Nitrogen-Doped Porous Waste Biomass as a Sustainable Adsorbent for CO2 Capture: The Influence of Preparation Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Activated Carbon Enhanced with Functional Groups
2.1.1. Carbonization Process for Activated Carbon Synthesis
2.1.2. Treatment of Activated Carbon
2.1.3. Method Preparation
2.1.4. Impregnation Ratio Preparation
2.1.5. Carbonization Temperature Preparation
2.2. CO2 Capture Capacity
2.3. Material Analysis Procedures
3. Results and Discussion
3.1. CO2 Adsorption Performance of Banana Peel-Impregnated Activated Carbon
3.1.1. Effect of the Method Preparation
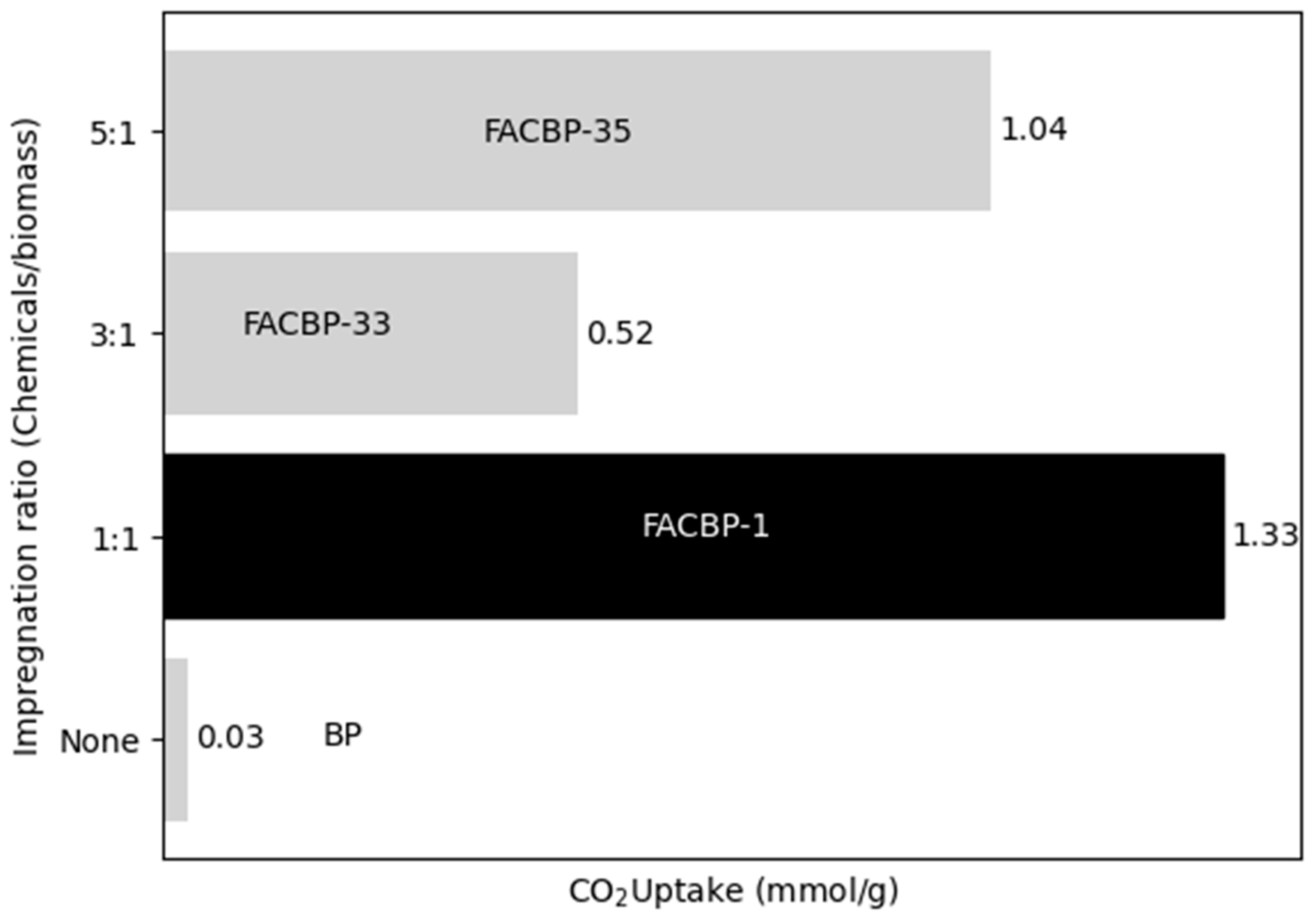
3.1.2. Effect of the Impregnation Ratio
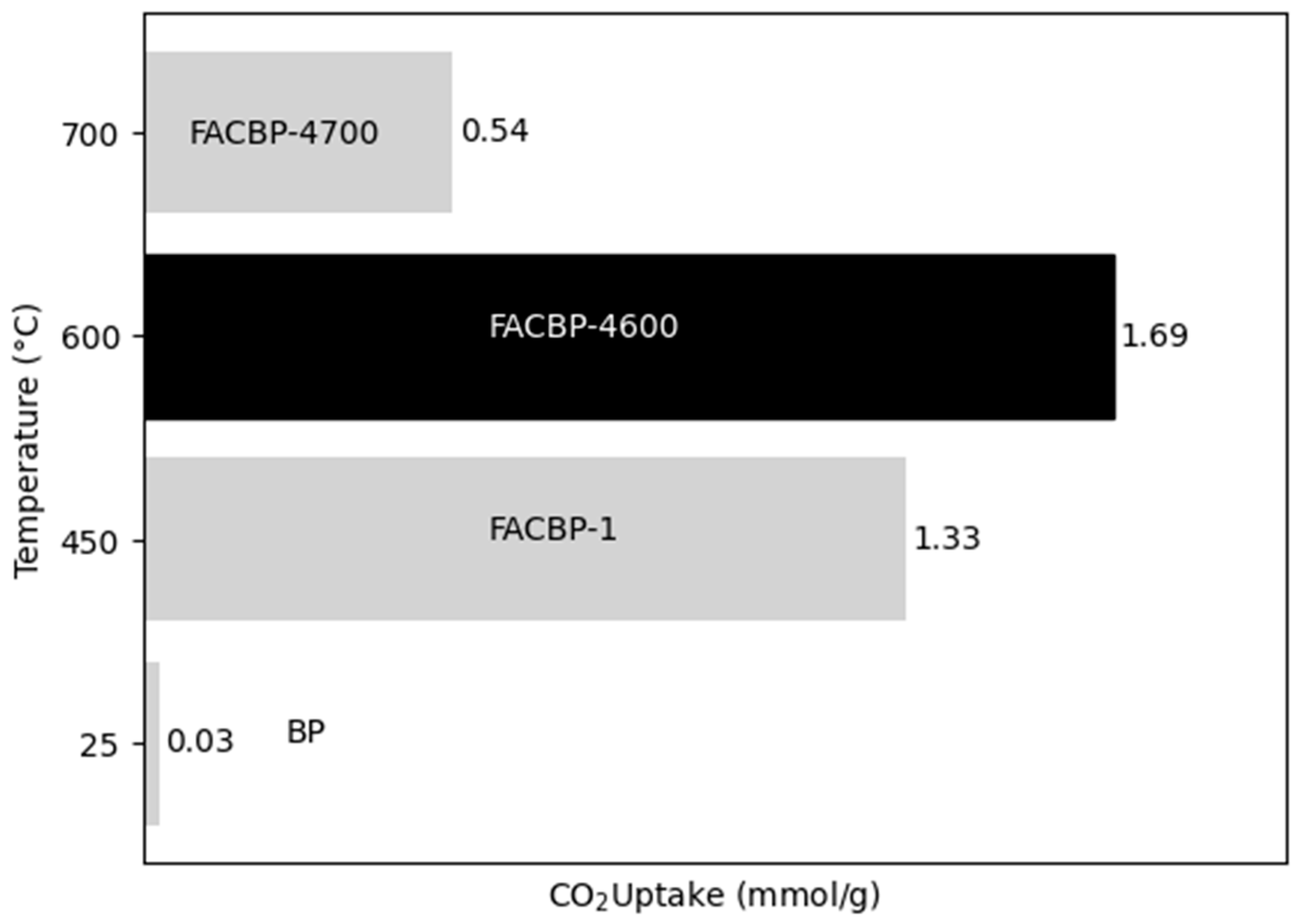
3.1.3. Effect of the Carbonization Temperature
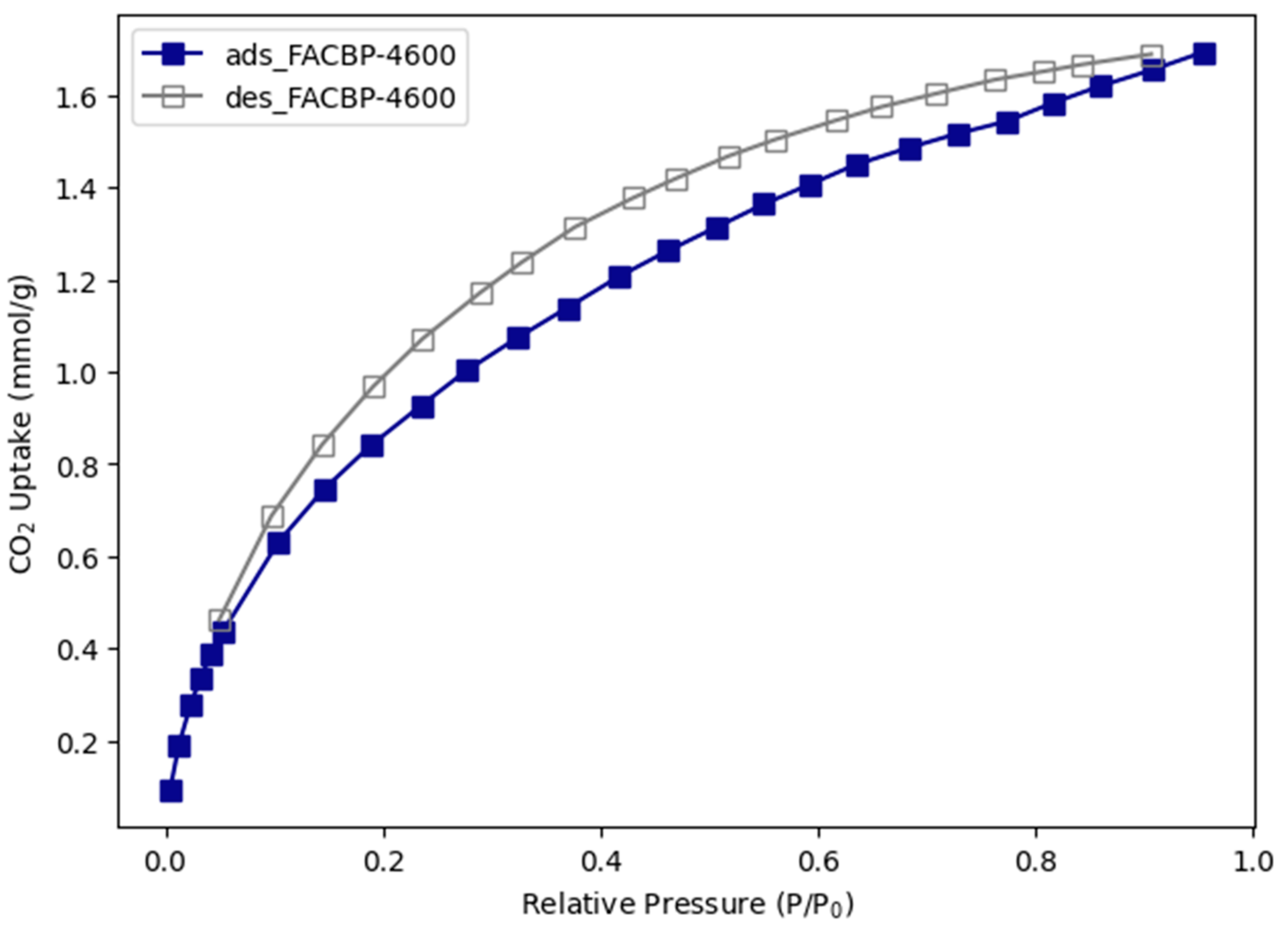
3.1.4. CO2 Adsorption Performance of Banana Peel Functionalized Impregnated Activated Carbon
3.2. Characterization of the Study
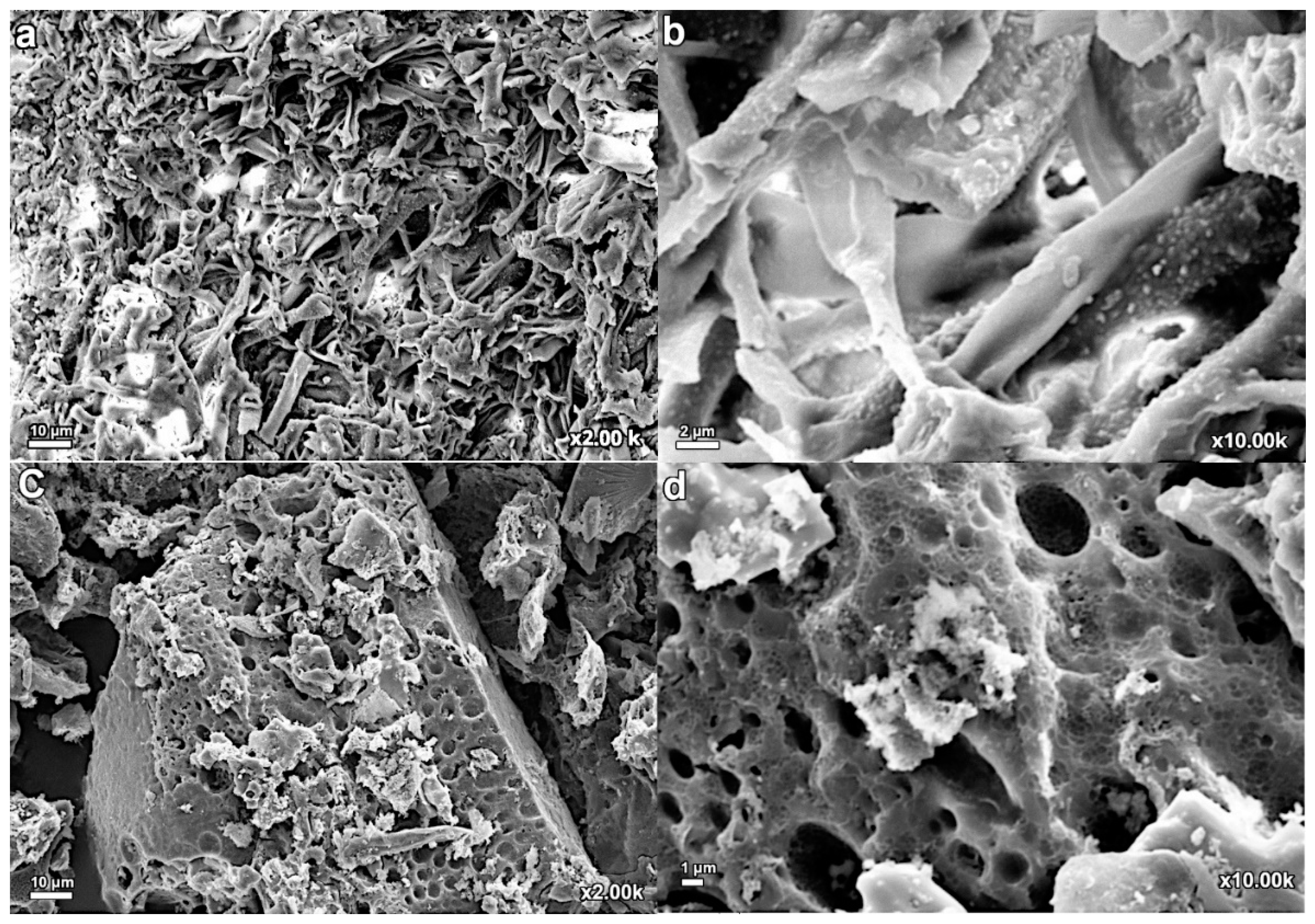
3.2.1. Scanning Electron Microscope Image Analysis (SEM)
3.2.2. Fourier-Transform Infrared (FTIR) Spectroscopy
3.2.3. Thermal Gravimetric Analysis (TGA)
3.2.4. X-Ray Diffraction Analysis (XRD)
3.2.5. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, M.M.F.; Zantye, M.S.; Kazi, M.-K. Challenges and opportunities in carbon capture, utilization and storage: A process systems engineering perspective. Comput. Chem. Eng. 2022, 166, 107925. [Google Scholar] [CrossRef]
- Wang, S.; Lee, Y.-R.; Won, Y.; Kim, H.; Jeong, S.-E.; Hwang, B.W.; Cho, A.R.; Kim, J.-Y.; Park, Y.C.; Nam, H.; et al. Development of high-performance adsorbent using KOH-impregnated rice husk-based activated carbon for indoor CO2 adsorption. Chem. Eng. J. 2022, 437, 135378. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Ye, G.; Fan, J.; Yao, F.; Wang, Y.; Jiao, Y.; Zhu, W.; Huang, H.; Ye, D. Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review. Chemosphere 2022, 287, 131995. [Google Scholar] [CrossRef] [PubMed]
- Hatta, N.S.M.; Hussin, F.; Gew, L.T.; Aroua, M.K. Enhancing surface functionalization of activated carbon using amino acids from natural source for CO2 capture. Sep. Purif. Technol. 2023, 313, 123468. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Zhang, L.; Zhuang, Y.; Du, J. An optimization model for carbon capture utilization and storage supply chain: A case study in Northeastern China. Appl. Energy 2018, 231, 194–206. [Google Scholar] [CrossRef]
- Peres, C.B.; Resende, P.M.R.; Nunes, L.J.R.; de Morais, L.C. Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis. Clean Technol. 2022, 4, 1193–1207. [Google Scholar] [CrossRef]
- Shen, Y. Preparation of renewable porous carbons for CO2 capture—A review. Fuel Process. Technol. 2022, 236, 107437. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, G.; Wu, C.; Liu, J.; Lv, Y. Nitrogen-doped porous carbons derived from sustainable biomass via a facile post-treatment nitrogen doping strategy: Efficient CO2 capture and DRM. Int. J. Hydrogen Energy 2022, 47, 24388–24397. [Google Scholar] [CrossRef]
- Shao, J.; Ma, C.; Zhao, J.; Wang, L.; Hu, X. Effective nitrogen and sulfur co-doped porous carbonaceous CO2 adsorbents derived from amino acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127750. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. Biomass-based activated carbon for CO2 adsorption–A review. Energy Environ. 2022, 34, 1674–1721. [Google Scholar] [CrossRef]
- Serafin, J.; Sreńscek-Nazzal, J.; Kamińska, A.; Paszkiewicz, O.; Michalkiewicz, B. Management of surgical mask waste to activated carbons for CO2 capture. J. CO2 Util. 2022, 59, 101970. [Google Scholar] [CrossRef] [PubMed]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.-J. From chitosan to urea-modified carbons: Tailoring the ultra-microporosity for enhanced CO2 adsorption. 2020, 159, 625–637. 159 2020, 159, 625–637. [Google Scholar] [CrossRef]
- Sevilla, M.; Al-Jumialy, A.S.M.; Fuertes, A.B.; Mokaya, R. Optimization of the Pore Structure of Biomass-Based Carbons in Relation to Their Use for CO2 Capture under Low- and High-Pressure Regimes. ACS Appl. Mater. Interfaces 2018, 10, 1623–1633. [Google Scholar] [CrossRef]
- Quan, C.; Wang, H.; Jia, X.; Gao, N. Effect of carbonization temperature on CO2 adsorption behavior of activated coal char. J. Energy Inst. 2021, 97, 92–99. [Google Scholar] [CrossRef]
- Serafin, J.; Baca, M.; Biegun, M.; Mijowska, E.; Kaleńczuk, R.J.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Pietrzak, R.; Nowicki, P.; Kaźmierczak, J.; Kuszyńska, I.; Goscianska, J.; Przepiórski, J. Comparison of the effects of different chemical activation methods on properties of carbonaceous adsorbents obtained from cherry stones. Chem. Eng. Res. Des. 2014, 92, 1187–1191. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Johir, A.H.; Zhou, J.L.; Ngo, H.H.; Nghiem, L.D.; Richardson, C.; Moni, M.A.; Bryant, M.R. Activated carbon preparation from biomass feedstock: Clean production and carbon dioxide adsorption. J. Clean. Prod. 2019, 225, 405–413. [Google Scholar] [CrossRef]
- Pramanik, P.; Patel, H.; Charola, S.; Neogi, S.; Maiti, S. High surface area porous carbon from cotton stalk agro-residue for CO2 adsorption and study of techno-economic viability of commercial production. J. CO2 Util. 2021, 45, 101450. [Google Scholar] [CrossRef]
- Kaya, N.; Uzun, Z.Y. Investigation of effectiveness of pine cone biochar activated with KOH for methyl orange adsorption and CO2 capture. Biomass Convers. Biorefinery 2020, 11, 1067–1083. [Google Scholar] [CrossRef]
- Peres, C.B.; de Morais, L.C.; Resende, P.M.R. Carbon adsorption on waste biomass of passion fruit peel: A promising machine learning model for CO2 capture. J. CO2 Util. 2024, 80, 102680. [Google Scholar] [CrossRef]
- Faisal, M.; Pamungkas, A.Z.; Krisnandi, Y.K. Study of Amine Functionalized Mesoporous Carbon as CO2 Storage Materials. Processes 2021, 9, 456. [Google Scholar] [CrossRef]
- de Sousa, P.A.R.; Furtado, L.T.; Neto, J.L.L.; de Oliveira, F.M.; Siqueira, J.G.M.; Silva, L.F.; Coelho, L.M. Evaluation of the Adsorption Capacity of Banana Peel in the Removal of Emerging Contaminants present in Aqueous Media—Study based on Factorial Design. Braz. J. Anal. Chem. 2019, 6, 14–28. [Google Scholar] [CrossRef]
- Tahir, M.H.; Zhao, Z.; Ren, J.; Rasool, T.; Naqvi, S.R. Thermo-kinetics and gaseous product analysis of banana peel pyrolysis for its bioenergy potential. Biomass Bioenergy 2019, 122, 193–201. [Google Scholar] [CrossRef]
- Xiao, J.; Yuan, X.; Zhang, T.C.; Ouyang, L.; Yuan, S. Nitrogen-doped porous carbon for excellent CO2 capture: A novel method for preparation and performance evaluation. Sep. Purif. Technol. 2022, 298, 121602. [Google Scholar] [CrossRef]
- Xie, L.; Li, Q.; Demir, M.; Yu, Q.; Hu, X.; Jiang, Z.; Wang, L. Lotus seed pot-derived nitrogen enriched porous carbon for CO2 capture application. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130226. [Google Scholar] [CrossRef]
- Liu, S.-H.; Huang, Y.-Y. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Clean. Prod. 2018, 175, 354–360. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Xin, C.; Kong, X.; Hu, X.; Guo, Q. The development of activated carbon from corncob for CO2 capture. RSC Adv. 2022, 12, 33069–33078. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-G.; Liu, Y.-F.; Wu, X.-X.; Cai, J.-J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Kilic, M.; Altay, B.N.; Hu, X.; Wang, L. N-doped porous carbon derived from macadamia nut shell for CO2 adsorption. Fuel Process. Technol. 2023, 249, 107854. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Du, J.; Chu, X.; Ren, F.; Zheng, S.; Shao, Z. Study on CO2 adsorption performance of biocarbon synthesized in situ from nitrogen-rich biomass pomelo peel. Fuel 2023, 352, 129163. [Google Scholar] [CrossRef]
- Sigiro, M. Natural biowaste of banana peel-derived porous carbon for in-vitro antibacterial activity toward Escherichia coli. Ain Shams Eng. J. 2021, 12, 4157–4165. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Perangin-Angin, S.; Simanungkalit, S.R.; Zega, N.P.; Sitepu, E.K. Utilization of waste banana peels as heterogeneous catalysts in room-temperature biodiesel production using a homogenizer. RSC Adv. 2023, 13, 6217–6224. [Google Scholar] [CrossRef]
- Li, W.; Tu, W.; Cheng, J.; Yang, F.; Wang, X.; Li, L.; Shang, D.; Zhou, X.; Yu, C.; Yuan, A.; et al. Tuning N-doping thermal-process enables biomass-carbon surface modification for potential separation effect of CO2/CH4/N2. Sep. Purif. Technol. 2022, 282, 120001. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz, O.F.; Sreńscek-Nazzal, J.; Gómez, I.C.; Azar, F.-Z.; Mafull, C.A.R.; Hotza, D.; Rambo, C.R. Conversion of fruit waste-derived biomass to highly microporous activated carbon for enhanced CO2 capture. Waste Manag. 2021, 136, 273–282. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, G.; Wu, C.; Liu, J.; Li, G.; Zhao, Y.; Wang, Y.; Xu, Y. A cost-effective synthesis of heteroatom-doped porous carbon by sulfur-containing waste liquid treatment: As a promising adsorbent for CO2 capture. J. Environ. Chem. Eng. 2021, 9, 105165. [Google Scholar] [CrossRef]
- Mohamed, G.R.; Mahmoud, R.K.; Shaban, M.; Fahim, I.S.; El-Salam, H.M.A.; Mahmoud, H.M. Towards a circular economy: Valorization of banana peels by developing bio-composites thermal insulators. Sci. Rep. 2023, 13, 12756. [Google Scholar] [CrossRef]
- Peres, C.B.; Rosa, A.H.; de Morais, L.C. CO2 adsorption of bagasse waste feedstock using thermogravimetric analyses. J. Therm. Anal. Calorim. 2021, 147, 5973–5984. [Google Scholar] [CrossRef]
- Hussien, N.A.; Al Malki, J.S.; Al Harthy, F.A.R.; Mazi, A.W.; Al Shadadi, J.A.A. Sustainable Eco-Friendly Synthesis of Zinc Oxide Nanoparticles Using Banana Peel and Date Seed Extracts, Characterization, and Cytotoxicity Evaluation. Sustainability 2023, 15, 9864. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.C.; Maia, A.A.D.; Guandique, M.E.G.; Rosa, A.H. Pyrolysis and combustion of sugarcane bagasse. J. Therm. Anal. Calorim. 2017, 129, 1813–1822. [Google Scholar] [CrossRef]
- Balogun, A.I.; Padmanabhan, E.; Abdulkareem, F.A.; Gebretsadik, H.T.; Wilfred, C.D.; Soleimani, H.; Viswanathan, P.M.; Wee, B.S.; Yusuf, J.Y. Optimization of CO2 Sorption onto Spent Shale with Diethylenetriamine (DETA) and Ethylenediamine (EDA). Materials 2022, 15, 8293. [Google Scholar] [CrossRef]
- Correia, L.B.; Fiuza, R.A.; de Andrade, R.C.; Andrade, H.M.C. CO2 capture on activated carbons derived from mango fruit (Mangifera indica L.) seed shells. J. Therm. Anal. Calorim. 2018, 131, 579–586. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. Valorization of orange peel waste to tunable heteroatom-doped hydrochar-derived microporous carbons for selective CO2 adsorption and separation. Sci. Total. Environ. 2022, 849, 157805. [Google Scholar] [CrossRef] [PubMed]
- Meriatna; Husin, H.; Riza, M.; Faisal, M.; Ahmadi; Sulastri. Biodiesel production using waste banana peel as renewable base catalyst. Mater. Today Proc. 2023, 87, 214–217. [Google Scholar] [CrossRef]
- Kobo, G.K.; Kaseke, T.; Fawole, O.A. Micro-Encapsulation of Phytochemicals in Passion Fruit Peel Waste Generated on an Organic Farm: Effect of Carriers on the Quality of Encapsulated Powders and Potential for Value-Addition. Antioxidants 2022, 11, 1579. [Google Scholar] [CrossRef]
- Tabarkhoon, F.; Abolghasemi, H.; Rashidi, A.; Bazmi, M.; Alivand, M.S.; Tabarkhoon, F.; Farahani, M.V.; Esrafili, M.D. Synthesis of novel and tunable Micro-Mesoporous carbon nitrides for Ultra-High CO2 and H2S capture. Chem. Eng. J. 2023, 456, 140973. [Google Scholar] [CrossRef]
- Ma, X.; Su, C.; Liu, B.; Wu, Q.; Zhou, K.; Zeng, Z.; Li, L. Heteroatom-doped porous carbons exhibit superior CO2 capture and CO2/N2 selectivity: Understanding the contribution of functional groups and pore structure. Sep. Purif. Technol. 2021, 259, 118065. [Google Scholar] [CrossRef]


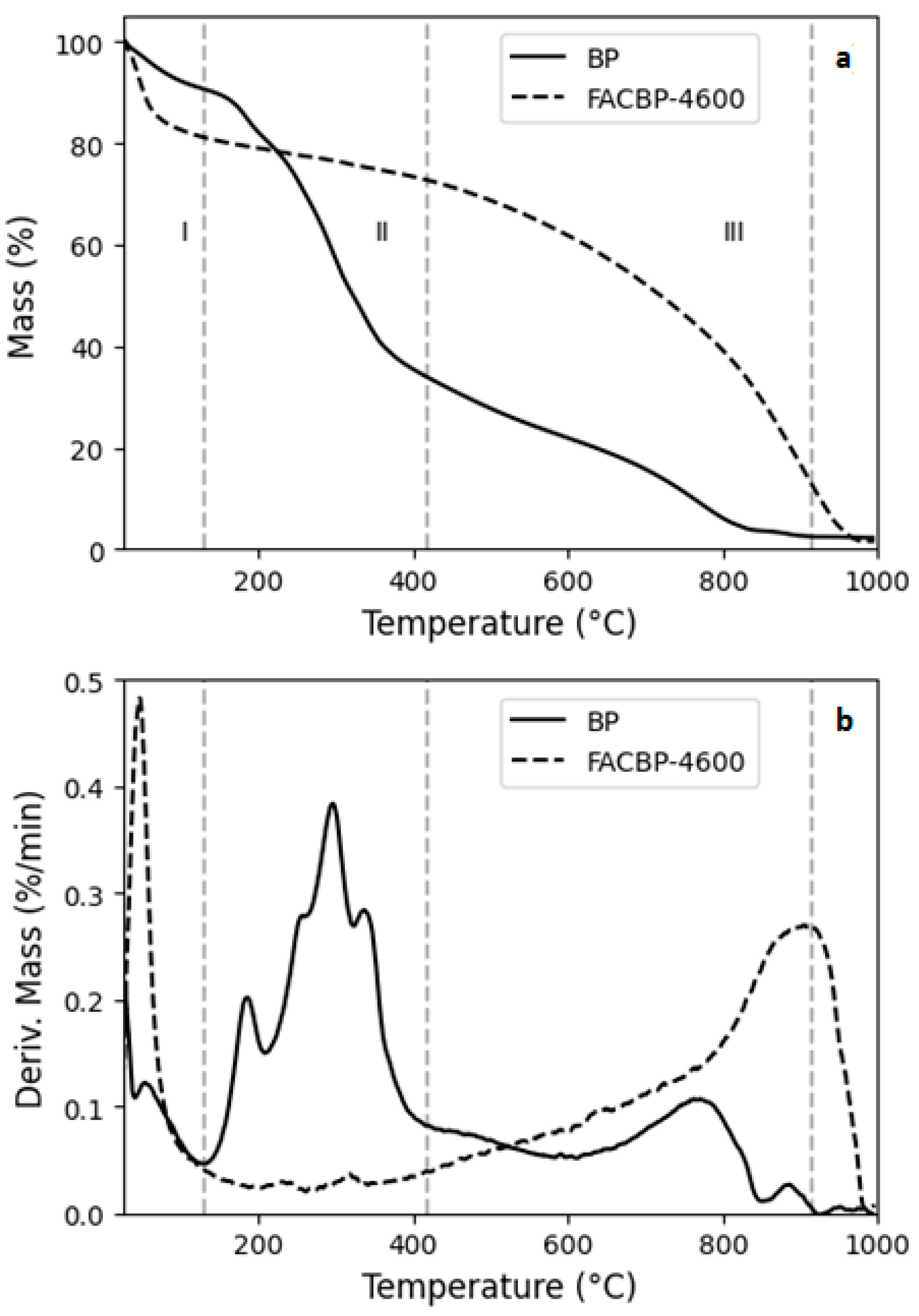
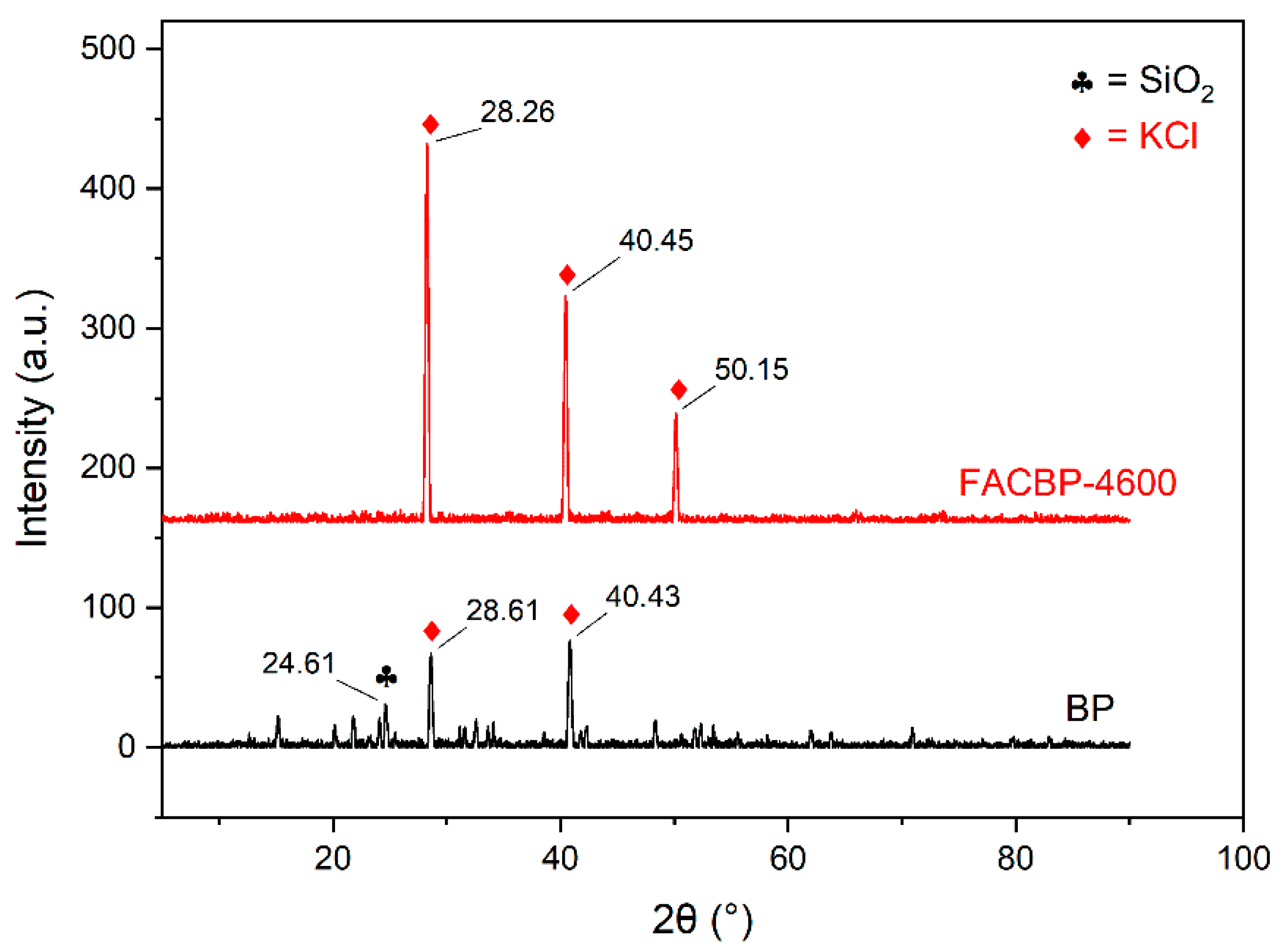

| Sample | Textural Properties | ||||||
|---|---|---|---|---|---|---|---|
| CO2 Purge | N2 Purge | ||||||
| SBET a (m2/g) | Pore Size b (Å) | Vpc (cm3/g) | Vmicro d (cm3/g) | SBET a (m2/g) | Vp c (cm3/g) | Vmicro d (cm3/g) | |
| BP | 0.02 | 4.10 | 0 | <0.01 | 1.68 | <0.01 | <0.01 |
| FACBP-1 | 57.85 | 3.13 | 0.02 | 0.03 | <0.01 | <0.01 | <0.01 |
| FACBP-2W | 15.49 | 3.13 | 0.02 | 0 | <0.01 | <0.01 | <0.01 |
| FACBP-33 | 11.49 | 2.99 | <0.01 | 0 | <0.01 | <0.01 | <0.01 |
| FACBP-35 | 47.83 | 2.86 | 0.02 | 0.03 | <0.01 | <0.01 | <0.01 |
| FACBP-4600 | 71.12 | 2.99 | 0.02 | 0.04 | <0.01 | <0.01 | <0.01 |
| FACBP-4700 | 41.61 | 2.99 | 0.01 | 0.02 | 56–63. | < 0.01 | 0.03 |
| Samples | Chemical Composition (%) | |||
|---|---|---|---|---|
| O | C | H | N | |
| BP | 49.9 | 42~61 | 6.19 | 1.30 |
| FACBP-4600 | 51.38 | 41.26 | 6.09 | 1.27 |
| Carbonaceous Precursor | CO2 Uptake (mmol/g) at 0 °C | Ref. |
|---|---|---|
| Activated coal char | 3.7 | Quan et al. [21] |
| LOTUS | 5.72 | Xie et al. [32] |
| Macadamia nut shell | 6.61 | Bai et al. [36] |
| Palm fruit bunch | 1.3 | Parshetti et al. [37] |
| Pomelo? | 4.83 | Du et al. [38] |
| banana | 1.69 | This work |
| Samples | C (at. %) | O (at. %) | K (at. %) | Cl (at. %) | Si (at. %) |
|---|---|---|---|---|---|
| BP | 63.90 | 75. | 2.31 | 0.78 | 0.14 |
| FACBP-4600 | 55:24 | 34 (69%) | 4.68 | 1.85 | 1.48 |
| Samples | C (at. %) | O (at. %) | N (at. %) | K (at. %) | Si (at. %) |
|---|---|---|---|---|---|
| BP | 88.8 | 10 | 0.65 | - | 0.34 |
| FACBP-4600 | 56–76 | 26.54 | 3.34 | 3.04 | 5.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, C.B.; Morais, L.C.; Resende, P.R. Nitrogen-Doped Porous Waste Biomass as a Sustainable Adsorbent for CO2 Capture: The Influence of Preparation Conditions. Clean Technol. 2025, 7, 25. https://doi.org/10.3390/cleantechnol7010025
Peres CB, Morais LC, Resende PR. Nitrogen-Doped Porous Waste Biomass as a Sustainable Adsorbent for CO2 Capture: The Influence of Preparation Conditions. Clean Technologies. 2025; 7(1):25. https://doi.org/10.3390/cleantechnol7010025
Chicago/Turabian StylePeres, Christiano B., Leandro C. Morais, and Pedro R. Resende. 2025. "Nitrogen-Doped Porous Waste Biomass as a Sustainable Adsorbent for CO2 Capture: The Influence of Preparation Conditions" Clean Technologies 7, no. 1: 25. https://doi.org/10.3390/cleantechnol7010025
APA StylePeres, C. B., Morais, L. C., & Resende, P. R. (2025). Nitrogen-Doped Porous Waste Biomass as a Sustainable Adsorbent for CO2 Capture: The Influence of Preparation Conditions. Clean Technologies, 7(1), 25. https://doi.org/10.3390/cleantechnol7010025






