitating global trade and sustaining economic growth worldwide, mounting concerns regarding its continuously rising emissions emphasize the critical and immediate need for effective mitigation strategies. Therefore, decarbonizing the shipping sector is an essential and immediate priority for mitigating global warming.
Efforts to reduce carbon emissions within the maritime sector, particularly those associated with conventional fossil fuels, typically encompass three main approaches: technical improvements, operational enhancements, and market-based measures (MBMs) [9]. Technical measures involve the adoption of energy-efficient engines, advanced hull and propeller designs, the use of low-carbon fuels such as liquefied natural gas (LNG), and alternative zero-carbon fuels like ammonia and hydrogen. Operational measures focus on optimizing vessel speed, improving fleet scheduling, and enhancing supply chain efficiency. In contrast, market-based measures (MBMs) include instruments such as Emissions Trading Schemes (ETS) and carbon pricing mechanisms, including fines or levies on CO2 emissions [9,10].
The International Maritime Organization (IMO) introduced its initial strategy for reducing greenhouse gas emissions in 2018, with a revision planned for 2023 [11,12]. This strategy sets forth ambitious targets: a 50% reduction in total CO2 emissions from international shipping by 2050 and at least a 40% reduction in carbon intensity by 2030, both relative to 2008 levels [13,14]. While multiple pathways and integrated strategies have been proposed to achieve carbon-neutral shipping, the adoption of alternative fuels consistently emerges as a fundamental and cross-cutting solution within all dimensions of the decarbonization framework [15]. This involves advancing research and development efforts to introduce a broad spectrum of zero-carbon and low-carbon alternative fuels, along with cutting-edge technologies, with the aim of enhancing maritime energy efficiency as part of the short-term measures targeted before 2023 [6,16]. The mid-term measures aimed to evaluate the commercial and operational feasibility of effectively integrating low- and zero-carbon alternative marine fuels. This also involved updating national action plans to specifically address the adoption and implementation of these emerging fuel technologies [17]. However, the shift toward alternative fuels is marked by considerable uncertainty, as many candidate fuels remain technologically underdeveloped or commercially unviable, presenting significant challenges to large-scale implementation [18,19]. Consequently, in the near term, biofuels appear to be the only alternative that is both commercially viable and technologically mature [20]. As such, they hold significant potential as a transitional solution, facilitating the gradual shift toward hydrogen-based fuels in the long run [12,20,21].
Among the alternative fuels, hydrogen is considered one of the most promising sustainable options for the maritime sector [22], owing to its highest energy content per unit mass [23] and its ability to produce zero emissions during operation [24,25]. Nonetheless, its widespread adoption is hindered by major technical challenges related to storage and transportation, primarily due to its low volumetric energy density and the need for extensive supporting infrastructure [26]. The debate over the future of sustainable fuels like hydrogen fundamentally hinges on striking a balance between cost-effectiveness and the practicality of onboard energy storage, widely recognized as a key technical barrier [27]. However, in addition to challenges related to infrastructure and economic feasibility, the scalability and capacity of hydrogen production must also be critically considered [28]. It is essential to recognize that hydrogen’s significance extends well beyond its role as a zero-emission fuel [29]. As the only sufficiently accessible and scalable technology for enabling sector coupling, hydrogen plays a pivotal role in optimizing energy systems by aligning production and consumption across diverse sectors [30]. Without the integration of hydrogen, achieving economy-wide deep decarbonization would not only be highly impractical but also prohibitively expensive [31].
Although numerous review papers [32,33,34] have investigated hydrogen technologies across various transportation sectors, this study sets itself apart through its dedicated focus on the maritime industry and the incorporation of a life cycle assessment (LCA) specifically tailored to hydrogen-powered marine vessels. Unlike broader studies that aggregate findings across sectors such as aviation, trucking, and general energy systems, this review delves deeply into the distinct challenges and opportunities associated with maritime decarbonization. It offers a thorough comparative evaluation of alternative marine fuels, including hydrogen, ammonia, LNG, and methanol, based on key factors such as physicochemical characteristics, greenhouse gas (GHG) emissions, and energy density. Additionally, the analysis is framed within the regulatory landscape of the International Maritime Organization (IMO), aligning with its emission reduction targets to provide meaningful guidance for policymakers and industry stakeholders. By integrating sector-specific LCA, regulatory context, and techno-economic analysis, this review delivers a targeted and insightful contribution, addressing a significant gap in the existing literature on hydrogen-driven maritime decarbonization.
Section 2 presents an overview of greenhouse gas (GHG) emissions from the maritime sector and their environmental and societal impacts, emphasizing the need for decarbonization. Section 3 explores the various sources and production methods of hydrogen, highlighting their suitability and limitations for marine applications. Section 4 reviews hydrogen-based technologies, including hydrogen fuel cells and hydrogen internal combustion engines (H2ICEs), focusing on their operational principles and potential integration in shipping. Section 5 discusses current storage methods and onboard fuel systems, with an emphasis on the technical challenges of implementing hydrogen in maritime vessels. Section 6 provides a life cycle assessment (LCA) comparing hydrogen-powered ships to conventional fossil-fuel-driven vessels, quantifying environmental impacts across different hydrogen sources. Finally, Section 7 outlines key challenges, regulatory considerations, and potential strategies to support the transition to a hydrogen-based marine sector.
2. Maritime GHG Emissions and Their Impact
To comprehend GHG emissions in the marine sector, it is worthwhile to examine the data from the Fourth IMO study [35]. Maritime GHG emissions consists of carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), oxides of nitrogen (NOx), carbon monoxide (CO), non-methane volatile organic compounds (NMVOC), particulate matter (PM2.5, PM), and black carbon (BC). Table 1 shows the various GHG emission in year 2017 by total international shipping, domestic navigation, and fishing for all fuel types.

Table 1.
GHG emissions data (tonnes) for international, domestic and fishing for year 2017, data from [35].
Even though there are data from the year 2012–2017, only later data (2017) was chosen to compare the amount of the various emissions from shipping sector. Among the emissions, CO2 covers the ≈ 96% of the total emissions whereas NOx and, and SOx fill the ≈2.17% and ≈1.12%, respectively. Excess of CO2 not only responsible for climate change but also reduce the pH of sea water and hampers the marine life [36]. Figure 1 demonstrates the global shipping industries increases the CO2 emission from 2012 to 2018 which shows the necessity of taking IMO GHG strategy [35] more seriously. Also, emissions of NOx and other ozone precursor such as NMVOC and CO lead to formation of tropospheric ozone (O3) by photochemical reactions [37].
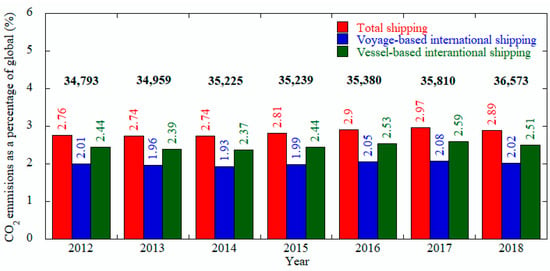
Figure 1.
GHG and other relevant substances emission by top-down methodology, data from [35]; the number on the top (black) shows the total.
Emissions lead to alterations of many trace species within the atmosphere that leads to radiative forcing (RF), a conventional climate metric. A positive RF number suggested warming while cooling is expressed as negative RF. Hence a significant CO2 emission results in warming the environment (positive RF) whereas the SOx, NMVOC, PM will lead through the cooling effect (negative RF). However, NOx emission has both warming and cooling effect by producing O3 and CH4, respectively [38,39].
These emitted species can undergo various atmospheric processes, such as reactions, changes in microphysical properties, or removal through wet and dry deposition onto surfaces like land and water. As a result, these altered trace species can subsequently influence the atmospheric radiative balance, the composition of the atmosphere, and the characteristics of clouds and aerosols [38,40] as can be seen by a conceptual summary in Figure 2.
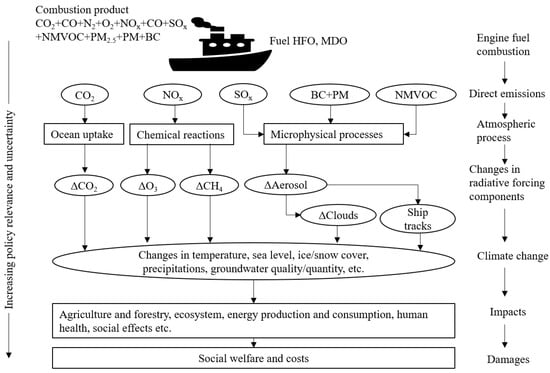
Figure 2.
Schematic depicting GHG emission from shipping sector, atmospheric process that is responsible for further changes to radiative forcing components and their impacts on environment, human and social welfare, adapted from [38,39,41,42].
These changes in radiative forcing (RF) can then have diverse effects on the climate, including impacts on global and local mean surface temperatures, sea levels, precipitation patterns, snow, and ice coverage, and more. Consequently, these physical effects have societal consequences by affecting areas such as agriculture, forestry, energy production, and human health, among others. Ultimately, all these impacts entail a social cost, which can be challenging to quantify accurately [42].
3. Hydrogen Sources, Technologies and Fuels in Shipping
3.1. Hydrogen Sources and Production Processes
Hydrogen (H2) being one of the most abundant and simple elements in the world with no CO2 and SOx emissions, having an environmentally benign oxidation product (water) and acting as an energy carrier that transports energy from one place to another [43]. However, high energy consumption during hydrogen production and lower volumetric density then heavy fuel oil (HFO), the price is about 2.7 to 3.5 times high then HFO [17]. Table 2 shows the comparison of alternate fuels available for shipping industries regarding physiochemical properties, GHG emissions and their potential of reducing CO2.

Table 2.
Analyzing the material properties, emissions and potential CO2 reductions in different shipping fuels for comparison, adapted from [6,44,45,46,47,48].
The majority of hydrogen is currently produced from fossil fuels, water, methane (approximately 6%), and coal (approximately 2%) through processes such as steam reforming, electrolysis, biomass conversion, and coal gasification [49,50,51]. H2 produced from renewable energy, unabated fossil fuels and fossil fuels with a combination with carbon capture and storage are referred to as green, grey, and blue hydrogen, respectively [52]. H2 can be stored physically as compressed gas, cold/cryo-compressed gas, or as a liquid [53]. Additionally, hydrogen can be stored in solid-state form by physically or chemically bonding with specific hydride materials [54]; when required, hydrogen can be released through thermal catalysis [55]. Figure 3 shows the overall technical aspects of H2 in shipping.
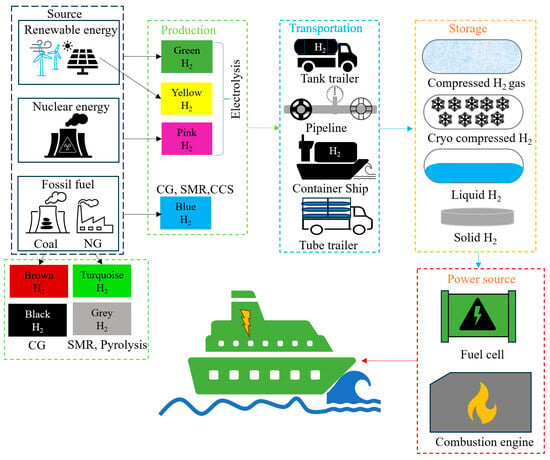
Figure 3.
Overall technical aspects of H2 in shipping industry.
The production methods for hydrogen can be broadly categorized into conventional (fossil-based), renewable, and nuclear-based processes, each with distinct technological and environmental characteristics [56,57].
3.1.1. Conventional Hydrogen Production
The majority of current hydrogen production is derived from fossil fuels—primarily through:
Steam Methane Reforming (SMR): Steam methane reforming (SMR) accounts for approximately 50% of global hydrogen production. This process involves reacting methane with steam to produce hydrogen and carbon monoxide, followed by a water-gas shift reaction that yields additional hydrogen and carbon dioxide. While SMR is economically efficient, it results in significant greenhouse gas emissions unless integrated with carbon capture and storage (CCS) technologies, in which case the output is classified as “blue hydrogen”.
Coal Gasification: Coal is oxidized at high temperature and pressure to form syngas (CO and H2). A shift reactor enhances hydrogen yield. When integrated with CCS, this process also yields blue hydrogen but remains carbon intensive.
Autothermal Reforming (ATR) and Partial Oxidation (POX): Alternative thermochemical processes using oxygen and/or steam to convert hydrocarbons into hydrogen.
3.1.2. Water Electrolysis (Green Hydrogen)
Electrolysis splits water into hydrogen and oxygen using electricity. When powered by renewable sources, the result is green hydrogen:
Alkaline Electrolyzers (ALK): Mature and cost-effective, suitable for steady loads.
Proton Exchange Membrane (PEM) Electrolyzers: Offer fast response and compact design, suitable for variable renewable input.
Solid Oxide Electrolyzers (SOE): Operate at high temperatures, offering high efficiency but lower technological readiness.
These methods produce zero emissions at the point of hydrogen generation and are gaining interest as electrolyzer and renewable electricity costs continue to decline.
3.1.3. Renewable Hydrogen Production Pathways
Renewable energy-driven hydrogen production includes:
Solar Energy: Can be used via photovoltaic (PV) panels to power electrolysis or through solar thermochemical cycles and photoelectrochemical methods.
Wind Energy: Wind turbines generate electricity that drive electrolyzers. Onshore and offshore wind farms both have strong hydrogen production potential.
Hydropower: Reliable and widely available, hydroelectricity can also power electrolysis with minimal emissions.
Biomass Conversion: Biomass can be gasified or pyrolyzed into syngas, followed by WGS to increase hydrogen yield. Biomass gasification with CCS is a promising carbon-negative option.
Ocean Thermal Energy Conversion (OTEC): Uses thermal gradients in ocean water to produce electricity for hydrogen electrolysis.
Geothermal Energy: Provides heat and electricity for high-efficiency hydrogen production systems.
3.1.4. Emerging and Alternative Sources
Nuclear-Assisted Hydrogen Production: High-temperature steam electrolysis (HTSE) and thermochemical cycles (e.g., sulfur-iodine) leverage nuclear heat to produce hydrogen with low carbon intensity.
Photobiological Methods: Using algae or bacteria in photobioreactors to biologically produce hydrogen, though this remains in early research stages.
Microbial Electrolysis Cells (MECs): A bioelectrochemical system generating hydrogen through bacterial digestion of organic matter.
With the increasing deployment of variable renewable energy (VRE) such as solar and wind, managing intermittency and ensuring grid stability have become central challenges. Green hydrogen, produced via water electrolysis powered by renewable electricity, offers a viable means of capturing excess electricity during periods of surplus and storing it chemically for later use. This functionality not only enhances energy security and flexibility but also facilitates sector coupling—linking electricity with transport, heating, and industrial sectors [58].
Hydrogen can be used as fuel in fuel cell, internal combustion engines or as blend in existing conventional diesel fuel such as HFO [51,59,60]. Figure 4 depicts various forms of fuel cell and IC engines available for H2 ship propulsions.
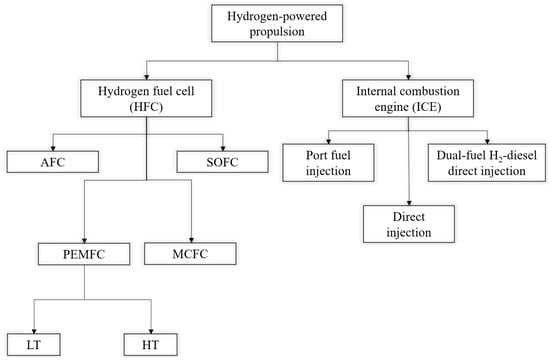
Figure 4.
Available hydrogen powered propulsion system for marine vessels, adapted from [60,61]. AFC—Alkaline Fuel cell, SOFC—Solid Oxide Fuel Cell, PEMFC—Proton Exchange Membrane Fuel Cell, MCFC—Molten Carbonate Fuel Cell, LT—Low Temperature, HT—High Temperature.
3.2. Hydrogen Fuel Cell (HFC)
In principle, HFC runs like a battery, it continuously produces the electrical energy from chemical energy as long as the fuel (H2) is supplied with water and heat as by product [50]. Depending on the electrolyte, HFC categorizes into Alkaline fuel cell (AFC), Proton Exchange Membrane Fuel Cell (PEMFC), Molten carbonate fuel cell (MCFC), and Solid oxide fuel cell (SOFC). Table 3 shows the comparison between different types of fuel cells available for the marine industries.

Table 3.
Commonly applied fuel cells in marine sector and their parameters [51,62,63].
Despite significant advancements in fuel cell technologies, a notable gap persists between their theoretical performance parameters and practical engineering outcomes. This deviation stems from several intrinsic and operational limitations. For instance, LT-PEMFCs, while demonstrating high power densities and rapid transient response in controlled environments, are constrained by their low operating temperatures (65–85 °C), which necessitate the use of platinum catalysts and strict water management protocols to maintain membrane hydration [64,65]. These factors introduce complexity and cost that are not reflected in theoretical models, which often assume ideal operating conditions and perfect reactant utilization. Moreover, sensitivity to fuel impurities such as CO, which irreversibly adsorb on catalyst surfaces at low temperatures, further reduces performance in practical scenarios—particularly in marine or heavy-duty applications where fuel purity may vary.
In contrast, PAFCs and HT-PEMFCs, which operate at higher temperatures, show improved CO tolerance and reduced platinum dependency [63]. However, their real-world viability is hindered by lower power densities and limited durability, outcomes not typically evident in short-term laboratory tests. Similarly, MCFCs and SOFCs, although theoretically capable of high efficiencies (>70%) and able to utilize waste heat in combined cycles, often underperform due to mechanical fragility, high system complexity, and material degradation over long operational periods [66]. For example, thermal cycling and sealing issues in SOFCs lead to mechanical failure, which is rarely captured in performance predictions derived from steady-state lab tests [67].
Furthermore, while laboratory-scale prototypes often use ultra-pure gases, precisely controlled environments, and idealized current loads, real-world systems must contend with load variability, start-stop cycles, impurity-laden fuels, and aging components—all of which exacerbate the performance gap. The lack of comprehensive degradation models and insufficient long-term field testing further widens this divide. Hence, bridging the gap between theoretical efficiency and practical performance requires not only technological refinements—such as the development of impurity-tolerant catalysts, durable membranes, and robust thermal management systems—but also extensive validation through realistic operational simulations and pilot-scale deployments.
Fuel cells have the potential of reaching energy conversion efficiency up to 60% with no acid rain and smog-causing pollutants emissions [50,68,69]. Also, there is little noise due to no moving parts in HFC in addition to better modularity and good part load characteristics unlike diesel engines [63,70]. In maritime industries, HFC systems have been implemented for main propulsion power and auxiliary power unit (APU) due to its high energy efficiency and C free emission. Nonetheless, at present, fuel cells exhibit a higher cost per kilowatt (kW) compared to internal combustion engines (ICE) [71,72]. Figure 5 shows the pros and cons of current fuel cell technology.
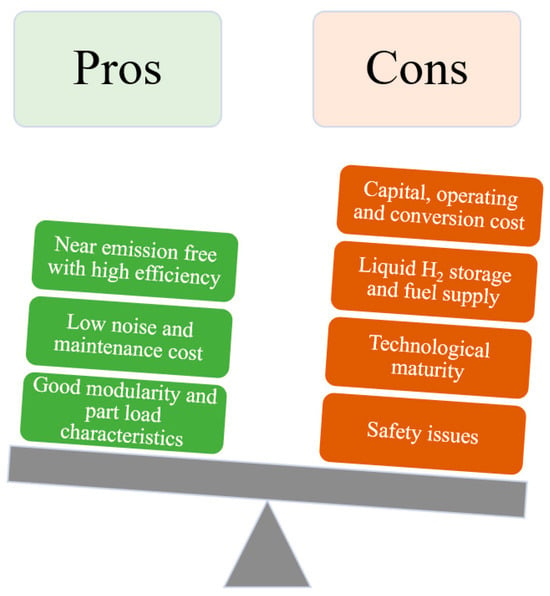
Figure 5.
Pros and cons of integrating HFC into maritime; adapted from [73,74].
As part of the broader technical maturity challenges, HFC such as PEMFCs, face notable durability limitation that hinder their widespread adoption in maritime shipping [75]. While they offer high efficiency and clean operation, PEMFCs degrade over time due to factors such as catalyst degradation, membrane thinning, and carbon support corrosion—especially under the dynamic load conditions typical of marine operations [76]. Exposure to impurities in hydrogen fuel, along with thermal and humidity cycling, further accelerates performance decline. These degradation mechanisms reduce operational lifespan and increase maintenance demands, highlighting the need for more resilient materials and improved system-level durability before PEMFCs can be reliably deployed in long-term maritime service.
3.3. Hydrogen Internal Combustion Engine (H2ICE)
Generally, H2ICE is categorized into 3 different types of injection method: 1. Port fuel injection, (PFI), 2. Direct injection (DI), and 3. Dual fuel H2-diesel direct injection as shown in Figure 3 [60,77].
In comparison to the conventional fuel in IC engines, it is not easy to utilize H2 for its unique physicochemical properties in PFI as shown in Table 4.

Table 4.
Key properties of H2 in comparison to CNG, gasoline, ammonia and diesel, adapted from [51,60,78,79,80].
Despite being clean fuel with zero carbon emissions and high specific energy density (almost 3 times than other fossil fuels), several limitation including low density and volumetric energy content (MJ/m3) hinders the H2 to become commercially available for IC engines [60]. Also, engine modification is needed to facilitate the compressed gaseous H2 unlike the liquid fuels [81]. In addition to that abnormal combustion characteristics such as high-pressure rise, pre-ignitions, backfire, knocking, and spontaneous ignitions are some of the challenging issues regarding H2ICE [82,83]. The pre-ignition initiates from the pre-mature ignition of H2 in the several hot spots in engine cylinder due to its low ignition energy while backfire occurs in the opening of the intake valves. To avoid the pre-ignition and backfire problem, a potential solution would be injecting H2 directly into the cylinder in the time of compression stroke [84] which is the basis of direct injection (DI). In addition to that DI also possess a very flexible engine operation for optimizing engine performance because of a number of adjustable parameters such as injection pressure, duration, timing and injection orientation [60]. However, DI is still limited by the high auto ignition temperature of H2 [85]. Nevertheless, DI H2ICE is an upgrade of the PFI, the efficiency is not up to the mark as the current diesel CI engine. In this regard, Dual-fuel H2-Diesel direct injection (H2DDI) would be a better option where a small amount of diesel pilot fuel is injected as an ignition source into the combustion chamber. This new combustion method for hydrogen internal combustion engines helps reduce knock and enables the engine to operate at a higher compression ratio, enhancing thermal efficiency to levels comparable to modern compression ignition engines [60]. However, more research is needed to utilize H2DDI as a commercial ICE engine for different applications as the underlying mechanism and effect on engine performance are yet to be fully understood. Currently H2 in H2ICE yields 40% of efficiency [86]. Figure 6 shows the tradeoffs for utilizing H2 in IC engines.
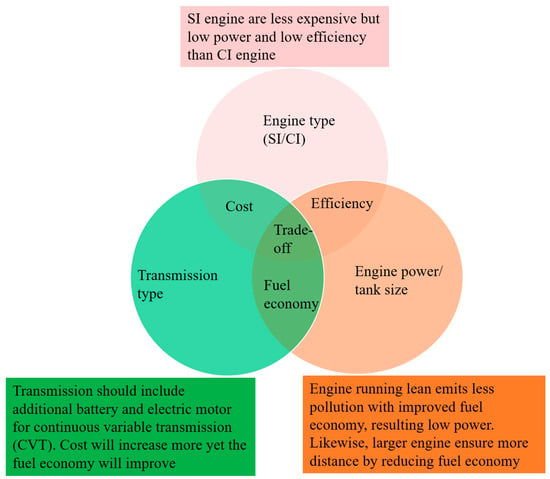
Figure 6.
Relevant tradeoffs for H2ICE, adapted from [87].
3.4. Hydrogen Storage Technology
Hydrogen storage remains a critical technological bottleneck limiting its widespread adoption. Efficient, safe, and cost-effective storage is essential for enabling hydrogen’s practical use across sectors. Current technologies fall into three broad categories: compressed gas storage, liquid hydrogen storage, and solid-state storage, each offering unique advantages and facing specific limitations [88,89].
3.4.1. Compressed Hydrogen Storage
Compressed hydrogen gas storage is the most established and widely deployed method, particularly in demonstration projects and early-stage deployments. Hydrogen is typically stored in high-strength composite tanks at pressures of 350 to 700 bar [90]. This approach is relatively mature, with a robust safety record and rapid fueling capabilities. However, it imposes a considerable weight and volume penalty, especially problematic in maritime contexts where vessel space is limited. High-pressure systems also demand strict safety protocols and incur significant costs in tank manufacturing, installation, and maintenance.
3.4.2. Cryogenic Liquid Hydrogen Storage (LH2)
To improve storage density, liquid hydrogen storage (LH2) has gained increasing attention. Hydrogen is liquefied by cooling it to cryogenic temperatures near −253 °C, resulting in a much higher volumetric density than compressed gas [56]. This makes it particularly attractive for large-scale, long-distance marine transport and onboard storage for ocean-going vessels. Nonetheless, cryogenic storage presents its own challenges: the liquefaction process is energy-intensive—consuming up to 30% of hydrogen’s usable energy—and boil-off losses from evaporation are inevitable [91]. Additionally, the required insulation systems and cryogenic handling infrastructure raise both capital and operational costs.
3.4.3. Solid-State Hydrogen Storage
Solid-state hydrogen storage offers an alternative route by incorporating hydrogen into materials such as metal hydrides, chemical hydrides, or nanoporous adsorbents. These materials can absorb hydrogen either chemically or physically and release it under controlled thermal conditions. Metal hydrides like magnesium or rare-earth alloys offer high volumetric densities and inherent safety due to low operating pressures [92]. However, they are typically heavy, slow to absorb or desorb hydrogen, and require thermal management systems. Chemical hydrides, such as ammonia borane or sodium borohydride, provide high gravimetric storage capacities, but often require complex regeneration processes that are still not cost-effective or environmentally friendly. Nanoporous materials, including metal–organic frameworks (MOFs) and carbon-based sorbents, show promise at laboratory scales but face scalability, performance consistency, and durability challenges. Table 5 shows the summary of these storage technologies.

Table 5.
Summary of hydrogen storage technologies.
Figure 7 presents an overview of hydrogen storage technologies, plotting gravimetric and volumetric capacities [93]. Storage solutions were categorized by physical state—gas, liquid, or solid—and represented as data points, with elliptical groupings indicating variability across sources. DOE target values [94] were also included for comparison. The analysis shows that while compressed gas storage falls short of the 2020 targets, liquid hydrogen meets them, and cryo-compressed storage aligns with 2025 goals. Solid-state storage technologies show promise in reaching or surpassing the DOE’s ultimate targets.
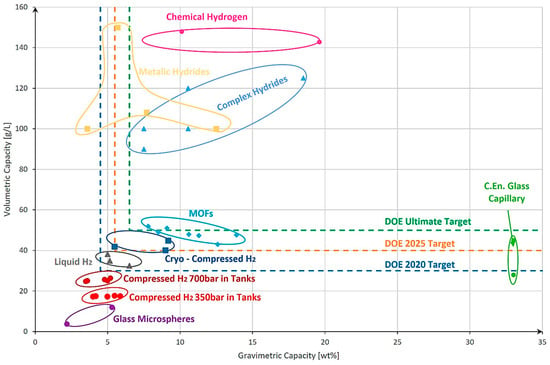
Figure 7.
Comparison of various hydrogen storage methods, highlighting their respective gravimetric and volumetric storage capacities, along with the U.S. Department of Energy (DOE) target benchmarks and deadlines: reproduced with permission [93,94]; 2023 MDPI.
Despite technological progress, limitations remain. For maritime applications in particular, the trade-offs between energy density, weight, safety, and cost are more acute [22]. Ships must store large quantities of fuel without compromising cargo capacity, structural integrity, or vessel balance. Moreover, international regulations concerning hydrogen bunkering, storage, and onboard safety are still under development, creating uncertainty for shipbuilders and port authorities. The integration of hydrogen storage systems with fuel cell propulsion, auxiliary systems, and onboard energy management also requires further innovation [95].
4. Challenges of Hydrogen Integration in Shipping
Although there are a number of marine vessels that are already implementing both H2 fuel cell and H2ICE engine as shown in Table 6, there are some challenging issues for achieving market competitiveness.

Table 6.
Prominent showcases of marine fuel cell systems and implementations since the year 2000, adapted from [62,63,96,97,98,99,100,101,102].
4.1. Ensure Zero Emission During Production of H2 for Shipping
Over 90% of global H2 comes from reforming fossil fuels, known as grey H2 and after capturing carbon, it becomes Blue H2. In both cases the CO2 emissions are around 830 million tons CO2 eq per year globally which is very high [103,104]. Unless the H2 is not produced from emission free sources such renewable energy (known as green H2) it shouldn’t be considered as carbon neutral [105]. However green H2 is very expensive as the renewable power generation is still very costly [104].
4.2. On-Board End-Use Limitations of HFC and H2ICE
Even though HFC is called emission free at the point of use, in addition to water some NOx can be released at high temperatures [106]. In case of H2ICE, owing to the lower efficiency and reduced density of hydrogen fuel, larger engine dimensions are required to generate equivalent power at the same speed as a diesel engine. This suggests that considerable efforts are still needed for hydrogen engines to reach a competitive phase. Nevertheless, endeavors to replace diesel-fueled engines with hydrogen-fueled ones should persist in order to surmount these challenges [59].
4.3. Safety Issues for Using HFC in Shipping
Any kind of leakage is difficult to notice as the H2 is odorless, non-toxic and invisible [107]. Also, high flammability of H2 (between 4 and 77% when mixed with air) could be results in explosion due to sudden exposure to air [86]. Hence safety is one of the primary concerns for passengers, crew, and the ship due to the risk of electrical hazards and the consequences of fire explosions resulting from the installation of a fuel cell system using low flashpoint fuels, such as H2, on the ship [108]. To ensure safety, DNV-GL [108] proposes certain rules and guidelines to install FC on board.
4.4. H2 Storage in Ship
Since H2 can be stored as a compressed gas, liquefied form or as a metal hydride, different types of storage unit and corresponding issues should be considered. In the case of compressed gas, hydrogen can be stored in a highly pressurized container, up to around 700 bars. However, this will require a larger space in the ship deck to accommodate such storage facilities [48,105,109]. Another potential storage system would be a number of H2 tanks (20–40 feet container) mounted on each other, known as cassette-type fuel system [48]. This could be a viable solution for smaller vessels such as ferries. However, for larger ships, the time required to load and unload numerous containers would significantly extend the duration of port calls. For liquid H2, ”boil-off phenomenon” could be an issue though it can use the experience from the LNG tank that had already been implemented in the marine vessel in a similar way [110]. In terms of storage, solid state storage such as metal H2 would be a better choice. However, the technical hurdles related to the dehydrogenation of fuel, complications of powder transportation with potential hazard needs to be overcome for using solid H2 in shipping [111].
4.5. Refueling of H2
When it comes to the space allocated for H2 storage on ships, the volumetric energy of liquid H2 is approximately one-fourth that of oil-based fuels for the same size of tank. Hence, transitioning from oil-based fuels to liquid hydrogen requires more frequent refilling of fuel tanks. First, its low volumetric energy density—particularly in gaseous form—necessitates more frequent refueling, which can be logistically and economically burdensome for long-haul vessels [88].
These challenges are not unique to shipping; they have been extensively studied in aerospace and space launch systems where hydrogen has long served as a primary rocket fuel. The space sector has developed strategies to manage thermal conditioning, multi-stage pressurization, and boil-off vapor handling, which could be adapted for maritime hydrogen refueling systems.
In this regards, port-based refueling infrastructure can adopt liquid hydrogen (LH2) bunkering terminals similar to LNG, incorporating high-throughput cryogenic pumps and pre-cooled pipelines to minimize transfer time. As previous studies [112,113] suggest, modular buffer tanks and high-capacity cryo-compressed storage systems can increase refueling capacity and reduce turnaround time.
Genovese et al. [112] describe the importance of multi-stage pre-cooling, which gradually lowers the temperature of onboard tanks and refueling lines, preventing thermal stress. Additionally, active vapor recovery systems and pressure cascade refueling sequences (as used in FCVs) can be scaled up for marine applications to manage vented gases and reduce pressure spikes.
In order to tackle structural stress on storage tanks and piping due to rapid temperature gradients during refueling at supercooled temperatures (~20 K for LH2), Aerospace research [114] on transcritical injection and supercritical phase behavior provides relevant insights. These challenges are not unique to shipping; they have been extensively studied in aerospace and space launch systems where hydrogen has long served as a primary rocket fuel. The space sector has developed strategies to manage thermal conditioning, multi-stage pressurization, and boil-off vapor handling, which could be adapted for maritime hydrogen refueling systems. The aerospace sector uses gradual pressurization, high-performance insulation, and flexible materials with low thermal expansion coefficients. These approaches can be adopted for shipboard fuel systems to handle thermal cycling during bunkering.
Therefore, the reduction in the carbon footprint within the naval sector through the adoption of hydrogen-fueled ships must be accompanied by the establishment of an accessible infrastructure for swift and practical refueling [115].
4.6. Development of Reactor
Solid state H2 storage method requires additional equipment such as a reactor system to facilitate onboard chemical conversion step for dehydrogenation [116] in ship. Although chemical reactors such as tubular and continuously stirred reactor are already implemented for stationary applications, conventional reactor needs to consider the ship motion effect [48].
5. Cost Analysis of Hydrogen Power Vessels
5.1. Well to Tank Cost
The well-to-tank cost projections for hydrogen production pathways from 2026 to 2050 were developed using a techno-economic dataset compiled for open energy system modeling in developing countries, with a focus on Colombia [117]. The dataset integrates capital expenditure (CAPEX), fixed and variable operational expenditures (OPEX), and technology-specific assumptions drawn from reputable international sources including the International Renewable Energy Agency (IRENA), International Energy Agency (IEA), National Renewable Energy Laboratory (NREL), and the Intergovernmental Panel on Climate Change (IPCC).
Cost values were expressed in constant 2020 USD per petajoule (PJ) and normalized for consistency. For future projections, cost decline trajectories were modeled using learning curves and compound annual reduction rates, based on expert consensus and empirical benchmarks. Hydrogen production technologies—such as Steam Methane Reforming with Carbon Capture and Storage (SMR + CCS), Coal and Biomass Gasification with CCS, and PEM Electrolyzers—were parameterized using pathway-specific inputs including energy feedstock requirements, efficiency factors, carbon capture rates, and deployment scenarios.
This dataset supports temporal projections from 2026 through 2050 and is structured to align with energy system models such as OSeMOSYS [118]. It offers transparent, regionally adaptable data to facilitate robust cost–benefit analysis and strategic decarbonization planning as shown in Figure 8.
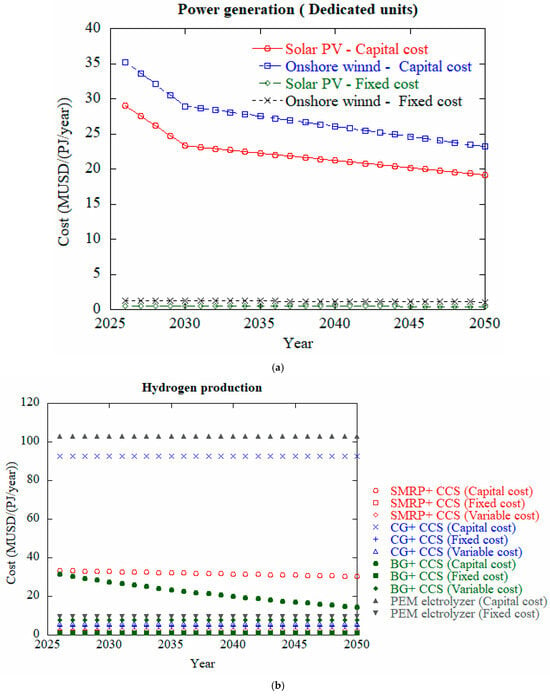
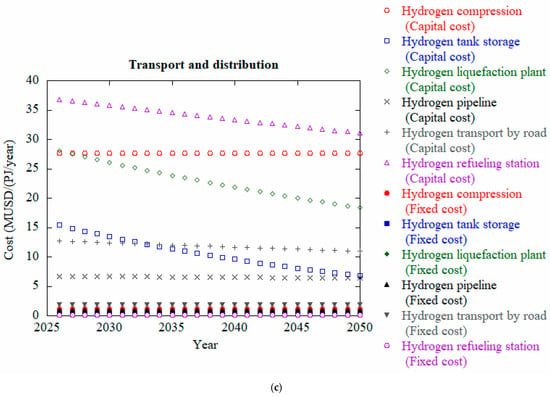
Figure 8.
Projected cost analysis for well to tank of hydrogen of 2026–2050 for (a) power distribution, (b) hydrogen production and (c) transport and distribution, data from [117].
Figure 8a compares the capital and fixed costs of solar PV and onshore wind power from 2026 to 2050. Both technologies show a declining trend in capital costs over time. Initially, onshore wind has a higher capital cost than solar PV, but the gap narrows by 2050. Fixed costs for both technologies remain very low and nearly constant throughout the period. Overall, solar PV becomes slightly more cost-effective over time, though both options show promising reductions in investment costs for dedicated renewable power generation. Figure 8b depicts comparison of capital, fixed, and variable costs of different hydrogen production methods from 2026 to 2050. Biomass gasification with CCS (BG + CCS) shows the most significant cost reduction over time, making it a promising low-carbon option. Steam methane reforming with CCS (SMRP + CCS) remains relatively stable but still cost-effective. In contrast, coal gasification with CCS (CG + CCS) has very high variable costs, making it the most expensive overall. PEM electrolysis has the highest capital and fixed costs, remaining economically uncompetitive without major technological breakthroughs. Among all components, hydrogen refueling stations show the highest capital costs, which gradually decline over time as shown in Figure 8c. Hydrogen compression and liquefaction plants also represent significant capital expenditures, with the cost of liquefaction plants showing a noticeable downward trend. Capital costs for hydrogen tank storage and pipeline infrastructure decrease steadily, indicating technological improvements and potential economies of scale. In contrast, fixed costs for all components—such as compression, storage, road transport, and pipeline systems—remain relatively low and stable throughout the time period. Overall, the graph highlights that while fixed costs remain a minor portion of total expenses, capital investment—particularly in refueling infrastructure and liquefaction—is the dominant cost factor in hydrogen transport and distribution systems.
5.2. Retrofitting Costs of Hydrogen-Powered Vessels
The transition to hydrogen-powered propulsion systems in maritime vessels presents both a promising decarbonization pathway and a significant economic challenge. Retrofitting conventional ships to accommodate hydrogen fuel cells involves high capital costs due to the unique requirements of hydrogen storage, processing, and safety infrastructure. These costs are magnified by indirect effects such as loss of cargo capacity, operational downtime, and design constraints.
5.2.1. Cost Components of Hydrogen Retrofitting
Hydrogen retrofits typically involve:
Replacement of main propulsion systems with fuel cell stacks, installation of cryogenic or high-pressure hydrogen storage tanks, piping, ventilation, and fire suppression systems to meet safety regulations, upgrades to auxiliary power systems and electrical integration, shipyard labor and refitting logistics, lost opportunity cost due to reduced cargo capacity or vessel downtime.
According to Lagemann et al. [119], the retrofit cost from a conventional LNG or VLSFO configuration to a liquid hydrogen-based power system is among the most expensive transitions, estimated to reach $22.6 million per vessel. This figure includes machinery, tank installation, safety retrofitting, and associated downtime. These costs are substantially higher than retrofits to other fuels such as LNG or ammonia, mainly due to hydrogen’s low energy density and cryogenic handling needs.
5.2.2. Influence of Design and Modular Flexibility
Vessels not initially designed with modularity or retrofit flexibility incur even higher costs. The absence of space allocation, tank reinforcement [120], or electrical integration pathways during the initial build phase means that retrofitting often requires extensive structural modifications. This not only increases direct capital expenditure but also extends shipyard time, increasing lost revenue from charter service interruptions.
As reported in Lagemann et al. [119], the lost opportunity cost from reduced payload capacity in a hydrogen retrofitted Supramax vessel was modeled to be approximately $2.7 million per 10 years, assuming typical dry bulk operational patterns. When combined with direct retrofit costs, the effective 10-year retrofit cost for hydrogen systems can exceed $25 million per vessel under realistic utilization scenarios.
5.2.3. Degradation-Driven Replacement Costs
Another key factor in retrofit cost modeling is the lifetime and replacement schedule of hydrogen fuel cells and batteries. Under high-degradation maritime conditions [121]:
Fuel cells may require replacement every 6–8 years,
Batteries may degrade faster, with replacement intervals of 4–6 years.
If the base retrofit cost is $9.4 million (a mid-range estimate for hybrid systems), and replacements are modeled at 30% of initial cost for fuel cells and 20% for batteries, the 10-year Net Present Value (NPV) of retrofitting costs increases to $13.2 million in high-degradation scenarios, compared to $11.3 million under low-degradation assumptions.
5.2.4. Utilization-Adjusted Infrastructure Amortization
The economic feasibility of hydrogen retrofitting is closely linked to the sunk cost of port-side infrastructure. Shared hydrogen bunkering infrastructure—including electrolyzers, storage, and compression units—requires capital investments between $13–22 million. However, the per-vessel amortized cost over 10 years varies significantly based on annual port utilization [122]:
Low utilization (5 ships/year): ~$340,000 per vessel (NPV)
High utilization (40+ ships/year): ~$42,000 per vessel (NPV)
These findings highlight the importance of coordinated fleet conversion strategies and shared infrastructure development to make retrofitting economically viable. Table 7 shows the summary of this cost and corresponding strategy.

Table 7.
Summary and strategic considerations.
Retrofitting to hydrogen remains a capital-intensive endeavor, requiring strategic timing, policy incentives, and robust port-side infrastructure to approach economic competitiveness. However, when framed within lifecycle emissions reductions and long-term decarbonization mandates, these investments may be justified for achieving regulatory compliance and corporate sustainability targets.
6. Life Cycle Analysis of Hydrogen Powered Vessels
A life cycle analysis (LCA) of hydrogen-powered vessels provides a comprehensive evaluation of the environmental impacts associated with different hydrogen propulsion technologies over their entire life span—from raw material extraction to end-of-life disposal as shown in Figure 9.
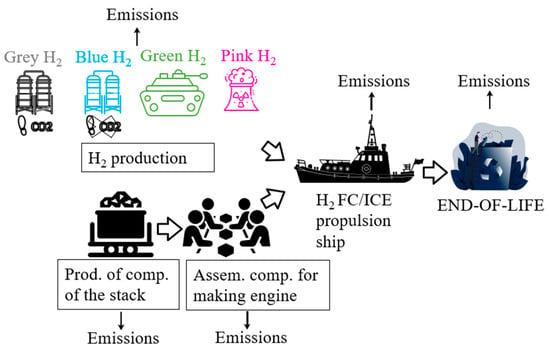
Figure 9.
System boundaries for cradle to grave LCA of hydrogen powered vessels.
Among the two hydrogen-based technologies, H2 ICEs were shown to offer the lowest environmental burdens across ten out of eleven impact categories, achieving reductions of up to 99% in categories such as eutrophication potential in comparison to diesel ICE as shown in Table 8 [123]. PEMFCs also demonstrated strong performance in certain areas, notably reducing photochemical ozone creation and acidification, but showed increased impacts in categories like human toxicity and global warming potential due to the upstream emissions from hydrogen production via steam methane reforming (SMR), known as grey H2. The environmental benefits of hydrogen technologies heavily depend on the hydrogen source as can be seen on Figure 10. If hydrogen is produced using renewable methods (electrolysis using wind energy, green H2) and SMR with CCS (blue H2), the overall life cycle emissions could be significantly reduced. However, at the current early stage of technology readiness, especially for maritime applications, these results are sensitive to production assumptions and deployment scale. The LCA thus supports hydrogen technologies as promising pathways for maritime decarbonization, emphasizing the need for continued innovation and supportive policy to realize their full potential.

Table 8.
Total environmental impacts are presented per functional unit (1 kWh). Blue cells indicate impact categories where hydrogen technologies outperform the diesel ICE baseline, while purple cells denote categories where impacts are higher than those of diesel ICE, adapted from [123].
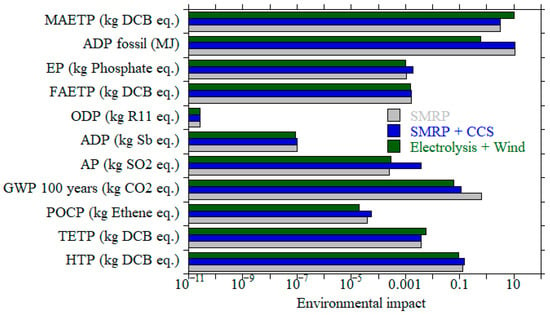
Figure 10.
Environmental impacts per functional unit (1 kWh) for hydrogen fuel cells (H2FC) are shown for each hydrogen production pathway with color, data from [123].
7. The Role of Governments in Supporting Hydrogen Adoption
Transitioning the world’s shipping fleet to hydrogen will not happen through industry efforts alone, government action is pivotal. Public sector involvement is needed to overcome the economic and infrastructural barriers in the early stages of this transition. Key responsibilities and tools for governments include:
7.1. Funding Research, Development and Demonstration (RD&D)
Governments can accelerate innovation by investing in hydrogen maritime R&D programs. This includes funding the development of safer storage systems, more durable fuel cells, and efficient hydrogen production methods. In recent years, public investment in hydrogen research, development, and demonstration (RD&D) has significantly increased, with many countries allocating substantial funds to build hydrogen infrastructure, such as refueling stations, pipelines, and storage facilities—to support its widespread adoption [124]. For example, the EU and national governments have co-financed pilot projects (through mechanisms like Horizon 2020, Horizon Europe, and Important Projects of Common European Interest) to build prototype hydrogen vessels and port facilities [125]. Such funding helps de-risk new technologies and bridge the gap from laboratory to full-scale deployment. Demonstration projects like the MV Sea Change ferry, 360 kW fuel cell powertrain and a 250 bar 246 kg capacity hydrogen storage system in California were enabled by public grants alongside private investment, showing the impact of a public–private approach [126].
7.2. Regulations and Standards
Governments set a regulatory framework that can either hinder or help hydrogen vessels. A crucial role is updating maritime safety and fuel regulations to accommodate hydrogen. International codes (like the IGF Code, which governs gaseous fuels on ships) need to be expanded to include hydrogen-specific provisions, a process that national maritime authorities and the IMO are now actively working on with input from industry. Clear safety standards and certification processes for hydrogen systems will give shipowners confidence and ensure operations are safe. Governments can also mandate emissions reductions that effectively compel industry to consider hydrogen. Norway’s decision that all cruise ships and ferries in certain fjords must be zero-emission by 2026 is a prime example of regulation driving adoption [127]. That requirement led operators to invest in batteries and hydrogen fuel cells to comply. Similarly, the IMO’s upcoming mid-2020s measures (potentially a fuel greenhouse gas standard or carbon levy) could create a regulatory push toward hydrogen and other zero-carbon fuels [128]. By signaling an endgame for fossil fuels, regulations can align the entire industry toward developing alternative fuels.
7.3. Economic Incentives and Carbon Pricing
Because of hydrogen’s current cost premium, government incentives are critical to stimulate early adoption. These can take the form of subsidies, tax credits, grants, or contracts-for-difference that make hydrogen fuels financially viable for shipping companies. For instance, several countries are implementing hydrogen fuel incentives through their clean energy programs. Over time, several federal initiatives and programs have played a key role in advancing hydrogen (H2) development in the United States. To support this progress, the U.S. Department of Energy (DOE) offers a range of incentives, such as tax credits and subsidies. These initiatives aim to enhance the economic viability of clean hydrogen production and to attract early-stage investments in hydrogen technologies [129]. Also, launched in 2023, the European Hydrogen Bank introduced two key support mechanisms to promote renewable hydrogen production. First, it aims to boost domestic hydrogen uptake through fixed premium auctions that support production within the EU. Second, it seeks to ensure a diversified supply by offering similar fixed premium payments to international producers of hydrogen and its derivatives, encouraging imports from outside the EU [130]. Specific to maritime, governments could offer rebates or extra credits for hydrogen use under low-carbon fuel standards or reduce port fees for zero-emission vessels. Another approach is co-financing the operational cost gap on certain routes: essentially paying the difference between hydrogen fuel and heavy fuel oil for early adopters. A 2024 global corridor report identified the lack of national policy incentives to bridge the fuel cost gap as the number one bottleneck for zero-emission shipping projects [131]. It recommended governments step in with operational subsidies (such as via Germany’s H2Global model or other auction schemes) to help make the business case for using hydrogen on pioneer routes [89]. In parallel, implementing or tightening carbon pricing for shipping (as the EU is doing and IMO is considering) will inherently reward use of fuels like hydrogen by internalizing fossil fuels’ environmental costs. Such market-based measures, alongside direct support, create financial pull for companies to invest in hydrogen vessels sooner rather than later [132].
7.4. Infrastructure and Industrial Strategy
Building the hydrogen supply infrastructure for maritime use is a massive undertaking that benefits from government coordination and investment. This includes supporting the construction of hydrogen production plants (electrolyzers or other methods) near ports, installing liquefaction and storage facilities, and developing bunker delivery systems (pipeline, truck, or bunker barge). Governments are beginning to roll out plans for hydrogen hubs that often center on port complexes—for example, Rotterdam, Singapore, and Los Angeles/Long Beach are among ports with government-backed studies on becoming hydrogen fueling hubs in the future. Public investments in these hubs, or incentives for the private sector to build them, are crucial to break the chicken-and-egg dilemma between fuel availability and demand. International coordination can ensure that standards for hydrogen fuel quality, refueling protocols, and handling are harmonized, which will facilitate global shipping operations. Several countries with abundant renewables (Australia, Chile, Middle East nations) have national hydrogen strategies aimed at becoming exporters of green hydrogen or ammonia by ship [89]. Diplomatic and trade arrangements by governments will shape how a global hydrogen supply chain develops and how easily maritime operators can procure fuel around the world. Additionally, governments can integrate maritime hydrogen plans with broader energy and industrial policy, for example, leveraging synergies with hydrogen use in trucking, rail, or industry to create larger markets that drive costs down.
7.5. International Collaboration and Agreements
Shipping is inherently a global industry, so no single government can enable the transition alone. International collaboration is therefore a key government responsibility. Through the IMO, countries work together on setting safety rules and climate policies for shipping—continued pressure and leadership in IMO negotiations is needed to set ambitious targets and enforceable measures that promote fuels like hydrogen. Beyond the IMO, coalitions of willing nations are forming partnerships: the aforementioned Clydebank Declaration sees countries jointly establishing green corridors, and initiatives like the Zero-Emission Shipping Mission (under Mission Innovation) bring together governments to share R&D and coordinate demonstration projects across borders. Governments can also collaborate on research (for instance, the EU, U.S., Japan and others frequently exchange findings on hydrogen safety and technology in maritime via forums and bilateral agreements). Such coordination helps avoid duplication and ensures that early efforts are aligned on technical standards. Financially, multi-country funds or climate finance mechanisms could support developing countries in building hydrogen-ready port infrastructure, ensuring the transition is inclusive. In essence, governments must provide not just national policies but also cooperate internationally to create a consistent and supportive environment for hydrogen in maritime. This ranges from agreeing on rules for transporting hydrogen (or ammonia) by sea to jointly investing in pilot routes that demonstrate zero-emission shipping. As noted by the Global Maritime Forum, if industry and governments share the costs and risks, the leading pilot projects can pave the way for broader deployment before 2030 [131].
In fulfilling these roles, governments act as enablers to tip hydrogen shipping from a niche experiment to a mainstream solution. The experience with LNG as a marine fuel has shown that supportive policies (like Norway’s tax exemptions for LNG, or EU co-funding of LNG bunkering terminals) were instrumental in adoption; an even stronger push will be needed for hydrogen given its greater challenges. The coming decade is pivotal: many governments (58 countries as of 2024) have published hydrogen strategies, and if they implement the right mix of R&D support, incentives, and regulations specifically targeting maritime use, hydrogen-fueled ships could become increasingly common in the 2030s [132].
8. Conclusions
Decarbonizing the marine sector is a critical step toward achieving global climate goals, especially given the industry’s significant contribution to greenhouse gas emissions. This review has examined the technological readiness, economic feasibility, and environmental impact of various hydrogen-based solutions for marine applications, including hydrogen internal combustion engines, fuel cells, and supporting refueling infrastructure. Among the fuel cell technologies, LT-PEMFCs have demonstrated promising performance but face durability and cost barriers, while high-temperature fuel cells such as SOFCs and MCFCs offer higher efficiencies yet struggle with material limitations and system complexity. Furthermore, the analysis of hydrogen production, distribution, and refueling highlights substantial capital investment requirements and the need for scalable, cost-effective supply chains.
A key barrier to implementation remains the lack of mature infrastructure and the need for large-scale demonstration projects, particularly in the maritime domain where reliability and longevity are paramount. The life cycle assessment (LCA) results presented in this study reinforce that green hydrogen produced via renewable pathways offers a significantly lower environmental footprint compared to conventional marine fuels and even other hydrogen sources. To accelerate the transition, a coordinated effort involving regulatory frameworks, incentives for innovation, and international collaboration is essential.
Ultimately, while considerable challenges persist—particularly in technical maturity, cost competitiveness, and operational integration—hydrogen technologies present a viable and promising path toward a low-emission maritime future. Continued research, pilot deployments, and supportive policy mechanisms will be crucial to bridging the gap between current potential and practical implementation.
Author Contributions
Conceptualization, R.K. and A.M.C.; methodology, R.K.; analysis, R.K. and A.M.C.; investigation, R.K.; data curation, R.K.; writing—original draft preparation, R.K. and A.M.C.; writing—review and editing, R.K. and A.M.C.; supervision, R.K.; project administration, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karl, M.; Jonson, J.E.; Uppstu, A.; Aulinger, A.; Prank, M.; Sofiev, M.; Jalkanen, J.-P.; Johansson, L.; Quante, M.; Matthias, V. Effects of ship emissions on air quality in the Baltic Sea region simulated with three different chemistry transport models. Atmos. Chem. Phys. 2019, 19, 7019–7053. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Song, D.-P. Ocean container transport in global supply chains: Overview and research opportunities. Transp. Res. Part B Methodol. 2017, 95, 442–474. [Google Scholar] [CrossRef]
- Fuglestvedt, J.; Berntsen, T.; Eyring, V.; Isaksen, I.; Lee, D.S.; Sausen, R. Shipping Emissions: From Cooling to Warming of Climate—And Reducing Impacts on Health. Environ. Sci. Technol. 2009, 43, 9057–9062. [Google Scholar] [CrossRef] [PubMed]
- Aakko-Saksa, P.T.; Lehtoranta, K.; Kuittinen, N.; Järvinen, A.; Jalkanen, J.-P.; Johnson, K.; Jung, H.; Ntziachristos, L.; Gagné, S.; Takahashi, C. Reduction in greenhouse gas and other emissions from ship engines: Current trends and future options. Prog. Energy Combust. Sci. 2023, 94, 101055. [Google Scholar] [CrossRef]
- ABS. Setting the Course to Low-Carbon Shipping: Pathways to Sustainable Shipping; American Bureau of Shipping: Houston, TX, USA, 2019. [Google Scholar]
- Ampah, J.D.; Yusuf, A.A.; Afrane, S.; Jin, C.; Liu, H. Reviewing two decades of cleaner alternative marine fuels: Towards IMO’s decarbonization of the maritime transport sector. J. Clean. Prod. 2021, 320, 128871. [Google Scholar] [CrossRef]
- Endresen, Ø.; Sørgård, E.; Sundet, J.K.; Dalsøren, S.B.; Isaksen, I.S.; Berglen, T.F.; Gravir, G. Emission from international sea transportation and environmental impact. J. Geophys. Res. Atmos. 2003, 108, 4560. [Google Scholar] [CrossRef]
- Eyring, V.; Köhler, H.; Lauer, A.; Lemper, B. Emissions from international shipping: 2. Impact of future technologies on scenarios until 2050. J. Geophys. Res. Atmos. 2005, 110, D17306. [Google Scholar] [CrossRef]
- Psaraftis, H.N. (Ed.) Green Maritime Transportation: Market Based Measures, in Green Transportation Logistics: The Quest for Win-Win Solutions; Springer International Publishing: Cham, Switzerland, 2016; pp. 267–297. [Google Scholar]
- ITF. Decarbonising Maritime Transport: Pathways to Zero-Carbon Shipping by 2035; International Transport Forum: Paris, France, 2018. [Google Scholar]
- Joung, T.-H.; Kang, S.-G.; Lee, J.-K.; Ahn, J. The IMO initial strategy for reducing Greenhouse Gas (GHG) emissions, and its follow-up actions towards 2050. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Serra, P.; Fancello, G. Towards the IMO’s GHG goals: A critical overview of the perspectives and challenges of the main options for decarbonizing international shipping. Sustainability 2020, 12, 3220. [Google Scholar] [CrossRef]
- Bullock, S.; Mason, J.; Larkin, A. The urgent case for stronger climate targets for international shipping. Clim. Policy 2022, 22, 301–309. [Google Scholar] [CrossRef]
- Ezinna, P.C.; Nwanmuoh, E.; Ozumba, B.U.I. Decarbonization and sustainable development goal 13: A reflection of the maritime sector. J. Int. Marit. Saf. Environ. Aff. Shipp. 2021, 5, 98–105. [Google Scholar] [CrossRef]
- Inal, O.B.; Zincir, B.; Deniz, C. Investigation on the decarbonization of shipping: An approach to hydrogen and ammonia. Int. J. Hydrogen Energy 2022, 47, 19888–19900. [Google Scholar] [CrossRef]
- IMO. Resolution MEPC.304(72): Initial IMO Strategy on Reduction of GHG Emissions From Ships; IMO: London, UK, 2018; Volume 304, pp. 1–12. [Google Scholar]
- Kim, H.; Koo, K.Y.; Joung, T.-H. A study on the necessity of integrated evaluation of alternative marine fuels. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 26–31. [Google Scholar] [CrossRef]
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905. [Google Scholar] [CrossRef]
- Deniz, C.; Zincir, B. Environmental and economical assessment of alternative marine fuels. J. Clean. Prod. 2016, 113, 438–449. [Google Scholar] [CrossRef]
- Solakivi, T.; Paimander, A.; Ojala, L. Cost competitiveness of alternative maritime fuels in the new regulatory framework. Transp. Res. Part D Transp. Environ. 2022, 113, 103500. [Google Scholar] [CrossRef]
- Harahap, F.; Nurdiawati, A.; Conti, D.; Leduc, S.; Urban, F. Renewable marine fuel production for decarbonised maritime shipping: Pathways, policy measures and transition dynamics. J. Clean. Prod. 2023, 415, 137906. [Google Scholar] [CrossRef]
- Karvounis, P.; Tsoumpris, C.; Boulougouris, E.; Theotokatos, G. Recent advances in sustainable and safe marine engine operation with alternative fuels. Front. Mech. Eng. 2022, 8, 994942. [Google Scholar] [CrossRef]
- Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Inal, O.B.; Zincir, B.; Dere, C. Hydrogen as maritime transportation fuel: A pathway for decarbonization. In Greener and Scalable E-Fuels for Decarbonization of Transport; Springer: Singapore, 2022; pp. 67–110. [Google Scholar]
- Kaiser, R.; Ahn, C.-Y.; Kim, Y.-H.; Park, J.-C. Performance and mass transfer evaluation of PEM fuel cells with straight and wavy parallel flow channels of various wavelengths using CFD simulation. Int. J. Hydrogen Energy 2023, 51, 1326–1344. [Google Scholar] [CrossRef]
- Mekonnin, A.S.; Wacławiak, K.; Humayun, M.; Zhang, S.; Ullah, H. Hydrogen Storage Technology, and Its Challenges: A Review. Catalysts 2025, 15, 260. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Venkateswaran, P.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Ball, M.; Wietschel, M. The future of hydrogen–opportunities and challenges. Int. J. Hydrogen Energy 2009, 34, 615–627. [Google Scholar] [CrossRef]
- French, S. The Role of Zero and Low Carbon Hydrogen in Enabling the Energy Transition and the Path to Net Zero Greenhouse Gas Emissions: With global policies and demonstration projects hydrogen can play a role in a net zero future. Johns. Matthey Technol. Rev. 2020, 64, 357–370. [Google Scholar] [CrossRef]
- Loschan, C.; Schwabeneder, D.; Maldet, M.; Lettner, G.; Auer, H. Hydrogen as Short-Term Flexibility and Seasonal Storage in a Sector-Coupled Electricity Market. Energies 2023, 16, 5333. [Google Scholar] [CrossRef]
- Yang, X.; Nielsen, C.P.; Song, S.; McElroy, M.B. Breaking the hard-to-abate bottleneck in China’s path to carbon neutrality with clean hydrogen. Nat. Energy 2022, 7, 955–965. [Google Scholar] [CrossRef]
- Gray, N.; McDonagh, S.; O’Shea, R.; Smyth, B.; Murphy, J.D. Decarbonising ships, planes and trucks: An analysis of suitable low-carbon fuels for the maritime, aviation and haulage sectors. Adv. Appl. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- Otto, M.; Chagoya, K.L.; Blair, R.G.; Hick, S.M.; Kapat, J.S. Optimal hydrogen carrier: Holistic evaluation of hydrogen storage and transportation concepts for power generation, aviation, and transportation. J. Energy Storage 2022, 55, 105714. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.; Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- International Maritime Organization. Fourth IMO GHG Study 2020; IMO: Chiba, Japan, 2021; p. 524. [Google Scholar]
- Haugan, P.M.; Drange, H. Effects of CO2 on the ocean environment. Energy Convers. Manag. 1996, 37, 1019–1022. [Google Scholar] [CrossRef]
- Sharma, S.; Chatani, S.; Mahtta, R.; Goel, A.; Kumar, A. Sensitivity analysis of ground level ozone in India using WRF-CMAQ models. Atmos. Environ. 2016, 131, 29–40. [Google Scholar] [CrossRef]
- Lee, D.S.; Fahey, D.W.; Forster, P.M.; Newton, P.J.; Wit, R.C.; Lim, L.L.; Owen, B.; Sausen, R. Aviation and global climate change in the 21st century. Atmos. Environ. 2009, 43, 3520–3537. [Google Scholar] [CrossRef]
- Prather, M.; Sausen, R.; Grossman, A.; Haywood, J.; Rind, D.; Subbaraya, B. Potential climate change from aviation. In Aviation and the Global Atmosphere. A Special Report of IPCC 185; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Ramanathan, V.; Callis, L.; Cess, R.; Hansen, J.; Isaksen, I.; Kuhn, W.; Lacis, A.; Luther, F.; Mahlman, J.; Reck, R.; et al. Climate-chemical interactions and effects of changing atmospheric trace gases. Rev. Geophys. 1987, 25, 1441–1482. [Google Scholar] [CrossRef]
- Wuebbles, D.; Gupta, M.; Ko, M. Evaluating the impacts of aviation on climate change. Eos Trans. Am. Geophys. Union 2007, 88, 157–160. [Google Scholar] [CrossRef]
- Buhaug, Ø.; Corbett, J.; Endresen, Ø.; Eyring, V.; Faber, J.; Hanayama, S.; Lee, D.S.; Lee, D.; Lindstad, H.; Markowska, A.Z. Second IMO GHG Study 2009; International Maritime Organization: London, UK, 2009. [Google Scholar]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Alternative fuel options for low carbon maritime transportation: Pathways to 2050. J. Clean. Prod. 2021, 297, 126651. [Google Scholar] [CrossRef]
- ITFDM Transport. Pathways to Zero-Carbon Shipping by 2035; International Transport Forum: Paris, France, 2018. [Google Scholar]
- Bicer, Y.; Dincer, I. Clean fuel options with hydrogen for sea transportation: A life cycle approach. Int. J. Hydrogen Energy 2018, 43, 1179–1193. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kim, W.; Chun, K. A preliminary study on an alternative ship propulsion system fueled by ammonia: Environmental and economic assessments. J. Mar. Sci. Eng. 2020, 8, 183. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Dincer, I. Hydrogen and fuel cell technologies for sustainable future. Jordan J. Mech. Ind. Eng. 2008, 2, 1–14. [Google Scholar]
- Mallouppas, G.; Yfantis, E.A. Decarbonization in shipping industry: A review of research, technology development, and innovation proposals. J. Mar. Sci. Eng. 2021, 9, 415. [Google Scholar] [CrossRef]
- Agency, I.E. Towards Hydrogen Definitions Based on Their Emissions Intensity; OECD: Paris, France, 2023. [Google Scholar]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef]
- Kusadome, Y.; Ikeda, K.; Nakamori, Y.; Orimo, S.; Horita, Z. Hydrogen storage capability of MgNi2 processed by high pressure torsion. Scr. Mater. 2007, 57, 751–753. [Google Scholar] [CrossRef]
- Hamedani, E.A.; Alenabi, S.A.; Talebi, S. Hydrogen as an energy source: A review of production technologies and challenges of fuel cell vehicles. Energy Rep. 2024, 12, 3778–3794. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Ma, N.; Zhao, W.; Wang, W.; Li, X.; Zhou, H. Large scale of green hydrogen storage: Opportunities and challenges. Int. J. Hydrogen Energy 2024, 50, 379–396. [Google Scholar] [CrossRef]
- Seddiek, I.S.; Elgohary, M.M.; Ammar, N.R. The hydrogen-fuelled internal combustion engines for marine applications with a case study. Brodogr. Teor. I Praksa Brodogr. I Pomor. Teh. 2015, 66, 23–38. [Google Scholar]
- Yip, H.L.; Srna, A.; Yuen, A.C.Y.; Kook, S.; Taylor, R.A.; Yeoh, G.H.; Medwell, P.R.; Chan, Q.N. A review of hydrogen direct injection for internal combustion engines: Towards carbon-free combustion. Appl. Sci. 2019, 9, 4842. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Liu, W.; Mao, L. Diagnosing Improper Membrane Water Content in Proton Exchange Membrane Fuel Cell Using Two-Dimensional Convolutional Neural Network. Energies 2022, 15, 4247. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Han, M. Industrial Development Status and Prospects of the Marine Fuel Cell: A Review. J. Mar. Sci. Eng. 2023, 11, 238. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Çögenli, M.; Mukerjee, S.; Yurtcan, A.B. Membrane electrode assembly with ultra low platinum loading for cathode electrode of PEM fuel cell by using sputter deposition. Fuel Cells 2015, 15, 288–297. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Yuan, X.-Z.; Martin, J.J.; Yang, D.; Qiao, J.; Ma, J. A review on water balance in the membrane electrode assembly of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 9461–9478. [Google Scholar] [CrossRef]
- Huijsmans, J.; Kraaij, G.; Makkus, R.; Rietveld, G.; Sitters, E.; Reijers, H.T.J. An analysis of endurance issues for MCFC. J. Power Sources 2000, 86, 117–121. [Google Scholar] [CrossRef]
- Pellegrino, S.; Lanzini, A.; Leone, P. Techno-economic and policy requirements for the market-entry of the fuel cell micro-CHP system in the residential sector. Appl. Energy 2015, 143, 370–382. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Williams, M.C.; Strakey, J.P.; Singhal, S.C. US distributed generation fuel cell program. J. Power Sources 2004, 131, 79–85. [Google Scholar] [CrossRef]
- Borroni-Bird, C.E. Fuel cell commercialization issues for light-duty vehicle applications. J. Power Sources 1996, 61, 33–48. [Google Scholar] [CrossRef]
- Korberg, A.D.; Brynolf, S.; Grahn, M.; Skov, I.R. Techno-economic assessment of advanced fuels and propulsion systems in future fossil-free ships. Renew. Sustain. Energy Rev. 2021, 142, 110861. [Google Scholar] [CrossRef]
- Sjölin, K.; Holmgren, E. A Proton Exchange Membrane & Solid Oxide Fuel Cell Comparison. Bachelor Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2019. [Google Scholar]
- Sürer, M.G.; Arat, H.T. Advancements and current technologies on hydrogen fuel cell applications for marine vehicles. Int. J. Hydrogen Energy 2022, 47, 19865–19875. [Google Scholar] [CrossRef]
- Yuan, J.; Sun, J.; Sun, P.; Nakazawa, T.; Sunde’n, B. Marine applications of fuel cell technology. In Proceedings of the International Conference on Fuel Cell Science, Engineering and Technology, Rochester, NY, USA, 14–16 June 2004. [Google Scholar]
- Phogat, P.; Chand, B.; Jha, R.; Singh, S. Hydrogen and methanol fuel cells: A comprehensive analysis of challenges, advances, and future prospects in clean energy. Int. J. Hydrogen Energy 2025, 109, 465–485. [Google Scholar] [CrossRef]
- Östling, E. Model on Degradation of PEM Fuel Cells in Marine Applications. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Switzerland, 2021. [Google Scholar]
- Hong, S.T. Decarbonizing the Global Shipping Industry: Evaluating Pathways for Alternative Fuels. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2022. [Google Scholar]
- Pyrc, M.; Gruca, M.; Tutak, W.; Jamrozik, A. Assessment of the co-combustion process of ammonia with hydrogen in a research VCR piston engine. Int. J. Hydrogen Energy 2023, 48, 2821–2834. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Bréquigny, P.; Medina, A.V.; Boulet, E.; Emberson, D.; Løvås, T. Ammonia as fuel for transportation to mitigate zero carbon impact. In Engines and Fuels for Future Transport; Springer: Berlin/Heidelberg, Germany, 2021; pp. 257–279. [Google Scholar]
- Karan, A.; Dayma, G.; Chauveau, C.; Halter, F. High-pressure and temperature ammonia flame speeds. In Proceedings of the 13th Asia-Pacific Conference on Combustion (ASPACC), ADNEC, Abu Dhabi, UAE, 4–9 December 2021. [Google Scholar]
- Teoh, Y.H.; How, H.G.; Le, T.D.; Nguyen, H.T.; Loo, D.L.; Rashid, T.; Sher, F. A review on production and implementation of hydrogen as a green fuel in internal combustion engines. Fuel 2023, 333, 126525. [Google Scholar] [CrossRef]
- Rosati, M.; Aleiferis, P. Hydrogen SI and HCCI combustion in a direct-injection optical engine. SAE Int. J. Engines 2009, 2, 1710–1736. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Mohammadi, A.; Shioji, M.; Nakai, Y.; Ishikura, W.; Tabo, E. Performance and combustion characteristics of a direct injection SI hydrogen engine. Int. J. Hydrogen Energy 2007, 32, 296–304. [Google Scholar] [CrossRef]
- Wittek, K.; Cogo, V.; Prante, G. Development of a pneumatic actuated low-pressure direct injection gas injector for hydrogen-fueled internal combustion engines. Int. J. Hydrogen Energy 2023, 48, 10215–10234. [Google Scholar] [CrossRef]
- Goldmann, A.; Sauter, W.; Oettinger, M.; Kluge, T.; Schröder, U.; Seume, J.R.; Friedrichs, J.; Dinkelacker, F. A study on electrofuels in aviation. Energies 2018, 11, 392. [Google Scholar] [CrossRef]
- Gillingham, K. Hydrogen Internal Combustion Engine Vehicles: A prudent Intermediate Step or a Step in the Wrong Direction; Department of Management Science & Engineering Global Climate and Energy Project Precourt Institute for Energy Efficiency of Stanford University: Stanford, CA, USA, 2007. [Google Scholar]
- Li, J.-C.; Xu, H.; Zhou, K.; Li, J.-Q. A review on the research progress and application of compressed hydrogen in the marine hydrogen fuel cell power system. Heliyon 2024, 10, e25304. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Adeel, S.; Chkoniya, V. Hydrogen as a Sustainable Fuel: Transforming Maritime Logistics. Energies 2025, 18, 1231. [Google Scholar] [CrossRef]
- Magliano, A.; Perez Carrera, C.; Pappalardo, C.M.; Guida, D.; Berardi, V.P. A comprehensive literature review on hydrogen tanks: Storage, safety, and structural integrity. Appl. Sci. 2024, 14, 9348. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Li, Y.; Zhao, Y.; Shu, X.; Zhang, G.; Yang, T.; Liu, Y.; Wu, P.; Ding, Z. Rare-Earth Metal-Based Materials for Hydrogen Storage: Progress, Challenges, and Future Perspectives. Nanomaterials 2024, 14, 1671. [Google Scholar] [CrossRef]
- Ratoi, A.; Munteanu, C.; Eliezer, D. Maximizing Onboard Hydrogen Storage Capacity by Exploring High-Strength Novel Materials Using a Mathematical Approach. Materials 2024, 17, 4288. [Google Scholar] [CrossRef]
- The U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. 2015. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storagelightduty-vehicles (accessed on 25 January 2024).
- Zhou, Z.; Tao, J. Hydrogen-powered vessels in green maritime decarbonization: Policy drivers, technological frontiers and challenges. Front. Mar. Sci. 2025, 12, 1601617.4. [Google Scholar] [CrossRef]
- AFCEnergy. AFC Energy PLC Product HydroX-Cell(L). 2021. Available online: https://www.afcenergy.com/products/hydrox-cell_l/ (accessed on 28 January 2021).
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Fuel cell power systems for maritime applications: Progress and perspectives. Sustainability 2021, 13, 1213. [Google Scholar] [CrossRef]
- McConnell, V.P. Now, voyager? The increasing marine use of fuel cells. Fuel Cells Bull. 2010, 2010, 12–17. [Google Scholar] [CrossRef]
- Tronstad, T.; Åstrand, H.H.; Haugom, G.-P.; Langfeldt, L. Study on the Use of Fuel Cells in Shipping; European Maritime Safety Agency: Lisbon, Portugal, 2017. [Google Scholar]
- Markowski, J.; Pielecha, I. The potential of fuel cells as a drive source of maritime transport. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 012019. [Google Scholar]
- Dvorak, D.; Sigfusson, T.I.; Gunnarsson, B. International Graduate Education in Fuel Cells and Hydrogen Energy in Iceland. ECS Trans. 2011, 30, 281. [Google Scholar] [CrossRef]
- Suda, R. Japan’s Tsuneishi, CMB to Build Hydrogen-Powered Tug. 2021. Available online: https://www.argusmedia.com/pt/news-and-insights/latest-market-news/2207781-japan-s-tsuneishi-cmb-to-build-hydrogen-powered-tug (accessed on 22 April 2022).
- Kommula, N.D.S. Analysis of Performance and Features of Hydrogen as an Energy Carrier. Master’s Thesis, Universita Politechnica delle Marche, Ancona, Italy, 2022. [Google Scholar]
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Turnock, S.; Hudson, D. A Comparison of Hydrogen and Ammonia for Future Long Distance Shipping Fuels. In Proceedings of the International Conference on LNG/LPG and Alternative Fuel Ships 2020, London, UK, 29–30 January 2020. [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Shakeri, N.; Zadeh, M.; Nielsen, J.B. Hydrogen fuel cells for ship electric propulsion: Moving toward greener ships. IEEE Electrif. Mag. 2020, 8, 27–43. [Google Scholar] [CrossRef]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Pratt, J.W. Feasibility of the SF-BREEZE: A Zero-Emission Hydrogen Fuel Cell High-Speed Passenger Ferry; Sandia National Lab. (SNL-CA): Livermore, CA, USA, 2017. [Google Scholar]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Genovese, M.; Cigolotti, V.; Jannelli, E.; Fragiacomo, P. Hydrogen refueling process: Theory, modeling, and in-force applications. Energies 2023, 16, 2890. [Google Scholar] [CrossRef]
- Sadi, M.; Deymi-Dashtebayaz, M. Hydrogen refueling process from the buffer and the cascade storage banks to HV cylinder. Int. J. Hydrogen Energy 2019, 44, 18496–18504. [Google Scholar] [CrossRef]
- Oschwald*, M.; Smith, J.; Branam, R.; Hussong, J.; Schik, A.; Chehroudi, B.; Talley, D. Injection of fluids into supercritical environments. Combust. Sci. Technol. 2006, 178, 49–100. [Google Scholar] [CrossRef]
- Bonacina, C.N.; Gaskare, N.B.; Valenti, G. Assessment of offshore liquid hydrogen production from wind power for ship refueling. Int. J. Hydrogen Energy 2022, 47, 1279–1291. [Google Scholar] [CrossRef]
- Aardahl, C.L.; Rassat, S.D. Overview of systems considerations for on-board chemical hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 6676–6683. [Google Scholar] [CrossRef]
- Plazas-Niño, F.A.; Ortiz-Pimiento, N.R.; Quirós-Tortós, J. Supporting energy system modelling in developing countries: Techno-economic energy dataset for open modelling of decarbonization pathways in Colombia. Data Brief 2023, 48, 109268. [Google Scholar] [CrossRef]
- Howells, M.; Rogner, H.; Strachan, N.; Heaps, C.; Huntington, H.; Kypreos, S.; Hughes, A.; Silveira, S.; DeCarolis, J.; Bazillian, M. OSeMOSYS: The open source energy modeling system: An introduction to its ethos, structure and development. Energy Policy 2011, 39, 5850–5870. [Google Scholar] [CrossRef]
- Lagemann, B.; Lindstad, E.; Fagerholt, K.; Rialland, A.; Erikstad, S.O. Optimal ship lifetime fuel and power system selection. Transp. Res. Part D Transp. Environ. 2022, 102, 103145. [Google Scholar] [CrossRef]
- Mestemaker, B.; van den Heuvel, H.; Castro, B.G. Designing the zero emission vessels of the future: Technologic, economic and environmental aspects. Int. Shipbuild. Prog. 2020, 67, 5–31. [Google Scholar] [CrossRef]
- Mylonopoulos, F.; Durgaprasad, S.; Coraddu, A.; Polinder, H. Lifetime design, operation, and cost analysis for the energy system of a retrofitted cargo vessel with fuel cells and batteries. Int. J. Hydrogen Energy 2024, 91, 1262–1273. [Google Scholar] [CrossRef]
- Semchukova, V.; Polemis, K.; Abdin, Z.; Solanki, B.; Cary, S. Hydrogen Infrastructure Analysis for the Port Applications; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2024. [Google Scholar]
- Fernández-Ríos, A.; Santos, G.; Pinedo, J.; Santos, E.; Ruiz-Salmón, I.; Laso, J.; Lyne, A.; Ortiz, A.; Ortiz, I.; Irabien, Á. Environmental sustainability of alternative marine propulsion technologies powered by hydrogen-a life cycle assessment approach. Sci. Total Environ. 2022, 820, 153189. [Google Scholar] [CrossRef]
- Wang, J.; Lobo, J.; Shutters, S.T.; Strumsky, D. Fueling a net-zero future: The influence of government-funded research on climate change mitigation inventions. Environ. Innov. Soc. Transit. 2024, 51, 100836. [Google Scholar] [CrossRef]
- Pisacane, G.; Sannino, G.; Carillo, A.; Struglia, M.V.; Bastianoni, S. Marine energy exploitation in the mediterranean region: Steps forward and challenges. Front. Energy Res. 2018, 6, 109. [Google Scholar] [CrossRef]
- Van Sickle, E.; Ralli, P.; Pratt, J.; Klebanoff, L. MV Sea Change: The first commercial 100% hydrogen fuel cell passenger ferry in the world. Int. J. Hydrogen Energy 2025, 105, 389–404. [Google Scholar] [CrossRef]
- Peeters, P.; Papp, B. Envisioning Tourism in 2030 and Beyond. The Changing Shape of Tourism in a Decarbonising World. 2023. Available online: https://pure.buas.nl/ws/portalfiles/portal/27136592/Peeters_Papp_EnvisionTourism_report.pdf (accessed on 17 February 2025).
- Lee, H.; Lee, J.; Roh, G.; Lee, S.; Choung, C.; Kang, H. Comparative life cycle assessments and economic analyses of alternative marine fuels: Insights for practical strategies. Sustainability 2024, 16, 2114. [Google Scholar] [CrossRef]
- Bade, S.O.; Tomomewo, O.S. Economic, social, and regulatory challenges of green hydrogen production and utilization in the US: A review. Int. J. Hydrogen Energy 2024, 49, 314–335. [Google Scholar] [CrossRef]
- Penttinen, S.L. Navigating the hydrogen landscape: An analysis of hydrogen support mechanisms in the US and the EU. Rev. Eur. Comp. Int. Environ. Law 2024, 33, 397–411. [Google Scholar] [CrossRef]
- Talalasova, E.; Boyland, J.; Garvin, B.; Fahnestock, J. Annual Progress Report on Green Shipping Corridors 2022. Global Maritime Forum. Available online: https://www.globalmaritimeforum.org/content/2022/11/The-2022-AnnualProgress-Report-on-Green-Shipping-Corridors.pdf (accessed on 17 November 2022).
- Corbeau, A.-S.; Kaswiyanto, R.P. National Hydrogen Strategies and Roadmap Tracker; Center on Global Energy Policy at Columbia University, School of International and Public Affairs. Available online: https://www.energypolicy.columbia.edu/publications/national-hydrogen-strategies-and-roadmap-tracker/ (accessed on 15 May 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).