Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem
Abstract
:1. Introduction
2. Materials and Methods
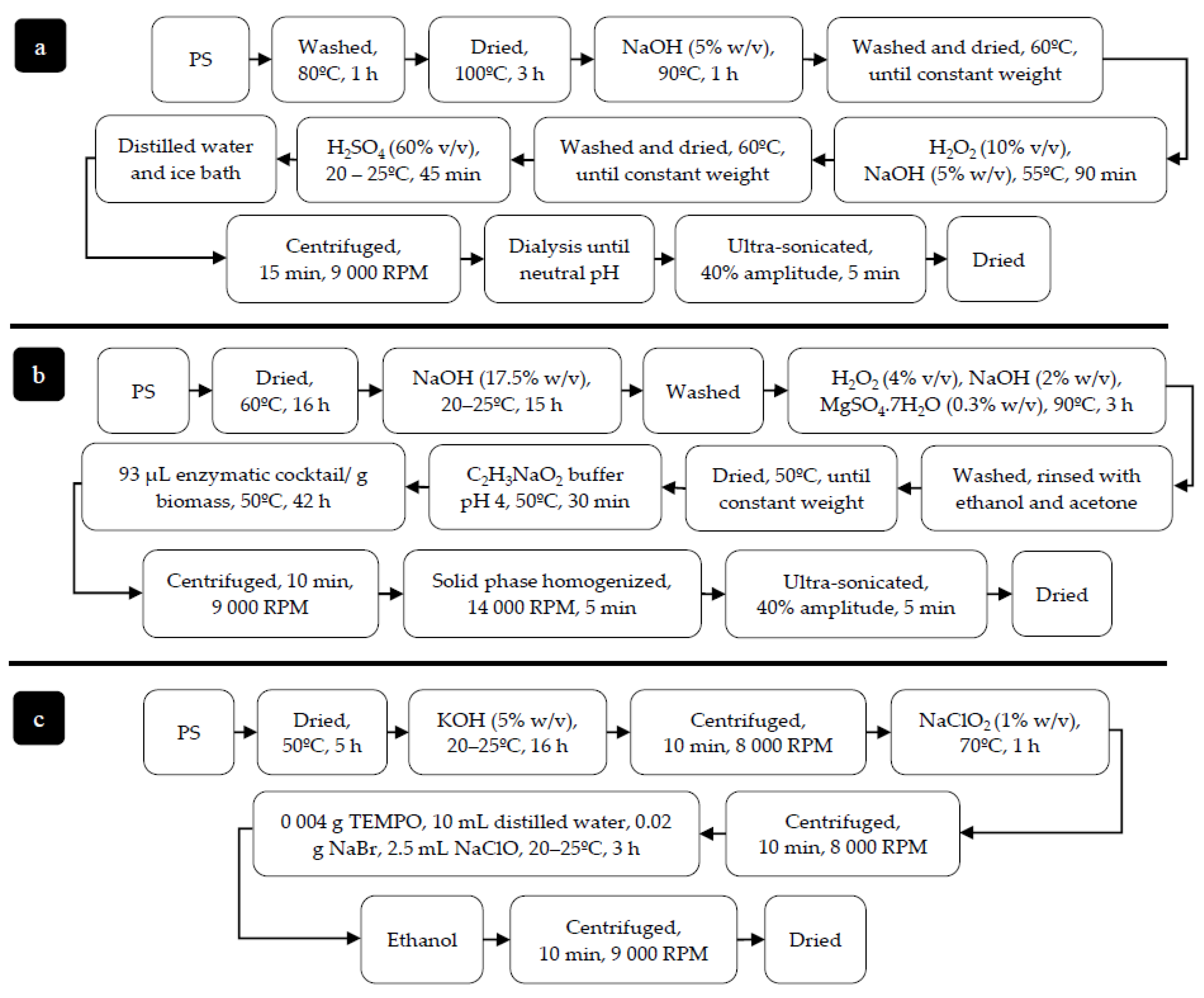
2.1. Biomass Preparation
2.2. Cellulose Extraction
2.3. Extracts Characterization
2.3.1. Sugars Quantification
2.3.2. Chemical Composition Identification
2.3.3. Crystallinity Determination
2.3.4. Thermal Stability Analysis
3. Results and Discussion
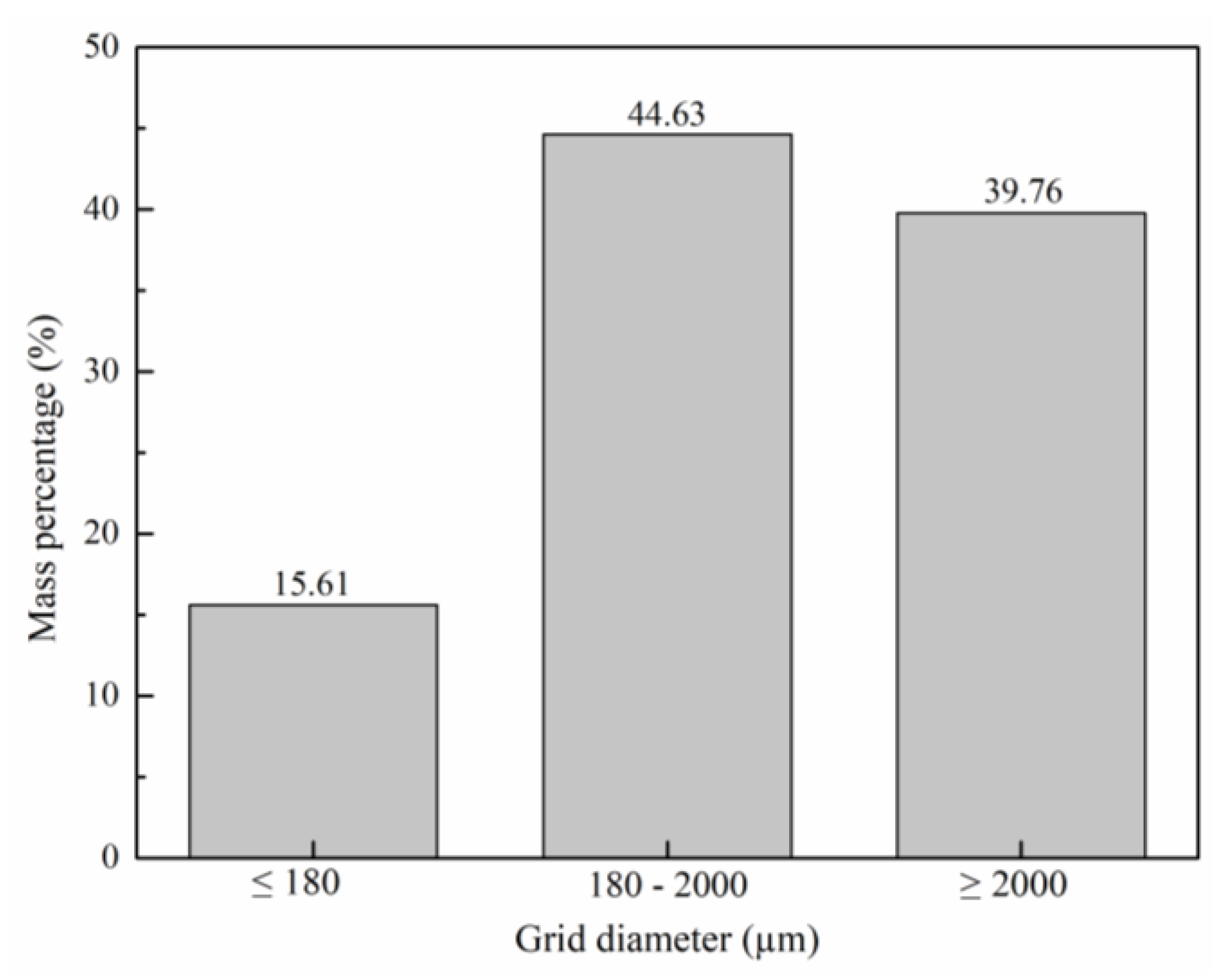
3.1. Biomass Preparation
3.2. Extracts Characterization
3.2.1. Sugars Quantification
3.2.2. Obtained Extracts
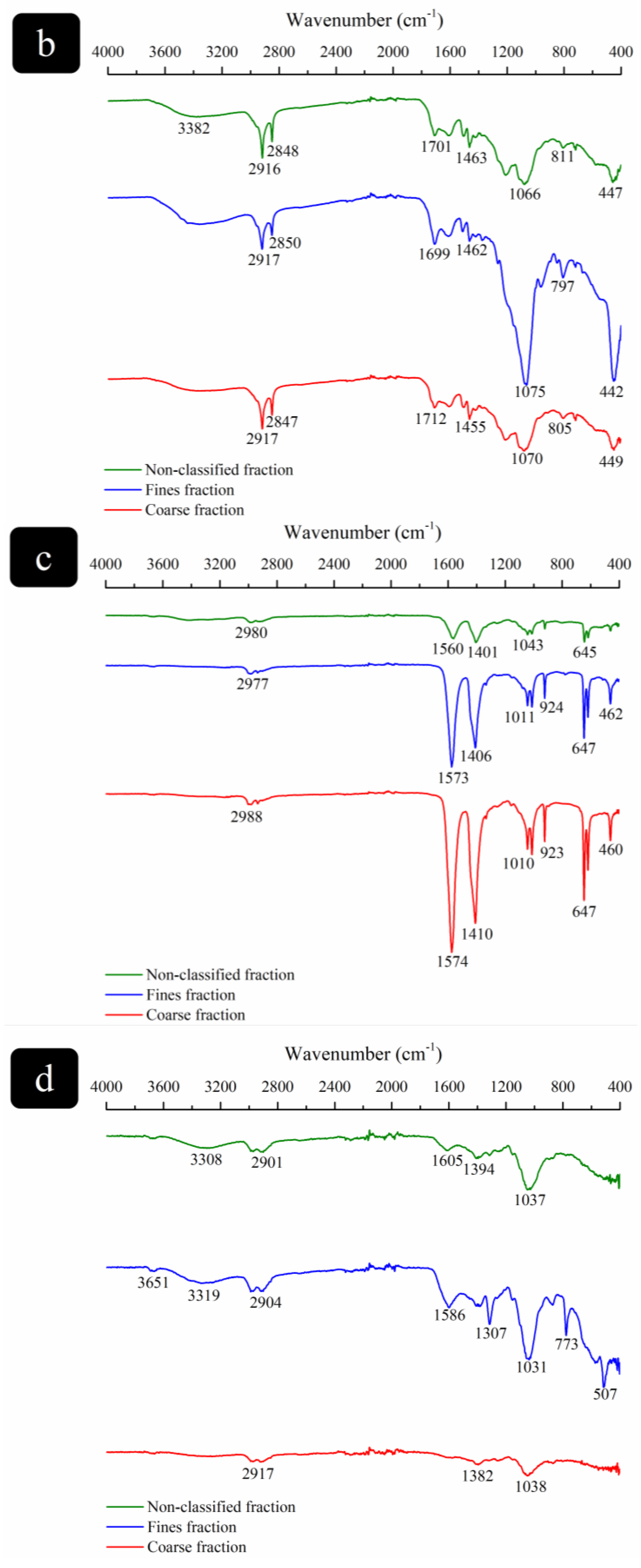
3.2.3. Chemical Composition Determination
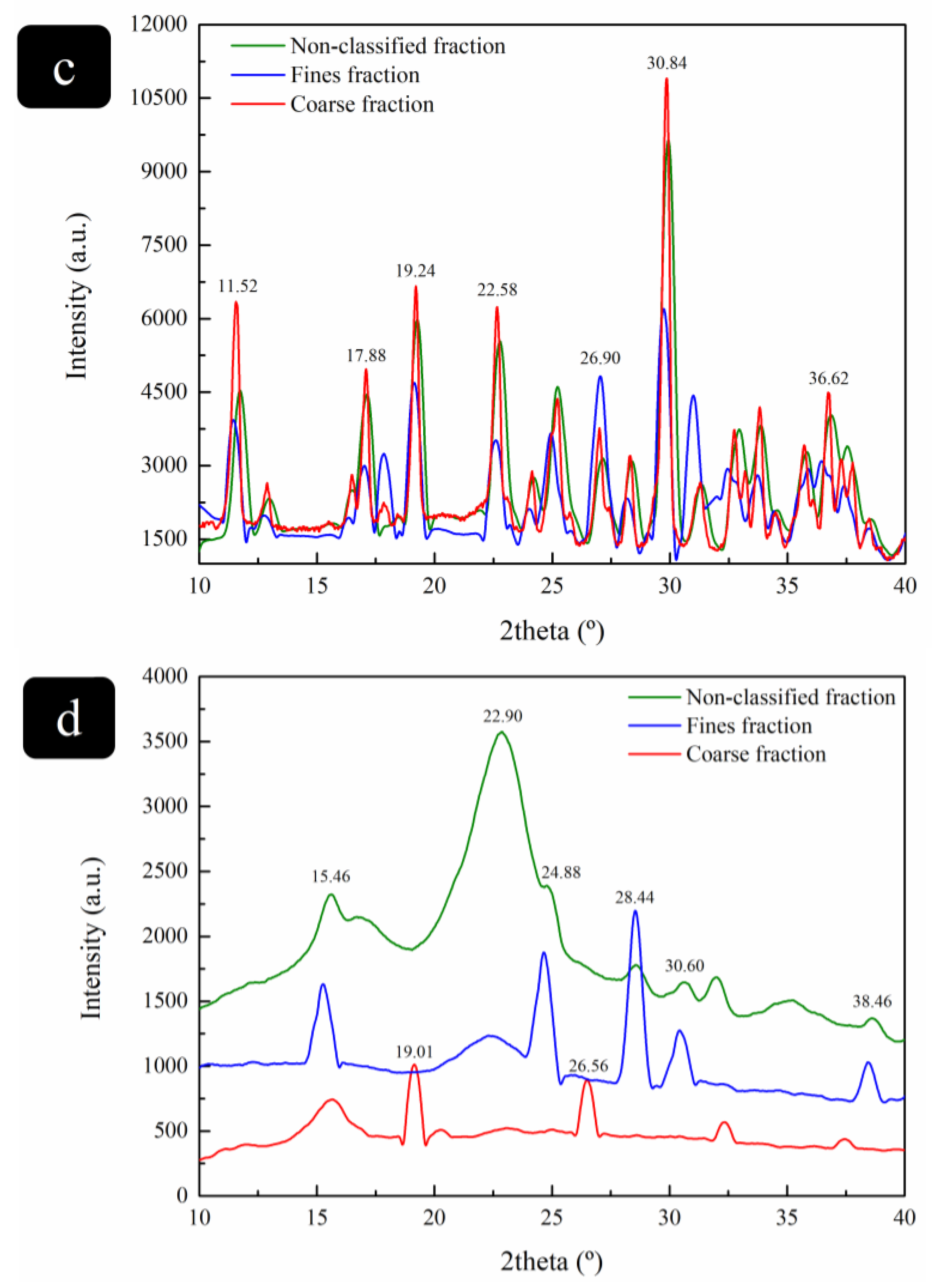
3.2.4. Crystallinity Determination
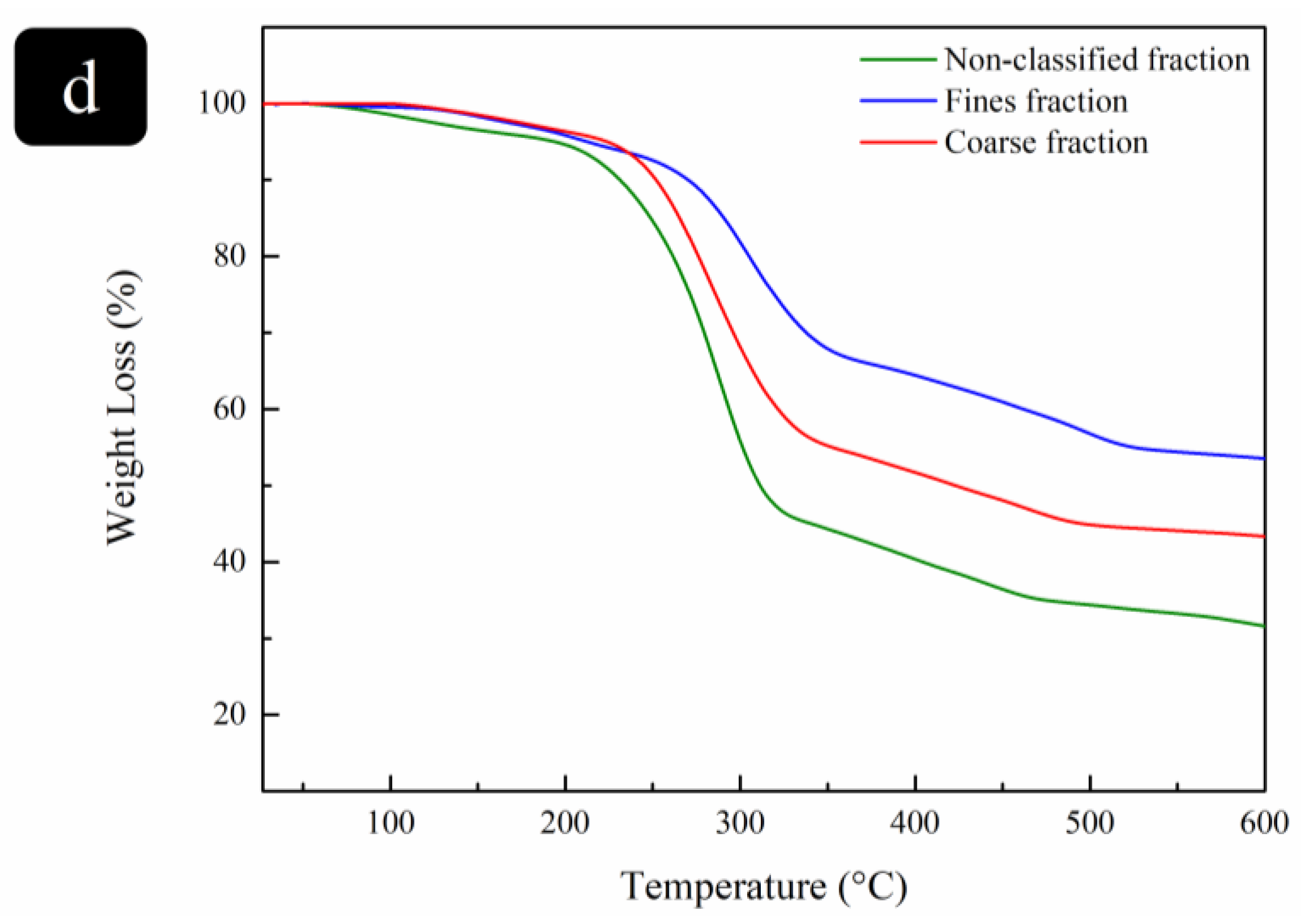
3.2.5. Thermal Stability Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, S.A.F.; Febriyanti, M.; Farzadillah, N. Natural Oral Anti-Dysentery from Pseudostem of Klutuk (Musa Balbisiana Colla) and Kepok (Musa Paradisiaca L.) Banana Plant from Indonesia. J. Pharm. Sci. Res. 2018, 10, 2443–2447. [Google Scholar]
- Statista. Production Volume of Bananas Worldwide from 2010 to 2021. Available online: https://www.statista.com/statistics/716037/global-banana-market-volume/#:~:text=Volume%20of%20bananas%20produced%20worldwide%202010-2021&text=In%202021%2C%20the%20volume%20of,been%20generally%20increasing%20since%202010 (accessed on 19 April 2023).
- de Oliveira Maia, B.G.; de Oliveira, A.P.N.; de Oliveira, T.M.N.; Marangoni, C.; Souza, O.; Sellin, N. Characterization and Production of Banana Crop and Rice Processing Waste Briquettes. Environ. Prog. Sustain. Energy 2018, 37, 1266–1273. [Google Scholar] [CrossRef]
- Nations, U. Sustainable Development Goals. Available online: https://unric.org/en/united-nations-sustainable-development-goals/ (accessed on 12 December 2022).
- Suárez-Eiroa, B.; Fernández, E.; Méndez-Martínez, G.; Soto-Oñate, D. Operational Principles of Circular Economy for Sustainable Development: Linking Theory and Practice. J. Clean. Prod. 2019, 214, 952–961. [Google Scholar] [CrossRef]
- Chopra, L.; Manikanika. Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2021, 48, 1265–1270. [Google Scholar] [CrossRef]
- Boufi, S. 6—Agricultural crop residue as a source for the production of cellulose nanofibrils. In Cellulose-Reinforced Nanofibre Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–152. [Google Scholar]
- Del Mar Verde Méndez, C.; Forster, M.P.; Rodríguez-Delgado, M.Á.; Rodríguez-Rodríguez, E.M.; Romero, C.D. Content of Free Phenolic Compounds in Bananas from Tenerife (Canary Islands) and Ecuador. Eur. Food Res. Technol. 2003, 217, 287–290. [Google Scholar] [CrossRef]
- Idicula, M.; Boudenne, A.; Umadevi, L.; Ibos, L.; Candau, Y.; Thomas, S. Thermophysical Properties of Natural Fibre Reinforced Polyester Composites. Compos. Sci. Technol. 2006, 66, 2719–2725. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.N.; Torres, I.C.; Moura, J.C.V.P. Chemical Composition and Pulping of Banana Pseudo-Stems. Ind. Crops Prod. 2004, 19, 147–154. [Google Scholar] [CrossRef]
- Pereira, N.R.L.; Lopes, B.; Fagundes, I.V.; de Moraes, F.M.; Morisso, F.D.P.; Parma, G.O.C.; Zepon, K.M.; Magnago, R.F. Bio-Packaging Based on Cellulose Acetate from Banana Pseudostem and Containing Butia Catarinensis Extracts. Int. J. Biol. Macromol. 2022, 194, 32–41. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Liu, C.; Liu, X.; Wang, J.; Li, S.; Fan, G.; Zhang, L. Alkaline Pretreatment for Enhancement of Biogas Production from Banana Stem and Swine Manure by Anaerobic Codigestion. Bioresour. Technol. 2013, 149, 353–358. [Google Scholar] [CrossRef]
- Prado, K.S.; Spinacé, M.A.S. Isolation and Characterization of Cellulose Nanocrystals from Pineapple Crown Waste and Their Potential Uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.G.; Tapia-Blácido, D.R. Soybean Straw Nanocellulose Produced by Enzymatic or Acid Treatment as a Reinforcing Filler in Soy Protein Isolate Films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Faradilla, R.H.F.; Lee, G.; Arns, J.Y.; Roberts, J.; Martens, P.; Stenzel, M.H.; Arcot, J. Characteristics of a Free-Standing Film from Banana Pseudostem Nanocellulose Generated from TEMPO-Mediated Oxidation. Carbohydr. Polym. 2017, 174, 1156–1163. [Google Scholar] [CrossRef]
- Nascimento, R.E.A.; Monte, J.; Cadima, M.; Alves, V.D.; Neves, L.A. Rendering Banana Plant Residues into a Potentially Commercial Byproduct by Doping Cellulose Films with Phenolic Compounds. Polymers 2021, 13, 843. [Google Scholar] [CrossRef]
- Brindha, R.; Narayana, C.K.; Vijayalakshmi, V.; Nachane, R.P. Effect of Different Retting Processes on Yield and Quality of Banana Pseudostem Fiber. J. Nat. Fibers 2019, 16, 58–67. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Chemical and Enzymatic Treatment. LWT 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Meng, F.; Wang, G.; Du, X.; Wang, Z.; Xu, S.; Zhang, Y. Extraction and Characterization of Cellulose Nanofibers and Nanocrystals from Liquefied Banana Pseudo-Stem Residue. Compos. Part B Eng. 2019, 160, 341–347. [Google Scholar] [CrossRef]
- Ajao, O.; Jeaidi, J.; Benali, M.; Restrepo, A.M.; El Mehdi, N.; Boumghar, Y. Quantification and Variability Analysis of Lignin Optical Properties for Colour-Dependent Industrial Applications. Molecules 2018, 23, 377. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Cheng, H.; Zhou, H. Hemicellulose Degradation: An Overlooked Issue in Acidic Deep Eutectic Solvents Pretreatment of Lignocellulosic Biomass. Ind. Crops Prod. 2022, 187, 115335. [Google Scholar] [CrossRef]
- Dube, A.M. Isolation and characterization of cellulose nanocrystals from Ensete ventricosum pseudo-stem fiber using acid hydrolysis. Biomass Convers. Biorefiney 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Zope, G.; Goswami, A.; Kulkarni, S. Isolation and Characterization of Cellulose Nanocrystals Produced by Acid Hydrolysis from Banana Pseudostem. Bionanoscience 2022, 12, 463–471. [Google Scholar] [CrossRef]
- Perez-Pimienta, J.A.; Lopez-Ortega, M.G.; Chavez-Carvayar, J.A.; Varanasi, P.; Stavila, V.; Cheng, G.; Singh, S.; Simmons, B.A. Characterization of Agave Bagasse as a Function Ofionic Liquid Pretreatment. Biomass Bioenergy 2015, 75, 180–188. [Google Scholar] [CrossRef]
- Solikhin, A.; Murayama, K. Enhanced Properties of Poly(Vinyl Alcohol) Composite Films Filled with Microfibrillated Cellulose Isolated from Continuous Steam Explosion. Int. J. Plast. Technol. 2018, 22, 122–136. [Google Scholar] [CrossRef]
- Nunes, R.C.R. 13—Rubber nanocomposites with nanocellulose. In Progress in Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 463–494. [Google Scholar]
- Kafle, K.; Greeson, K.; Lee, C.; Kim, S.H. Cellulose Polymorphs and Physical Properties of Cotton Fabrics Processed with Commercial Textile Mills for Mercerization and Liquid Ammonia Treatments. Text. Res. J. 2014, 84, 1692–1699. [Google Scholar] [CrossRef]
- Diarsa, M.; Gupte, A. Preparation, characterization and its potential applications in Isoniazid drug delivery of porous microcrystalline cellulose from banana pseudostem fibers. 3 Biotech 2021, 11, 334. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Enzymatic Treatment: Study of Process Conditions. Ind. Crops Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Zuluaga, R.; Luc, J.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis : Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Shrestha, P.; Bilal, M.; Kumar, A. Development of Antibacterial Biocomposites Reinforced with Cellulose Nanocrystals Derived from Banana Pseudostem. Carbohydr. Polym. Technol. Appl. 2021, 2, 100112. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of Polyvinyl Alcohol/Xylan Composite Films with Citric Acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- De Paiva, J.M.F.; Frollini, E. Unmodified and Modified Surface Sisal Fibers as Reinforcement of Phenolic and Lignophenolic Matrices Composites: Thermal Analyses of Fibers and Composites. Macromol. Mater. Eng. 2006, 291, 405–417. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal Decomposition of Wood: Influence of Wood Components and Cellulose Crystallite Size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Bullard, J.W.; Jin, Q.; Snyder, K.A. How Do Specific Surface Area and Particle Size Distribution Change When Granular Media Dissolve. Chem. Eng. J. 2021, 406, 127098. [Google Scholar] [CrossRef]




| Extraction Method | Cellulose Content (mg/g Biomass, Dried Basis) | Cellulose Yield (%, Dried Basis) |
|---|---|---|
| PS | 2.68 × 10−1 ± 7.60 × 10−2 | - |
| Alkaline-acid hydrolysis | 9.09 × 10−3 ± 1.06 × 10−3 | 3.40 ± 0.11 |
| Enzymatic hydrolysis | 3.90 × 10−2 ± 3.0 × 10−3 | 14.58 ± 0.30 |
| TEMPO oxidation | 6.76 × 10−2 ± 7.76 × 10−4 | 25.25 ± 0.08 |
| Particle Fraction | Non-Classified | Fines | Coarse | |
|---|---|---|---|---|
| Method | ||||
| Alkaline-acid hydrolysis |  |  |  | |
| Enzymatic hydrolysis |  |  |  | |
| TEMPO oxidation |  |  |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, R.E.A.; Carvalheira, M.; Crespo, J.G.; Neves, L.A. Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem. Clean Technol. 2023, 5, 1028-1043. https://doi.org/10.3390/cleantechnol5030052
Nascimento REA, Carvalheira M, Crespo JG, Neves LA. Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem. Clean Technologies. 2023; 5(3):1028-1043. https://doi.org/10.3390/cleantechnol5030052
Chicago/Turabian StyleNascimento, Rosa E. A., Mónica Carvalheira, João G. Crespo, and Luísa A. Neves. 2023. "Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem" Clean Technologies 5, no. 3: 1028-1043. https://doi.org/10.3390/cleantechnol5030052
APA StyleNascimento, R. E. A., Carvalheira, M., Crespo, J. G., & Neves, L. A. (2023). Extraction and Characterization of Cellulose Obtained from Banana Plant Pseudostem. Clean Technologies, 5(3), 1028-1043. https://doi.org/10.3390/cleantechnol5030052








