Abstract
The use of alternatives for petroleum-based products is becoming more and more important, especially considering the new and constantly changing geopolitical context, where excessive energy dependence is not desirable. Thus, biodiesel could play an important role in contributing to the implementation of biorefineries, which represent desirable goals in terms of sustainability, green chemistry and the circular economy. However, one challenge related to biodiesel based on vegetable oils is its low oxidative stability, which can alter the properties of these products during storage. To avoid this problem, interesting antioxidants, such as propyl gallate (PG), could be added to biodiesel to allow it to keep its main properties during oxidation. Additionally, monitoring PG content during oxidation is interesting, and the use of voltammetry could be suitable for this purpose. The aim of this work was to assess the effectiveness of PG during cardoon biodiesel oxidation, while monitoring the process through cyclic voltammetry (CV). As a result, it was proven that PG was highly effective, increasing the length of oxidative stability to more than 10 h at low concentrations (600 mg·L−1) and retaining its main properties (viscosity and acidity) during oxidation. Regarding CV, this technique was successfully optimized to determine PG concentration in cardoon biodiesel during oxidation.
1. Introduction
1.1. The Role of Biodiesel in Global Energy Scenario
The role of biofuels is becoming more and more important, especially taking into account the current geopolitical context, where the shortage/cost of fuels is considerable in certain regions, such as Europe [1]. Consequently, energy and fuel transition is vital, as well as a real challenge [2]. Thus, the implementation of biofuels as a form of renewable energy could contribute to changing the current geopolitical status of some countries [3]. In addition, biofuels as a replacement of petrol-derived fuels could contribute to a necessary green transition, where sustainability, green chemistry and the circular economy play an important role [4,5]. Specifically, biodiesel production based on vegetable oils has considerably increased in recent years, attracting the attention of international agencies and governments as a real alternative for petrol-based fuel dependence. In parallel, the improvement of biodiesel production, in order to make this process as competitive as possible compared to petrol-based refineries, has gained attention from researchers from many fields. Thus, the use of different catalysts (both homogeneous and heterogeneous) [6], feedstocks (including microalgae) [7] or additives [8] to improve the performance of biodiesel, among other things, have been popular topics of research.
1.2. Biodiesel Based on Vegetable Oils
Biodiesel can be produced through the transesterification of fatty acids with methanol or ethanol, with vegetable oils as one of the main sources of these fatty acids. In that sense, there is a wide variety of vegetable oils that have been studied in the literature for this purpose, offering interesting results regarding yield and global quality. Additionally, some wastes, such as frying oil, could be used to produce biodiesel, offering a viable alternative for a product that is difficult to manage in an environmentally friendly way.
One of the advantages of this product is the possibility of adapting its production to different regions, even those with extreme climate conditions, which could aid the economic growth of developing countries. Thus, economic independence (with less import-dependence and an increase in renewable sources) and the promotion of local economies (through local raw materials or wastes) could be an important aspect to be taken into account [9]. In addition, biodiesel production is cleaner than the corresponding petrol-based process, as it results in zero net CO2 emissions and low pollutant emissions, among others.
Regarding crop adaptation to climate and soils, cardoon could be a suitable crop for biodiesel production, as it presents good adaptation to dry areas and poor soils and could be an alternative to rotation crops, as it has a long main root, which facilitates the recovery of soils in which other crops, such as wheat, had previously been grown. Additionally, the oil yields of its seeds are considerable, which makes it an attractive crop for biodiesel production [10,11].
However, many vegetable oils, due to their fatty acid profiles, present low oxidation stability values, which is a disadvantage, especially when these products are stored for a long time. This is mainly due to the high presence of specific fatty acids, such as linoleic or linolenic acids (methyl or ethyl linoleate or linolenate in final biodiesel), which present two or three double bounds that act as starting points for free radical generation and the subsequent auto-oxidation process [12]. When free radicals are generated, termination stages when producing stable products can take place, with the possible polymerization or free-fatty acid generation, resulting in an increase in viscosity or acid number in biodiesel during storage, which are undesirable effects that can worsen the performance of biofuel in Diesel engines. This way, oxidative stability in biodiesel is vital, as it maintains the main properties of biodiesel, such as viscosity or acid number during storage. On the other hand, low oxidative stabilities could result in an increase in viscosity or acid number during storage, with the subsequent quality loss of biodiesel for its use in Diesel engines, provoking obstructions and corrosion. To avoid this, the use of antioxidants is advisable, along with other techniques such as the use of sources with low linoleic or linolenic acid levels [13]. In the following subsection, some of the main antioxidants utilized biodiesel are discussed.
1.3. Antioxidants to Improve Oxidative Stability in Biodiesel
As previously outlined, the use of antioxidants (both natural and synthetic) could be a real alternative for the improvement of oxidation stability in biodiesel, especially in the cases of those biofuels with high linoleic or linolenic acid levels. They can act via scavenging or chelation, cutting off or delaying the initiation or propagation steps during the auto-oxidation of biodiesel [14]. Essentially, these antioxidants are phenolic compounds, and there is a wide range of them that can be used in biodiesel, such as tert-butylhydroquinone (TBHQ), butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl gallate (PG), etc. Depending on the product or the combination with other additives, the efficiency of these antioxidants may vary [15].
Concerning the latter (which is used in food and biofuel conservation to avoid oxidation), its effectiveness in increasing the oxidation stability of biodiesel has been widely studied. Thus, several biodiesel samples (from tallow, neem, poultry fat, jetropha, pongamia, safflower or residual cooking oil) had a considerable increase in oxidative stability with 1000 ppm of PG, clearly exceeding 10 h [15,16,17]. Even at lower concentrations (500 ppm), biodiesel obtained from Tilapia oil exceeded 10 h of oxidative stability [18]. Additionally, PG at high concentrations (5000 ppm) displayed other positive effects, such as the inhibition of biodiesel crystallization at low temperatures [19].
Consequently, PG could be another parameter to be monitored during biodiesel storage or oxidation, as its decrease in content might provide interesting information regarding the optimum antioxidant concentration that should be used or the service life of these products. This way, voltammetry could be a useful way to determine PG evolution in biodiesel during oxidation, as explained in the following subsection.
1.4. Voltammetry Applied to Antioxidant Determination in Biodiesel
Voltammetry is an electroanalytical method that can be used to obtain information about an analyte by measuring the current as potential. There are mainly two kinds of voltammetry, i.e., cyclic voltammetry (CV) and differential pulse voltammetry (DPV):
- Cyclic voltammetry: a linear potential scan (triangular shape) as a function of time is carried out, from an initial to a final potential (Ei and Ef, respectively) and the scan is usually inverted to Ei. The signal is obtained when a stationary electrode is immersed in a chemical solution without stirring. This technique is useful to carry out studies concerning electrochemical reaction mechanisms, electrode processes or organic compounds. It is mainly used for quantification, presenting low sensitivity values (LOD 10−5 M).
- Differential pulse voltammetry: DPV is applied as a function of potential versus time, and the signal consists of a series of pulses (shaped like stairs), and the base potential gradually increases at small intervals between 5–10 mV. The signal is obtained through the difference between the intensity obtained before the pulse and the intensity obtained before the end of the pulse application. Consequently, a decrease in the capacitive current is obtained, with a higher sensitivity (LOD 10−7 M) compared to CV.
The most important parameters in CV are the anode and cathode peak potentials (Eap and Ecp, respectively) and the corresponding intensity peaks (Iap and Icp). Regarding DPV, the most important values are Ip and Ep, used to obtain the peak height (and antioxidant content, as it is proportional to antioxidant concentration in biodiesel). This technique could be useful to determine PG concentration in cardoon biodiesel. PG provides an electrochemical signal with different electrodes and mediums. Previous studies have covered electroanalytical methods to determine antioxidants such as TBHQ or BHA in biodiesel samples, such as soybean biodiesel [20,21,22,23,24,25].
In our previous experiments, we have successfully used voltammetry to determine TBHQ in different samples (such as cardoon, rapeseed or safflower biodiesel) during oxidation [26], revealing an interesting basis for adapting this method for PG determination in biodiesel. In this work, emulsified medium was selected as it does not require any pre-treatment for PG quantification in biodiesel.
1.5. Objective and Novelty of This Work
Considering the above, the aim of this work was to assess the effect of propyl gallate addition to cardoon biodiesel, focusing on its effectiveness in retaining the main properties of this biofuel (especially viscosity and acid number) during extreme oxidation conditions.
Additionally, the characterization of the main properties of cardoon biodiesel was carried out, including the optimum propyl gallate concentration in cardoon biodiesel. Finally, the voltammetric method to determine propyl gallate in cardoon biodiesel was optimized, in order to assess the evolution of this antioxidant during oxidation. This is an important point, as the characterization of biodiesel and its additives is vital for the correct implementation of this kind of biofuel in the industry. Thus, as there are few studies that perform a thorough characterization of PG in biodiesel, including voltammetry, it could be an interesting method to be applied in quality laboratories devoted to biodiesel characterization.
2. Materials and Methods
2.1. Raw Material
The raw material used in this study was cardoon oil, which was collected and mechanically extracted in 2021 by the Agricultural Research Institute “Finca La Orden–Valdesequera”, belonging to CICYTEX (Centro de Investigaciones Científicas y Tecnológicas de Extremadura, Guadajira, Badajoz, Spain). The oil was filtered, and its acidity was checked (below 1%, which allowed the process to continue without any further treatment). Afterwards, it was collected in 25 L containers at room temperature for further characterization and biodiesel and biolubricant production.
2.2. Fatty acid Methyl Ester Production
Fatty acid methyl ester (FAME) production was carried out via the transesterification of cardoon oil with methanol. Chemical condition optimization (paying attention to methanol/oil ratio, catalyst or reaction time, among others) is vital in order to obtain a high yield of high-quality biodiesel [27]. The chemical conditions to obtain a high yield (exceeding 96%) in this reaction were based on previous studies [28], where thorough research on the influence of the main chemical condition factors on biodiesel production was performed. Thus, the main chemical conditions according to this study were the following: methanol/oil ratio, 6:1; catalyst concentration (sodium methylate), 0.5% w/w; reaction temperature, 65 °C; stirring rate, 350 rpm; reaction time, 120 min. The final product (cardoon biodiesel, CARBD) was separated from glycerol through decantation and purified via washing treatments with ultrapure water. The sample was dried at 110 °C to avoid moisture and was stored in 2.5 L opaque containers for further characterization.
2.3. Antioxidant Addition
Once CARBD was obtained, propyl gallate (PG, >98%, Sigma-Aldrich, Saint Louis, MO, USA) was added to the samples to improve their oxidative stability. Different concentrations, which are typically studied in the literature, of other fatty acid methyl esters (from 0 to 1000 mg·L−1), were added to CARBD. Thus, the right amount of PG was added to 100 mL of sample, dissolving it via ultrasound for 5 min. The doped samples were stored for further study, such as oxidation stability determination via the Rancimat method. The optimum concentration needed to reach an oxidation stability of 8 h (taking the lower limit of the UNE-EN standard as a reference) was selected to undergo extreme oxidation conditions.
2.4. Extreme Oxidation Conditions
Extreme oxidation conditions were selected to assess the evolution of three main characteristics of CARBD, that is, viscosity, acid number and TBHQ content (for those samples that were doped). These conditions were carried out according to previous studies (similar to Rancimat method) [26,29], and the procedure was the following: 30 g of biolubricant was heated at 110 °C, bubbling synthetic air (100 L·h−1) for 8 h. During this process, samples at 0, 1, 3, 5 and 8 h were collected to measure their viscosity and acid number.
2.5. Sample Characterization
2.5.1. Fatty Acid Methyl Ester Content and Composition
Fatty acid methyl ester (FAME) content and composition in CARBD was measured through gas chromatography coupled to FID detection (Varian 3900, Agilent, Santa Clara, CA, USA), using a polyethylene glycol column (Zebron ZB-WAX PLUS, Phenomenex, length: 30 m, film thickness: 0.5 µm and i.d.: 0.32 mm). The main FAMEs determined were methyl oleate, linoleate, linolenate and palmitate, using methyl heptadecanoate as an internal standard—all provided by Sigma-Aldrich (Saint Louis, MO, USA). The chromatographic conditions were based on previous studies, according to UNE-EN 14214 standard [28,30].
2.5.2. Oxidation Stability
The oxidative stability of CARBD was obtained using the Rancimat method, as described in previous works [29,31]. Briefly, 3 g of biodiesel was heated at 110 °C, synthetic air (10 L·h−1) was bubbled into biodiesel during heating, and the resulting stream was connected to 50 mL of distilled water. Afterwards, water conductivity increased due to the dilution of oxidation by-products in the biodiesel, which was recorded using a conductivity meter (Mettler Toledo, Columbus, OH, USA). The oxidative stability (in hours) was the time when conductivity abruptly increased.
2.5.3. Viscosity
Viscosity was measured at 40 °C by using an Ostwald viscometer, following the corresponding standard [32].
2.5.4. Acid and Iodine Number Determination
Acid and iodine numbers were determined according to UNE-EN 12634:1999 and UNE-EN 14111:2003 standards [33,34].
2.5.5. PG Content through Voltammetry
PG content in CARBD was determined by using cyclic (CV) and differential pulse (DPV) voltammetry. Voltammetric equipment (µAutolab, ECO Chemie, Utrecht, Netherlands) was used, coupled to a 663 VA-Stand Metrohm unit (Herisau, Switzerland) with a three-electrode system: a working electrode (glassy carbon), a reference electrode (Ag/AgCl) and an auxiliary electrode (Pt) with a measuring cell. The cleaning of the glassy carbon electrode after each measure consisted of rubbing it with cotton dipped in dimethylformamide. Afterwards, the electrode was rubbed with cotton dipped in Milli-Q water. The procedure to analyze PG content in CARBD was as follows: in a 50-mL flask, 1 mL of sample (with the corresponding amount of PG), 8 mL of ethanol, 4 mL of buffer (pH 2.5 NaH2PO4/H3PO4 0.5 mol·L−1) and 2 mL of surfactant (0.8% cetyl trimethyl ammonium, CTAB) were added, and finally, the solution was diluted to the mark with Milli-Q water, using ultrasound for 15 min to homogenize the final solution. For statistical analysis, the ACOC V 2.0. program [35], a statistical tool for analytical chemistry in a MATLAB 5.3 environment, was used, which carried out univariate calibration and quality parameter calculations for calibration lines.
Once the sample was prepared, the voltammetry was performed, introducing the sample in the electrochemical cell and registering its cyclic voltammograms (150 mV·s−1). Afterwards, another sample composed of the same quantities, but with the addition of suitable aliquots of PG standard, was prepared for each experiment. Each sample was determined in duplicate. For PG quantification, the standard addition method was used, adding volumes of PG standard and assessing each addition in duplicate and each standard addition in triplicate. Moreover, the sample with the lowest PG amount was also analyzed using differential pulse voltammetry, and the same results when compared to CV were obtained.
2.5.6. Other Quality Parameters
Moisture and density were determined according to UNE-EN ISO 12937:2000 and UNE-EN-ISO 3675 standards, respectively. For moisture, Metrohm 870 trinitro plus equipment was used, and density was measured by a pycnometer (Pobel, Madrid, Spain) [36,37].
The cold filter plugging point (CFPP) was determined using the EN 116 standard [38]. Flash and combustion points were obtained via the Cleveland open-cup method using a semi-automatic instrument (Herzog Cleveland semi-automatic, Herzog, Landa-Königshofen, Germany), according to the UNE 51-023-90 standard [39].
To sum up, the main steps taken during this research are given in Figure 1.
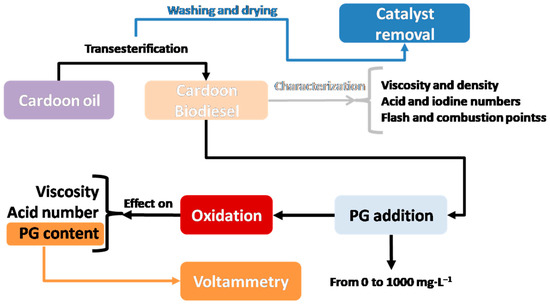
Figure 1.
Experimental design for this study.
3. Results and Discussion
3.1. Cardoon Biodiesel Characterization
As observed in Table 1, cardoon biodiesel (CARBD) presented acceptable parameters compared to the UNE-EN 14214 standard [40]. Thus, it complied with almost all the parameters covered in this study, especially regarding high flash and combustion points (exceeding 170 °C), and can be considered very suitable for storage and handling. Regarding the iodine number, its value is related to the presence of double bonds, being similar to other biodiesel samples derived from palm or sunflower oil (ranging from 55 to 130 g I2·100 g−1). Additionally, the cold filter plugging point was relatively low compared to biodiesel obtained from diverse raw materials, such as corn, coconut, palm or moringa (from −8 to 18 °C) [41]. Additionally, the acid number was relatively low and similar to other cardoon biodiesels found in the literature (0.26 mg KOH·g−1), which is important in order to assess the presence of free fatty acid in biodiesel. In that sense, the sample in this study is less corrosive than other vegetable oil biodiesels found in the literature made from coffee or brassica (0.6 and 0.7 mg KOH·g−1, respectively) [42]. These results were comparable to previous studies and other works found in the literature for fatty acid methyl esters obtained from cardoon oils, where they proved the suitability of this oil for producing biodiesel [43,44]. However, as was found in previous works, oxidative stability (or the induction point) was reduced. Although it was slightly higher compared to previous studies (where induction points of 1.35 h were obtained), the oxidative stability of cardoon biodiesel (around 2 h) is quite far below the lower limit established by the standard (8 h). This could be a considerable disadvantage, as it implies faster degradation during storage with an increase in viscosity and acid number as the main quality loss [45].

Table 1.
Cardoon biodiesel characterization and comparison with the standard.
This low oxidative stability could be due to the fatty acid profile of cardoon oil, which determines the fatty acid methyl ester (FAME) composition of biodiesel (as the conversion of these fatty acids to FAMEs is considered to be the same and, therefore, equivalent, regardless the kind of fatty acid). As can be seen in Figure 2, the majority FAME in cardoon biodiesel was methyl linoleate, at around 60%, followed by methyl oleate (25%), palmitate (11%) and linolenate (2%). These results are in accordance with other fatty acid compositions observed in different cardoon accessions, with linoleic acid ranging from 52.3 to 67.4% and oleic acid from 18.4 to 31.3% [44]. Previous studies pointed out the influence of majority FAMEs on biodiesel characteristics, especially concerning oxidation stability [12,46]. Thus, methyl linoleate, whose molecular structure presents two double bonds, is more unstable than methyl oleate (with one double bond), due to the capability of free radical generation in double bonds during the initiation process in auto-oxidation. Thus, after propagation and termination processes, FAMEs can interact with each other to generate polymers (increasing viscosity due to the molecular complexity and higher intermolecular interactions) or free fatty acids (with the subsequent increase in acid number) [45].
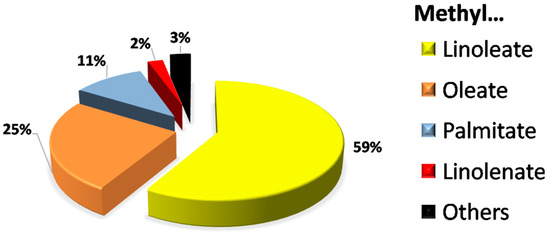
Figure 2.
FAME profile of CARBD.
The results obtained in Table 1 and Figure 2 were similar to those of previous works, where the majority FAME was methyl linoleate with a percentage range of 58–60% and oxidative stability values below 2 h, proving the influence of FAME composition on this parameter [43,47]. Other works, where sunflower biodiesel with a similar FAME composition (around 60% and 30% of methyl linoleate and oleate, respectively), pointed out the need for additives to improve oxidation stability [48]. Consequently, the addition of antioxidants, such as the one selected in this study (PG), could represent an interesting solution for improving the oxidative stability of cardoon biodiesel.
3.2. Effect of PG on Oxidation Stability of Biodiesel
Figure 3 shows the effect of PG addition on the oxidative stability of CARBD. As expected, the effectiveness and efficiency of PG was outstanding, with a linear increase in oxidative stability in the studied concentration range, and cardoon biodiesel complied with the standard at around 400–450 mg·L−1.
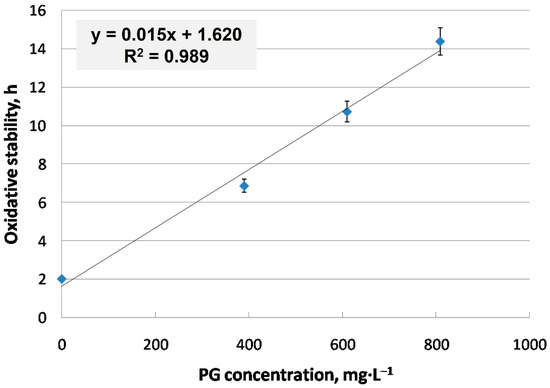
Figure 3.
Effect of PG on the oxidative stability of cardoon biodiesel.
As outlined in the literature, PG, along with other antioxidants, such as BHA or TBHQ, presented a high efficiency at increasing oxidative stability in fatty acid methyl esters from several vegetable oils, exceeding 8 h of the induction point at 500–1000 ppm [45]. However, there is no clear consensus on which antioxidant is the most efficient. Thus, some studies present a higher effectiveness for PG over TBHQ, for instance, whereas the opposite trend has also been reported [45,49]. Regarding this study, a concentration of 425 mg·L−1 was selected for the further study of biodiesel under extreme oxidation conditions.
3.3. PG Determination through Voltammetry
Figure 4 shows voltammograms related to PG. Thus, a control sample (cardoon biodiesel without any PG addition) and biodiesel doped with PG were compared. The PG signal can be observed in CV by using a glassy carbon electrode. In the case of the reverse scan, there was no signal, which indicated the irreversible nature of the electrochemical process. Biodiesel did not present any signal without PG addition, showing it only when it was doped. Thus, biodiesel analysis through this technique was possible, avoiding interferences with the blank due to a possible overlapping. For this purpose, different voltammetric studies were carried out. PG was quantified in emulsified medium using cetyltrimethyl ammonium through cyclic voltammetry. This technique uses faster scanning, avoids glassy carbon degradation and its mechanical cleaning is easier.
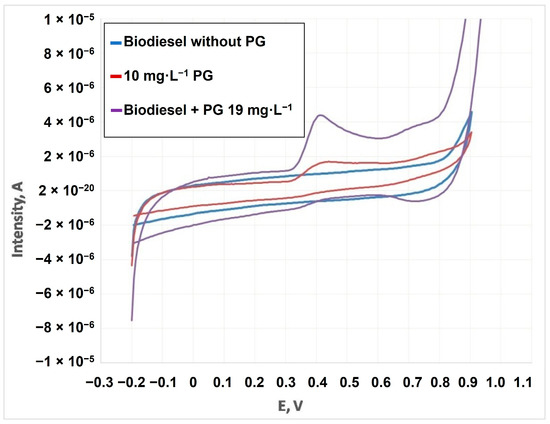
Figure 4.
Cyclic voltammograms of: PG, cardoon biodiesel without PG and biodiesel with PG.
The influence of PG concentration (from 5.1 to 20.4 mg·L−1) was studied (each concentration was assessed in duplicate, showing some of the cyclic voltammograms in Figure 5). As expected, the intensity peak increased with concentration, making the calibration of PG in cardoon biodiesel possible.
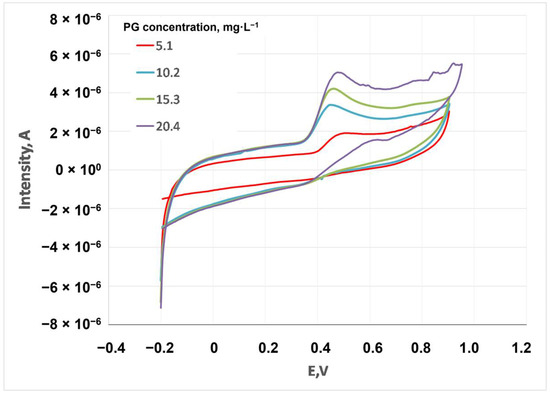
Figure 5.
Cyclic voltammogram of different concentrations of PG.
The optimum PG concentration was added to cardoon biodiesel (doped sample, with 425 mg·L−1 of PG), in compliance with the UNE-14214 standard. Thus, its content was analyzed using the standard addition method in triplicate, registering the cyclic voltammograms in duplicate. Figure 6 shows intensity peaks (Ip) versus PG concentration, and Table 2 shows the main quality parameters obtained.
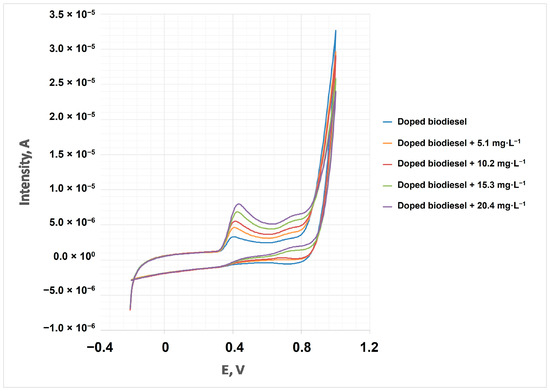
Figure 6.
Cyclic voltammograms of addition standard method (biodiesel doped with PG 450 mg·L−1) and successive additions of the PG standard.

Table 2.
Figures of merit for the standard addition of PG in CARBD.
As observed in this figure, there was a constant increase in intensity peak with propyl gallate concentration, which could be a suitable parameter for quantifying PG content in cardoon biodiesel.
According to Table 2, where the main quality parameters of standard addition are included, an acceptable linearity was found, with a coefficient of determination exceeding 0.9, presenting low detection and quantification limits. This way, the possibility of PG quantification in cardoon biodiesel using this technique and these working concentrations was proven.
3.4. Effect of PG on Biodiesel during Extreme Oxidation
In order to assess its main changes during oxidation, cardoon biodiesel underwent extreme oxidation conditions. Thus, a comparison of control samples (without any PG addition) and doped samples with an optimum PG concentration of 425 ppm (PG) was carried out, taking into account viscosity, acid number and antioxidant evolution (the latter for the doped sample only). In the following subsections, each parameter is explained.
3.4.1. Viscosity Evolution
Regarding viscosity, Figure 7 shows the evolution of this parameter during the oxidation of cardoon biolubricant both for control and doped (PG) samples. This way, there was a considerable increase in viscosity for control samples, from 5 h, when viscosity increased from 6.85 to 20.01 mm2·s−1. This fact could be due to auto-oxidation processes, where FAMEs in biodiesel can result in polymers with higher molecular complexity, resulting in an increase in intermolecular interactions and, consequently, viscosity [49,50].
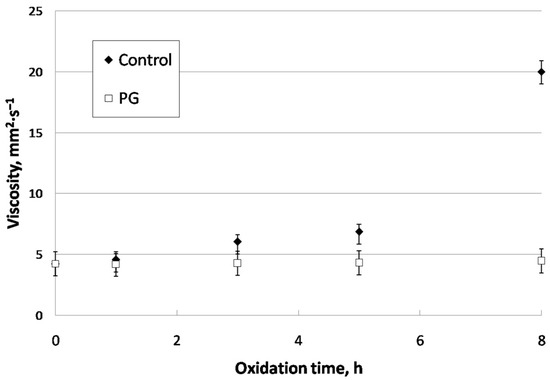
Figure 7.
Viscosity evolution for control and doped cardoon biodiesel.
In that sense, the use of PG was effective at retaining the viscosity of original cardoon biodiesel. Thus, after 8 h of oxidation, there was only a 5% increase in viscosity, whereas in the control sample, this increase was around 375%. Previous studies concerning corn and sunflower biodiesel with TBHQ and BHA additions showed a similar trend, proving the effectiveness of these antioxidants during the oxidation of the doped sample [29]. As a result, viscosity in biodiesel was constant during extreme oxidation, which could be an advantageous situation during storage, where auto-oxidation could also be inhibited. Thus, obstruction (due to deposition) in Diesel engines is avoided [48].
3.4.2. Acid Number Evolution
Regarding acid number (Figure 8), there were clear changes during cardoon biodiesel oxidation, especially in the case of the control sample, where a considerable increase took place from 0.26 at the beginning of the experiment to 7.31 mgKOH·g−1 after 8 h of oxidation. Similarly, this could be due to biodiesel auto-oxidation, where free fatty acids are generated apart from products derived from polymerization [50]. On the other hand, the use of PG implied a less prominent increase in acid number (0.75 mgKOH·g−1 at 8 h of oxidation), proving the high effectiveness of this antioxidant at retaining the main properties of biodiesel during oxidation. Again, previous studies showed a similar trend, with a 25% increase after 7 h of oxidation. On the other hand, control samples showed higher increases of around 100% [29]. Thus, the use of antioxidants helped to keep acid number in biodiesel during oxidation (which usually takes place during storage), thus avoiding problems in Diesel engines, such as corrosion.
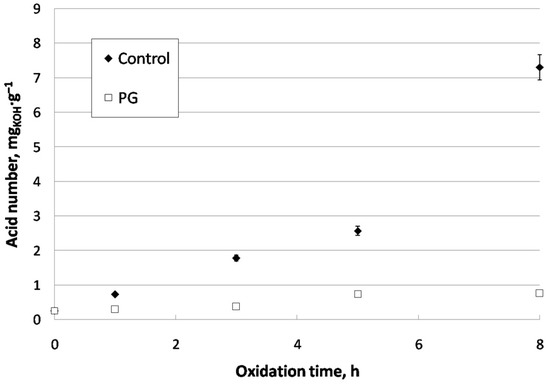
Figure 8.
Acid number evolution for control and doped CARBD during oxidation.
3.4.3. PG Content Evolution
Once the voltammetric determination of PG was optimized, the evolution of optimum PG concentration in cardoon biodiesel during oxidation was assessed. To achieve this, different samples of doped cardoon biodiesel were measured through cyclic voltammetry and the standard addition method in extreme oxidation conditions at different oxidation times (0, 2, 3, 5 and 8 h). To quantify PG content at different oxidation times, the addition standard method in triplicate, with voltammogram registration in duplicate, was carried out. Figure 9 depicts some of the voltammograms obtained. The results obtained for the corresponding recoveries are included in Figure 10.
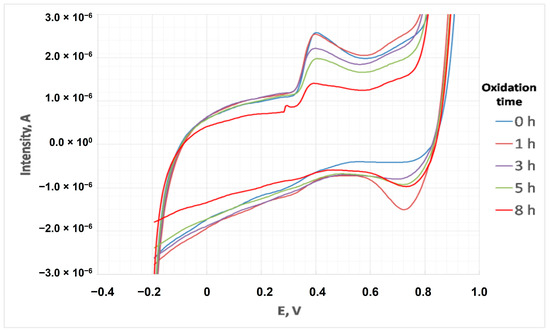
Figure 9.
Voltammograms of doped biodiesel during oxidation.
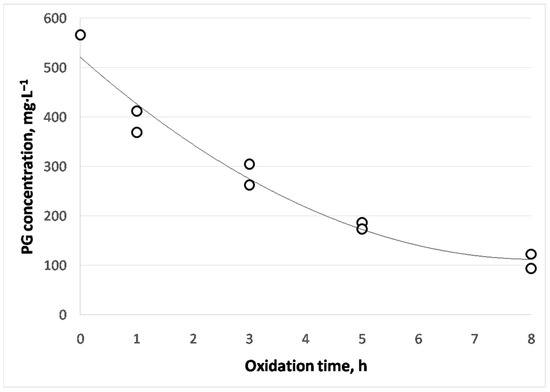
Figure 10.
PG evolution in doped cardoon biodiesel during oxidation.
As observed, the intensity peaks decreased during oxidation, due to PG degradation during oxidation. After 8 h of oxidation, the peak was negligible, implying low PG concentrations at the end of the experiment. According to Figure 10, PG concentration (with relative standard deviations of 10%) constantly decreased during extreme oxidation, reaching 100 mg·L−1 at 8 h of oxidation. Thus, the optimum concentration of PG for cardoon biodiesel applied in this work (425 mg·L−1) was enough to retain the main properties of biodiesel, as at the end of the oxidation experiment there was still some PG (at low concentration, that is, 100 mg·L−1), which was generally oxidized instead of cardoon biodiesel. The same behavior was observed in a similar product (fatty acid esters from waste cooking oil, used as biolubricant) with TBHQ addition, whose content decreased during oxidation [51].
4. Conclusions
A summary of the main findings of this work is presented below:
- Cardoon biodiesel could be a suitable biofuel, as it presents characteristics within the UNE-EN 14214 standards, except for its low oxidative stability.
- Thus, the use of antioxidants, such as propyl gallate, is justified. In this case, propyl gallate showed a high effectiveness in increasing the oxidative stability of cardoon biodiesel, complying with the standard (induction time = 8 h) at low concentrations of this antioxidant.
- Therefore, with the optimum concentration of propyl gallate (425 mg·L−1), cardoon biodiesel showed good resistance to oxidation, retaining properties such as viscosity or acid number during extreme oxidation conditions.
- It was thus proven that voltammetry can be a suitable method for determining propyl gallate in cardoon biodiesel, with the subsequent monitoring of this antioxidant during oxidation. This application could be an important aspect when determining the global quality of biodiesels, as the use of additives such as antioxidants (for instance, PG) is often necessary.
- Moreover, a decrease in propyl gallate during oxidation was observed, especially during the first 3 h, with low amounts of this antioxidant remaining at the end of the extreme oxidation test. Consequently, the optimum concentration ensures that the quality of cardoon biodiesel is maintained.
- Further studies in this field should consider the application of voltammetry to determine the concentrations of combined antioxidants, such as PG or TBHQ, whose synergistic effects can enhance biodiesel properties during storage. Additionally, the possible combination of this technique with kinetic models could provide valuable information regarding the effects of antioxidants during the auto-oxidation of biodiesel.
Author Contributions
Conceptualization, S.N.-D., A.G.C. and J.M.E.M.; methodology, S.N.-D., A.G.C. and J.M.E.M.; validation, S.N.-D., A.G.C. and J.M.E.M.; formal analysis, S.N.-D. and J.P.M.; investigation, S.N.-D., A.G.C. and J.P.M.; resources, A.G.C. and J.M.E.M.; data curation, S.N.-D., A.G.C. and J.P.M.; writing—original draft preparation, S.N.-D.; writing—review and editing, S.N.-D., A.G.C. and J.M.E.M.; visualization, A.G.C. and J.M.E.M.; supervision, J.M.E.M.; project administration, A.G.C. and J.M.E.M.; funding acquisition, J.M.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación de España (Project PID2020-112996GB-I00), Junta de Extremadura and FEDER, grant numbers GR21139, IB18028 and IB20016.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Junta de Extremadura and FEDER (GR21139 and IB18028) for their financial support. A. Guiberteau acknowledges the Financial support provided by the Ministerio de Ciencia e Innovación de España (Project PID2020-112996GB-I00 funded by MCIN/AEI/10.13039/501100011033) and Junta de Extremadura (Project IB20016) co-financed by European Funds for Regional Development. Sergio Nogales would like to thank all the members of “José Luís Sotelo” building for their assistance and support during these past years.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Costantini, V.; Morando, V.; Olk, C.; Tausch, L. Fuelling the Fire: Rethinking European Policy in Times of Energy and Climate Crises. Energies 2022, 15, 7781. [Google Scholar] [CrossRef]
- Gitelman, L.; Kozhevnikov, M. Energy Transition Manifesto: A Contribution towards the Discourse on the Specifics Amid Energy Crisis. Energies 2022, 15, 9199. [Google Scholar] [CrossRef]
- Overland, I.; Bazilian, M.; Ilimbek Uulu, T.; Vakulchuk, R.; Westphal, K. The GeGaLo index: Geopolitical gains and losses after energy transition. Energy Strategy Rev. 2019, 26, 100406. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the crossroads: A critical review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Androniceanu, A.; Sabie, O.M. Overview of Green Energy as a Real Strategic Option for Sustainable Development. Energies 2022, 15, 8573. [Google Scholar] [CrossRef]
- Ideris, F.; Zamri, M.F.M.A.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Fattah, I.M.R.; Mahlia, T.M.I. Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies 2022, 15, 7190. [Google Scholar] [CrossRef]
- Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Miranda da Silva, L.R.; Castro, A.P.; Sanders Lopes, A.A.; Chaves de Lima, R.K.; de Sousa Rios, M.A.; dos Santos, J.C.S. Sustainable Feedstocks and Challenges in Biodiesel Production: An Advanced Bibliometric Analysis. Bioengineering 2022, 9, 539. [Google Scholar] [CrossRef]
- Haq, M.U.; Jafry, A.T.; Ahmad, S.; Cheema, T.A.; Ansari, M.Q.; Abbas, N. Recent Advances in Fuel Additives and Their Spray Characteristics for Diesel-Based Blends. Energies 2022, 15, 7281. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable energy and geopolitics: A review. Renew. Sustain. Energy Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Curt, M.D.; Sánchez, G.; Fernández, J. The potential of Cynara cardunculus L. for seed oil production in a perennial cultivation system. Biomass Bioenergy 2002, 23, 33–46. [Google Scholar] [CrossRef]
- Ciancolini, A.; Alignan, M.; Pagnotta, M.A.; Vilarem, G.; Crinò, P. Selection of Italian cardoon genotypes as industrial crop for biomass and polyphenol production. Ind. Crops Prod. 2013, 51, 145–151. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Aini, N.; Bello, U.; Sya, M.; Ruslan, H. Heliyon The role of antioxidants in improving biodiesel ’ s oxidative stability, poor cold fl ow properties, and the effects of the duo on engine performance: A review. Heliyon 2022, 8, e09846. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Kumar, D.; Singh, B.; Shahbeig, H.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biodiesel antioxidants and their impact on the behavior of diesel engines: A comprehensive review. Fuel Process. Technol. 2022, 232, 107264. [Google Scholar] [CrossRef]
- Serqueira, D.S.; Pereira, J.F.S.; Squissato, A.L.; Rodrigues, M.A.; Lima, R.C.; Faria, A.M.; Richter, E.M.; Munoz, R.A.A. Oxidative stability and corrosivity of biodiesel produced from residual cooking oil exposed to copper and carbon steel under simulated storage conditions: Dual effect of antioxidants. Renew. Energy 2021, 164, 1485–1495. [Google Scholar] [CrossRef]
- Xin, J.; Imahara, H.; Saka, S. Kinetics on the oxidation of biodiesel stabilized with antioxidant. Fuel 2009, 88, 282–286. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; do Valle, C.P.; Uchoa, A.F.J.; Ramos, D.M.; da Ponte, F.A.F.; de S. Rios, M.A.; de Queiroz Malveira, J.; Pontes Silva Ricardo, N.M. Comparative study of synthetic and natural antioxidants on the oxidative stability of biodiesel from Tilapia oil. Renew. Energy 2020, 156, 1100–1106. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Yang, S.; Bi, Y. Inhibitory effect of antioxidants on biodiesel crystallization: Revealing the role of antioxidants. Fuel 2021, 297, 120782. [Google Scholar] [CrossRef]
- de Araujo, T.A.; Barbosa, A.M.J.; Viana, L.H.; Ferreira, V.S. Electroanalytical determination of TBHQ, a synthetic antioxidant, in soybean biodiesel samples. Fuel 2011, 90, 707–712. [Google Scholar] [CrossRef]
- de Araújo, T.A.; Barbosa, A.M.J.; Viana, L.H.; Ferreira, V.S. Voltammetric determination of tert-butylhydroquinone in biodiesel using a carbon paste electrode in the presence of surfactant. Colloids Surf. B Biointerfaces 2010, 79, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Tormin, T.F.; Cunha, R.R.; Richter, E.M.; Munoz, R.A.A. Fast simultaneous determination of BHA and TBHQ antioxidants in biodiesel by batch injection analysis using pulsed-amperometric detection. Talanta 2012, 99, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Caramit, R.P.; de Freitas Andrade, A.G.; Gomes de Souza, J.B.; de Araujo, T.A.; Viana, L.H.; Trindade, M.A.G.; Ferreira, V.S. A new voltammetric method for the simultaneous determination of the antioxidants TBHQ and BHA in biodiesel using multi-walled carbon nanotube screen-printed electrodes. Fuel 2013, 105, 306–313. [Google Scholar] [CrossRef]
- Goulart, L.A.; Teixeira, A.R.L.; Ramalho, D.A.; Terezo, A.J.; Castilho, M. Development of an analytical method for the determination of tert-butylhydroquinone in soybean biodiesel. Fuel 2014, 115, 126–131. [Google Scholar] [CrossRef]
- Squissato, A.L.; Richter, E.M.; Munoz, R.A.A. Voltammetric determination of copper and tert-butylhydroquinone in biodiesel: A rapid quality control protocol. Talanta 2019, 201, 433–440. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Guiberteau, A.; Encinar, J.M. Effect of tert-butylhydroquinone on biodiesel properties during extreme oxidation conditions. Fuel 2022, 310, 122339. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Kalthum Ibrahim, U.; Abdullah, S.; Haron, N. Biodiesel production from waste cooking oil: A brief review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower biodiesel: Improvement of its oxidative stability by using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. J. Am. Oil Chem. Soc. 2019, 97, 201–212. [Google Scholar] [CrossRef]
- UNE-EN ISO 12966-2:2011; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters Of Fatty Acids. ISO: Geneva, Switzerland, 2011.
- Focke, W.W.; Van Der Westhuizen, I.; Oosthuysen, X. Biodiesel oxidative stability from Rancimat data. Thermochim. Acta 2016, 633, 116–121. [Google Scholar] [CrossRef]
- UNE-EN ISO 3104/AC:1999; Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and calculation of dynamic Viscosity (ISO 3104:1994). ISO: Geneva, Switzerland, 1999.
- UNE-EN-12634:1999; Productos Petrolíferos y Lubricantes. Determinación del Índice de Ácido. Método de Valoración Potenciométrica en un Medio no Acuoso. AENOR: Madrid, Spain, 1999.
- UNE-EN 14111:2003; Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Iodine Value. AENOR: Madrid, Spain, 2003.
- Espinosa-Mansilla, A.; Muñoz de la Peña, A.; González Gómez, D. Using Univariate Linear Regression Calibration Software in the MATLAB Environment. Application to Chemistry Laboratory Practices. Chem. Educ. 2005, 10, 1–9. [Google Scholar] [CrossRef]
- UNE-EN-ISO 3675; Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method. ISO: Geneva, Switzerland, 1999.
- UNE-EN-ISO-12937:2000; Productos Petrolíferos. Determinación de Agua. Método de Karl Fischer por Valoración Culombimétrica. ISO: Geneva, Switzerland, 2001.
- UNE-EN 116:2015; Diesel and Domestic Heating Fuels—Determination of Cold Filter Plugging Point—Stepwise Cooling Bath Method. German Institute for Standardization: Berlin, Germany, 2015.
- UNE-EN 51023:1990; Petroleum Products. Determination of Flash and Fire Points. Cleveland Open Cup Method. AENOR: Madrid, Spain, 1990.
- UNE-EN 14214:2012; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Biodiesel Engines and Heating Applications—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2013.
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Jaliliantabar, F.; Ghobadian, B.; Carlucci, A.P.; Najafi, G.; Ficarella, A.; Strafella, L.; Santino, A.; De Domenico, S. Comparative evaluation of physical and chemical properties, emission and combustion characteristics of brassica, cardoon and coffee based biodiesels as fuel in a compression-ignition engine. Fuel 2018, 222, 156–174. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies 2020, 13, 5085. [Google Scholar] [CrossRef]
- Mancini, M.; Lanza Volpe, M.; Gatti, B.; Malik, Y.; Moreno, A.C.; Leskovar, D.; Cravero, V. Characterization of cardoon accessions as feedstock for biodiesel production. Fuel 2019, 235, 1287–1293. [Google Scholar] [CrossRef]
- Saluja, R.K.; Kumar, V.; Sham, R. Stability of biodiesel—A review. Renew. Sustain. Energy Rev. 2016, 62, 866–881. [Google Scholar] [CrossRef]
- Tang, H.; De Guzman, R.C.; Salley, S.O.; Ng, S.K.Y. The oxidative stability of biodiesel: Effects of FAME composition and antioxidant. Lipid Technol. 2008, 20, 249–252. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M. Cardoon biolubricant through double transesterification: Assessment of its oxidative, thermal and storage stability. Mater. Lett. 2021, 302, 130454. [Google Scholar] [CrossRef]
- Khan, E.; Ozaltin, K.; Spagnuolo, D.; Bernal-ballen, A.; Piskunov, M.V.; Di Martino, A. Biodiesel from Rapeseed and Sunflower Oil: Effect of the Transesterification Conditions and Oxidation Stability. Energies 2023, 16, 657. [Google Scholar] [CrossRef]
- Sia, C.B.; Kansedo, J.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez, M.; Aracil, J. Long term storage stability of biodiesel: Influence of feedstock, commercial additives and purification step. Fuel Process. Technol. 2013, 116, 135–141. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Cabanillas, A.G.; Romero, Á.G.; Encinar Martín, J.M. Monitoring tert-Butylhydroquinone Content and Its Effect on a Biolubricant during Oxidation. Molecules 2022, 27, 8931. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).