Study of Sorption Activity of Carbon Nanomaterials for Capture of Chlorine-Containing Gases
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
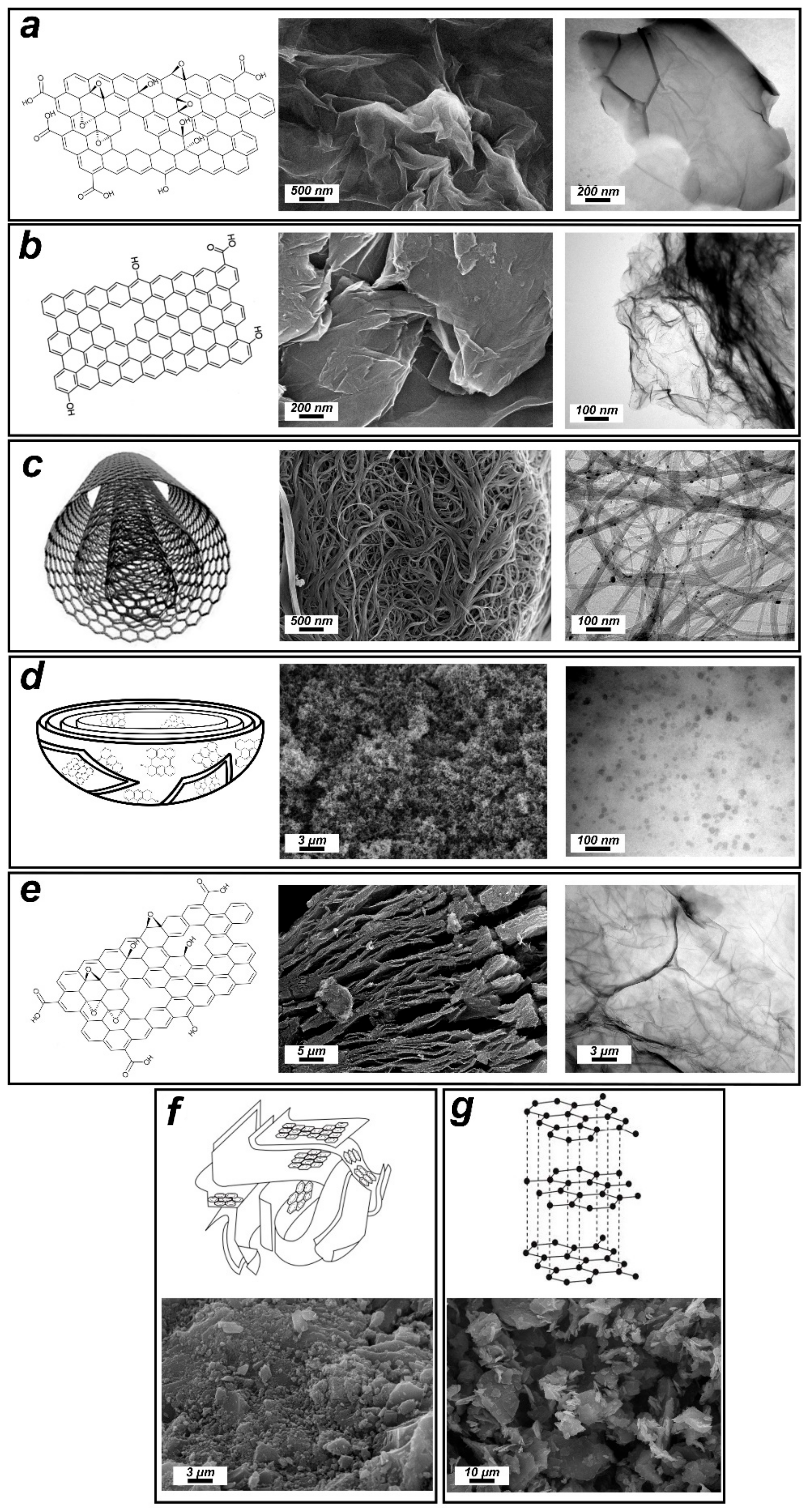
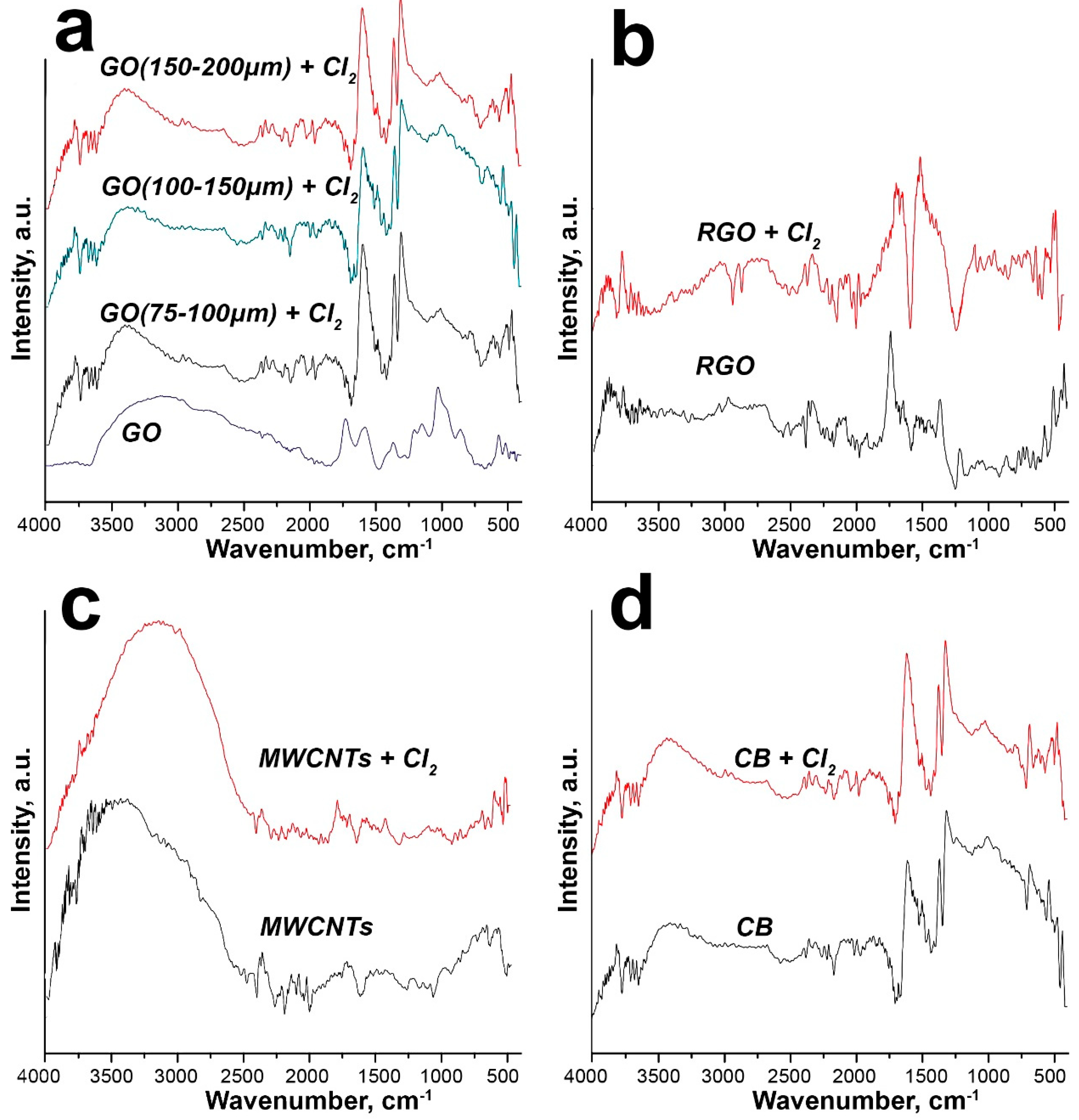
2.2. Characterization
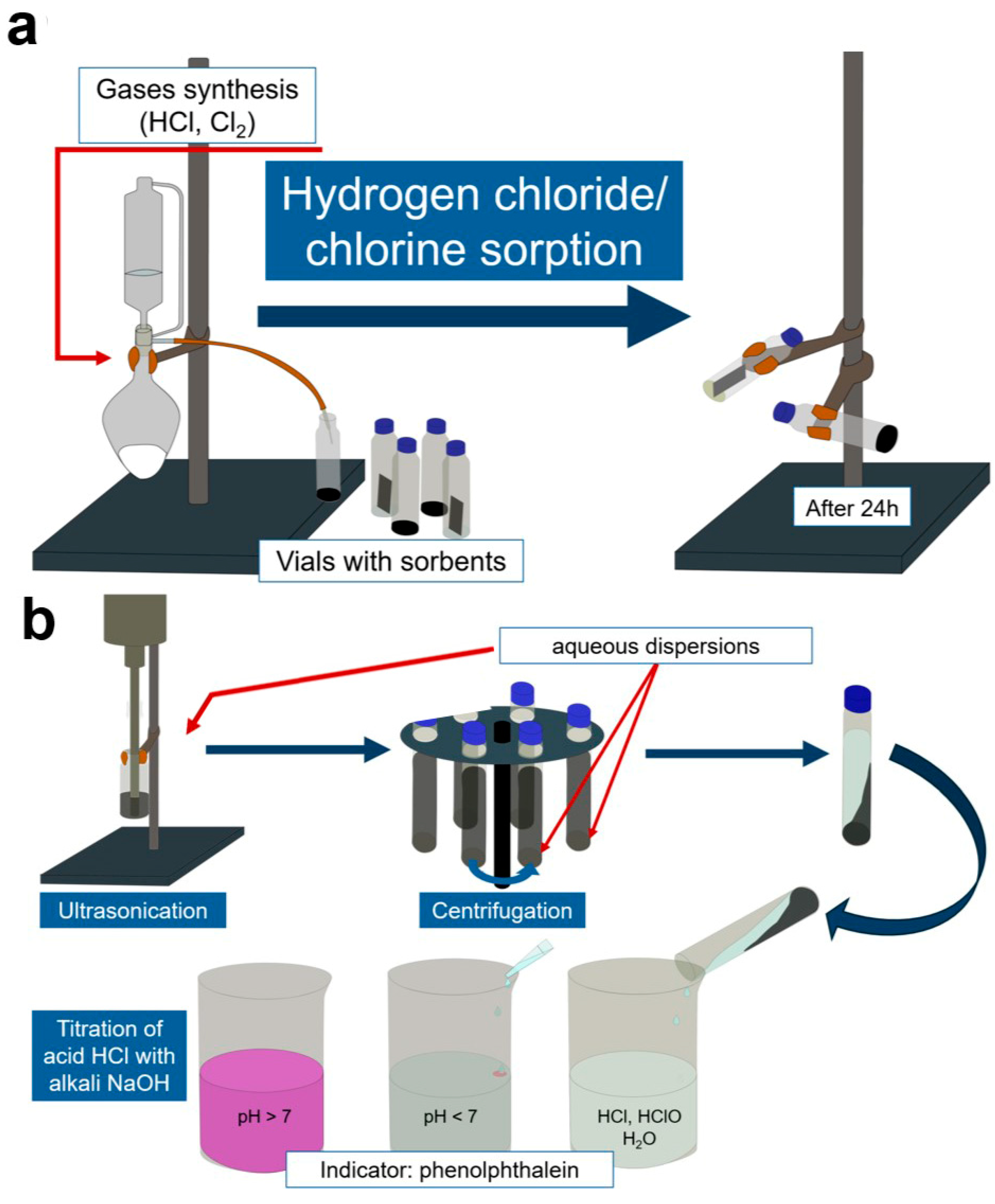
2.3. Sorption Experiments
2.3.1. Chlorine Sorption
2.3.2. Hydrogen Chloride Sorption
2.3.3. Quantitative Measurement of Absorbed Gases
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Fu, X.; Peng, X.; Wang, W.; Badia, A.; Fernandez, R.P.; Cuevas, C.A.; Mu, Y.; Chen, J.; Jimenez, J.L.; et al. Halogens Enhance Haze Pollution in China. Environ. Sci. Technol. 2021, 55, 13625–13637. [Google Scholar] [CrossRef] [PubMed]
- Salkinoja-Salonen, M.; Uotila, J.; Jokela, J.; Laine, M.; Saski, E. Organic Halogens in the Environment: Studies of Environmental Biodegradability and Human Exposure. Environ. Health Perspect. 1995, 103, 63–69. [Google Scholar]
- Addis, D.R.; Aggarwal, S.; Lazrak, A.; Jilling, T.; Matalon, S. Halogen-Induced Chemical Injury to the Mammalian Cardiopulmonary Systems. Physiology 2021, 36, 272–291. [Google Scholar] [CrossRef]
- Sivey, J.D.; Roberts, A.L. Assessing the Reactivity of Free Chlorine Constituents Cl2, Cl2O, and HOCl Toward Aromatic Ethers. Environ. Sci. Technol. 2012, 46, 2141–2147. [Google Scholar] [CrossRef]
- Lim, S.H.; Feng, L.; KemLing, J.W.; Musto, C.J.; Suslick, K.S. An optoelectronic nose for the detection of toxic gases. Nat. Chem. 2009, 1, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Lopez, A.; von Glasow, R. Reactive halogen chemistry in the troposphere. Chem. Soc. Rev. 2012, 41, 6448–6472. [Google Scholar] [CrossRef]
- Tratnyek, P.G.; Edwards, E.; Carpenter, L.; Blossom, S. Environmental occurrence, fate, effects, and remediation of halogenated (semi)volatile organic compounds. Environ. Sci. Process. Impacts 2020, 22, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Anderson, T.A.; Redder, A.; Jackson, W.A. Perchlorate Formation by Ozone Oxidation of Aqueous Chlorine/Oxy-Chlorine Species: Role of ClxOy Radicals. Environ. Sci. Technol. 2010, 44, 2961–2967. [Google Scholar] [CrossRef]
- Baljinnyam, T.; Niimi, Y.; Salsbury, J.R.; Fukuda, S.; Ouellette, C.M.; Andersen, C.R.; Hirasawa, Y.; Prough, D.A.; Garner, C.E.; Salzman, A.L.; et al. Dose and gender dependence of chlorine inhalation in a conscious ovine model. Sci. Rep. 2023, 13, 22367. [Google Scholar] [CrossRef]
- Govier, P.; Coulson, J.M. Civilian exposure to chlorine gas: A systematic review. Toxicol. Lett. 2018, 293, 249–252. [Google Scholar] [CrossRef]
- de Genaro, I.S.; de Almeida, F.M.; Hizume-Kunzler, D.C.; Moriya, H.T.; Silva, R.A.; Cruz, J.C.G.; Lopes, R.B.; Righetti, R.F.; de Paula Vieira, R.; Saiki, M.; et al. Low dose of chlorine exposure exacerbates nasal and pulmonary allergic inflammation in mice. Sci. Rep. 2018, 8, 12636. [Google Scholar] [CrossRef] [PubMed]
- Dragstedt, C.A. The Halogenated Hydrocarbons: Their Toxicity and Potential Dangers. AMA Arch. Intern. Med. 1956, 97, 261–262. [Google Scholar] [CrossRef]
- Council, N.R. Assessment of Exposure-Response Functions for Rocket-Emission Toxicants. “Appendix D: Acute Toxicity of Hydrogen Chloride”; The National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Kohsaka, Y.; Matsuura, D.; Kimura, Y. Sustainable synthesis of fine chemicals and polymers using industrial chlorine chemistry. Commun. Chem. 2024, 7, 265. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mondelli, C.; Schmidt, T.; Schlüter, O.F.K.; Wolf, A.; MLeczko, L.; Dreier, T. Sustainable chlorine recycling via catalysed HCl oxidation: From fundamentals to implementation. Energy Environ. Sci. 2011, 4, 4786–4799. [Google Scholar] [CrossRef]
- Kaur, S.; Ranjeesh, K.C.; Garg, K.; Gaber, S.; Mehta, S.; Nagaiah, T.C.; Shetty, D. Electrochemical valorization of HCl for the production of chlorine via a proton-filter functional covalent organic framework. J. Mater. Chem. A 2024, 12, 8516–8525. [Google Scholar] [CrossRef]
- Shen, T.; Xu, Y.; Jiang, C.; Lai, Y.; Wu, L.; Liu, S.; Zhang, L.; Qian, C.; Zhou, S. Electrochemical Synthesis of Aryl Chlorides Using HCl as the Chlorine Source. ACS Sustain. Chem. Eng. 2024, 12, 3289–3297. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, C.; Mao, K.; Jiang, W.; Low, J.; Duan, D.; Ju, H.; Liu, D.; Wang, K.; Zang, Y.; et al. Sustainable methane utilization technology via photocatalytic halogenation with alkali halides. Nat. Commun. 2023, 14, 1410. [Google Scholar] [CrossRef]
- Le, G.T.; Ta, N.T.; Pham, T.Q.; Dao, Y.H. Response Surface Analysis of Fenobucarb Removal by Electrochemically Generated Chlorine. Water 2019, 11, 899. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Kumari N, S.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- Zellner, T.; Eyer, F. Choking agents and chlorine gas—History, pathophysiology, clinical effects and treatment. Toxicol. Lett. 2020, 320, 73–79. [Google Scholar] [CrossRef]
- Valentukeviciene, M.; Andriulaityte, I.; Karczmarczyk, A.; Zurauskiene, R. Removal of Residual Chlorine from Stormwater Using Low-Cost Adsorbents and Phytoremediation. Environments 2024, 11, 101. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Qu, Z.; Li, X.; Zhang, Z.; Liang, X.; Chen, J.; Li, J. A Review of Traditional and Emerging Residual Chlorine Quenchers on Disinfection By-Products: Impact and Mechanisms. Toxics 2023, 11, 410. [Google Scholar] [CrossRef]
- Collins, T. A call for a chlorine sunset. Nature 2000, 406, 17–18. [Google Scholar] [CrossRef]
- Shah, K.B.; Kim, D.; Pinakana, S.D.; Hobosyan, M.; Montes, A.; Raysoni, A.U. Evaluating Indoor Air Quality in Residential Environments: A Study of PM2.5 and CO2 Dynamics Using Low-Cost Sensors. Environments 2024, 11, 237. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Grazhulene, S.S.; Zolotareva, N.I.; Hodos, I.I. Efficiency of Adsorbents Containing Various Carbon Allotropes, Including Modified Carbon Nanotubes. J. Anal. Chem. 2024, 79, 1399–1407. [Google Scholar] [CrossRef]
- Pavlenko, V.; Khosravi H, S.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; et al. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Lee, M.; Park, S. Silica-Coated Multi-Walled Carbon Nanotubes Impregnated with polyethyleneimine for Carbon Dioxide Capture under Flue Gas Condition. J. Solid State Chem. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified carbon nanotubes/tetraethylenepentamine for CO2 capture. Fuel 2017, 206, 10–18. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Arami-Niya, A. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation. Appl. Surf. Sci. 2011, 257, 3936–3942. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Impact of tailored textural properties of activated carbons on methane storage. Powder Technol. 2021, 394, 336–352. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, H.; Zhong, Q. Preparation and characterization of aminated graphite oxide for CO2 capture. Appl. Surf. Sci. 2012, 258, 4301–4307. [Google Scholar] [CrossRef]
- Attia, Y.A.; Ezet, A.E.; Saeed, S.; Galmed, A.H. Nano carbon-modified air purification filters for removal and detection of particulate matters from ambient air. Sci. Rep. 2024, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yamashita, K.; Nabeshi, H.; Yoshikawa, T.; Yoshioka, Y.; Tsunoda, S.I.; Tsutsumi, Y. Carbon Nanomaterials: Efficacy and Safety for Nanomedicine. Materials 2012, 5, 350–363. [Google Scholar] [CrossRef]
- Ioni, Y.; Khamidullin, T.; Sapkov, I.; Brysko, V.; Dimiev, A.M. Revealing the effect of graphite source on the properties of synthesized graphene oxide. Carbon Lett. 2024, 34, 1219–1228. [Google Scholar] [CrossRef]
- Ioni, Y.; Sapkov, I.; Kirsanova, M.; Dimiev, A.M. Flame modified graphene oxide: Structure and sorption properties. Carbon 2023, 212, 118122. [Google Scholar] [CrossRef]
- Ioni, Y.; Popova, A.; Maksimov, S.; Kozerozhets, I. Ni Nanoparticles on the Reduced Graphene Oxide Surface Synthesized in Supercritical Isopropanol. Nanomaterials 2023, 13, 2923. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Eigler, S. Graphene Oxide: Fundamentals and Applications; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Chaudhuri, H.; Lin, X.; Yun, Y.-S. Graphene oxide-based dendritic adsorbent for the excellent capturing of platinum group elements. J. Hazard. Mater. 2023, 451, 131206. [Google Scholar] [CrossRef]
- Ioni, Y.V.; Ivannikova, A.S.; Shapovalov, S.S.; Gubin, S.P. Study of the interaction of graphene oxide with chlorine. Russ. Chem. Bull. 2022, 71, 675–679. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Grebenko, A.K.; Krasnikov, D.V.; Bubis, A.V.; Stolyarov, V.S.; Vyalikh, D.V.; Makarova, A.A.; Fedorov, A.; Aitkulova, A.; Alekseeva, A.A.; Gilshtein, E.; et al. High-Quality Graphene Using Boudouard Reaction. Adv. Sci. 2022, 9, 2200217. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ma, S. Precise synthesis of graphene by chemical vapor deposition. Nanoscale 2024, 16, 4407–4433. [Google Scholar] [CrossRef]
- Novikov, I.V.; Krasnikov, D.V.; Shestakova, V.S.; Rogov, I.P.; Dmitrieva, V.A.; Goldt, A.E.; Kallio, T.; Nasibulin, A.G. Boosting CO-based synthesis of single-walled carbon nanotubes with hydrogen. Chem. Eng. J. 2023, 476, 146527. [Google Scholar] [CrossRef]
- Zamansky, K.K.; Fedorov, F.S.; Shandakov, S.D.; Chetyrkina, M.; Nasibulin, A.G. A gas sensor based on free-standing SWCNT film for selective recognition of toxic and flammable gases under thermal cycling protocols. Sens. Actuators B Chem. 2024, 417, 136116. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Vershinina, A.I.; Lomakin, M.V.; Russakov, D.M.; Gordaya, O.R.; Chirkova, I.M.; Kosobutsky, A.V.; Krasnikov, D.V.; Chetyrkina, M.R.; Nasibulin, A.G.; et al. SWCNT fibers decorated with Au nanoparticles as voltammetric sensors for arsenic (III) determination. Microchem. J. 2024, 206, 111482. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Ioni, Y.; Ibragimova, V.; Sapkov, I.; Dimiev, A. Graphene oxide with different oxygen content produced from natural and synthetic graphite sources for methylene blue sorption. Diam. Relat. Mater. 2024, 149, 111550. [Google Scholar] [CrossRef]
- Pavlenko, V.V.; Zakharov, A.Y.; Ayaganov, Z.E.; Mansurov, Z.A. Nanoporous carbon materials: Modern production methods and applications. Russ. Chem. Rev. 2024, 93, RCR5122. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Riwu, A.A.P.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Darmokoesoemo, H.; Kusuma, H.S. Potential of activated carbon from various sources as a low-cost adsorbent to remove heavy metals and synthetic dyes. Results Chem. 2023, 5, 100711. [Google Scholar] [CrossRef]
- Scholz-Böttcher, B.M.; Bahadir, M.; Hopf, H. The Beilstein Test: An Unintentional Dioxin Source in Analytical and Research Laboratories. Angew. Chem. Int. Ed. 1992, 31, 443. [Google Scholar] [CrossRef]
- Tomkins, D.F.; Frank, C.W. Atomic absorption determination of chloride utilizing the Beilstein test. Anal. Chem. 1972, 44, 1451–1454. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Highly efficient, rapid and selective CO2 capture by thermally treated graphene nanosheets. J. CO2 Util. 2016, 13, 50–60. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Zheng, Q.R. Methane adsorption on the graphene sheets, activatedcarbon and carbon black. Appl. Therm. Eng. 2016, 108, 605–613. [Google Scholar] [CrossRef]
- Yan, T.; Li, T.X.; Wang, R.Z.; Jia, R. Experimental investigation on the ammonia adsorption and heat transfer characteristics of the packed multi-walled carbon nanotubes. Appl. Therm. Eng. 2015, 77, 20–29. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Enhanced Adsorption of Ammonia on Metal-Organic Framework/Graphite Oxide Composites: Analysis of Surface Interactions. Adv. Funct. Mater. 2010, 20, 111–118. [Google Scholar] [CrossRef]
- Babu, D.J.; Puthusseri, D.; Kühl, F.G.; Okeil, S.; Bruns, M.; Hampe, M.; Schneider, J.J. SO2 gas adsorption on carbon nanomaterials: A comparative study. Beilstein J. Nanotechnol. 2018, 9, 1782–1792. [Google Scholar] [CrossRef] [PubMed]

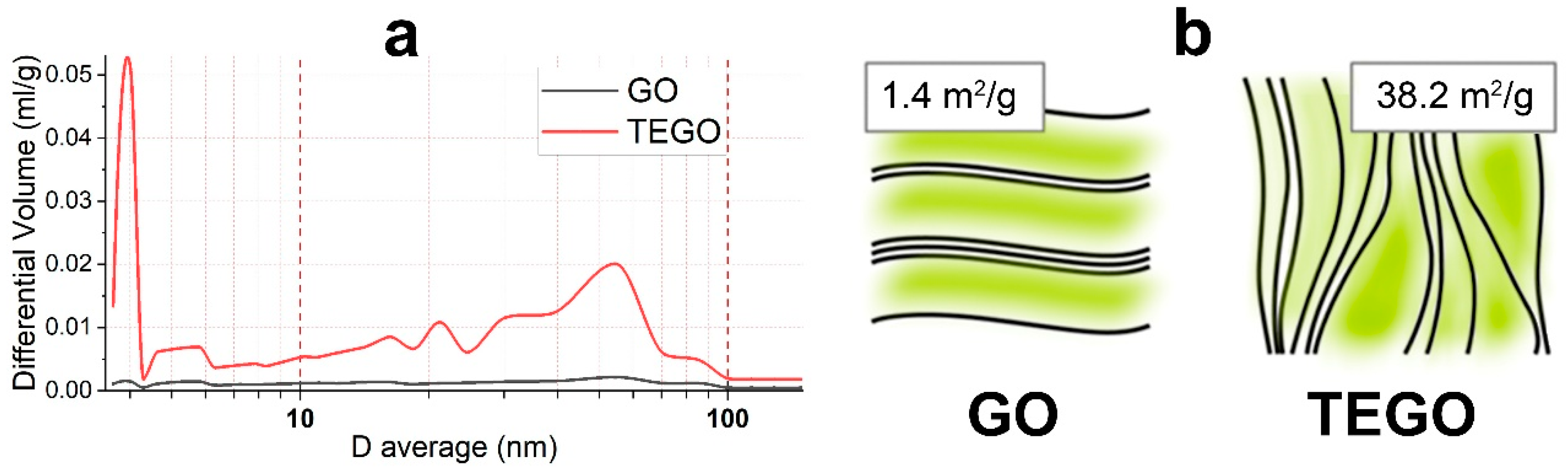
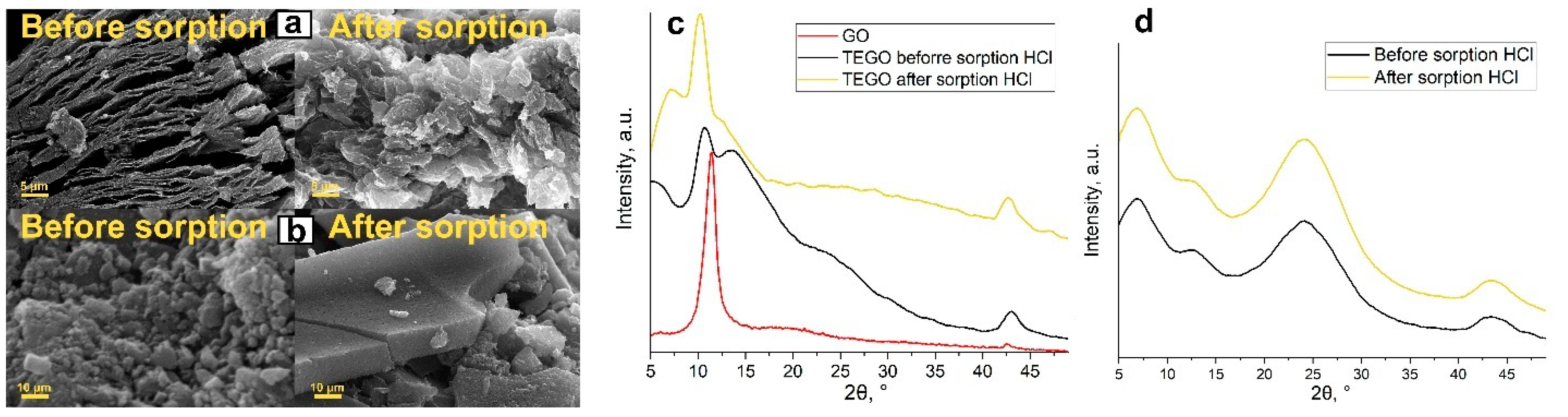
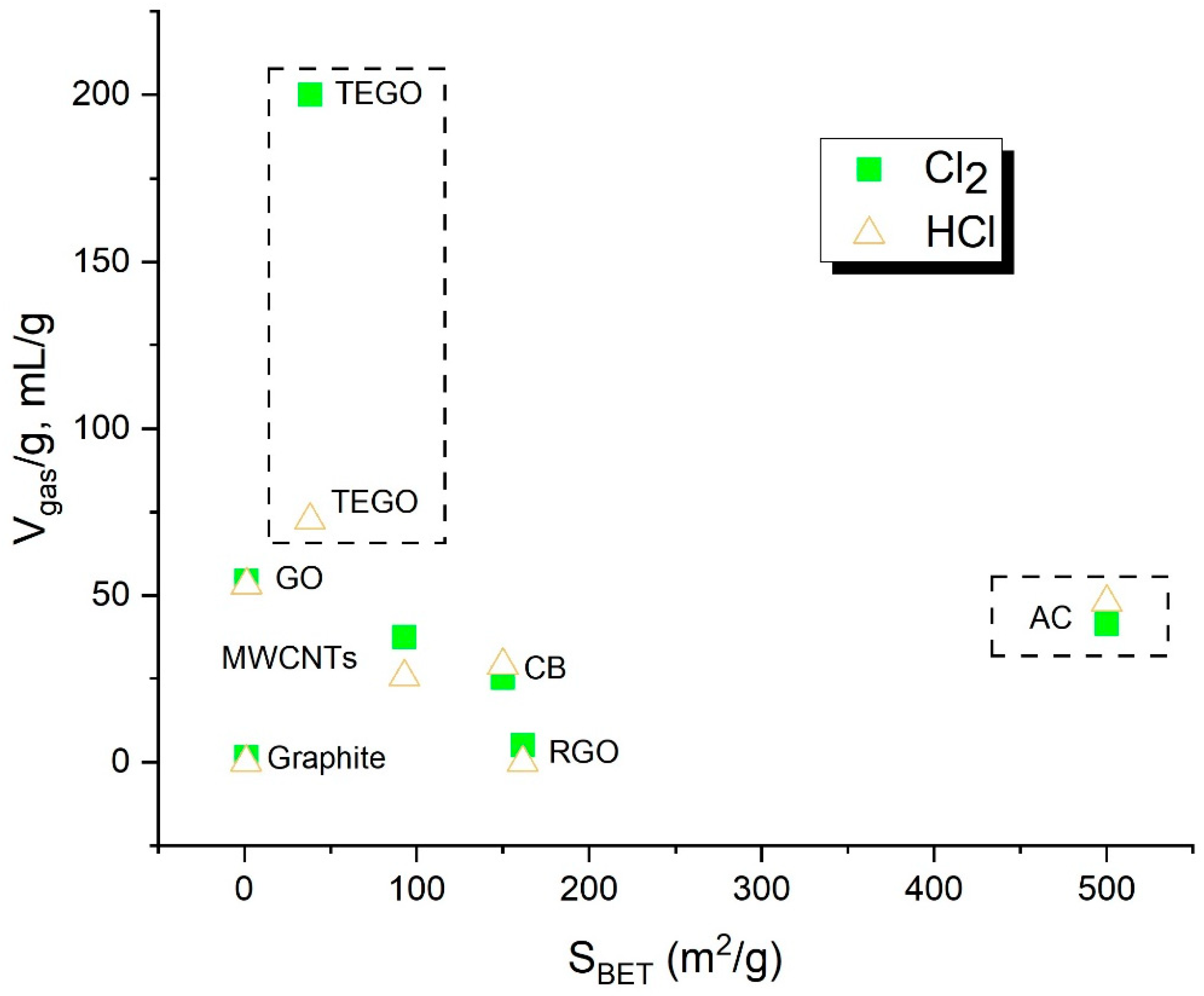
| Sample | SBET (m2/g) * |
|---|---|
| Graphite | 1.1 |
| AC | 500.1 |
| GO | 1.4 |
| RGO | 161.5 |
| MWCNT | 92.9 |
| CB | 150.0 |
| TEGO | 38.2 |
| Sorbent | Beilstein Test | XRF | ||||||
|---|---|---|---|---|---|---|---|---|
| After Sorption | After US Treatment | After Sorption | After US Treatment | |||||
| Cl2 | HCl | Cl2 | HCl | Cl2 | HCl | Cl2 | HCl | |
| GO | + | + | − | − | + | + | − | − |
| RGO | + | − | − | − | + | − | − | − |
| MWCNTs | + | + | − | − | + | + | − | − |
| CB | + | + | − | − | + | + | − | − |
| TEGO | + | + | − | − | + | + | − | − |
| AC | + | + | − | − | + | + | − | − |
| Graphite | + | − | − | − | + | − | − | − |
| Sorbent | Gas | Conditions | Capture Capacity (mmol·g−1) | Reference |
|---|---|---|---|---|
| AC | CO2 | 105 °C, 100 kPa | 2.04 | [32] |
| MWCNTs | 25 °C, 100 kPa | 1.88 | [30] | |
| Aminated GO | 100 °C, 100 kPa | 2.9 | [34] | |
| RGO | 0 °C, 100 kPa | 2.89 | [60] | |
| MWCNT modified | 60 °C, 100 kPa | 5 | [31] | |
| AC | CH4 | 25 °C, 3.5 MPa | 9.7 | [33] |
| AC | 0 °C, 2 MPa | 6 | [61] | |
| RGO | 1.25 | |||
| CB | 0.6 | |||
| MWCNTs | NH3 | 35 °C, 744 kPa | 5.29 | [62] |
| GO | 25 °C, 100 kPa | 2.46 | [63] | |
| SWCNTs | SO2 | 25 °C, 375 kPa | 22.8 | [64] |
| GO | 25 °C, 25 kPa | 4 | ||
| AC | Cl2 | 20 °C, 100 kPa | 1.88 | This study |
| GO | 2.42 | |||
| TEGO | 8.93 | |||
| AC | HCl | 20 °C, 100 kPa | 2.14 | This study |
| GO | 2.37 | |||
| TEGO | 3.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioni, Y.; Ibragimova, V. Study of Sorption Activity of Carbon Nanomaterials for Capture of Chlorine-Containing Gases. Clean Technol. 2025, 7, 39. https://doi.org/10.3390/cleantechnol7020039
Ioni Y, Ibragimova V. Study of Sorption Activity of Carbon Nanomaterials for Capture of Chlorine-Containing Gases. Clean Technologies. 2025; 7(2):39. https://doi.org/10.3390/cleantechnol7020039
Chicago/Turabian StyleIoni, Yulia, and Victoria Ibragimova. 2025. "Study of Sorption Activity of Carbon Nanomaterials for Capture of Chlorine-Containing Gases" Clean Technologies 7, no. 2: 39. https://doi.org/10.3390/cleantechnol7020039
APA StyleIoni, Y., & Ibragimova, V. (2025). Study of Sorption Activity of Carbon Nanomaterials for Capture of Chlorine-Containing Gases. Clean Technologies, 7(2), 39. https://doi.org/10.3390/cleantechnol7020039






