Torrefaction of Pine Using a Pilot-Scale Rotary Reactor: Experimentation, Kinetics, and Process Simulation Using Aspen Plus™
Abstract
1. Introduction
2. Materials and Methods
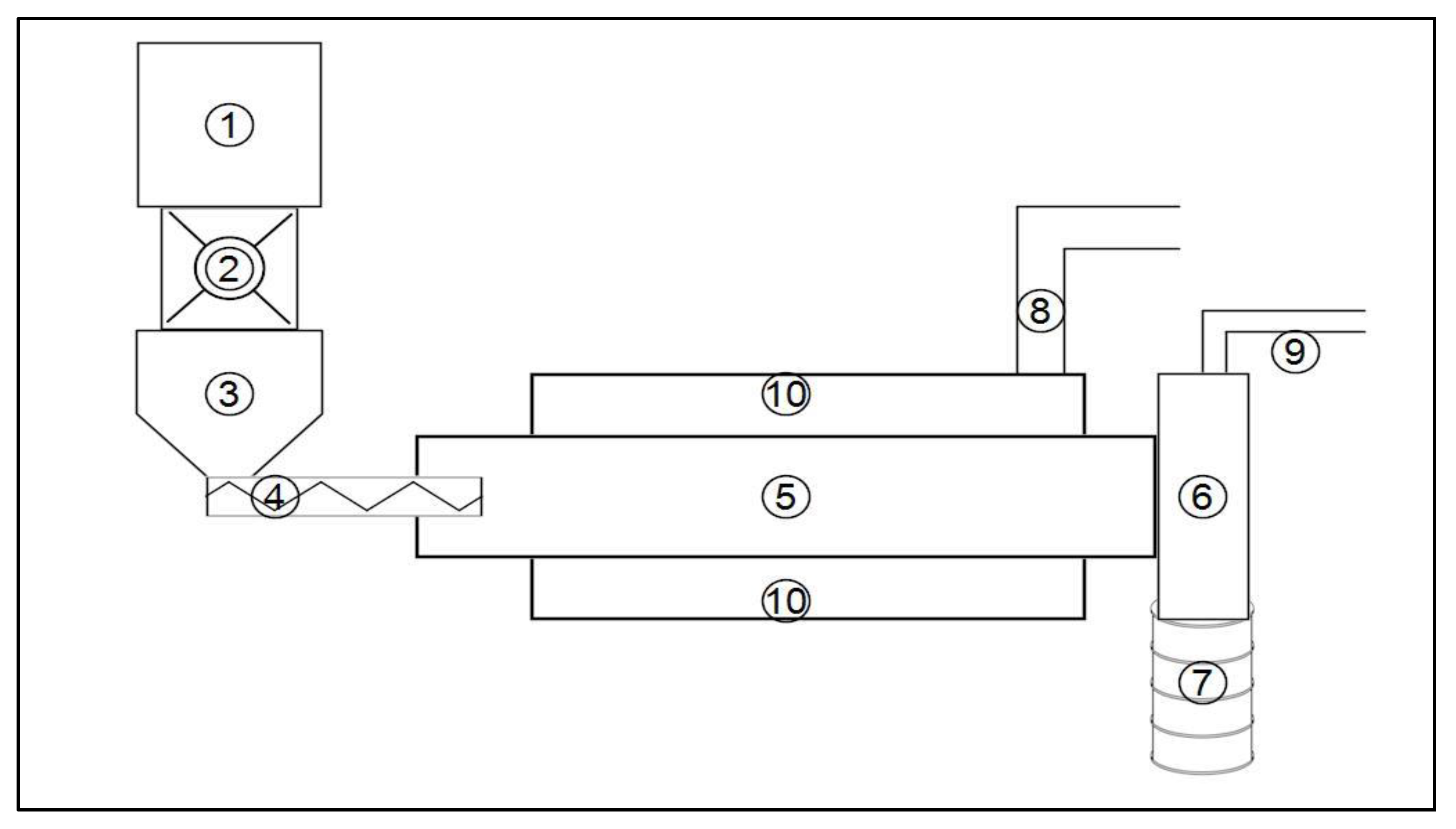
2.1. Pilot-Scale Experiments
2.1.1. Materials and Experimental Procedure
2.1.2. Pilot-Scale Experimental Procedure
2.1.3. Post-Experimental Analysis
2.2. Aspen Plus™ Simulation
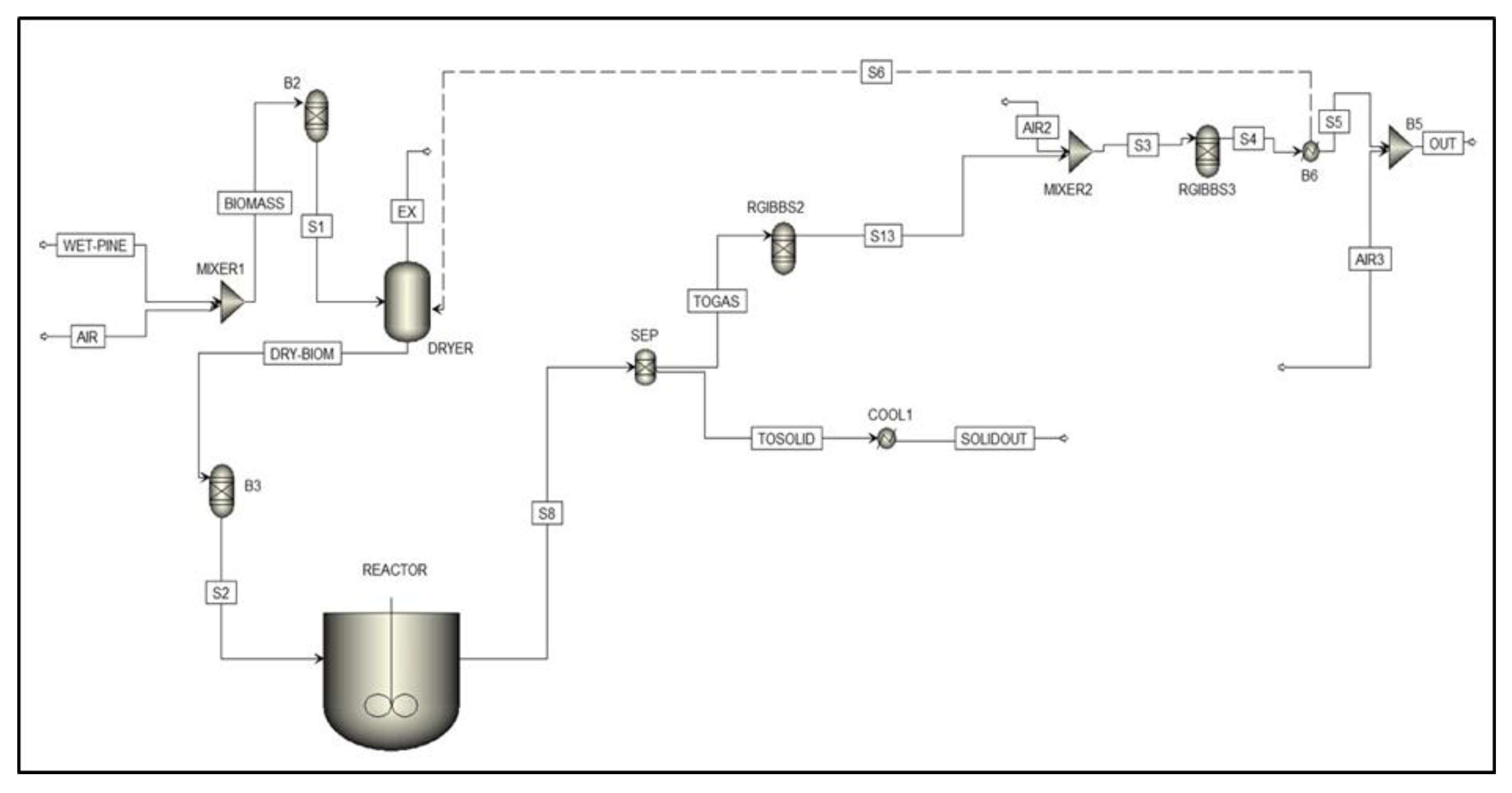
Process Flow Sheeting
- The process being continuous and steady-state, with the mechanical aspects of the equipment being disregarded. The model does not consider the movement of the material within the dryer or reactor.
- The simulation process was conducted at atmospheric pressure (1 atm).
- The air used for drying and combustion was at a temperature of 250 °C and 25 °C, respectively.
- The ultimate and proximate analysis data were used to define the non-conventional biomass feedstock. For the enthalpy and density calculations, the solid property models of HCOALGEN and DCOALIGT for coal were used.
- Due to the low or near atmospheric processing pressures (1 atm) and the presence of conventional gaseous compounds (such as H2O, CO, and CO2), the ideal gas law equation was adopted for calculating thermodynamic properties.
- B2 is a first stage torrefaction with a RYield block to ensure the final drying of pine to 0% wt. moisture.
- DRYER block separates the moisture from the biomass, which is subsequently released through the exhaust stream along with air.
- B3 uses the elemental composition of the biomass to convert the incoming non-conventional dry biomass into a conventional form.
- The reactor is a RCSTR block with a specified temperature and residence time. In this block, the kinetic model that was developed based on the experimental data was used to determine the yield of torrefied solid product. Reaction kinetic parameters are shown in Table 2.
- SEP separates the solid torrefied product (TOSOLID) from torrefied gas (TOGAS).
- RGIBBS2 recomposes the gaseous products in stream TOGAS from its elemental constituents.
- B4, RGIBBS3, and B6 are used in the combustion section. Exhaust gases after torrefaction are mixed with air to ensure the complete combustion of the torrefied gases. The heat generated from combustion is then recycled into the dryer to minimize the energy requirement for the drying process.
- Final step involves the cooling of both TOGAS and torrefied solids. This section is implemented in Aspen Plus™ by means of conventional “heat exchanger” blocks (Figure 2). After combustion, the torrefied gases are mixed with air. This dilutes the exhaust gases and decreases the temperature before releasing them into the atmosphere.
2.3. Torrefaction Kinetic Modelling
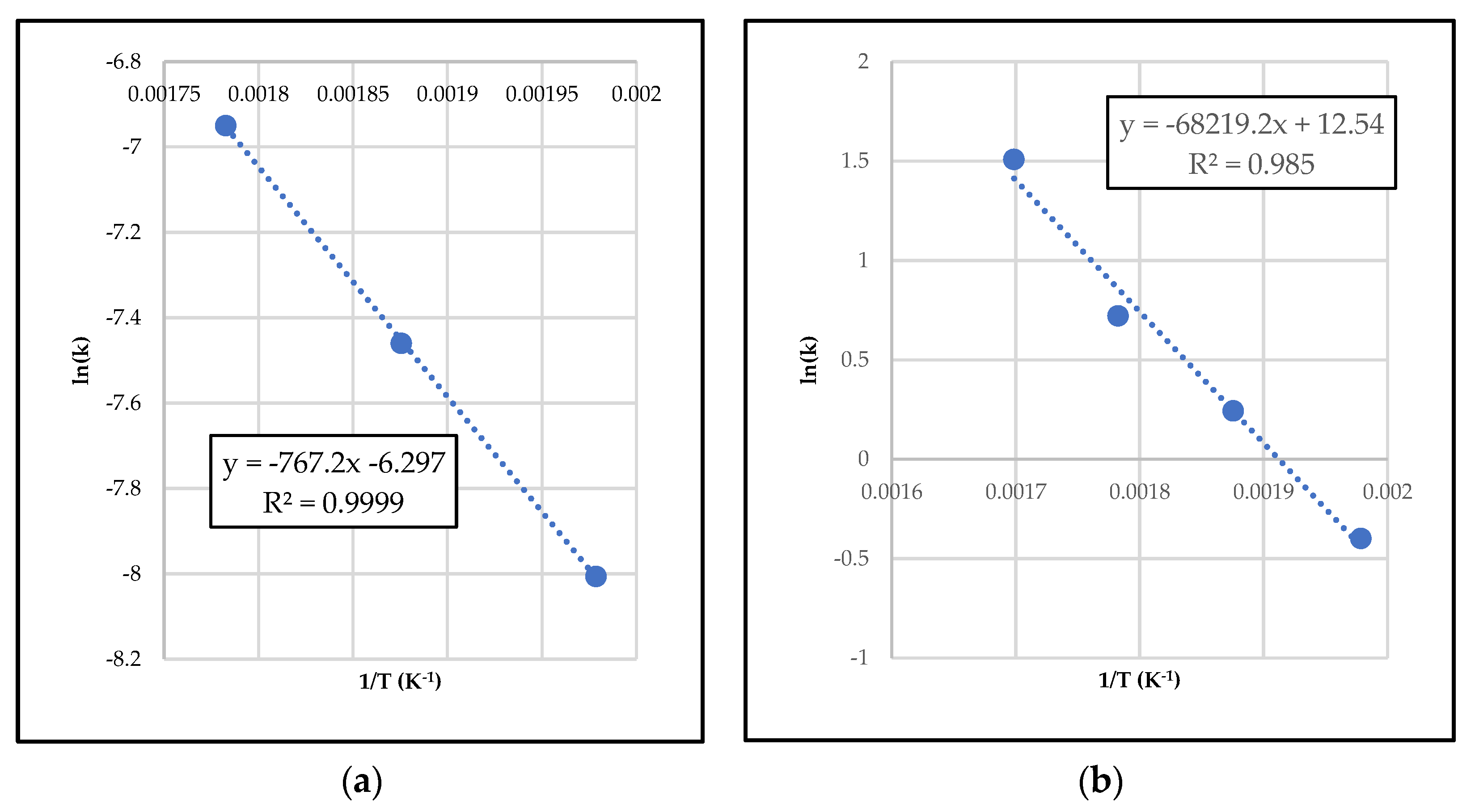
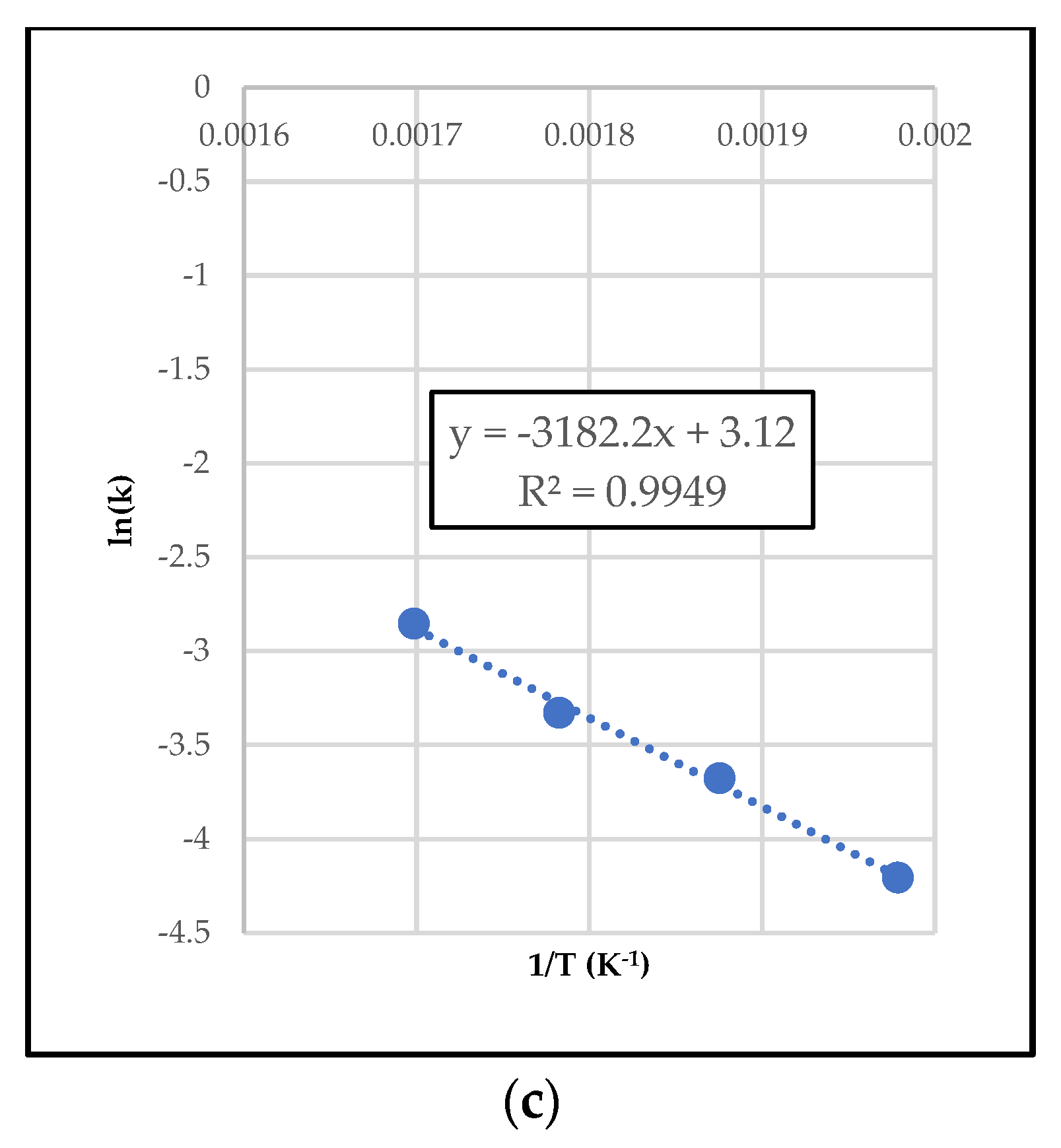
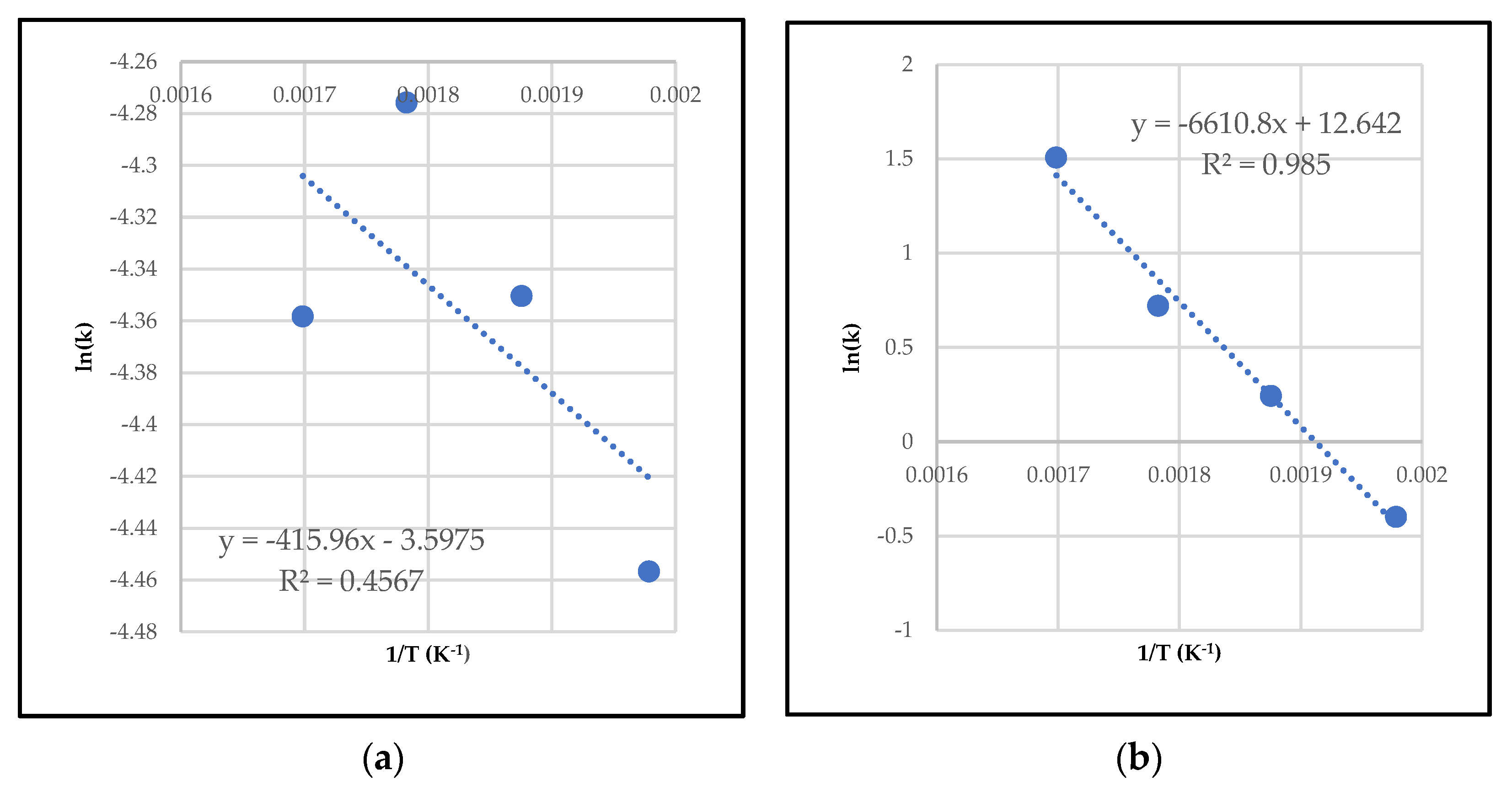
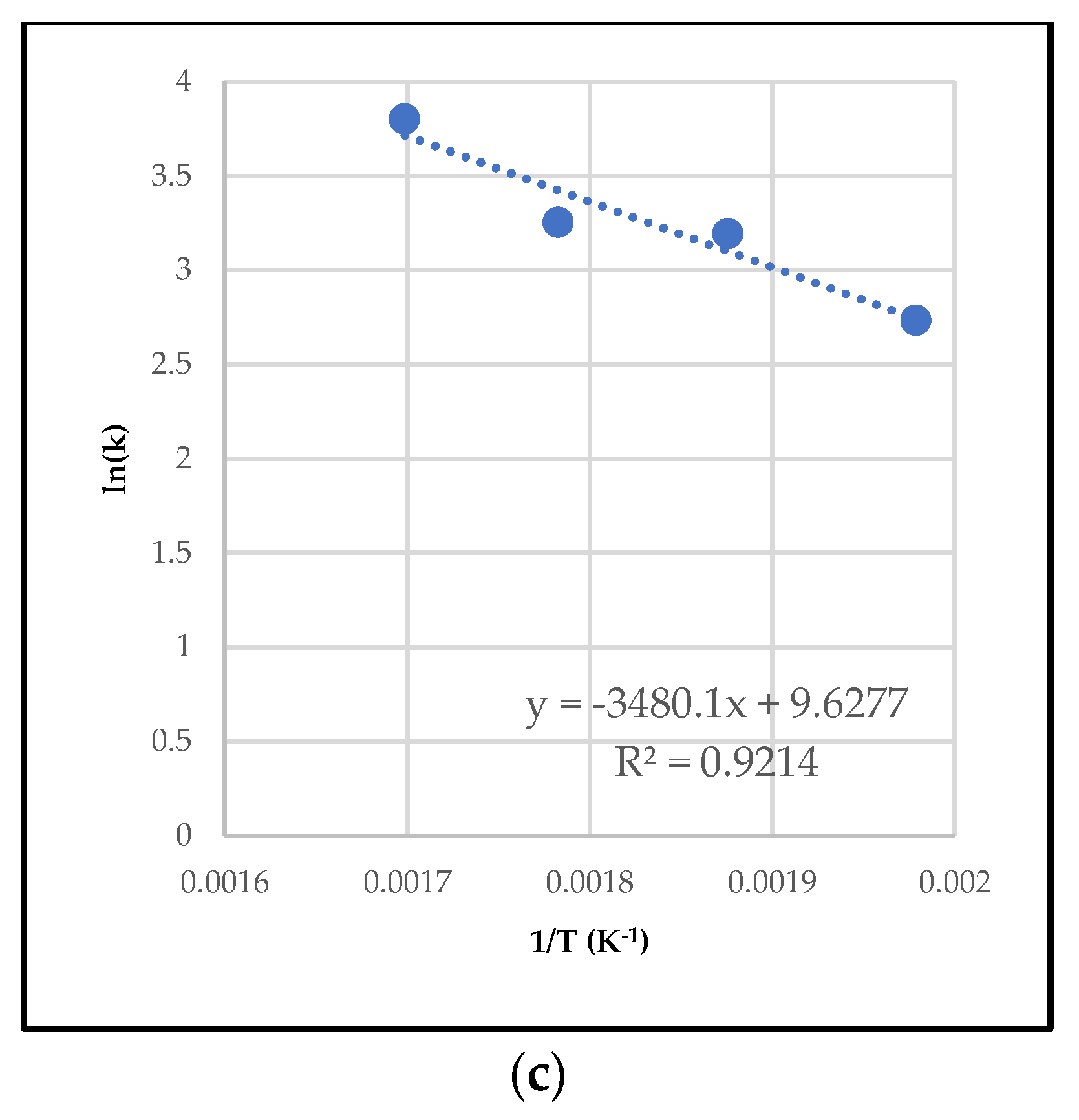
2.3.1. Differential Method of Rate Law
2.3.2. Integral Method of Rate Law
2.4. Simulation Runs and Data Validation
3. Results
3.1. Proximate and Ultimate Analysis of Pine
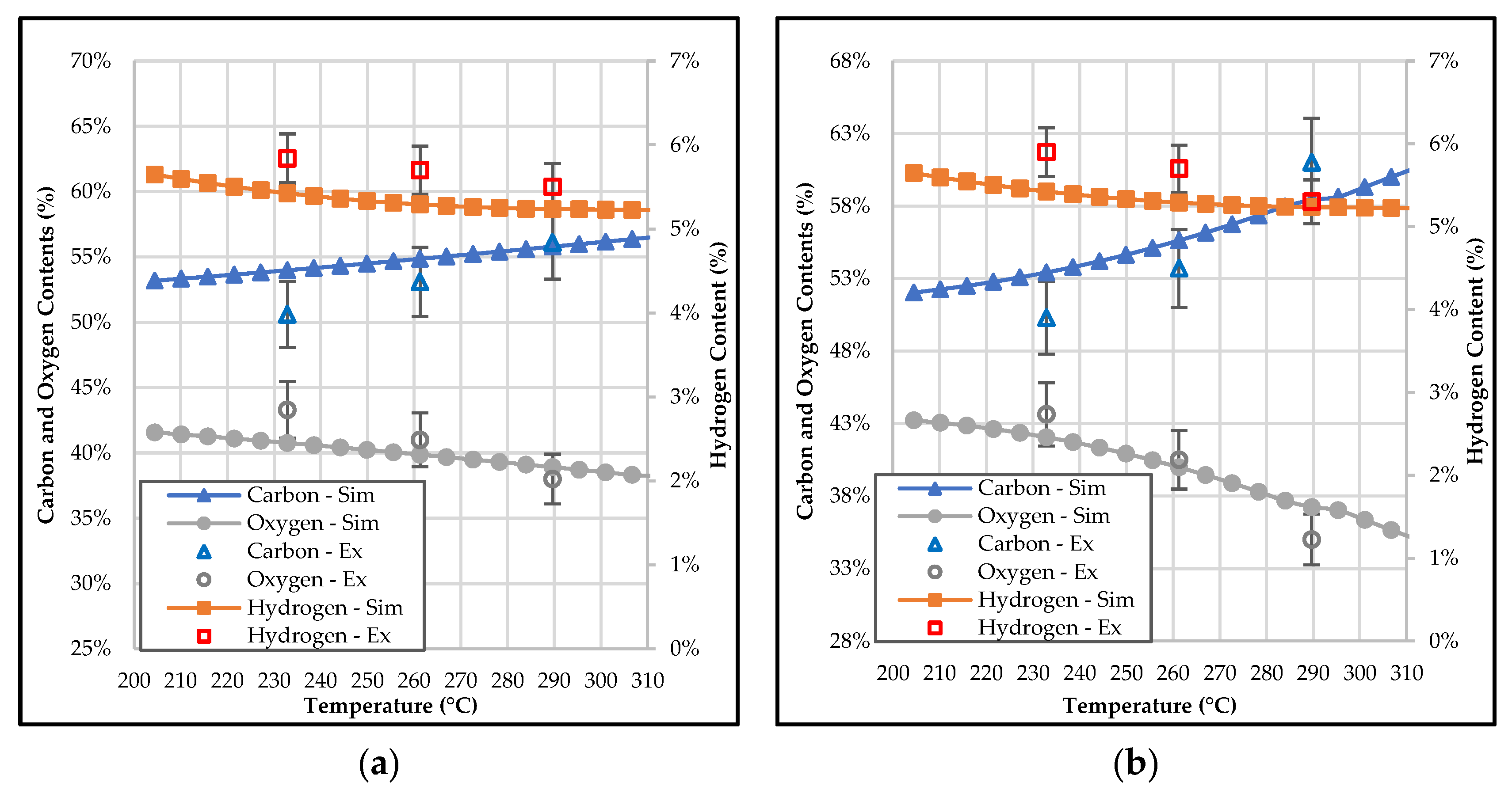
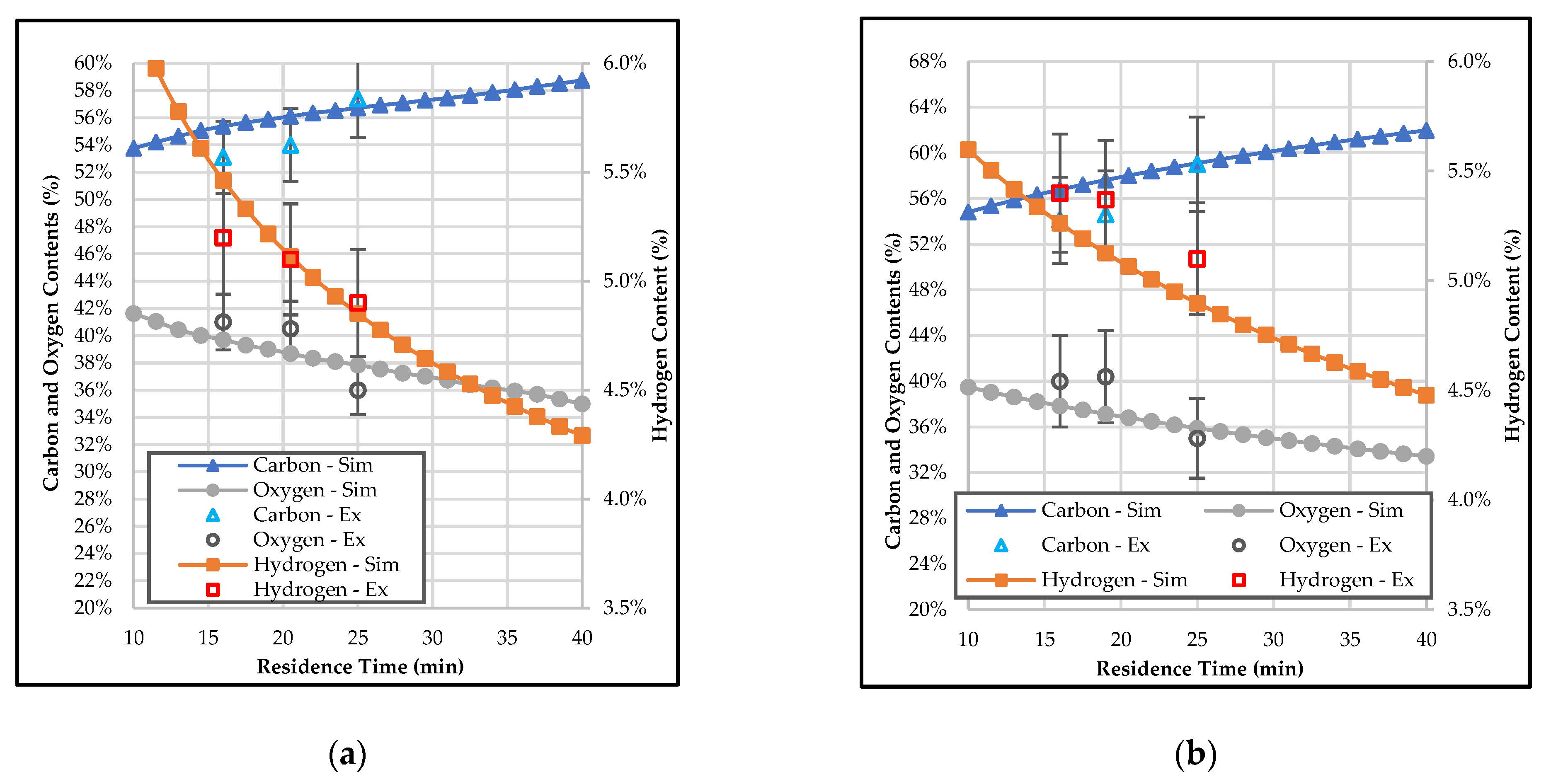
3.2. Effect of Temperature and Residence Time on the Composition of Bio-Coal
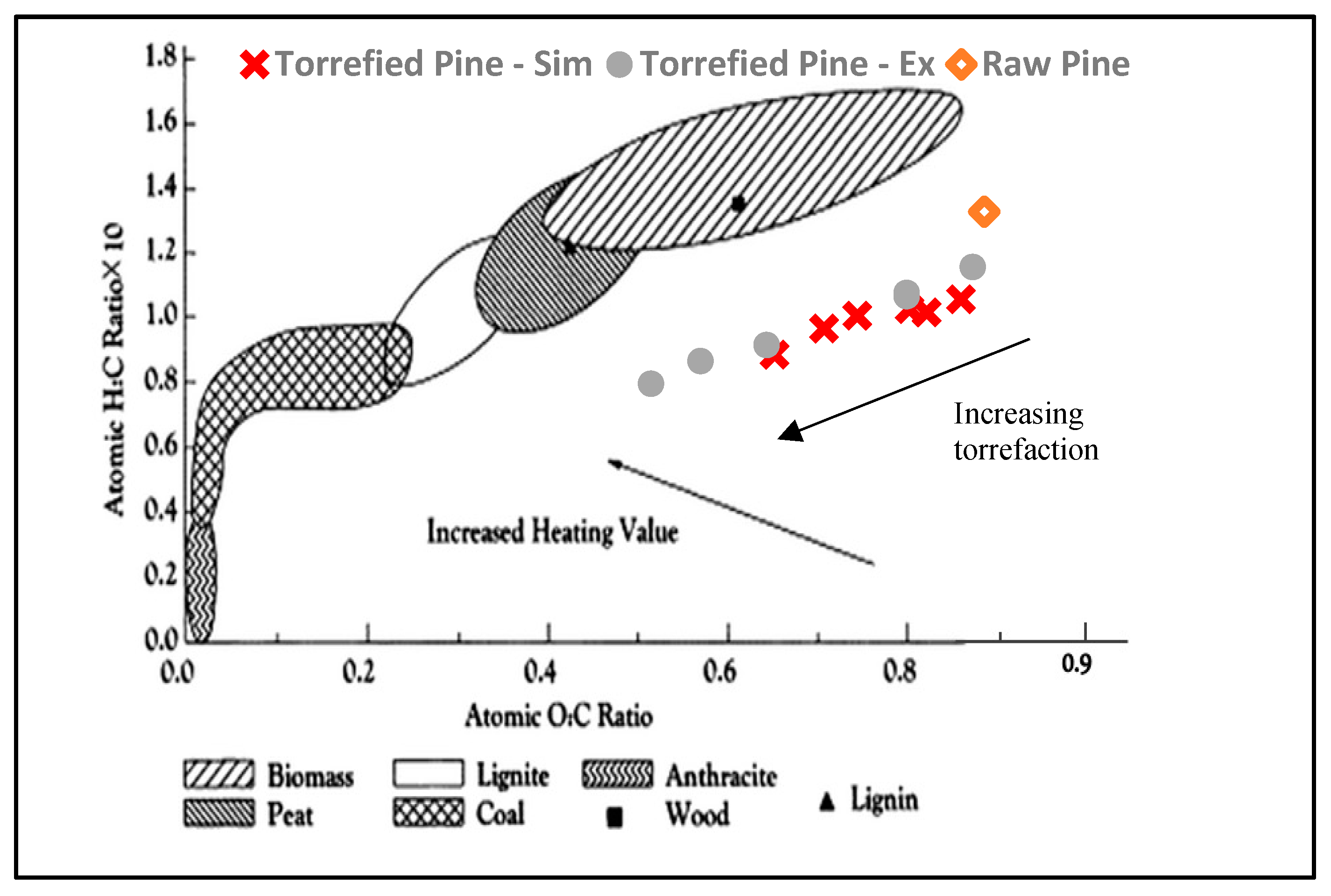
3.3. Van Krevelen Plot
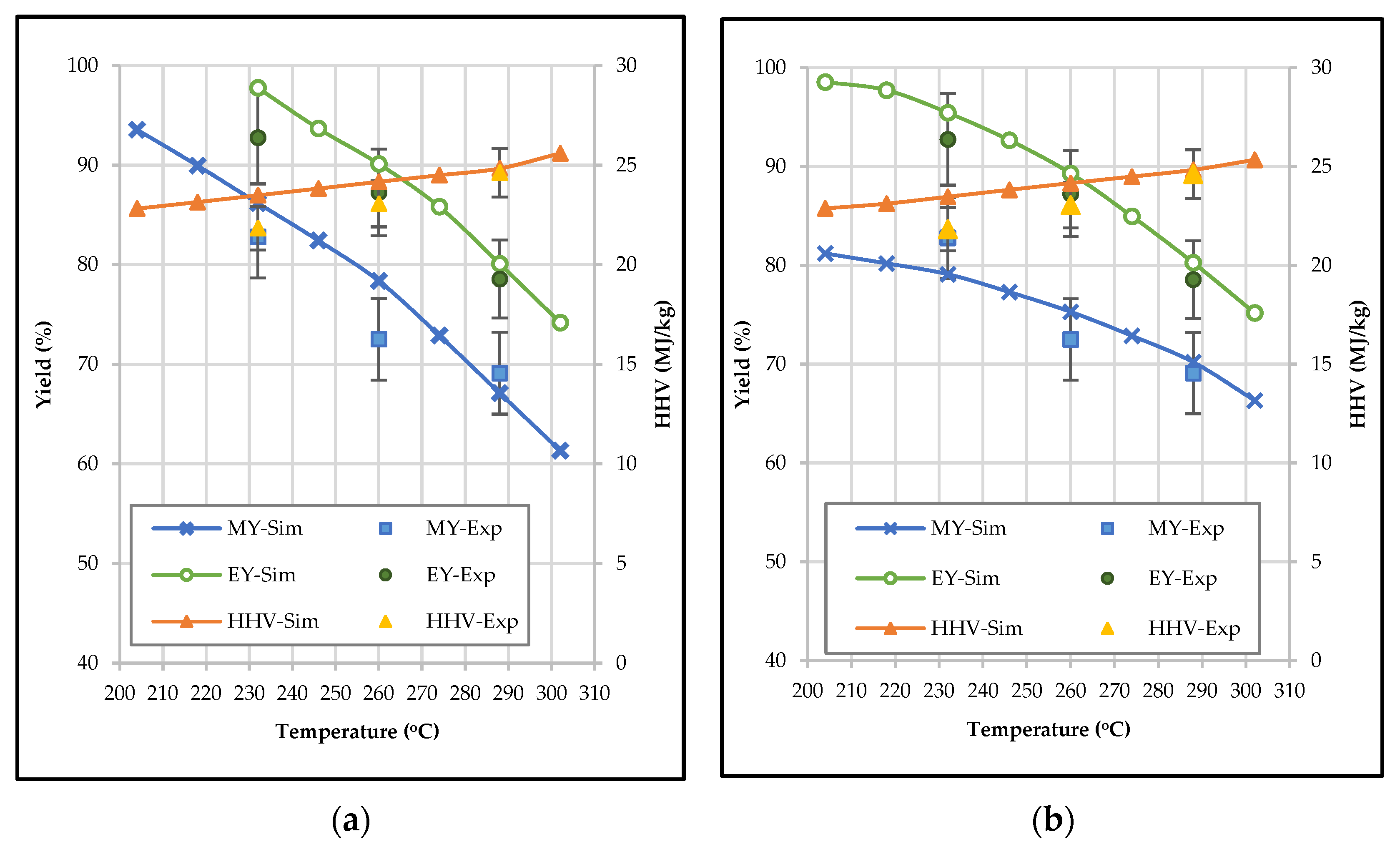
3.4. Effect of Temperature and Residence Time on the Properties of Bio-Coal in Terms of Mass Yield, Energy Yield, and HHV
3.5. Effect of Temperature and Residence Time on Product Distribution
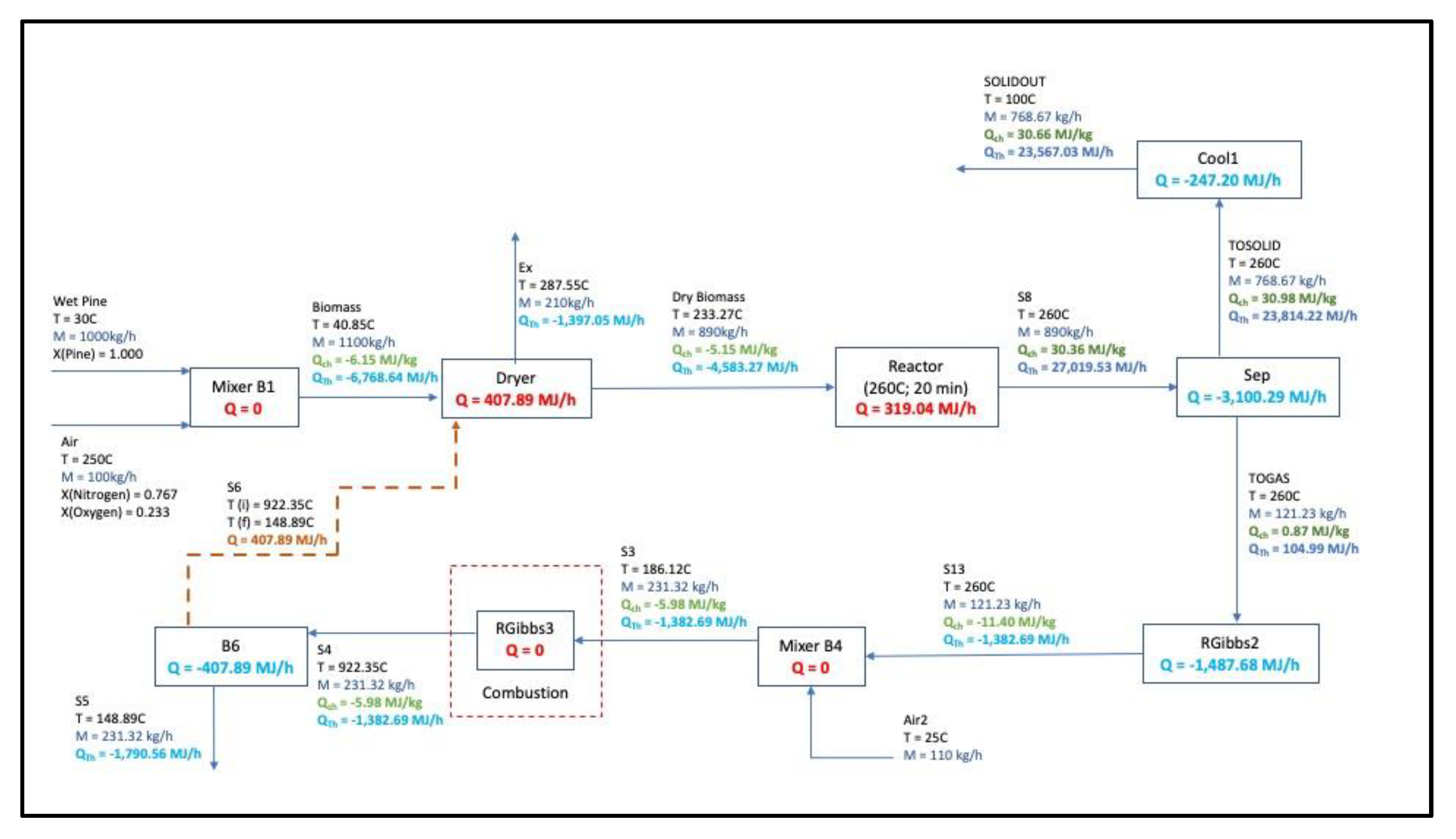
3.6. Mass and Energy Balance Flowsheet
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Prabir Basu: Kolkata, India, 2013; ISBN 9780123965431. Available online: https://www.elsevier.com/books/biomass-gasification-pyrolysis-and-torrefaction/basu/978-0-12-396488-5 (accessed on 13 March 2022).
- REN21. Renewables 2020 Global Status Report; REN21: Paris, France, 2020; ISBN 978-3-948393-00-7. [Google Scholar]
- Faleeva, J.M.; Sinelshchikov, V.A.; Sytchev, G.A.; Zaichenko, V.M. Exothermic Effect during Torrefaction. J. Phys. Conf. Ser. 2018, 946, 012033. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass Upgrading by Torrefaction for the Production of Biofuels: A Review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Balat, M. Mechanisms of Thermochemical Biomass Conversion Processes. Part 2: Reactions of Gasification. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 636–648. [Google Scholar] [CrossRef]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of Biomass: A Review of Production Methods for Biocoal from Cultured and Waste Lignocellulosic Feedstocks. Renew. Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Zappi, M.E.; Buchireddy, P. Part B: Torrefaction of Lignocellulosic Agricultural Waste into Biocoal. In Biomass and Waste Energy Applications; ASME Press: New York, NY, USA, 2021; pp. 3–83. [Google Scholar]
- Di Blasi, C.; Lanzetta, M. Intrinsic Kinetics of Isothermal Xylan Degradation in Inert Atmosphere. J. Anal. Appl. Pyrolysis 1997, 40–41, 287–303. [Google Scholar] [CrossRef]
- Thurner, F.; Mann, U. Kinetic Investigation of Wood Pyrolysis. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 482–488. [Google Scholar] [CrossRef]
- Shang, L.; Ahrenfeldt, J.; Holm, J.K.; Barsberg, S.; Zhang, R.Z.; Luo, Y.H.; Egsgaard, H.; Henriksen, U.B. Intrinsic Kinetics and Devolatilization of Wheat Straw during Torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 145–152. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Gajek, M.; Zajemska, M.; Jayaraman, K.; Gokalp, I. Combustion and Kinetic Parameters Estimation of Torrefied Pine, Acacia and Miscanthus Giganteus Using Experimental and Modelling Techniques. Bioresour. Technol. 2017, 243, 304–314. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, H.C.; Lu, K.M.; Lee, W.J.; Lin, T.C. Thermal Pretreatment of Wood (Lauan) Block by Torrefaction and Its Influence on the Properties of the Biomass. Energy 2011, 36, 3012–3021. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, W.Y.; Lu, K.M.; Huang, Y.P. An Evaluation on Improvement of Pulverized Biomass Property for Solid Fuel through Torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood: Part 1. Weight Loss Kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood. Part 2. Analysis of Products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of Torrefaction Process Parameters on Biomass Feedstock Upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of Torrefaction on the Grindability and Reactivity of Woody Biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Cremers, M.; Koppejan, J.; Sokhansanj, S.; Melin, S.; Madrali, S. Status Overview of Torrefaction Technologies—A Review of the Commercialization Status of Biomass Torrefaction; IEA Bioenergy: Paris, France, 2015; ISBN 978-1-910154-23-6. Available online: https://www.ieabioenergy.com/wp-content/uploads/2015/11/IEA_Bioenergy_T32_Torrefaction_update_2015b.pdf (accessed on 13 March 2022).
- Wilèn, C.; Jukola, P.; Järvinen, T.; Sipilä, K.; Verhoeff, F.; Kiel, J. Wood Torrefaction–Pilot Tests and Utilization; TT Technical Research Centre of Finland: VOstolaskut, Finland, 2013; Volume 122. [Google Scholar]
- Hardianto, T.; Pasek, A.D.; Suwono, A.; Azhari, R.; Ardiansyah, W. The Aspen TM Software Simulation of a Peat Torrefaction System Using RYield and SSplit Block as Reactor Model. In Proceedings of the International Symposium on Sustainable Energy and Environmental Protection (ISSEEP), Yogyakarta, Indonesia, 23–26 November 2009; pp. 23–26. [Google Scholar]
- Nikolopoulos, N.; Isemin, R.; Atsonios, K.; Kourkoumpas, D.; Kuzmin, S.; Mikhalev, A.; Nikolopoulos, A.; Agraniotis, M.; Grammelis, P.; Kakaras, E. Modeling of Wheat Straw Torrefaction as a Preliminary Tool for Process Design. Waste Biomass Valorization 2013, 4, 409–420. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.; Zwart, R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations; Energy Research Centre of the Netherlands ECN: Petten, The Netherlands, 2005; p. 71.
- Arteaga-Pérez, L.E.; Segura, C.; Espinoza, D.; Radovic, L.R.; Jiménez, R. Torrefaction of Pinus Radiata and Eucalyptus Globulus: A Combined Experimental and Modeling Approach to Process Synthesis. Energy Sustain. Dev. 2015, 29, 13–23. [Google Scholar] [CrossRef]
- Dudgeon, R. An Aspen Plus Model of Biomass Torrefaction; University Turbine Systems Research (UTSR) Fellowship 2009; Electric Power Research Institute: Charlotte, NC, USA, 2009; p. 12.
- Lisboa, M.H.; Alves, M.; Vitorino, D.; Delaiba, W.B.; Finzer, J.R.D.; Barrozo, M.A.S. Study of the Performance of the Rotary Dryer with Fluidization. In Proceedings of the 14th International Drying Symposium, Saõ Paulo, Brazil, 22–25 August 2004; Volume 3, pp. 1668–1675. [Google Scholar]
- Cherry, R.S.; Wood, R.A.; Westover, T.L. Analysis of the Production Cost for Various Grades of Biomass Thermal Treatment; INL/EXT-13-30348; U.S. Department of Energy Office of Scientific and Technical Information: Idaho Falls, ID, USA, 2013. [CrossRef]
- Visconti, A.; Miccio, M.; Juchelková, D. An Aspen Plus® Tool for Simulation of Lignocellulosic Biomass Pyrolysis via Equilibrium and Ranking of the Main Process Variables. Int. J. Math. Model. Methods Appl. Sci. 2015, 9, 71–86. [Google Scholar]
- Bach, Q.V.; Skreiberg, Ø.; Lee, C.J. Process Modeling and Optimization for Torrefaction of Forest Residues. Energy 2017, 138, 348–354. [Google Scholar] [CrossRef]
- Scott Fogler, H. Elements of Chemical Reaction Engineering; Prentice Hall: Hoboken, NJ, USA, 2017. [Google Scholar]
- Morampudi, P. Pilot Scale Evaluation of Torrefaction Operating Process Parameters on Thermal Properties of Biomass; University of Louisiana at Lafayette: Lafayette, LA, USA, 2019. [Google Scholar]
- Sofia, D.; Coca Llano, P.; Giuliano, A.; Iborra Hernández, M.; García Peña, F.; Barletta, D. Co-Gasification of Coal–Petcoke and Biomass in the Puertollano IGCC Power Plant. Chem. Eng. Res. Des. 2014, 92, 1428–1440. [Google Scholar] [CrossRef]
- Montagna, J.M.; Iribarren, O.A. A New Strategy for Process Simulation with the Sequential Modular Approach. Comput. Ind. 1989, 12, 23–29. [Google Scholar] [CrossRef]
- Ferrentino, G.; Barletta, D.; Balaban, M.O.; Ferrari, G.; Poletto, M. Measurement and Prediction of CO2 Solubility in Sodium Phosphate Monobasic Solutions for Food Treatment with High Pressure Carbon Dioxide. J. Supercrit. Fluids 2010, 52, 142–150. [Google Scholar] [CrossRef]
- Diego, B.; Paola, B.; Antonio, G.; Michele, M. Simulation and Flowsheeting of Agro Industrial Residues Torrefaction the Case of Tomato Peels Waste. In Proceedings of the Fifth International Conference on Advances in Civil, Structural and Environmental Engineering—ACSEE 2017, Rome, Italy, 27–28 May 2017; Institute of Research Engineers and Doctors, LLC: New York, NY, USA, 2017; pp. 27–31. [Google Scholar]
- EverGreenRenewable, LLC. Biomass Torrefaction as a Preprocessing Step for Thermal Conversion. 2009, pp. 1–4. Available online: https://www.scribd.com/document/348196215/Biomass-Torrefaction-as-a-Preprocessing-Step-for-Thermal-Conversion-Evergreen (accessed on 13 March 2022).
- Pirraglia, A.; Gonzalez, R.; Saloni, D.; Wright, J.; Denig, J. Fuel Properties and Suitability of Eucalyptus Benthamii and Eucalyptus Macarthurii for Torrefied Wood and Pellets. BioResources 2012, 7, 217–235. [Google Scholar]
- Lê Thành, K.; Commandré, J.M.; Valette, J.; Volle, G.; Meyer, M. Detailed Identification and Quantification of the Condensable Species Released during Torrefaction of Lignocellulosic Biomasses. Fuel Process. Technol. 2015, 139, 226–235. [Google Scholar] [CrossRef]
- Commandré, J.M.; Leboeuf, A. Volatile Yields and Solid Grindability after Torrefaction of Various Biomass Types. Environ. Prog. Sustain. Energy 2015, 34, 1180–1186. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Mehrabian, R.; Scharler, R.; Obernberger, I. Kinetic Scheme to Predict Product Composition of Biomass Torrefaction. Chem. Eng. Trans. 2014, 37, 43–48. [Google Scholar] [CrossRef]
- Portilho, G.R.; de Castro, V.R.; de Cássia Oliveira Carneiro, A.; Zanuncio, J.C.; Zanuncio, A.J.V.; Surdi, P.G.; Gominho, J.; de Araújo, S.O. Potential of Briquette Produced with Torrefied Agroforestry Biomass to Generate Energy. Forests 2020, 11, 1272. [Google Scholar] [CrossRef]
- Candelier, K.; Chaouch, M.; Dumaray, S.; Pétrissans, A.; Pétrissans, M.; Gérardin, P. Utilization of Thermodesorption Coupled to GC–MS to Study Stability of Different Wood Species to Thermodegradation. J. Anal. Appl. Pyrolysis 2011, 92, 376–383. [Google Scholar] [CrossRef]
- Chang, S.; Zhao, Z.; Zheng, A.; He, F.; Huang, Z.; Li, H. Characterization of Products from Torrefaction of Sprucewood and Bagasse in an Auger Reactor. Energy Fuels 2012, 26, 7009–7017. [Google Scholar] [CrossRef]
- Nocquet, T.; Dupont, C.; Commandre, J.M.; Grateau, M.; Thiery, S.; Salvador, S. Volatile Species Release during Torrefaction of Wood and Its Macromolecular Constituents: Part 1-Experimental Study. Energy 2014, 72, 180–187. [Google Scholar] [CrossRef]
- Repellin, V.; Govin, A.; Rolland, M.; Guyonnet, R. Modelling Anhydrous Weight Loss of Wood Chips during Torrefaction in a Pilot Kiln. Biomass Bioenergy 2010, 34, 602–609. [Google Scholar] [CrossRef]
- Bourgois, J.; Guyonnet, R. Characterization and Analysis of Torrefied Wood. Wood Sci. Technol. 1988, 22, 143–155. [Google Scholar] [CrossRef]
- Strandberg, M.; Olofsson, I.; Pommer, L.; Wiklund-Lindström, S.; Åberg, K.; Nordin, A. Effects of Temperature and Residence Time on Continuous Torrefaction of Spruce Wood. Fuel Process. Technol. 2015, 134, 387–398. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of Reed Canary Grass, Wheat Straw and Willow to Enhance Solid Fuel Qualities and Combustion Properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Radics, R.I.; Gonzalez, R.; Bilek, E.M.; Kelley, S.S. Systematic Review of Torrefied Wood Economics. BioResources 2017, 12, 6868–6884. [Google Scholar] [CrossRef]
- Manouchehrinejad, M.; Mani, S. Process Simulation of an Integrated Biomass Torrefaction and Pelletization (IBTP) Plant to Produce Solid Biofuels. Energy Convers. Manag. X 2019, 1, 100008. [Google Scholar] [CrossRef]
- Mckendry, P. Energy Production from Biomass (Part 2): Conversion Technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of Biomass Torrefaction Based on Three Major Components: Hemicellulose, Cellulose, and Lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Sinnott, R.; Towler, G. Chemical Engineering Design; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081025994. [Google Scholar]
- Deng, J.; Wang, G.; Kuang, J.; Zhang, Y.; Luo, Y. Pretreatment of Agricultural Residues for Co-Gasification via Torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]



| Capacity | 10,000 tons/year |
| Plant type | Stand alone |
| Feed stock | Pine, 11% moisture content |
| Torrefaction conditions | Residence time: 10–40 min Torrefaction temperature: 204–304 °C (400–580 °F) Flow rate: 8760 ton/year |
| Production rates | 7000–8000 ton/year |
| Available utilities | Natural gas, water, air |
| Raw pine softwood HHV (MJ/kg) (db) | 20.5 |
| Apparent density of raw pinewood (kg/m3) | 440 |
| Density of dry pine wood (kg/m3) | 250 |
| Parameters | Carbon | Oxygen | Hydrogen |
|---|---|---|---|
| Reaction order (α) | 1.05 | 1.98 | 1.97 |
| Activation energy | 6378.501 | 68,334.5 | 26,457.6 |
| Pre-exponential factor | 0.00184 (min−1) | 2799.52 (g·mol−1·min−1) | 22.64 (g·mol−1·min−1) |
| Reaction Order | Integral Form of the Equation | |
|---|---|---|
| Zero-order | (8) | |
| First-order | (9) | |
| Second-order | (10) | |
| Third-order | (11) | |
| Proximate analysis—pine | |
| Volatile matter (%) | 82.5 |
| Fixed carbon (%) | 17.0 |
| Ash (%) | 0.45 |
| Ultimate analysis—pine | |
| C (%) | 50 |
| H (%) | 6.6 |
| O (%) | 43.2 |
| N (%) | 0.1 |
| S (%) | 0.0 |
| higher heating value (MJ/Kg) * db (Btu/lb) | 20.3 (8680) |
| Biomass type | Softwood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazra, S.; Morampudi, P.; Prindle, J.C.; Fortela, D.L.B.; Hernandez, R.; Zappi, M.E.; Buchireddy, P. Torrefaction of Pine Using a Pilot-Scale Rotary Reactor: Experimentation, Kinetics, and Process Simulation Using Aspen Plus™. Clean Technol. 2023, 5, 675-695. https://doi.org/10.3390/cleantechnol5020034
Hazra S, Morampudi P, Prindle JC, Fortela DLB, Hernandez R, Zappi ME, Buchireddy P. Torrefaction of Pine Using a Pilot-Scale Rotary Reactor: Experimentation, Kinetics, and Process Simulation Using Aspen Plus™. Clean Technologies. 2023; 5(2):675-695. https://doi.org/10.3390/cleantechnol5020034
Chicago/Turabian StyleHazra, Suchandra, Prithvi Morampudi, John C. Prindle, Dhan Lord B. Fortela, Rafael Hernandez, Mark E. Zappi, and Prashanth Buchireddy. 2023. "Torrefaction of Pine Using a Pilot-Scale Rotary Reactor: Experimentation, Kinetics, and Process Simulation Using Aspen Plus™" Clean Technologies 5, no. 2: 675-695. https://doi.org/10.3390/cleantechnol5020034
APA StyleHazra, S., Morampudi, P., Prindle, J. C., Fortela, D. L. B., Hernandez, R., Zappi, M. E., & Buchireddy, P. (2023). Torrefaction of Pine Using a Pilot-Scale Rotary Reactor: Experimentation, Kinetics, and Process Simulation Using Aspen Plus™. Clean Technologies, 5(2), 675-695. https://doi.org/10.3390/cleantechnol5020034






