1. Introduction
The Food and Agriculture Organization (FAO) has published that around a third of global food production is wasted every year, which corresponds to around 1.3 billion tons of food. In Europe, around 90 million tons of food waste is produced annually, which is the equivalent of 170 million tons of CO
2 released into the environment every year. The food industry is responsible for 60% of total food losses during production, distribution and sales [
1,
2,
3].
One of the industries with the highest percentage of waste and by-products is the fruit and vegetable processing industry. The final product is often less than 50% of the source material and the processing of this material results in a devastating balance of waste and final product. For this reason, there is a growing need to convert fruit and vegetable by-products into commercially valuable products, especially in the form of bioactive compounds and fiber. Sustainable management of fruit and vegetable waste is, therefore, essential, as is the development of new techniques that enable efficient recycling and reuse for value-added food production [
2].
For years, red beetroots were mostly consumed as a vegetable (in about 90% of cases) and only minimally processed [
4]. Due to numerous findings on the beneficial composition of red beetroot and the possibility of using not only the pulp, but also other associated parts and its individual components, red beetroot has recently been used in the development and production of various foods with positive effects on the human body. The processing of red beetroot produces sufficient by-products and waste, consisting of peel, pulp, pomace, leaves and stems, depending on the situation. This means that in addition to a significant increase in red beetroot production, the production of processing waste and by-products also increases considerably. Igual et al. [
5] reported in their study that around 40% of red beetroot is generated as waste during the extraction of liquefied beet for pulping. A Croatian producer of fruit and vegetable products processes about 1000 tons of red beetroot annually, with about 35% of the waste generated during the process (A. Samardžija, personal communication, 30 March 2025). Certain by-products of red beetroot processing, such as its peel, have significant potential for recycling and reuse to enrich certain food products. The peel is a by-product of any red beetroot processing process (especially minimal processing), although it has a good nutritional composition. Red beetroot peel contains around 2–33% fiber, 4–18% protein and 10–12% minerals (depending on the extraction process), with potassium being the most abundant mineral. Recently, its antioxidant and general bioactive properties have been increasingly studied, as it is considered rich in polyphenols and antioxidants [
4,
6]. Red beetroot is a rich source of bioactive compounds, including betalains (betacyanin and betaxanthin), flavonoids (such as rutin, astragalin, kaempferol and quercetin), terpenoids, saponins, vitamins, phenolic acids (such as gallic acid, p-coumaric acid and caffeic acid), steroids, alkaloids, tannins, dietary fiber, and sugars [
7]. Red beetroot is characterized by high concentrations of nitrates and nitrites, with an average of 1379 mg/kg, making it the highest value among root vegetables. These compounds play an important role in supporting respiratory and cardiovascular health, highlighting the potential benefits of red beetroot and its supplements for improving these physiological systems [
8].
Dietary fiber is an essential part of an adequate and balanced diet and plays an important role in maintaining overall health, as the benefits of consuming food rich in dietary fiber are numerous and varied. One of the most well-known benefits of dietary fiber is its role in promoting digestive health. Other benefits include promoting gut health, aiding weight control and chronic disease prevention, and improving blood sugar control [
7].
Betaine plays several roles in mammalian physiology, which include three main functions. First, as an organic osmolyte, betaine plays a crucial role in maintaining normal cellular volume under osmotic stress conditions that may arise from various environmental or physiological factors. This function is essential for cellular homeostasis and the proper functioning of cells in response to fluctuating osmotic pressures. Secondly, betaine provides protection against protein denaturation, which is an essential aspect of maintaining cellular integrity and function, especially under stressful conditions such as heat shock or oxidative stress. Thirdly, betaine is significantly involved in the remethylation of homocysteine to methionine as part of the one-carbon metabolism. Together with methylfolate, betaine is one of the few compounds that can donate methyl groups to this important biochemical process, emphasizing its importance in maintaining metabolic balance and reducing the risk of homocysteine-related cardiovascular problems. Although betaine is not classified as an essential nutrient due to its ability to be endogenously synthesized from free choline via the enzyme choline dehydrogenase, the body’s ability to produce sufficient amounts of betaine is often inadequate to meet the body’s daily requirements. Consequently, dietary betaine intake is necessary to ensure proper physiological function. Foods rich in betaine or its precursor choline serve as the main sources for the body’s betaine pool. In particular, cereal grains, pseudocereals (especially amaranth and quinoa), whole grain products (e.g., whole wheat flour, bread, pasta, couscous and breakfast cereals) and certain vegetables such as spinach and red beetroot contribute significantly to the absorption of betaine. In addition, betaine is often found in various shellfish, including mussels, oysters, clams and scallops [
9]. This paper focuses on two bioactive components found in beetroot, the previously mentioned dietary fiber and betaine.
The development of innovative food processing methods can increase the competitiveness in the food industry market by improving product quality, introducing new products to the market, and reducing production costs [
10].
The effects of reversible and irreversible electromechanical destruction of the cell membrane have numerous applications in the food industry, mainly because they are not thought to alter the sensory properties of food or denature proteins and most enzymes. The pulsed electric field (PEF) is often used to improve the extraction yield of desired components from various natural substances. A review of the existing literature shows that the extraction of sugar from sugar beet, betalains from red beetroot, inulin from chicory, anthocyanins from red cabbage, polyphenols from fresh tea leaves and many others has been successful. PEF technology is based on the electrical treatment of the sample in an extremely short time (from a few nanoseconds to a few milliseconds), with the strength of the electric field pulses typically ranging between 15–80 kV/cm. The treatment of food with PEF leads to a non-thermal electroplasmolysis of the cell contents, which breaks down the cell membrane [
11].
The most common extraction methods are still conventional techniques such as maceration and digestion (a process similar to maceration in which slight heating is applied during extraction), but these have numerous disadvantages due to the nature of the process. Conventional extraction methods require the use of large volumes of organic solvents, are time-consuming and energy consuming, and yield lower quantities of the desired product. They also often have a toxic effect on the environment. To overcome the limitations of conventional methods, environmentally friendly green extraction techniques are currently being developed. These techniques require less use of solvents or the use of “green” solvents, lower process temperatures and shorter extraction times. These improvements, combined with optimized process parameters, result in lower energy consumption, higher yields and less environmental impact. Ultrasonic-assisted extraction, a non-thermal process in which acoustic energy is used to increase the release and diffusion rates of the target substances by cavitation of the solvent, is one of these environmentally friendly extraction techniques. The impact of ultrasonic wave-induced acoustic cavitation on the treated matrix contributes significantly to the disruption of cell walls. This process improves both mass and heat transfer, allowing the desired components to diffuse more easily into the extraction solvent, ultimately leading to a higher extraction yield. Ultrasonic waves and their effects alter the structure of the cellular material by causing fragmentation, erosion, tissue tension and dexturization while stimulating redox reactions in the system. Numerous studies have been conducted on the efficacy of ultrasonic extraction, especially of plant metabolites due to the above mechanisms, such as the extraction of chlorophyll from spinach, polyphenols from apple pomace, betalain antioxidants from beet pomace, oil from caraway seeds and oil from fresh yeast cells [
3,
12,
13].
This study focuses on sustainability by utilizing industrial waste materials as a source of betaine and dietary fiber, combined with nonthermal techniques that minimize environmental impact. The pretreatment of beetroot was carried out using PEF technology. PEF pretreatment was used to facilitate the peeling of beets, thereby reducing waste, and maximizing the utilization of by-products from red beetroot processing. The efficacy of ultrasound-assisted extraction (UAE) and conventional thermal extraction (CE) of betaine and dietary fiber from beetroot peel was evaluated by varying the applied amplitude, solvent, and treatment time for UAE extraction, as well as the applied solvent and treatment time for CE extraction. To our current knowledge, there is no reference to the UAE extraction of betaine from beetroot peel. The aim was to obtain a high amount of betaine and dietary fiber, which could then be used as a source of bioactive components for functional food production and upcycling of red beetroot peel.
4. Discussion
The application of non-thermal extraction techniques, together with the use of environmentally friendly solvents, plays an important role in reducing costs and promoting an environmentally friendly approach compared to conventional extraction methods. The pretreatment of red beetroot was carried out using PEF technology to facilitate manual peeling. The proportion of red beetroot peel pre-treated with PEF was 11%, compared to a process without PEF pre-treatment, where the proportion of peel is 35%. The results of this study are consistent with the results of Giancaterino and Jaeger [
21], who investigated the effects of pulsed PEF treatment on the peeling effect of tomatoes and kiwis. Their research showed that PEF treatment significantly improved the peeling efficiency of both fruits, confirming the results of our own study. In the work of Giancaterino and Jaeger [
21], PEF treatment led to a reduction in peeling loss in tomatoes from 43% to 33%, demonstrating the potential of PEF to improve the efficiency of the peeling process. This reduction in peel loss suggests that PEF treatment may improve peel detachment from the fruit, likely due to the breakdown of cell wall structures and weakening of intercellular bonds, a mechanism often associated with the effects of PEF on plant tissue. In our study, an increase in the peeling effect was also observed, although the exact extent of the improvement may vary due to different experimental conditions, such as the specific PEF parameters used and the type of fruit. However, the benefits of PEF treatment were even more pronounced in kiwifruit. Giancaterino and Jaeger [
21] reported up to 66% less peel loss in the PEF-treated kiwifruit compared to the untreated samples, indicating a significant improvement in peeling efficiency. This result is particularly noteworthy, as kiwifruit has a relatively thick, fuzzy skin that is more difficult to remove compared to other fruits. The effectiveness of PEF in reducing peel loss in kiwifruit may indicate that PEF treatment is particularly beneficial for fruit with complex or tough peel structures. In addition, the evaluation methods used by Giancaterino and Jaeger [
21], which include both manual and mechanical peeling as well as the evaluation of weight loss during the peeling process, provide a comprehensive understanding of the effects of PEF treatment. Weight loss during peeling can be a critical factor in food processing as it has a direct impact on yield and the economic profitability of the process. The reduction in peel loss observed in both tomatoes and kiwifruit could, therefore, have important implications for the food industry, particularly in terms of improving fruit processing efficiency while minimizing waste.
As part of the research, samples of red beetroot peel were subjected to a UAE and a CE extraction to determine and optimize the yield of total dietary fiber in the extraction residue and betaine in the extract. UAE was used due to the possibility of achieving relatively low treatment temperatures, while the selected extraction solvents, distilled water and ethanol, represent a cheap, ecologically and technologically acceptable “green solvent” [
19,
22,
23].
In general, ultrasonically treated samples were found to have higher levels of TDF in the extract residues and betaine in the extracts compared to samples subjected to conventional thermal extraction. The TDF yield ranged from 25.55% to 63.55% in the ultrasonically treated samples. Optimum input values of amplitude, treatment time and ethanol content for the maximum output value of TDF were determined via the Response Optimized Model in STATAGRAFICS Centurion (StatPoint Technologies, Inc., Warrenton, VA, USA). For the maximum output value of 44.07%, the optimal parameters are 76.71% amplitude, 6.59 min and 50% ethanol solution. In general, consistent trends were observed; TDF in the extraction residue increased with treatment time, amplitude, and ethanol concentration in the solvent. Accordingly, an increase in these parameters led to a lower extraction yield in the extract, as the TDF content was determined in the extraction residue and not in the extract. Insoluble dietary fibers are not soluble in water anyway (and usually not in ethanol either), or the ethanol content in combination with other mild processing conditions does not create conditions under which they can be successfully extracted. On the other hand, ethanol causes precipitation of soluble fiber (less polar solvent than water), which prevents its transfer from the plant to the extract and makes permeability more difficult. Both phenomena could explain the abundance of TDF in the extraction residue of samples extracted with ultrasound using a higher ethanol content in the solvent compared to samples extracted under milder parameters (e.g., water), potentially improving mass transfer into the extract [
24]. From this point of view, the lack of a statistically significant influence of the ethanol content in the solvent is logical. Regarding the treatment time, it is important to emphasize that compared to previous studies and the optimization of the treatment duration parameter, it was found that the range of the time interval is much smaller. Indeed, most studies show that there is a positive correlation between the extended treatment time and the extraction yield when the treatment time exceeds 10 min, which is longer than the longest treatment time used in this study [
25,
26]. The optimum yield of plant metabolites, including fibers, is achieved in a time range of 10 to 60 min, after which the efficiency decreases [
27]. Therefore, it is possible that the treatment time interval used in this study (with a maximum of 9 min) is too short to achieve a statistically significant effect of the treatment time parameter. The same listed inconsistencies may also be an explanation for the lack of statistical significance of the effect of amplitude on extraction yield, as the trend of yield increase as a function of process duration and applied ultrasonic power are consistent with each other [
28]. Results such as our research findings were recorded in the study on the optimization of ultrasound-assisted extraction of dietary fiber from yellow dragon fruit peels, which resulted in the highest insoluble dietary fiber (IDF) (61.3%) and soluble dietary fiber (SDF) (10.8%) values. The best extraction conditions were achieved by maximizing IDF and SDF with the following parameters: pause time 1 s, liquid to solid ratio 30 mL/g and total treatment time 60 min [
29]. Another study investigated the potential of extracting dietary fiber from the Queen pineapple of the Tripura region using ultrasound-assisted extraction (UAE). The UAE method was compared with the traditional alkali extraction technique for extracting dietary fiber from the peel waste of the Queen pineapple. The results showed that the UAE method achieved a yield of 86.67% after 22.35 min of sonication, with a solid/liquid ratio of 27.5 g/mL and an ultrasonic amplitude of 46.9% [
30].
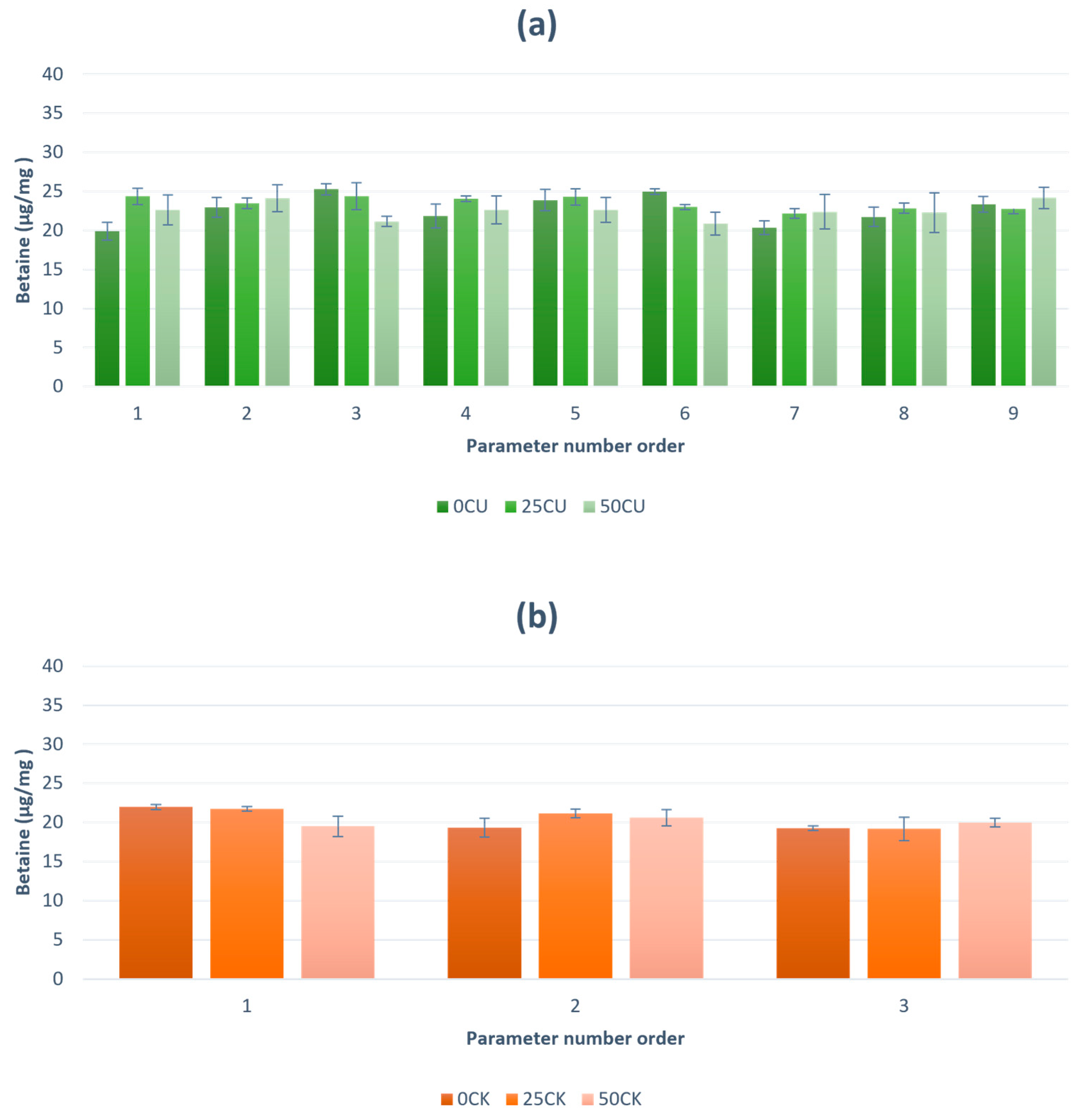
The total betaine yield ranges from 19.91 µg/mL to 24.37 µg/mL in the samples treated with ultrasound. In the samples treated with UAE, most of the two-way interactions and single quadratic interactions of amplitude, treatment time and solvent showed no statistically significant effect on betaine yield (
p > 0.05). A statistically significant influence of the mutual interaction of treatment time and solvent was found (
p = 0.01). Optimum input values of amplitude, treatment time and ethanol content for the maximum output value of betaine were determined using the Response Optimized Model in STATAGRAFICS Centurion (StatPoint Technologies, Inc., Warrenton, VA, USA). For the maximum output value of 24.80 µg/mL, the optimal parameters are 100% amplitude, 3 min, and distilled water. For the samples extracted with CE, the betaine yield was lower compared to the samples extracted with UAE. The decrease in betaine yield with conventional extraction (CE) can be attributed to the degradation of betaine due to prolonged exposure to heat. In general, most biologically active compounds are sensitive to heat. We found no data on betaine extraction from red beetroot and its by-products to compare. Most researchers investigated the extraction of betalain and betanin. For example, Aztatzi-Rugerio et al. [
31] investigated the betaine concentration at 75 °C over different time intervals and observed the degradation of betanins, a pigment group of betalains, which led to the formation of neobetanin, the main degradation product of betalains. In comparison, the highest average temperatures during ultrasound-assisted extraction (UAE) were 25.25, 28 and 29.3 °C, while CE was performed at a constant temperature of 60 °C, which probably contributed to the greater degradation of betaines. Despite the limited number of studies dealing with betaine extraction, one study presents an efficient and environmentally friendly method for the extraction of betaine from sugar beet waste (molasses) in the sugar industry. The method uses a sustainable surfactant and is based on the technique of cloud point extraction (CPE). Under optimized conditions, the extraction efficiency for betaine recovery reached up to 88%. Polyethylene glycol (PEG), an edible surfactant, was used in the process. In the final step, the extracted betaine was freeze-dried at −56 °C for 16 h under 0.5 bar ambient pressure. The results indicate that the final betaine powder product is suitable for direct use as a dietary supplement in animal nutrition given the safety of PEG for consumption [
32]. Borjan et al. [
33] compared Soxhlet, ultrasonic, cold and supercritical fluid extraction methods. Soxhlet extraction with water as solvent yielded the highest amount of betalain, while Soxhlet extraction with 50% ethanol as a solvent yielded the highest concentration of total phenols. The total phenol concentration ranged from 12.09 mg/g to 18.60 mg/g. Ultrasonic extraction with a solvent of 30% methanol gave the lowest concentration of total phenols, while Soxhlet extraction with 50% ethanol gave the highest concentration. Cold extraction with 50% methanol showed the highest anti-inflammatory activity. The authors concluded that Soxhlet extraction with 50% ethanol is the most suitable method for the extraction of bioactive compounds, as it has a higher yield and bioactivity. Red beetroot is a valuable source of betalains, natural red pigments, polyphenols, fiber and nitrates, and its popularity (especially in the form of juice) has resulted in a considerable amount of waste being generated. Fernando et al. [
3] focused their study on the recovery of betalains and polyphenols from dried whole red beetroot and beet pulp waste from the juicing industry. For UAE, ethanol/water-based solvent mixtures were used, which proved to be more effective than single solvents. Initially, enzyme-assisted extraction was tested for wet beet pulp, but this method failed to obtain the betalains. The results indicate that the betalains are more stable in dried pulp, making it a preferred starting material for extraction. Another study was focused on the UAE of betalains from red beetroot using water as a solvent. The effects of extraction time and temperature and the comparison of UAE with orbital shaking extraction (OSE) were investigated to evaluate the influence of ultrasound on the extraction process. The optimal conditions for UAE were determined and further studies on ultrasonic power and solvent/sample ratio were conducted. The results showed that the betalain content in the extract decreased with increasing temperature, with a similar decrease observed at higher extraction times. Consequently, the optimum conditions were set at 30 °C and 30 min. Under these conditions, UAE outperformed OSE in terms of betalain extraction efficiency. In addition, increasing the ultrasonic power improved the extraction process, with 83 W being sufficient for maximum removal of betalains. The solvent-to-sample ratio also played an important role, with the highest betalain content achieved at a ratio of 75 mL/g, which corresponded to the highest diffusion coefficient under these conditions [
34].
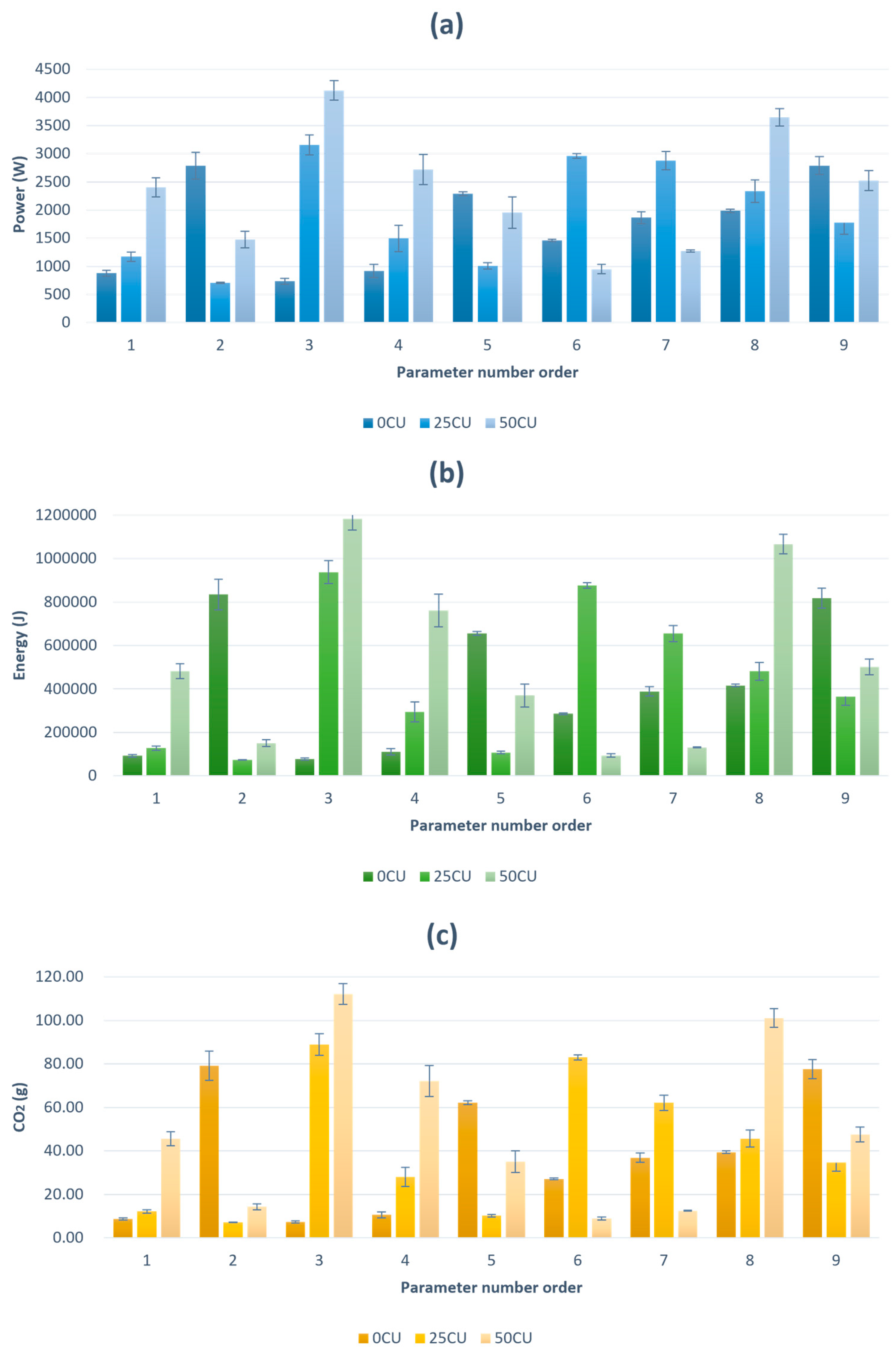
This study highlights the importance of the sustainability of extraction processes, particularly regarding the power and energy consumption associated with thermal and ultrasonic extraction techniques. The results show that ultrasonic treatment consumes less energy compared to thermal treatment. As a non-thermal extraction method, ultrasound operates at lower temperatures and significantly reduces the required extraction time, which directly contributes to lower energy consumption [
35]. Higher energy consumption is often associated with higher emissions of CO
2 and other pollutants, as reported by [
36].
In this study, ultrasonic treatment resulted not only in lower energy consumption but also in a reduction of CO2 emissions compared to conventional thermal extraction. For the samples treated with ultrasound, the interactions between amplitude, treatment time and solvent showed statistically significant effects on CO2 emissions (p < 0.05) and for the samples treated with CE, treatment time and solvent showed statistically significant effects on CO2 emissions (p < 0.05). This double benefit of lower energy consumption and reduced environmental impact makes ultrasound a more sustainable extraction method.
Red beetroot peel is becoming an increasingly popular source of bioactive compounds such as dietary fibers and betaine, which are extracted in a much larger quantity with ultrasound and whose antioxidant properties are better preserved than with CE extraction.
5. Conclusions
Red beetroot peel, which is a by-product from the vegetable processing industry, is a good source of dietary fiber and betaine. In this study, it was shown that dietary fibers and betaine can be extracted in a very efficient and economical way by using green solvents and non-thermal extraction techniques. PEF pretreatment of red beetroot facilitates manual peeling. UAE extraction proved to be more efficient than CE extraction for the extraction of total dietary fiber and betaine. For the samples treated with UAE, single quadratic interactions of amplitude, treatment time and solvent showed no statistically significant effect on the yield of betaine (p > 0.05). Betaine is more soluble in water than in ethanol. Ethanol causes precipitation of the soluble fiber (less polar solvent than water), preventing its transfer from the plant to the extract and leaving it in the extraction residue. Taken together, this suggests that distilled water could replace ethanol as a solvent in the UAE. This substitution offers environmental and economic benefits, as water is more environmentally friendly and less expensive than ethanol. In addition, the use of distilled water eliminates the need to evaporate ethanol, which is particularly advantageous if the extracted material is to be used to fortify or improve the technical and functional properties of food. Considering the nutritional value of dietary fibers and betaine, it is increasingly certain that they will be used in the production of functional products such as cookies, spreads, beverages, etc. For further research, it is essential to scale-up the process to an industrial level to evaluate its feasibility and sustainability under real production conditions. In addition, the health safety of the extraction residues and extract must be investigated to ensure their safety for human consumption.
The development of new products from food waste and by-products has the potential to address global food shortages while reducing environmental impact. This approach not only helps to reduce the amount of food waste, which represents a significant environmental impact, but also contributes to the reduction of greenhouse gas emissions and the conservation of natural resources.